Abstract
Glioblastomas are highly lethal cancers that contain cellular hierarchies with self-renewing cancer stem cells that can propagate tumors in secondary transplant assays. The potential significance of cancer stem cells in cancer biology has been demonstrated by studies showing contributions to therapeutic resistance, angiogenesis, and tumor dispersal. We recently reported that physiologic oxygen levels differentially induce hypoxia inducible factor-2α (HIF2α) levels in cancer stem cells. HIF1α functioned in proliferation and survival of all cancer cells but also was activated in normal neural progenitors suggesting a potentially restricted therapeutic index while HIF2α was essential in only in cancer stem cells and was not expressed by normal neural progenitors demonstrating HIF2α is a cancer stem cell specific target. We now extend these studies to examine the role of hypoxia in regulating tumor cell plasticity. We find that hypoxia promotes the self-renewal capability of the stem and non-stem population as well as promoting a more stem-like phenotype in the non-stem population with increased neurosphere formation as well as upregulation of important stem cell factors, such as OCT4, NANOG, and c-MYC. The importance of HIF2α was further supported as forced expression of non-degradable HIF2α induced a cancer stem cell marker and augmented the tumorigenic potential of the non-stem population. This novel finding may indicate a specific role of HIF2α in promoting glioma tumorigenesis. The unexpected plasticity of the non-stem glioma population and the stem-like phenotype emphasizes the importance of developing therapeutic strategies targeting the microenvironmental influence on the tumor in addition to cancer stem cells.
Keywords: Cancer Stem Cell, Hypoxia, Hypoxia Inducible Factor, HIF2α, Glioblastoma
INTRODUCTION
Glioblastomas are the most common and lethal primary brain tumor with current therapies offering only palliation.1 Standard-of-care includes maximal surgical resection, chemoradiotherapy, and adjuvant chemotherapy with median overall survival of 12–15 months.2 Targeted therapies have largely failed in clinical trial with the notable exception of bevacizumab (Avastin), a neutralizing antibody against vascular endothelial growth factor (VEGF).3,4 Despite substantial research efforts, the mechanisms underlying the overwhelming lethality of glioblastomas remain unclear. Glioblastomas frequently recur after therapy in a nodular pattern suggesting a clonal source of tumor growth. The functional cellular heterogeneity in the neoplastic compartment of cancers has been modeled with two proposed paradigms, a stochastic or random model in which every neoplastic cell has an equal chance of acquiring genetic changes required for tumor maintenance and a hierarchical model in which different populations have distinct capacities for tumor growth based on differentiation status.5
More than 150 years ago, Virchow proposed an embryonic rest theory for the origin of cancer. In the 1920’s, a relationship between gliomas and undifferentiated cells was proposed by Bailey and Cushing. In the interval years, other researchers were able to demonstrate that leukemias could be transmitted with a single cell and later Till and McCulloch demonstrated the existence of stem cells. With the generation of new tools and markers, Dick and co-workers demonstrated that the tumor initiating cells within leukemias could be prospectively enriched.6,7 Clarke and co-workers showed that similar cells were present in a solid cancer, breast carcinoma.8 Several groups demonstrated that brain tumors (gliomas, medulloblastomas, and ependymomas) display a functional cellular heterogeneity with a potential hierarchy of differentiation.9–14 Cancer stem cells -- also known as tumor initiating cells or tumor propagating cells -- are self-renewing tumor cells that propagate tumors phenotypically similar to the parental tumor. Glioblastoma cancer stem cells share some characteristics with normal neural stem cells: expression of neural stem cell markers, capacity for self-renewal and long term proliferation, formation of neurospheres, and ability for multi-lineage differentiation into nervous system lineages (neurons, astrocytes, and oligodendrocytes).15 In contrast, solid cancer stem cells differ from normal stem cells in frequency, proliferation, aberrant expression of differentiation markers, chromosomal abnormalities, and tumor formation. The potent tumor initiation of cancer stem cells together with their radioresistance and chemoresistance suggests that these cells contribute to tumor maintenance and recurrence and targeting cancer stem cells may be important cellular targets.16–22 The cancer stem cell hypothesis has been recently validated in a breast cancer clinical trial in which patients receiving cytotoxic chemotherapy displayed an increase in breast cancer stem cell frequency in residual tumor while a targeted therapeutic with an anti-cancer stem cell therapy stabilized the cancer stem cell population.23
Normal stem cells are physically located in specific physical and functional anatomical locations or niches that are essential for maintenance of self-renewal and an undifferentiated state.24,25 We and others have found that cancer stem cells in brain tumors reside in a perivascular niche17,26 that recapitulates a relationship between normal neural stem/progenitors and the vasculature.27,28 Cancer stem cells promote the development of their own perivascular niche through the secretion of pro-angiogenic factors, prominently VEGF, but remain dependent on the niche.29 Florid angiogenesis is a defining hallmark of glioblastomas but these tumors are also characterized by regions of pseudopallisading necrosis, which are hypoxic. Oxygen tension is tightly regulated in normal physiology and is an important signal in development with low oxygen tension associated with maintenance of an undifferentiated cell state. Hypoxia promotes the self-renewal of embryonic stem (ES) cells and prevents the differentiation of neural stem cells in vitro.30–32 In vivo, hypoxia is likely to be a functional component of a normal stem cell niche as well. Hematopoietic stem cells are maintained in bone marrow, which contains a hypoxic niche.33 However, the importance of hypoxia in CSC maintenance remains largely unknown.
Cellular differentiation has been classically perceived as unidirectional and irreversible, but increasing evidence supports potential plasticity of cellular differentiation. Multiple types of fully differentiated cells have been successfully reprogrammed into pluripotent state through defined molecules, including Oct4, Sox2, Klf4 and c-Myc.34,35 Notably, Oct4, Sox2, and c-Myc contribute to the survival and self renewal of brain tumor stem cells.36–39 Cancer stem cells also display overlap of transcriptional circuitry with ES cells.40,41 Stem cell signatures may also inform cancer prognosis.42,43 In comparison to normal tissues, tumor cells have greater plasticity and this suggests that they can dramatically change their phenotypes depending on microenvironmental context.44 Several studies have shown that restricted oxygen conditions expand the fraction of cells positive for a cancer stem cell marker or the side population in established cancer cell lines or cultures from human tumors may and increase expression of stem cell markers in stem cell marker positive cells.22,45–49 These descriptive studies have left two important unresolved questions: 1) how does hypoxia regulate cancer stem cell self renewal and tumor growth and 2) can non-stem cells be converted or reprogrammed towards a cancer stem cell phenotype? These questions are not only potentially important to basic tumor biology but also to the design of anti-cancer stem cell therapies as plasticity in cell state will inform the utility of these therapies.
The Role of Hypoxia Inducible Factors in Cancer Stem Cell Self-Renewal and Tumor Growth
In response to hypoxia, cells undergo modification of the transcriptome leading to many alterations in cell biology – regulation of cell survival pathways, proliferation, motility, and secretion of paracrine factors including pro-angiogenic factors. Multiple mechanisms mediate these effects of hypoxia but prominent are the hypoxia inducible factors (HIFs), which are a heteroduplex transcription factor consisting of an α subunit that is regulated in response to hypoxia and the constitutively expressed β subunit. While HIF1α is the target of most HIF studies, HIF2α serves a non-overlapping role in both normal physiology and cancer biology, particularly renal cell carcinomas with von Hippel Lindau (VHL, an E3 ligase for HIFα) mutations. We had previously demonstrated that glioblastoma cancer stem cells preferentially stimulated tumor angiogenesis compared to non-stem glioblastoma cells through VEGF secretion and that bevacizumab specifically targeted the pro-angiogenic effects of cancer stem cells.17
To build on these observations and investigate the molecular responses of cancer stem cells to hypoxia, we interrogated the expression and function of the HIFs in cancer stem cell models.50 Under hypoxic conditions, cancer stem cells displayed a specific pattern of gene expression relative to the non-stem cells. In addition to increased VEGF, cancer stem cells specifically regulated several targets (HIF2A and transcriptional targets of HIF2α: Oct4, Glut1, and Serpin B9) under hypoxia to a greater degree than non-stem cells.50 Conventional wisdom holds that hypoxia regulates HIF1α via post-translational modification and proteosomal degradation – which we saw in our studies as well – but HIF2α appears more complex with regulation at both transcriptional and post-translational levels. Of note, different oxygen levels had different effects when we measured HIF protein levels. Cancer stem cells displayed high levels of HIF2α under oxygen levels as high as 5% (within the normal physiologic range of oxygen in the brain and most tumor areas51,52) whereas HIF1α was present in both cancer stem cell and non-stem cells only at more severely hypoxic conditions (≤1%). The regulation of HIF2α was not a general stem cell phenomenon as normal neural progenitors expressed essentially no HIF2α mRNA or protein.50 To extend these studies into the original tumors, we performed dual labeling immunofluorescence studies of human glioblastoma surgical biopsy specimens and detected stem cell markers (CD133, Olig2) and HIF2α in two locations: perivascular locations and around areas of necrosis.50 Strikingly, nearly all HIF2α positive cells expressed stem cell markers but the HIF2α positive cells marked a subpopulation of cells that expressed stem cell markers suggesting that these cells are a subpopulation in stem or progenitor cells.
We interrogated the functions of HIF1α and HIF2α in both cancer stem cells and non-stem cells through RNA interference in functional assays. Depleting either HIF1α or HIF2α inhibited cancer stem serial neurosphere generation (an indication of attenuated self renewal) and proliferation while inducing apoptosis but only HIF1α regulated non-stem glioblastoma cell proliferation and survival.50 Further, we were able to address the regulation of VEGF in these studies and found that both HIF1α and HIF2α controlled cancer stem cell VEGF levels (indeed, in results not included in the paper we found this regulation to be non-overlapping and additive) and endothelial cell proliferation whereas only HIF1α functioned in non-stem glioblastoma cells. The most important assay in defining cancer stem cells is tumor propagation. We found that selected glioblastoma stem cells with targeted HIF1α or HIF2α did not initiate tumors on xenotransplantation supporting an essential role for both HIFs. Finally, in silico analysis of the HIFs at the mRNA level in a patient database (REMBRANDT) showed that HIF2α but not HIF1α levels informed negative survival.50 Taken together, these studies show that cellular responses to restricted oxygen levels are necessary for cancer stem cell maintenance with both HIF1α and HIF2α important but HIF2α more specific and selective for cancer stem cells.
Hypoxia Reprogams Non-Stem Cancer Cells towards a Stem-like Phenotype
Based on the association of hypoxia with cancer stem cells established in our previous study and indications from other studies that bulk tumor populations may increase the number of cancer stem cell marker positive cells, we hypothesized that hypoxia may induce a stem cell phenotype in non-stem cancer cells (inducing a plasticity of differentiation). We now demonstrate that extended exposure to hypoxia can result in a phenotypic shift in the non-stem population to mirror that of the stem-like subset and promote cell growth and self-renewal. We investigated how hypoxia contributes to the cancer stem cell phenotype and whether non-stem cancer cells area able to respond to changes in the hypoxic microenvironment by altering growth and gene expression patterns.
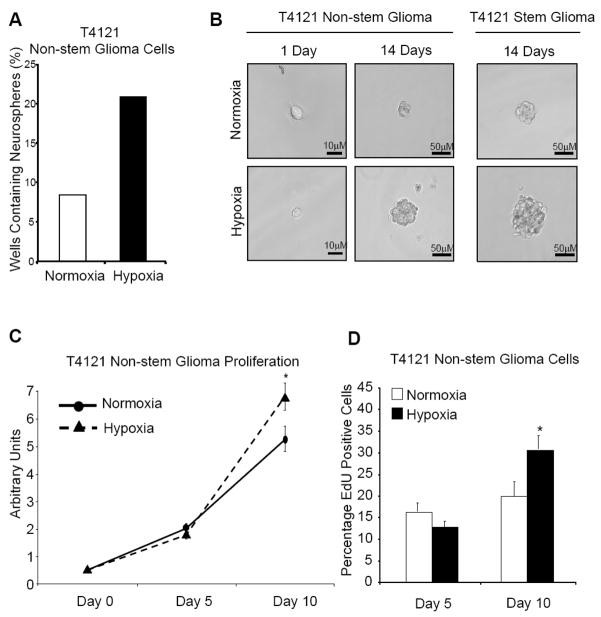
To determine the effect of hypoxia on stem and non-stem cells, we created cultures of cells enriched or depleted for glioma stem cells from patient biopsies using our previously described methodology.16,17,50 After separation of these distinct populations, we validated enrichment or depletion of the stem population by in vitro functional assays, including fluorescence-activated cell sorting (FACS) for known cancer stem cell markers such as CD133, and in vivo tumorigenic propagation. The conventional in vitro method of measuring self renewal is the neurosphere formation assay. This assay displays a cell’s ability to self renew by generating neurospheres (suspended spheroids of cells) starting with as little as a single cell. Cells were pretreated in hypoxia and sorted into low adhesion culture plates. We found that non-stem cells cultured under hypoxia were able to form neurospheres at twice the rate of control cells in normoxia (Fig. 1A). Additionally, when closely examining the spheroids, non-stem cells under hypoxia formed larger neurospheres compared to control cells grown in normoxia (Fig. 1B). This observation was true for both glioma cell populations. This suggests that a hypoxic microenvironment plays a critical role in promoting and maintaining the ability of stem-like cells to self-renew and can even confer self-renewal capability to the non-stem population. Self-renewal is closely tied to proliferation. We next investigated how cellular proliferation may play a role in this neurosphere forming phenotype and how hypoxia alters glioma cell proliferation.
Figure 1. Hypoxia enhances neurosphere formation and cell proliferation.
(A) Glioma non-stem and glioma stem cells were harvested from T4121 primary human patient specimen. Cells were plated in a low attachment 24 well plate at a density of 10 cells/well with 2 plates per condition for a total of 48 wells. Immediately following plating, the cells were subjected to hypoxia at 2% oxygen or 21% normoxia as negative control. At 9 days 24-well plates were analyzed for wells containing neurospheres. Wells containing at least one sphere were counted as positive. Values were recorded as a percentage of positive wells divided by total wells. (B) Follow 1 day and 14 days of treatment, representative phase contrast images were taken at 20× magnification on a Leica wide field microscope. Images of the glioma stem cells at the single cell level were not included due to space considerations. (C) T4121 Glioma non-stem and glioma stem cells were plated into 5 wells of a 96 well plate at a density of 500 cells/well. Cells were cultured at 2% or 21% oxygen immediately after plating for 0, 5, or 10 days per condition. At each time point, one 96 well plate per condition was collected and total ATP content was measured by Cell Titer Glo Assay (Promega). The subsequent luminescence was measured by illuminometer following Promega suggested protocol. *, p < 0.05 with Student’s t-test comparison of cells grown under hypoxia to cells grown in normoxia. Bars show standard error of the mean (SEM) for two separate reactions. (D) Following 5 or 10 days treatment, T4121 non-stem glioma cells were plated on glass cover slips and allowed to recover overnight in their respective treatment culture. The following day cells were dosed with 10 μM EdU (Invitrogen) for 1 hour. Staining and secondary antibody incubation was performed according to suggested Invitrogen protocol and reagents. After staining DNA by application of Hoescht 33342 dye, samples were mounted onto glass slides. Ten representative fields for each condition at Day 5 and Day 10 were analyzed by Image J software for number of EdU positive cells divided by the total cell number. *, p < 0.01 with Student’s t-test comparison of hypoxia cultured cells to cells grown under normoxia.
T4121 glioma stem and non-stem cells were pretreated in hypoxia for several days and then sorted onto 96-well culture plates. After long-term culture at normoxic or hypoxic conditions, distinct growth advantages were noted (Fig. 1). Cells grown in hypoxia had increased growth over long term observation compared to cells grown in normoxia, suggesting a cellular response sensitive to changes in the microenvironment. Interestingly, altered cell growth was observed in both the stem and non-stem population, indicating a conserved response mechanism between the two populations. We quantified the percentage of actively proliferating cells during hypoxic culture. This differential growth response was quantified by EdU labeling (Fig. 1D). The labeling showed that after 5 days, cells growing under hypoxia had slightly fewer proliferating cells than cells growing in normoxia, as was expected from our previous cell titer data. At day 10 the number of actively proliferating cells was significantly higher under hypoxia compared to cells grown under normoxia. Thus, in hypoxic conditions non-stem glioma cells acquire self-renewal and long term proliferative potential.
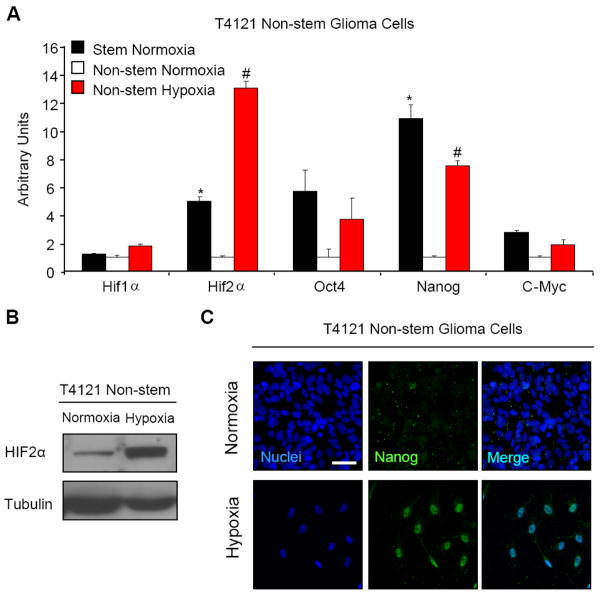
To elucidate potential mechanisms underlying these observations, we measured the change in gene expression in the non-stem population following long term culture at normoxia or hypoxia. Using semi-quantitative real time PCR, we measured the levels of several important genes related to stem cell function. Of the stem genes measured, OCT4, NANOG, and c-MYC displayed significant and consistent increase in T4121 non-stem glioma cells in addition to several other patient biopsy cell lines treated with hypoxia (Fig. 2A and data not shown) to basal levels in the matched cancer stem cell populations at normoxic conditions. This may indicate a role of stem gene expression in promoting growth and survival when experiencing microenvironment (hypoxic) stress. This finding is also important due to the known functions of OCT4, NANOG, and c-MYC in embryonic stem cells. c-MYC has been shown to be important in controlling a wide variety of stem cell functions such as proliferation, self-renewal, and apoptosis. It is also known as a potent oncogene and is known to be an important factor in the creation of induced pluriportent stem cells. NANOG is known to work in complex with OCT4 and plays a major role in maintaining pluripotency in embryonic stem cells.53,54 This finding is novel as it has yet to be shown that NANOG is downstream target of hypoxia. OCT4 is also required for maintenance of self-renewal in stem cells and is tightly regulated in order to maintain appropriate levels of self-renewal in the embryo.55 Additionally, recent studies have shown that OCT4 is a downstream target of HIF2α.56 This study shows that the non-stem cells are able to upregulate stem genes in a similar manner to the stem-like population. We confirmed the expression of HIF2α of the non-stem cells under long-term hypoxia by immunoblotting (Fig. 2B) and Nanog expression by immunofluoresence (Fig. 2C). These data confirm that hypoxia can induce plasticity in cellular differentiation and reprogram non-stem cells towards a more stem cell-like state.
Figure 2. Exposure to long-term hypoxia increases mRNA transcript levels of known stem genes.
(A) T4121 stem and non-stem glioma cells were cultured in hypoxia at 1% oxygen for 10 days. After treatment, total RNA was harvested by RNAeasy Kit (Qiagen) and used to generate cDNA. Semi-quantitative PCR was performed using an Applied Biosystems 7900 PCR Cycler. *, p < 0.05 with ANOVA comparison of transcript levels of glioma stem cell grown in normoxia to transcript levels of non-stem glioma cells grown in normoxia. #, p < 0.05 with ANOVA comparison of transcript levels of non-stem glioma cells grown in 1% hypoxia to transcript levels of non-stem glioma cells grown in normoxia. Bars show standard error of the mean (SEM) for two separate reactions. (B) T4121 non-stem glioma cells were treated by chemical hypoxia using 100 μM deferoxamine for 48 hours. Cell lysates were collected using Universal Lysis Buffer and immunoblotted. Left lane is control T4121 non-stem glioma cells treated with equal volume of water only. (C) T4121 stem and non-stem cells were cultured in hypoxia at 1% oxygen or normoxia at 21% oxygen in 8-well chamber slides (Nunc) for 10 days. Cells were fixed and incubated in primary and secondary antibodies according to protocol described in Experimental Methods. Images were taken on a Leica wide field fluorescent microscope and are representative fields. White scale bars denote 25 μM.
HIF2α Promotes a Cancer Stem Cell State
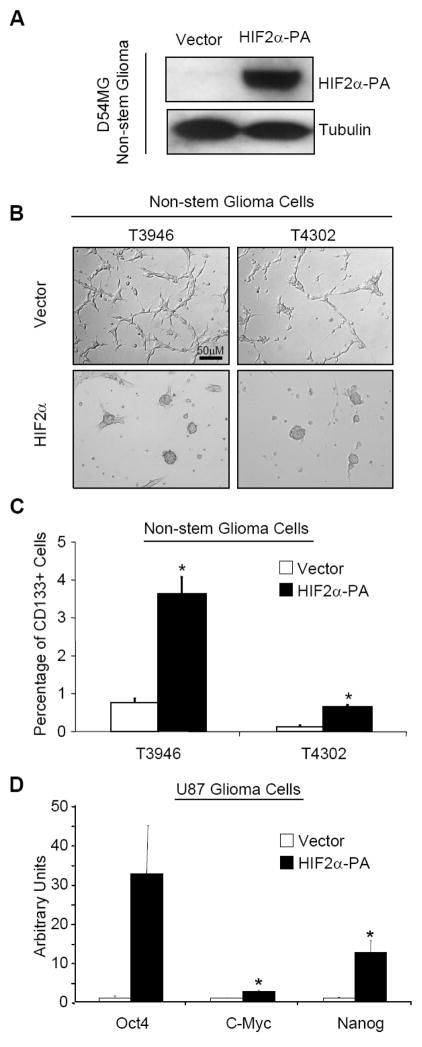
We previously demonstrated that HIF2α is necessary to maintain a cancer stem cell phenotype and HIF1α is expressed in all hypoxic tumor cells. We therefore hypothesized that HIF2α may be sufficient to induce a cancer stem cell phenotype in non-stem cancer cells. To determine the specific role of HIF2α in cellular reprogramming in cancer, we expressed HIF2α in normoxic conditions to avoid other hypoxia induced mechanisms. We utilized two complementary strategies: the first being delivery of ectopic HIF2α and the second being use of a non-degradable HIF2α mutant (referred to as HIF2α-PA, a kind gift of William Kaelin). The latter HIF2α construct contains two proline residues mutated to alanine.57 This mutation prevents prolyl hydroxylase from hydroxylating the HIF2α protein and targeting it for proteosomal degradation. The non-degradable form of the protein was transduced into three non-stem cell populations of patient biopsy lines: D456MG, T3946, and T4302. To demonstrate that the construct was functional we harvested lysates from transduced D456MG cells cultured under normoxia and immunoblotted for HIF2α (Fig. 3A). The cells showed strong protein expression of HIF2α at normoxic conditions, indicating successful delivery of stable HIF2α to the cells. T3946 and T4302 non-stem glioma cells were transduced with ectopic HIF2α and cultured for 7 days in serum-free media. Under standard conditions, glioma non-stem cells grow as adherent monolayers (Fig. 3B, “Vector”) while HIF2α transduction induced morphological changes (Fig. 3B, “HIF2α”) similar to the detached spheroids of glioma stem cells or hypoxic non-stem cells. Although imperfect58, CD133 has been employed as a cell surface marker to enrich for brain tumor stem cells.11,12,16,17,50 We quantified the CD133 positive fractions of T3946 and T4302 cultures transduced with either vector control or HIF2α and found that HIF2α induced an increase in the CD133 positive fraction (Fig. 4C). In data not shown, we find that HIF2α induces only a small direct increase in CD133 mRNA suggesting that the primary effect of HIF2α is not direct transcriptional control but rather a conversion to a more cancer stem cell state. Next, we measured levels of stem genes with HIF2α expression that were preferentially upregulated under hypoxia in the studies above. T4121 non-stem glioma cells were transduced with non-degradable HIF2α and 72 hours later we quantified the transcript levels of target stem genes we have previously showed to be increased under hypoxia using semi-quantitative PCR. In HIF2α expressing cells, OCT4, NANOG, and c-MYC transcripts were significantly upregulated (Fig. 3D). These data support our hypothesis that HIF2α is sufficient to induce a cancer stem cell phenotype.
Figure 3. Ectopic expression of non-degradable HIF2α causes morphological and phenotypic changes in non-stem glioma cells.
(A) D54MG glioma cells were transfected with non-degradable HIF2α or empty pBabe vector control. Cells were allowed to recover for 3 days then lysates were harvested by Universal Lysis Buffer. Lysates were immunoblotted for the presence of HIF2α and NDRG1, a known HIF2α target. Left lane is negative control cells containing empty pBbabe vector backbone. (B) T3946 and T4302 nonstem glioma cells were transfected with non-degradable HIF2α. 2 days following transfection culture media was changed from serum containing DMEM to Neurobasal plus growth factors (detailed in Methods). Phase contrast images were taken 7 days after media change. (C) Following transfection and culture conditions outlined in (B), cells were analyzed for presence of cell surface marker CD133 (Prominin-1). White bars denote cells transfected with empty pBabe vector backbone. Fold increase was calculated by cells positive for CD133 divided by total number of viable cells gated. *, p < 0.05 with Student’s t-test comparison of cells bearing the HIF2α-PA construct against cells transfected with vector alone. Bars show standard error of the mean (SEM) for three separate measurements. (D) U87MG glioma cells transfected with non-degradable HIF2α were harvested for total RNA after puromycin selection. Semi-quantitative PCR was used to measure mRNA transcript levels of OCT4, NANOG, and c-MYC. White bars denote cells transfected with vector alone while black bars show cells transfected with HIF2α-PA. PCR data was normalized to Actin for each measurement. *, p < 0.05 with Student’s t-test comparison of cells bearing the HIF2α-PA construct against cells transfected with vector alone. Bars show standard error of the mean (SEM) for two separate reactions.
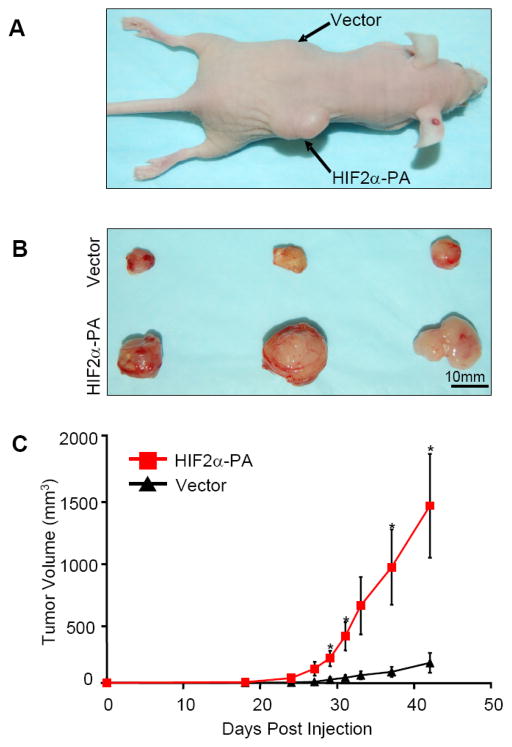
Figure 4. Overexpression of HIF2α in glioma non-stem cells increased tumorigenic capacity.
(A) Glioma non-stem cells isolated from D456MG glioma xenograft were transfected with vector alone or HIF2α constructs. 100,000 cells were injected into nude mouse subcutaneously. In each mouse, vector control cells were injected into the left side while HIF2α group were injected into the other side of the same mouse. Image shows representative mouse with apparent tumor from glioma non-stem cells that overexpress HIF2α but not vector alone. (B) Tumor-bearing mice were sacrificed at day 43 and tumors were excised and measured. (C) Tumors grew much faster from glioma non-stem cells with HIF2α overexpression. The growth of tumors were monitored and measured at a daily rate. *, p < 0.05 with Student’s t-test comparing cells bearing the HIF2α-PA construct against cells bearing vector alone. Error bars show standard error of the mean (SEM) for three separate measurements.
To determine the contribution of HIF2α in inducing a cancer stem cell phenotype in vivo, non-stem D456MG cells were transduced with either vector control or non-degradable HIF2α and grown in serum-free media for 7 days. To optimally control in vivo conditions, we implanted vector control or non-degradable HIF2α in contralateral flanks of immunocompromised mice. D456MG cells expressing non-degradable HIF2α formed significantly larger tumors than vector control (Fig. 4). In sum, these data show that HIF2α alone can reprogram differentiated, non-stem cancer cells towards an undifferentiated state.
DISCUSSION
Glioblastoma is among the most lethal cancers and current treatment modalities are limited in increasing the lifespan of patients.1,2 We and others have shown that cancer stem cells potentially contribute to glioblastoma treatment failure as cancer stem cells are resistant to radiotherapy and chemotherapy.16,20 The limited efficacy of cytotoxic therapies in most advanced cancers has led to the development of many novel molecularly targeted therapies. The targets of these therapies are varied but a common biologic target of the more successful of these therapies is angiogenesis.59 The neutralizing VEGF antibody bevacizumab has been approved for the treatment of several solid cancers, including recurrent glioblastoma. The mechanism by which bevacizumab functions has been an unresolved area of active investigation but recent work from our laboratory and others suggest that bevacizumab may target cancer stem cell induced angiogenesis17 and disrupt the functional perivascular niche in which cancer stem cells reside.26 These studies are important as the clinical application of anti-angiogenic therapies remains to be optimized and methods of resistance are being identified. For example, patients with recurrent glioblastomas treated with a low molecular weight VEGF receptor antagonist, cediranib (AZD2171), displayed increases in bFGF, the SDF1α chemokine, and viable circulating endothelial cells.60 As bFGF and SDF1α regulate cancer stem cell proliferation, maintenance, and motility, these escape mechanisms may indicate a contribution of cancer stem cells to resistance to yet another cancer treatment. Indeed, anti-angiogenic therapy may induce invasion and metastasis61,62, which are strongly linked to the cancer stem cell phenotype.63
Hypoxia is one of the most potent driving forces for tumor angiogenesis. Hypoxia has also been linked to tumor progression, metastasis, invasion, and therapeutic resistance in cancer biology. As these characteristics are enriched in cancer stem cells, it is not surprising that hypoxia may regulate cancer stem cells. Indeed, several studies have shown that hypoxia increases the number of cells that express cancer stem cell markers in bulk populations22,45–49, but these studies used bulk cells limiting the ability to distinguish effects of hypoxia on neoplastic subpopulations. Two potential explanations are evident: an increase in cancer stem cell proliferation or a conversion of non-stem cancer cells to a cancer stem cell phenotype. In our current studies, we see evidence of both effects – hypoxia augments maintenance of cancer stem cells and reprograms non-stem cells towards a more stem cell behavior. These results potentially inform the development of anti-cancer stem cell therapies. Targeting only cancer stem cells may be insufficient to improve patient outcomes because the non-stem cells may acquire stem cell characteristics due to effects of the microenvironment. Indeed, simultaneous targeting the tumor microenvironment may be essential for efficacy of cancer stem cell therapies.
These studies also suggest that standard culture conditions may fail to maintain the cellular heterogeneity present in parental tumors. Previous research has already shown that cancer cells grown on extracellular matrix display striking differences compared to plastic.64 Recent studies have now shown that similar approaches may improve maintenance of cancer stem cells.65,66 Serum has also been used in culture but serum induces differentiation and irreversibly alters gene expression of cancer cells.67 Our work and that of others suggest that cancer cells should be maintained under restricted oxygen conditions22,45–50 much like embryonic stem cell growth and in vitro fertilization can be facilitated with oxygen levels less than room air.
Hypoxia alters the expression levels of hundreds or thousands of genes so it is unlikely that a single mechanism will explain the cellular responses of cancer stem cells or reprogramming effects of hypoxia. However, we recently described essential roles of the hypoxia inducible factors (HIFs) in promoting tumorigenesis.50 While HIF1α was expressed in all neoplastic cells and normal neural progenitors suggesting a potentially limited therapeutic index, HIF2α functioned specifically in cancer stem cells without expression in the normal progenitors. In support, a recent study of neuroblastoma biopsy specimens showed that HIF2α marked a perivascular location with cells that express immature neural crest markers. Thus, HIF2α inhibitors may be a relatively specific anti-cancer stem cell therapy for nervous system cancers. Small molecule inhibitors of HIF2α translation are under development68 and may have utility for cancer stem cells.
In the current study, we have also shown that hypoxia induces the expression of key stem cell genes, specifically Nanog, Oct4, and c-Myc, in non-stem cancer cells. These genes have gained attention as Yamanaka and co-workers demonstrated that these factors with Sox2 reprogram fully differentiated fibroblasts into pluripotent stem cells (i.e. induced pluripotent stem cells, iPSCs).34,35 While Nanog has yet to be fully investigated in glioblastomas, Oct4 has been shown to be expressed by human gliomas and induces colony formation37 while we and the DePinho laboratory have shown that c-Myc is important in glioblastoma stem cell maintenance.38,39 Oct4 is a specific target of HIF2α in cell lines56, which we were able to confirm in cancer stem cells.50 HIF2α interacts with c-Myc by binding and stabilizing c-Myc containing complexes while HIF1α disrupts these complexes.69 Therefore, HIF2α may directly regulate core stem cell pathways that are essential in cancer stem cell maintenance.
There are a number of other potential molecular targets of hypoxia with direct relevance to cancer stem cells. Hypoxia requires Notch signaling to maintain an undifferentiated state and prevent neuronal and myogenic differentiation in normal cells70 and similar Notch dependence has been demonstrated in hypoxia-induced epithelial-mesenchymal transition (EMT) and invasion.71 Hypoxia and the HIFs may also attenuate the ability of bone morphogenic proteins (BMPs) to induce differentiation of gliomas.72 BMPs regulate cell fate decisions in the central nervous system and regulate brain tumor stem cell differentiation and proliferation.73
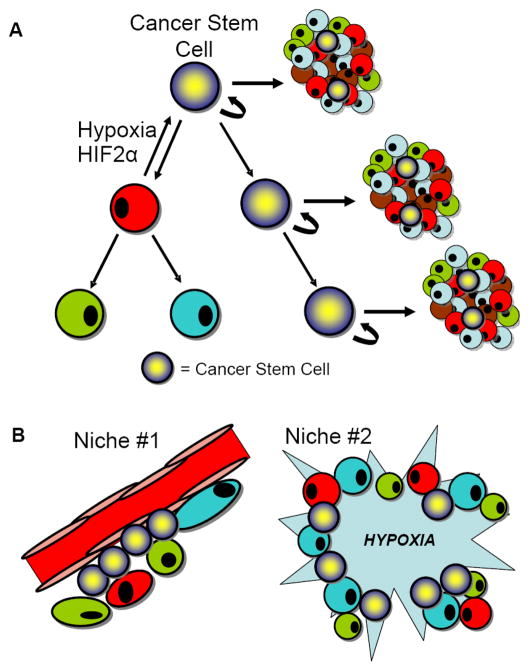
In conclusion, recent studies and these current data strongly support a novel significance in hypoxia and the hypoxia inducible factors in cancer biology through regulation of cellular differentiation and the cancer stem cell phenotype. We must now revise the hierarchical model to include a bidirectional relationship with differentiation potential as hypoxia and HIF2α may induce a cancer stem cell phenotype (Fig. 5A). It is also clear that cancer stem cells can reside within at least two niches within the same tumor type, a perivascular niche and a hypoxic niche (Fig. 5B). Integrating the cancer stem cell hypothesis into novel therapeutic paradigms will require incorporation of this added complexity.
Figure 5. The Role of Hypoxia in the Cancer Stem Cell Hypothesis.
(A) The hierarchical model of tumor heterogeneity includes plasticity of cellular differentiation based on the effects of the tumor microenvironment. (B) Cancer stem cells reside in two niches: (1) the perivascular niche and (2) areas of hypoxia.
EXPERIMENTAL PROCEDURES
Isolation of Glioma Stem Cells and Non-stem Glioma Cells
Matched cultures enriched or depleted for GSCs were isolated from primary human brain tumor patient specimens or human glioblastoma xenografts as previously described in accordance with a Duke University Institutional Review Board approved protocol concurrent with national regulatory standards with patients signing informed consent.16,17,50 Briefly, tumors were disaggregated by Papain Dissociation System (Miltenyi) and filtered by 70 μm cell strainer according to the manufacturer’s instructions. Cells were then cultured in stem cell culture medium supplemented as detailed below for at least four to five hours to recover surface antigens. Cells were then labeled with APC- or PE-conjugated CD133 antibody, and sorted by fluorescence-activated cell sorting (FACS). Alternatively, cells were separated by magnetic sorting column using microbead-conjugated CD133 antibodies. CD133 positive cells were designated as GSCs whereas CD133 negative cells utilized as non-stem glioma cells.
Tissue Culture and Hypoxia Induction
GSCs were cultured in Neurobasal media with B27 (without Vitamin A, Invitrogen), basic fibroblast growth factor (10 ng/ml) and epidermal growth factor (10 ng/ml). After trypsinizing, non-stem tumor cells were cultured overnight in Dulbecco’s minimal essential media (DMEM) and 10% serum to allow cell attachment and survival. Then, in some cases, DMEM medium was removed and the cells cultured in supplemental Neuralbasal medium in order for experiments to be performed in identical media. In order to induce hypoxia, cells were cultured in multi-gas chambers (Sanyo). Nitrogen gas was supplied to the chambers in order to compensate for the reduced percentage of oxygen. Alternatively, cells were treated by 200 μM hypoxia-mimetic deferoxamine mesylate (DFX, Sigma).
Neurosphere Culture Assay
T4121 Stem and Non-stem glioma cells were cultured in normoxia (21% oxygen) or hypoxia (2% oxygen) in Neurobasal media with B27 plus growth factors (stem cells) or DMEM plus 10% serum (non-stem cells) for 10 days prior to plating. At day 10 cells were trypsinized and sorted by trypan blue counting into 24 well low adhesion plates containing Neurobasal media with B27 plus growth factors at a density of 10 cells/well. Wells were observed over 14 days. Wells were counted as positive for neurosphere formation if they contained at least spheroid.
Cell Titer Assay
T4121 non-stem glioma cells were trypsinized and plated into 96 well plates contained DMEM plus 10% serum at a density of 500 cells/well. They were cultured in 2% or 21% oxygen for 0, 5, or 10 days. At each time point total ATP per well was measured by Cell Titer Glo kit (Promega) and subsequent luminescence was read by illuminometer (PerkinElmer).
Immunofluorescent Imaging
Cells were fixed in 4% paraformaldehyde (Sigma) for 15 minutes at room temperature. After fixation, cells were washed with PBS. Blocking buffer containing normal goat serum plus Triton X-100 in PBS were added to the washed cells for 30 minutes at room temperature. Following blocking, primary antibody, NANOG (Santa Cruz), was added at appropriate dilutions as noted by the manufacturer. Antibodies were incubated at 4° Celsius overnight. The next day secondary antibodies conjugated to fluorescent isotopes (Alexa Fluor, Invitrogen) were added to cells at appropriate dilutions. After incubation in secondary antibody, Hoescht 3342 nuclear stain was added to the cells for 5 minutes.
EdU Labeling and Imaging
Following 5 or 10 days culture in hypoxia, T4121 non-stem glioma cells were trypsinized and plated on glass cover slips at a density of 10,000 or 20,000 cells per slip. After 24 hours recovery in hypoxia or normoxia, cells were dosed for 1 hour with 10 μM of EdU (Invitrogen). Following dosing cells were fixed by 4% paraformaldehyde (PFA) and blocked using 3% BSA in PBS. The slides were then stained using fluorescent secondary antibody directed against Edu, and stained with Hoescht 33342 nuclear dye. All images were taken on a Leica wide field fluorescent microscope. Ten representative fields for each condition were quantified for number of Edu positive cells divided by total number of cells using Image J software.
Semi-Quantitative PCR
PCR was performed on cDNA generated by Superscript III reverse transcriptase (Invitrogen). Total RNA was harvested from cells using RNAeasy Kit (Qiagen). Primers used were as follows: OCT4 forward 5′-GAGAACCGAGTGAGAGGCAACC-3′ and reverse 5′-CATAGTCGCTGCTTGATCGCTTG-3′. NANOG forward 5 ′-AATACCTCAGCCTCCAGCAGATG-3′ and reverse 5′-TGCGTCACACCATTGCTATTCTTC-3′. c-MYC forward 5′-TCAAGAGGCGAACACACAAC-3′ and reverse 5′-GGCCTTTTCATTGTTTTCCA-3′. HIF1α forward 5′-TCCATGTGACCATGAGGAAA-3′ and reverse 5′-CCAAGCAGGTCATAGGTGGT-3′. HIF2α forward 5′-CCACCAGCTTCACTCTCTCC-3′ and reverse 5′-TCAGAAAAAGGCCACTGCTT-3′. All data was normalized to Actin or GAPDH transcript levels.
In Vivo Tumor Formation Assays
Intracranial or subcutaneous transplantation of GSCs into nude mice was performed as described in accordance with a Cleveland Clinic Foundation Institutional Animal Care and Use Committee approved protocol concurrent with national regulatory standards.50 Briefly, 72 hours after hypoxic treatment, cells were counted and certain number cells were implanted into the right frontal lobes of athymic BALB/c nu/nu mice. In some cases, 48 hours after infection, 1 mg/ml puromycin was applied to select infected cells for 48 hours before counting. Mice were maintained up to 25 weeks or until the development of neurological signs. Brains of euthanized mice were collected, fixed in 4% Paraformaldehyde (PFA), and paraffin embedded.
Statistical Analysis
Descriptive statistical analysis was generated for all repeated quantitative data with inclusion of means and standard error. Significance was tested by one-way analysis of variance (ANOVA) or Student’s t-Test using SigmaStat 3.5 (Chicago, IL).
Acknowledgments
We thank Dr. Mike Cook, Dr. Beth Harvat, Cathy Shemo, and Sage O’Bryant for assistance with flow cytometry and the members of the Rich laboratory for technical assistance and critical review of the manuscript. Work in the Rich laboratory is supported by Financial support was provided by the Childhood Brain Tumor Foundation, the Pediatric Brain Tumor Foundation of the United States, Accelerate Brain Cancer Cure, Alexander and Margaret Stewart Trust, Brain Tumor Society, Goldhirsh Foundation, Duke Comprehensive Cancer Center Stem Cell Initiative Grant, and NIH grants NS047409, NS054276, CA112958, and CA116659. J.N.R. is a Damon Runyon-Lilly Clinical Investigator supported by the Damon Runyon Cancer Research Foundation and a Sidney Kimmel Foundation for Cancer Research Scholar. A.H. is supported by the National Brain Tumor Society. J.D.L. is supported by an American Brain Tumor Association Basic Research Fellowship. The Duke University Brain Tumor Tissue Bank is supported by the Duke University Brain Cancer SPORE, and we thank D. Satterfield, L. Ehinger, J. Funkhouser, and J. Faison for technical assistance. Studies were supported by the Cleveland Clinic Foundation Tissue Procurement Service, and we thank S. Staugatis, R. Weil, and M. McGraw for their assistance.
References
- 1.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Dowell JM, Reardon DA, Quinn JA, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–9. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 4.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 5.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 6.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet D, Dick J. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 10.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–83. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 12.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 13.Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–35. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392–400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 15.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–36. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 16.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 17.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, et al. Stem Cell-like Glioma Cells Promote Tumor Angiogenesis through Vascular Endothelial Growth Factor. Cancer Res. 2006;66:7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 18.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–48. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wulf GG, Wang RY, Kuehnle I, Weidner D, Marini F, Brenner MK, et al. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–73. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- 20.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Blazek ER, Foutch JL, Maki G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133- cells, and the CD133+ sector is enlarged by hypoxia. Int J Radiat Oncol Biol Phys. 2007;67:1–5. doi: 10.1016/j.ijrobp.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 24.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 25.Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–6. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 26.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang ZJ, Wechsler-Reya RJ. Hit ‘em where they live: targeting the cancer stem cell niche. Cancer Cell. 2007;11:3–5. doi: 10.1016/j.ccr.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Silván U, Dìez-Torre A, Arluzea J, Andrade R, Silió M, Aréchaga J. Hypoxia and pluripotency in embryonic and embryonal carcinoma stem cell biology. Differentiation. 2009 doi: 10.1016/j.diff.2009.06.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Clarke L, van der Kooy D. Low Oxygen Enhances Primitive and Definitive Neural Stem Cell Colony Formation By Inhibiting Distinct Cell Death Pathways. Stem Cells. 2009 doi: 10.1002/stem.96. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–96. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–6. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, et al. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40–8. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 37.Du Z, Jia D, Liu S, Wang F, Li G, Zhang Y, et al. Oct4 is expressed in human gliomas and promotes colony formation in glioma cells. Glia. 2009;57:724–33. doi: 10.1002/glia.20800. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Wang H, Li Z, Wu Q, Lathia JD, McLendon RE, et al. c-Myc is required for maintenance of glioma cancer stem cells. PLoS One. 2008;3:e3769. doi: 10.1371/journal.pone.0003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–33. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu G, Kim J, Xu Q, Leng Y, Orkin SH, Elledge SJ. A genome-wide RNAi screen identifies a new transcriptional module required for self-renewal. Genes Dev. 2009;23:837–48. doi: 10.1101/gad.1769609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–44. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–26. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bissell MJ, Kenny PA, Radisky DC. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harb Symp Quant Biol. 2005;70:343–56. doi: 10.1101/sqb.2005.70.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jögi A, Øra I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, et al. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci U S A. 2002;99:7021–6. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tavaluc RT, Hart LS, Dicker DT, El-Deiry WS. Effects of low confluency, serum starvation and hypoxia on the side population of cancer cell lines. Cell Cycle. 2007;6:2554–62. doi: 10.4161/cc.6.20.4911. [DOI] [PubMed] [Google Scholar]
- 47.Giuntoli S, Rovida E, Gozzini A, Barbetti V, Cipolleschi MG, Olivotto M, et al. Severe hypoxia defines heterogeneity and selects highly immature progenitors within clonal erythroleukemia cells. Stem Cells. 2007;25:1119–25. doi: 10.1634/stemcells.2006-0637. [DOI] [PubMed] [Google Scholar]
- 48.Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26:1818–30. doi: 10.1634/stemcells.2007-0724. [DOI] [PubMed] [Google Scholar]
- 49.McCord AM, Jamal M, Shankavarum UT, Lang FF, Camphausen K, Tofilon PJ. Physiologic oxygen concentration enhances the stem-like properties of CD133+ human glioblastoma cells in vitro. Mol Cancer Res. 2009;7:489–97. doi: 10.1158/1541-7786.MCR-08-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–13. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 52.Cárdenas-Navia LI, Mace D, Richardson RA, Wilson DF, Shan S, Dewhirst MW. The pervasive presence of fluctuating oxygenation in tumors. Cancer Res. 2008;68:5812–9. doi: 10.1158/0008-5472.CAN-07-6387. [DOI] [PubMed] [Google Scholar]
- 53.Pan GJ, Pei DQ. Identification of two distinct transactivation domains in the pluripotency sustaining factor nanog. Cell Res. 2003;13:499–502. doi: 10.1038/sj.cr.7290193. [DOI] [PubMed] [Google Scholar]
- 54.Chambers I, Tomlinson SR. The transcriptional foundation of pluripotency. Development. 2009;136:2311–22. doi: 10.1242/dev.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niwa H, Masui S, Chambers I, Smith AG, Miyazaki J. Phenotypic complementation establishes requirements for specific POU domain and generic transactivation function of Oct-3/4 in embryonic stem cells. Mol Cell Biol. 2002;22:1526–36. doi: 10.1128/mcb.22.5.1526-1536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–70. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan Q, Bartz S, Mao M, Li L, Kaelin WG., Jr The hypoxia-inducible factor 2alpha N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Mol Cell Biol. 2007;27:2092–102. doi: 10.1128/MCB.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–5. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 59.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–22. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 60.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fael Al-Mayhani TM, Ball SL, Zhao JW, Fawcett J, Ichimura K, Collins PV, et al. An efficient method for derivation and propagation of glioblastoma cell lines that conserves the molecular profile of their original tumours. J Neurosci Methods. 2009;176:192–9. doi: 10.1016/j.jneumeth.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 66.Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–80. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 67.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 68.Zimmer M, Ebert BL, Neil C, Brenner K, Papaioannou I, Melas A, et al. Small-molecule inhibitors of HIF-2a translation link its 5′UTR iron-responsive element to oxygen sensing. Mol Cell. 2008;32:838–48. doi: 10.1016/j.molcel.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–47. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–28. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 71.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci U S A. 2008;105:6392–7. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pistollato F, Chen HL, Rood BR, Zhang HZ, D’Avella D, Denaro L, et al. Hypoxia and HIF1alpha repress the differentiative effects of BMPs in high-grade glioma. Stem Cells. 2009;27:7–17. doi: 10.1634/stemcells.2008-0402. [DOI] [PubMed] [Google Scholar]
- 73.Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–5. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]