Abstract
Purpose
Replication-selective oncolytic adenoviruses are a promising class of tumor-targeting agents with proven safety in hundreds of patients. However, clinical responses have been limited and viral mutants with higher potency are needed. Here we report on the generation of a novel set of mutants with improved efficacy in prostate and pancreatic carcinoma models. Currently no curative treatments are available for late stage metastatic prostate or rapidly progressing pancreatic cancers.
Experimental design
Ad5 mutants were created with deletions in the E1ACR2-region for tumor selectivity and/or the E1B19K-gene for attenuated replication in vivo; all constructs retain the E3-genes intact. Cell killing efficacy, replication and cytotoxicity in combination with chemotherapeutics were investigated in normal cells (PrEC, NHBE), seven carcinoma cell lines, and human (PC3, DU145) and murine (TRAMPC, CMT-64, CMT-93) tumor models in vivo.
Results
The double-deleted AdΔΔ (ΔE1ACR2 and ΔE1B19K) mutant had high cell killing activity in prostate, pancreatic and lung carcinomas. Replication was similar to wild-type in all tumor cells and was attenuated in normal cells to levels less than the single-deleted AdΔCR2 mutant. AdΔΔ combined with the chemotherapeutics docetaxel and mitoxantrone resulted in synergistically enhanced cell killing and greatly improved anti-tumor efficacy in prostate xenografts in vivo. In murine immunocompetent in vivo models efficacy was greater for mutants with the E3B-genes intact even in the absence of viral replication indicating attenuated macrophage-dependent clearance.
Conclusions
These data suggest that the novel oncolytic mutant AdΔΔ is a promising candidate for targeting of solid tumors specifically in combination with chemotherapeutics.
Keywords: adenovirus, oncolytic, E1ACR2, E1B19K, prostate, pancreas
INTRODUCTION
Replication-selective oncolytic adenoviruses represent a promising anti-cancer approach with proven efficacy in cancer cells and tumor xenografts in vivo without cross-resistance to conventional clinical therapies (1, 2). Numerous mutants have been constructed to target tumors specifically, enabling viral gene expression and amplification at the tumor site with minimal toxicity to normal cells (1, 3). Safety has been demonstrated in clinical trials with various adenoviral mutants in hundreds of patients (4). The majority of clinical trials evaluated mutants designed to complement the dysfunctional p53 activity frequently present in human tumours. The first clinical application of this group of biologicals was dl1520 (Onyx-015; ΔE1B55K and ΔE3B) (5-7). Recently a similar mutant, H101, was licensed for anti-cancer therapy in China (Shanghai Sunway Biotech, China) (8). While tumor selectivity was demonstrated for both mutants efficacy was only reported in combination with cytotoxic drugs (3, 8, 9). It was later demonstrated that essential functions of the deleted E1B55K- and E3B-genes (such as late viral RNA transport and protection against host-immune defence respectively) contributed to the attenuated efficacy of these viruses (7, 10).
Recently, oncolytic mutants have been constructed that retain functions essential for the viral life cycle by deletion of smaller gene-regions for example, the dl922-947 virus and mutants with similar CR2-deletions (Δ24) (11-13) and by incorporating selective promoters targeting prostate (14, 15) or the majority of solid tumors (16-19). Several of these mutants have also been tested in the clinic and promising outcomes were reported when combined with standard chemotherapy (14, 15, 20, 21). Various mutants with the E1ACR2-deletion were highly efficacious in preclinical studies (22-24). Their potency was superior to that of dl1520 in most models even when additional deletions in the E3-genes were included (11, 25, 26). The majority of oncolytic adenoviral mutants evaluated in the clinic to date lack the E3B-region, a deletion associated with high levels of macrophage infiltration at the injection sites in dl1520-treated glioblastoma patients (27). We previously demonstrated that higher levels of macrophage infiltration in xenografts infected with an E3B-deleted virus correlated with greater clearance of virus from the tissue while mutants with an intact E3-region had higher levels of replication and enhanced antitumor responses (10, 28). More recently, we found that the attenuated efficacy in vivo of E3B-deleted mutants could be rescued by combining virotherapy with suboptimal doses of cytotoxic drugs (29). These findings suggested that viral efficacy could be improved through several strategies including engineering of both E1 and E3 genes and through co-administration with cytotoxic agents. To this end we generated a set of replication-selective mutants based on the potent E1ACR2-deletion with intact E3-genes to enhance in vivo efficacy.
While the potency of previously constructed ΔCR2 viruses was clearly higher than that of other adenoviral mutants, replication could still proceed in proliferating normal cells (11). The E1ACR2-region is responsible for binding and inactivation of pRb thereby releasing E2F for S-phase induction. Consequently, in proliferating normal cells and in tumor cells with deregulated cell cycle control (mainly pRb and p16 alterations) the E1ACR2-region is redundant. To further improve on the selectivity by attenuating viral replication in cycling normal cells we included a deletion of the anti-apoptotic E1B19K-gene that sensitizes normal tissue to death receptor-induced signaling and apoptosis in vivo, pathways that are deregulated or inactivated in the majority of cancer cells. Previously we demonstrated that ΔE1B19K-mutants had increased therapeutic index and lower liver toxicity in vivo, while anti-tumor potency was retained (30, 31). The anti-apoptotic E1B19K protein promotes viral replication and spread by blocking Bax-Bak oligomerization and mitochondrial pore-formation analogous to the cellular Bcl-2 homologue (32, 33). In contrast to the E1B55K protein that mainly inhibits p53-dependent pathways, E1B19K inhibits both death receptor- and intrinsically induced apoptosis through p53-dependent and –independent mechanisms (34-38). Recently we demonstrated that the AdΔ19K mutant (E1B19K-deleted) could synergise with gemcitabine to kill pancreatic cancer cells (39). Based on these findings we hypothesized that a mutant deleted in both the E1B19K-gene and E1ACR2-region would not only improve safety in vivo but also promote cell death in response to cytoxic drug-induced apoptosis.
Here, we report that a replication-selective mutant (AdΔΔ) targeting alterations in pRb (ΔCR2) and apoptosis pathways (ΔE1B19K) with intact E3-region improved efficacy and selectivity both as a single agent and in combination with standard chemotherapeutics. Viral replication and oncolysis in prostate and pancreatic carcinoma cells were as potent as that of wild type virus with significant efficacy in human prostate cancer xenografts in athymic mice. In animals with intact immune responses higher efficacy was observed with E3-intact mutants compared to the corresponding E3B-deleted mutants. A trend towards decreased macrophage invasion was also observed in tumors infected with E3-intact mutants.
MATERIAL AND METHODS
Cancer and normal cells
Human carcinoma cell lines from prostate PC3, DU145, LNCaP, 22Rv1 (ATCC), pancreas PT45 and Suit2, and lung H460 (Cell Services, CRUK) were cultured in Dulbecco’s Modified Eagle Media (DMEM) supplemented with 10% fetal calf serum (FCS; Life Technologies). Normal human bronchial (NHBE) and prostate epithelial cells (PrEC) (Lonza) were cultured according to the manufacturer’s instructions.
Adenoviruses and mutant construction
Adenoviral type 5 mutants were generated by homologous recombination as previously described (40). The complete adenovirus type 5 (Ad5) genome was used as the backbone in all new mutants and was derived from the pTG3602 plasmid (a generous gift from Dr. M. Methali, Transgene, France). The following viruses were generated: Ad5tg (wild type Ad5), AdΔ19K (E1B19K-deleted), AdΔCR2 (E1ACR2-deleted) and the AdΔΔ (E1B19K- and CR2-deleted). All newly generated mutants were characterized for purity, sequence determination (E1-genes), gene expression, cell killing activity and replication as previously reported (10, 29, 39). The non-replicating dl312 (E1A- and E3B-deleted) and AdGFP (CMV-GFP replacing E1-region) mutants were used as controls. All viruses had a viral particle to infectious unit ratio of 10-40 vp/pfu.
Cell killing assay and synergistic interactions
Cells were infected with viruses and/or treated with docetaxel (Sanofis Aventis) or mitoxantrone (Baxter) 24h after seeding and assayed for viability 4 to 7 days later using the MTS assay (Promega). Dose response curves were generated to calculate the concentration of each agent killing 50% of cells (EC50) using untreated cells or cells treated with one agent only as control, isobolographs were generated from these data as previously described (29).
Adenovirus replication assay
Cells were infected with viral mutants at 1-100 ppc and 24-72h later cells and media were collected, freeze-thawed and titered on JH293 cells by the limiting dilution method (tissue culture inhibitory dose at 50%: TCID50) (29). Each assay was repeated 3 times, averaged and expressed as pfu/cell ±SD. An internal Ad5 control of known activity was included in each assay.
Immunoblot analysis
Cells were infected with viruses and harvested after 24, 48 and 72h, lysed in buffer (50mM Hepes pH 7.4, 250mM NaCl, 1mM EDTA, 1mM DTT, 1mM NaF, 1% Triton X-100) containing a protease inhibitor cocktail (Roche). Total protein, 10-20μg, was separated on SDS-polyacrylamide gels under reducing conditions and transferred to PVDF membranes (Millipore). Viral and cellular proteins were detected by the following antisera: rabbit anti-Ad2 E1A at 1:200 (SC-430; Santa Cruz Biotechnology), rabbit anti-hexon at 1:2000 (AutogenBioclear), mouse anti-caspase-3 at 1:500 (Alexis) and mouse anti-ß-tubulin at 1:20000 (Sigma-Aldrich). Detection was by horseradish peroxidase-conjugated secondary antibodies (Dako), chemiluminescence reagent (Amersham/Pharmacia) and autoradiography (BioMax film; Kodak).
Flow cytometric analysis
Mitochondrial depolarisation was analysed in cells infected with viruses at 100ppc for 2h and harvested 24-96h post-infection. Cells were stained with tetramethylrhodamine ethyl ester perchlorate (TMRE) (Molecular Probes/Invitrogen) at 60ng/ml in PBS containing 4-6-diamidino-2-phenylindole (DAPI) at 1μg/ml and analysed on an LSRI (Becton Dickinson). Data were expressed as percentages of decreases in mitochondrial membrane potential (Δψ).
In vivo tumor growth
Tumors were grown in one flank of C57BL athymic (ICRF nu/nu) by subcutaneous implantation of cells at 1×106 for DU145 and 1×107 for PC3. Murine carcinoma cells CMT-64, CMT-93 and TRAMPC (Cell Services, CRUK) were grown in immunocompetent C57BL mice at 1×105, 5×106 and 1×104 cells/flank respectively, as previously described (10, 28). Dose responses to viral mutants or docetaxel were determined by administration of virus intratumorally at 1×106 −1010 vp/injection x3 at 48 h intervals and docetaxel at 5.0-15.0mg/kg intraperitoneally twice on day 2 and 8 after virus injection. To determine efficacy in response to combination treatments suboptimal doses of each agent were administered. Tumor volumes were estimated twice weekly: volume = (length × width2 × π)/6. Treatments were initiated when tumors were 100±20 μl with tumor growth and progression followed until tumors reached 1.44 cm2 or until symptomatic tumor ulceration occurred (according to UK Home Office Regulations). Survival analysis was performed according to the method of Kaplan-Meier (log rank test for statistical significance). Tumor growth curves were compared using one-way Anova for significance.
In vivo replication and gene expression
Tumors grown as above but implanted in both flanks were each injected once with the respective viral mutant (1×1010vp) with and without one intraperitoneal dose of docetaxel 24h later, 10 or 15mg/kg for DU145 and TRAMPC respectively. The DU145 tumors were harvested 3, 6 and 10 days after initial virus administration and the TRAMPC tumors on days 4, 8 and 15. One tumor from each animal was processed for immunohistochemistry (IHC) of E1A and hexon expression, and for the TRAMPC tumors the macrophage marker CD68, as previously described (10, 28, 30). The second tumor was immediately frozen (N2(l)), homogenized and processed for viral genome analysis or replication.
Quantitative PCR (qPCR)
For quantification of viral genome amplification in tumors and monolayer cells, DNA was isolated from tumor homogenates and cell-lysates by extraction and purification using the QIAamp DNA Blood Mini Kit (Qiagen). Hexon-DNA was detected by SYBR Green and quantified as previously described (39). Viral genomes were expressed as ratio of particles in each tumor relative to total DNA, averages of three tumors per treatment group and three replicates per cell-lysate were presented as means/total DNA.
RESULTS
Sensitivity to the novel adenoviral mutants is cell line-dependent
To verify the identity of each novel virus (Fig. 1A) PCR-analysis and E1-gene sequencing were performed (Fig. S1). Cell killing efficacy of Ad5tg wild type, the AdΔ19K, AdΔCR2 and AdΔΔ mutants was determined in seven carcinoma cell lines including prostate (PC3, DU145, 22Rv, LNCaP), pancreas (PT45, Suit2) and lung (H460). The PC3 cells were the least sensitive to virus-induced cell death with an EC50 value of 220±90 for Ad5tg followed by Suit2 at 140±22 particles per cell (ppc) and H460 and PT45 with slightly higher sensitivity. Other cell lines were highly sensitive to Ad5tg with EC50 values of 1.1±0.3, 1.2±0.5 and 0.6±0.2 ppc for DU145, 22Rv and LNCaP cells, respectively. The deletion of E1B19K (AdΔ19K) enhanced cell-killing potency except in the PC3, DU145 and Suit2 cells where activity was similar to that of Ad5tg (Fig. 1B). The CR2 deletion (AdΔCR2) also increased cell-killing activity compared to wild type virus in all cell lines except the 22Rv and LNCaP cells. Interestingly, these two cell lines were more sensitive to the combination mutant AdΔΔ with a reduction in EC50 values of greater than 50% compared to both wild type virus and the ΔCR2 mutant (Fig. 1B). In fact, the AdΔΔ virus was also more potent than Ad5tg in H460, PT45 and Suit2 cells with a similar trend (not significant) in DU145 and the highly insensitive PC3 cells. The corresponding E3B-deleted mutants with ΔCR2 or ΔE1B19K (dl922-947 and dl337 respectively) were also at least as potent as wild type virus in these cell lines (data not shown). The non-replicating control viruses dl312 and AdGFP induced < 20% cell death at doses up to 1×105 ppc in all cell lines.
Figure 1. Deletions of the E1ACR2 region and the E1B19K gene improved cell killing potency of Ad5 in several tumor cell lines.
A) Adenovirus wild type 5 (Ad5tg) and the respective mutants with the E1B19K (AdΔ19K), the E1ACR2 (AdΔCR2) or both gene-regions deleted (AdΔΔ) were generated. All mutants had intact E3-region. B) Cell-killing efficacy determined as EC50 values in PC3, DU145, 22Rv1 and LNCaP prostate, PT45 and Suit2 pancreatic, and H460 lung carcinoma cell lines with Ad5tg, AdΔ19K, AdΔCR2 and AdΔΔ viruses. The decreases in EC50 values are presented as percentages of the EC50 for wild type Ad5tg, averages ±SEM, n ≥ 3, * p < 0.05.
Potent replication of the novel mutants in cancer cells
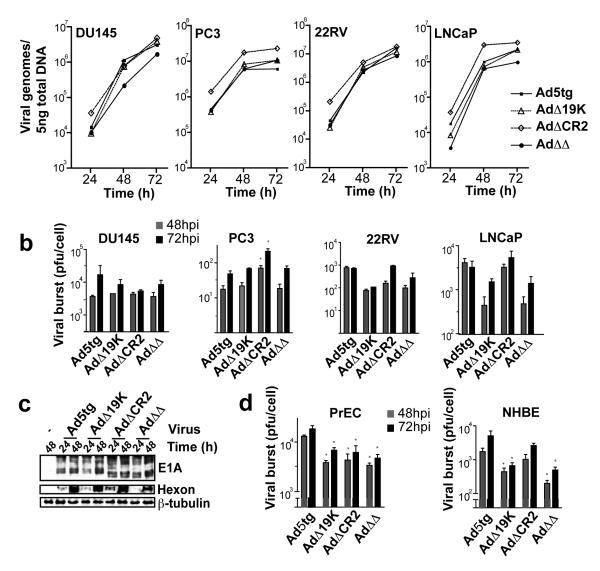
Viral DNA amplification and replication rates were determined in the four prostate cancer cell lines (Fig. 2A-B). For all mutants, viral DNA amplification over time paralleled that of wild type virus in each cell line while the levels of amplification varied and was slightly lower (not significant) for the AdΔΔ virus in DU145 and LNCaP cells (Fig. 2A). The highest levels of replication were observed in the DU145 cells 72h after infection with no significant differences between mutants except in the PC3 cells (Fig. 2B). While replication for all mutants was low in the PC3 cells, the AdΔCR2 mutant replicated at higher levels both at 48 and 72h after infection. In 22Rv and LNCaP cells the replication rates for the AdΔΔ and AdΔ19K mutants were lower than for the AdΔCR2 and wild type viruses.
Figure 2. Viral replication and gene expression of the novel mutants paralleled that of the wild type virus.
A) Amplification of viral genomes was determined over time for all mutants with qPCR of the hexon gene in DU145, 22Rv1 and LNCaP cells infected at 5ppc and in PC3 cells at 100ppc, only cells were included in the DNA analysis. Data expressed as averages of three samples ± SEM, differences between viruses were not significant (p>0.5). B) Viral replication determined in burst assays by TCID50 at 48 and 72h after infection with the respective mutants at 100ppc in all cell lines, both cells and media were analysed. C) Early E1A and late hexon gene expression in DU145 cells 24 and 48h after infection, representative immunoblot. D) Viral replication in the NHBE and PrEC normal cells determined by TCID50 assay at 48 and 72h after infection with 100ppc. (B and D) Averages of three samples ± SEM, * p< 0.05.
Potent viral gene expression in the cancer cell lines
To determine whether viral gene expression was affected by the deletions in the different mutants, cell lysates were probed for early (E1A) and late (hexon) viral proteins 24 and 48h after infection (Fig. 2C, and data not shown). The expression pattern was similar for wild type and mutants in all cell lines.
Attenuated replication of the novel mutants in normal cells
To test our hypothesis that inclusion of the E1B19K deletion would increase safety of replication-selective oncolytic mutants through inhibition of viral spread in non-tumor tissue, we determined the level of replication in normal cells. All mutants replicated to a significantly lesser degree (p<0.05) than Ad5tg in PrEC cells 48 and 72h after infection (Fig. 2D). In NHBE cells, replication of the AdΔΔ mutant was greatly attenuated and the AdΔ19K mutant to a lesser degree while replication for the ΔCR2-deletion was closer to that of Ad5tg. The attenuation of AdΔΔ compared to AdΔCR2 in normal cells was likely caused by premature induction of cell death in the absence of the E1B19K-gene as previously demonstrated for AdΔ19K in NHBE cells (39). This was reflected in the lower EC50 values for these mutants in normal cells; 10% of the Ad5tg value for E1B19K-deleted viruses compared to 40% for the single ΔCR2 mutant in NHBE cells (Table S1). Lower EC50 values for the corresponding E3B-deleted dl922-947 and dl337 mutants (Table S1) with parallel decreases in replication were also observed as previously reported (31).
The novel viral mutants synergistically enhance docetaxel-induced cell killing
Our previous observations suggest that the clinically used cytotoxic drugs docetaxel and mitoxantrone could synergistically increase cell killing when combined with Ad5 in prostate cancer cells (Radhakrishnan S, et al., unpublished data). Here, we demonstrate synergistic effects on cell death when the newly generated deletion-mutants were combined with docetaxel in PC3 and DU145 cells (Fig. 3A, Table 1). Synergistic interactions were observed in DU145 cells when docetaxel was combined with any of the mutants at all combinations tested while with the wild type virus, the majority (75%) of test conditions resulted in only additive effects. In PC3 cells, synergistic cell death was greater with the AdΔΔ mutant than with the single-deleted viruses while combinations with Ad5tg caused antagonistic effects (Table 1). Similar trends were seen in both DU145 and PC3 cells when the mutants were combined with mitoxantrone and to a lesser degree in the LNCaP cells (data not shown). In the LNCaP and 22Rv cells that were more sensitive to virus and drug induced cell death than the DU145 and PC3 cells, enhanced sensitisation to docetaxel was also observed with the AdΔΔ mutant compared to the single deleted mutants (Fig. S2). These data suggest that further improved anti-tumor efficacy of the potent AdΔΔ mutant can be achieved if combined with low doses of cytotoxic drugs e.g. 50% cell death was achieved with 1200-1900 fold and 1-2 fold reductions in docetaxel and virus doses respectively compared to each agent alone (DU145).
Figure 3. The novel viral mutants synergistically enhanced docetaxel-induced cell killing in prostate cancer cell lines.
A) DU145 and PC3 cells were treated with combinations of viral mutants and docetaxel at fixed ratios, EC50-values were calculated for each condition and isobolograms generated to determine synergistic interactions on cell death. The straight line represents the theoretical line for additive effects and each data point the respective combination ratio. B) Caspase 3 activation in response to infection with viral mutants at 100ppc after 24 and 48h in 22Rv1 and DU145 cells. C) Mitochondrial depolarisation (Δψ; TMRE staining) to determine proportion of live 22Rv1 and DU145 cells 24-96h after infection at 10ppc, averages ±SD, n=3.
Table 1.
Combination index (CI) for DU145 and PC3 cells treated with docetaxel and viral mutants at four constant dilution ratios.
| Cells | Ratio Ad/Doc (ppc/nM) |
Combination Index (CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ad5tg | AdΔ19K | AdΔCR2 | AdΔΔ | ||||||
| DU145 | 0.5 | 0.98 | ad | 0.68 | S | 0.57 | S | 0.46 | S |
| 2.5 | 1.19 | 0.63 | S | 0.50 | S | 0.91 | ad | ||
| 12.5 | 1.04 | ad | 0.75 | S | 0.48 | S | 0.88 | S | |
| 62.5 | 0.81 | S | 0.66 | S | 0.80 | S | 0.64 | S | |
| PC3 | 0.5 | 2.48 | 1.96 | 2.14 | 1.63 | ||||
| 2.5 | 3.20 | 1.7 | 1.86 | 1.19 | |||||
| 12.5 | 1.59 | 1.26 | 1.29 | 0.67 | S | ||||
| 62.5 | 1.88 | 0.78 | S | 0.76 | S | 0.49 | S | ||
CI values: <0.9=synergy (S), >1.1=antagonism (A), 0.9-1.1=additive effects (ad).
E1B19K-deleted mutants induce caspase 3 activation in 22Rv cells
In previous studies we found that the AdΔ19K virus could potently stimulate apoptotic cell death in combination with gemcitabine in pancreatic cancer cells (39). Surprisingly, both the AdΔ19K and AdΔΔ mutants induced procaspase 3 cleavage in 22Rv cells 48h after infection even in the absence of cytotoxic drugs (Fig. 3B, right panel). In combination with docetaxel caspase 3 activation was only observed when the 22Rv cells were simultaneously infected with the AdΔΔ mutant (Fig. S3). At the low doses used in this study neither docetaxel alone or in combination with Adtg induced procaspase cleavage. Caspase 3 activation was not observed in other prostate and pancreatic cell lines at these time points as shown for DU145 cells (Fig. 3B, left panel). Apoptotic death was further demonstrated to contribute to the enhanced cell-killing activity by the E1B19K-deleted mutants through increased mitochondrial depolarisation 48 - 96h after infection in the 22Rv cells but not in the DU145 (Fig. 3C).
Combinations of the double-deleted mutant (AdΔΔ) and docetaxel inhibit growth of DU145 and PC3 tumor xenografts in vivo
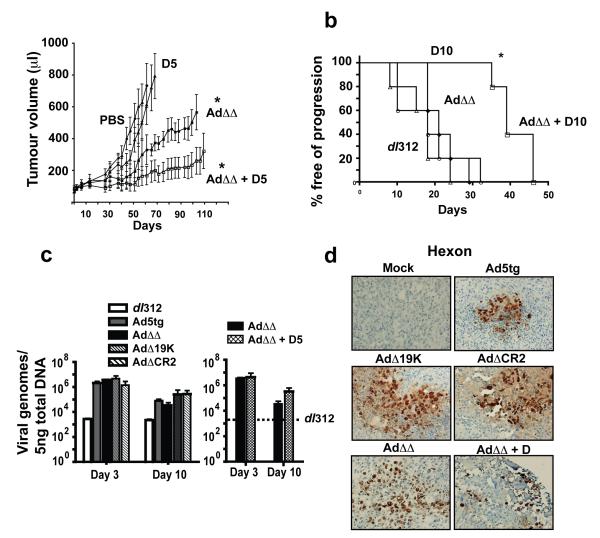
The potent response to combination treatments in cultured cells was verified in vivo in two human xenograft models. Subcutaneous tumors of DU145 and PC3 cells were treated with suboptimal doses of each agent (Fig. 4). Both the wild type and AdΔΔ viruses could potently inhibit tumor growth and prolong survival in combination with low doses of docetaxel in DU145 cells. Time to tumor progression was significantly (p<0.09) prolonged from 72d to 111d for AdΔΔ in combination with docetaxel (5mg/kg), compared to a median survival of 44d for docetaxel alone (p<0.02). The response to the combination treatment was greater than additive since this low dose of docetaxel had no efficacy in this model (Fig. 4A). In fact, the combination treatment resulted in a higher proportion of surviving animals at the end of the experiment than the higher dose of docetaxel at 10 mg/kg (70d) and was as efficient as the highest dose tested at 15mg/kg (113d). When the AdΔΔ mutant was combined with a higher dose of docetaxel (10mg/kg) more than 50% of animals were still alive when the study was terminated at day 170. Mutants and wild type virus had similar efficacy in the DU145 xenograft model with no significant differences between treatment groups (Fig. S4). Efficacy was also demonstrated in the PC3 xenograft model (Fig. 4B). Neither wild type nor the mutant viruses prolonged time to progression when administered alone at 1×109vp and only slightly inhibited tumor growth at a higher dose (Fig. 4B, S4 and data not shown). The median time to progression was 18-29d for all viruses at 1×109vp and was not significantly different from mock-, dl312- or docetaxel-treated animals. While docetaxel administered at 10mg/kg had no significant anti-tumor efficacy, when combined with AdΔΔ the median time to progression was increased from 18d to 39d and was significantly (p<0.002) different from single agent treatments. These results suggest that tumor regression and prolonged survival can be obtained with the AdΔΔ virus even in relatively treatment-resistant cancer cells such as PC3. No significant differences in efficacy were observed between AdΔΔ and Ad5tg in these models.
Figure 4. Combinations of low doses of the AdΔΔ mutant and docetaxel inhibited tumor progression in human prostate carcinoma xenografts in nude mice.
A) Animals with DU145 subcutaneous tumor xenografts were treated with PBS (phosphate buffered saline; filled circle), the AdΔΔ mutant at 1×109vp (intratumoral injections on day 1, 3, and 5) with and without docetaxel at 5mg/kg (intraperitoneal administration on day 2 and 8) (open and filled squares respectively) or docetaxel 5mg/kg alone (D5; triangle) and tumor growth was monitored. *p<0.001 for treatments compared to either single agent treatment and viral mutant compared to untreated animals. Significance determined by one-way Anova analysis. B) Animals with PC3 subcutaneous tumor xenografts were treated as above with the indicated suboptimal doses 1×109vp (dl312; open circles, and AdΔΔ; filled diamond) and docetaxel at 10mg/kg (D10; open triangle) or combination of AdΔΔ and docetaxel (open square). Median time to tumor progression (tumor volume >500μl) was determined by Kaplan-Meier survival analysis, 6-10 animals per group. *p<0.002 for combination treated compared to single agent groups. C) Viral genome amplification in DU145 tumors after intratumoral administration of virus on day 0, treated as indicated for 3 and 10 days. Total DNA was extracted and quantified by qPCR using specific hexon primers. Data averaged from 3 tumors/group, analysed in triplicates, and expressed as hexon DNA copies/5ng total DNA ±SD. Right panel: Viral amplification in DU145 tumors for the AdΔΔ mutant with and without simultaneous docetaxel administration at 5mg/kg. D) Viral hexon expression in DU145 tumor sections after intratumoral injection of 1×109vp of the respective mutant. One group treated with the AdΔΔ mutant and docetaxel at 10mg/kg. Three tumors were evaluated from each treatment group and analysed for expression of hexon 10 days after viral treatment, magnification 200x.
Anti-tumor efficacy in DU145 xenografts was caused by viral gene-expression and replication
In a separate study, DU145 tumors treated once with virus were analysed for viral replication and genome amplification in tumors 3-10d later (Fig. 4C). Higher levels of viral genomes were detected 3 days after treatment for all viruses compared to the non-replicating dl312 mutant. The amount of virus recovered after 10 days was slightly lower for all viruses. However, in animals treated with AdΔΔ in combination with docetaxel slightly higher levels of viral genome copies were retained (Fig. 4C, right panel). In parallel, viral replication assays (TCID50) were performed on three tumors per group. Three days after infection, viral replication per μg tumor was 1.45×106±3×105pfu (Ad5tg), 6.7×105±5×104pfu (AdΔ19K), 5.23×105±8×104pfu (AdΔCR2), 1.05×106±4×105pfu (AdΔΔ), and 5.1×105±2×105pfu (AdΔΔ + docetaxel 5mg/kg). After ten days a slight decrease in replication was detected for all viruses; Ad5tg was 5.23×105±1×104pfu and AdΔ19K and AdΔΔ were 90 and 80% respectively of the Ad5tg value. Other treatments including the AdΔΔ mutant in combination with docetaxel resulted in similar levels of replication to that of wild type alone, in agreement with the genome amplification data. Histological examination of the corresponding tumor tissue revealed increased levels of necrosis 10 days after treatment with Ad5tg or mutants compared to mock-infected tumors. Hexon expression was demonstrated with all viruses from 3-10 days and was paralleled by E1A expression (Fig. 4D and not shown).
The novel mutants with intact E3-region have higher efficacy in immune-competent animals
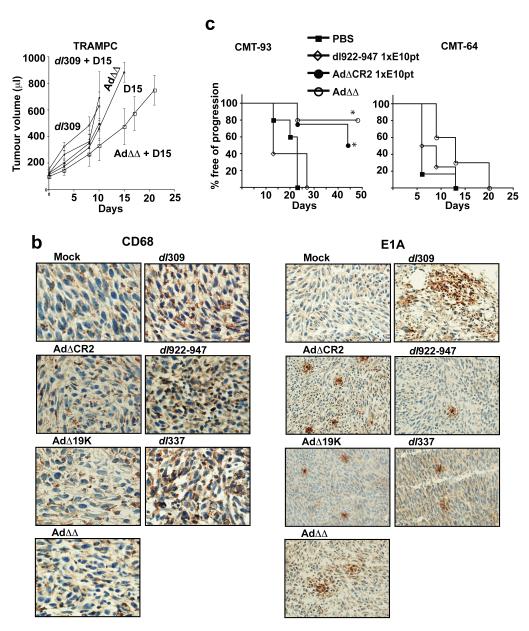
To evaluate efficacy of the novel mutants in hosts with functional immune responses the murine prostate cancer cells TRAMPC were implanted subcutaneously in intact mice. Anti-tumor efficacy was not significant for any mutant in this model likely due to the rapid growth progression and the lack of viral replication. However, when docetaxel (15mg/kg) was combined with the AdΔΔ mutant a trend towards reduced tumor growth was observed (Fig. 5A) although this was not significantly different from animals treated with docetaxel and dl309 (p>0.3) (Fig. 5A). Despite lack of replication, E1A expression was present in a heterogeneous pattern throughout the tumors (4-15d) treated with all tested mutants (Fig. 5B, day 15 right panels). Interestingly, both mock-infected and untreated TRAMPC tumors had high levels of CD68-positive macrophages making a quantitative determination of infiltrating macrophages in the presence of virus difficult (Fig. 5B, left panels). However, after infection with the E3B-deleted mutants dl309, dl922-947, and dl337 a further increase in macrophage immunoreactivity was observed. In contrast, in tumors treated with the novel mutants with intact E3-genes, no CD68-immunoreactivity above that of mock-infected tissue was seen (Fig. 5B, left panels). These data are in agreement with our previous findings that replication-selective mutants with intact E3B-genes attenuated host macrophage infiltration and gained anti-tumor efficacy both in replication-permissive and non-permissive models (10, 28). To further verify the increased efficacy in the presence of an intact immune system the AdΔΔ mutant was also evaluated in our previously developed models; the aggressive CMT-64 tumors (supporting low levels of replication) and the more antigenic CMT-93 tumors (not supporting viral replication) (Fig. 5C). A trend towards efficacy was also seen in the CMT-64 animals with the AdΔΔ mutant alone similar to the TRAMPC model with the combined treatment. However, in the CMT-93 animals both AdΔΔ and AdΔCR2 alone significantly prolonged survival (p<0.02 for AdΔΔ compared to dl922-947).
Figure 5. Retention of the E3B-genes in the AdΔΔ mutant improved efficacy in murine immunocompetent models.
A) C57BL intact mice with subcutaneous TRAMPC murine prostate tumor xenografts were treated with dl309 or AdΔΔ at 1×1010 vp/injection on day 1, 3, and 5 with and without docetaxel at 15mg/kg administered intraperitoneally on day 2 and 8. Treatments were not significantly different (p>0.3). B) Representative micrographs of TRAMPC tumors stained for macrophages (CD68) and viral proteins (E1A) 15 days after intratumoral administration of one dose of the respective virus intratumorally at 1×1010vp. Magnification: 400x for CD68 and 200x for E1A. C) Survival curves for C57BL intact mice with murine colorectal CMT-93 (left panel) and lung CMT-64 (right panel) subcutaneous tumors, treated with 1×1010 vp/injection on day 1, 3, and 5 with dl922-947 (ΔE3B) (open diamond), AdΔCR2 (closed circle) and AdΔΔ (open circle) for CMT-93, and with dl922-947 and AdΔΔ for CMT-64; mock treated animals (closed square). Survival of AdΔΔ treated animals was significantly different from animals treated with dl922-947 (*p<0.02) only in the CMT-93 model, 8 animals/group.
DISCUSSION
The aim of this study was to generate a novel oncolytic adenoviral deletion mutant with improved anti-tumor efficacy targeting solid cancers. We hypothesised that replication-selectivity would be greatly improved through elimination of E1B19K anti-apoptotic functions while limiting replication to cancer cells through the E1ACR2-deletion. Retention of the E1B55K-gene would prevent attenuation of the viral life cycle observed for the dl1520 mutant (7). We also included the entire E3-region to further enhance viral efficacy in vivo in the presence of intact anti-viral immune responses. The first generation of oncolytic adenoviruses tested in the clinic had the E3B-genes deleted to facilitate clearance of virus (4, 6, 8). However, later findings demonstrated that this deletion severely attenuated viral efficacy (10). Furthermore, retention of the E3B-genes did not appear to decrease safety. In this report we demonstrated that the double-deleted AdΔΔ mutant is highly potent in killing prostate, pancreatic and lung carcinoma cells and could efficiently replicate in all tested cell lines. In addition, we showed that replication in normal cells was significantly attenuated.
The cell killing potency of the AdΔΔ mutant was either superior or similar to wild type virus in all cancer cell lines tested. Similarly, the corresponding single-deleted mutants also had cell-killing activities similar to or better than the wild type virus. The order of potency for AdΔΔ, AdΔ19K, AdΔCR2 and Ad5tg in the different cell lines varied and was likely a consequence of specific gene alterations in each cell type. The choice of prostate and pancreatic cell lines covered a spectrum of gene mutations (for example K-ras activation, p53 and p16 deletions in PT45 and Suit-2 cells) (41). Each prostate cancer cell line had a unique combination of activating and inactivating mutations such as PTEN-deletions and various degrees of functionality of p53, pRb, p16 and HDM2 while all cell lines had activated Bcl-2 (e.g. LNCaP: pRb-, PTEN-; 22Rv: p16-, pAkt-; DU145: pRb-, p16-, p53-; and PC3: p16-, p53-, PTEN-) (42). Consequently, both the prostate and pancreatic cancer cells had deregulated pRb-p53 pathways enabling the ΔCR2-deleted viruses to replicate and kill these cells significantly more efficient than the normal cells with intact cell cycle regulation. Interestingly, the AdΔCR2 mutant was less potent in killing the LNCaP and 22Rv cells than mutants including the E1B19K-deletion while replication was similar to that of Ad5tg. In the DU145 and PC3 cells an opposite trend was noted with the single-deleted AdΔ19K having lower cell killing efficacy but no attenuation of replication for any mutant, rather an increase for the AdΔCR2 mutant in PC3 cells.
Reasons for these differences between the AR-positive and AR-negative cell lines are likely more complex than the AR-status alone. Both LNCaP and 22Rv cells have functional p53-genes, constitutively active androgen receptors and the 22Rv cells also have functional pRb expression (42). It is possible that in cells with intact p53 and/or pRb functions, elimination of one of the viral anti-apoptotic genes might suffice to advance the timing of virus-induced death and consequently reduce the amount of virus produced. However, the absolute levels of replication for AdΔΔ in LNCaP and 22Rv cells were decreased less than 5-fold after 72h and in PC3 and DU145 cells (with non-functional p53 and/or pRb) replication was closer to that of wild type virus. More importantly, replication of the AdΔΔ mutant in non-arrested normal cells was significantly attenuated compared to wild type virus, with the greatest decrease in NHBE cells. In contrast, the single-deleted AdΔCR2 mutant replicated to higher levels under these conditions. Hence the additional deletion of the E1B19K-function attenuated viral replication, suggesting improved safety by preventing spread in normal tissue. These findings are in agreement with a previous report demonstrating that the CR2-deleted dl922-947 mutant could still replicate in normal SAEC and MVEC cells (11). Most normal tissues have few proliferating cells and replication of the AdΔΔ mutant would likely be further attenuated in vivo due to its inability to bind to pRb, in addition to impaired defence against the anti-viral TNF-response. Taken together these results suggest that the AdΔΔ virus has potential as a new improved oncolytic candidate for future evaluation in clinical trials.
To this end, AdΔΔ was combined with cytotoxic drugs currently used in the clinic to treat prostate cancer. Enhanced cell killing was observed in DU145 and PC3 cells in combination with both docetaxel and mitoxantrone through synergistic interactions. Synergistic cell death also occurred with the single-deleted mutants and to a lesser degree with wild type virus. While it has previously been demonstrated that enhancement of virus and cytotoxic drug-induced cell death is dependent on E1A expression, the specific E1A regions necessary for the sensitisation have not yet been identified (29, 43-45). Here we show that viruses deleted in the E1ACR2-region could potently synergise with docetaxel both in the presence and absence of E1B19K expression, an interesting finding in light of the potential for future combination therapies. Docetaxel is currently the drug of choice in the UK for metastatic, hormone-refractory prostate cancers. Previous reports showed that antitumor efficacy could be enhanced by combining Δ24-mutants with DNA-damaging cytotoxins such as CPT-11 (46), radiotherapy (22) or gemcitabine (23). Recently, we demonstrated that the E1B19K-deletion alone could also greatly enhance tumor cell killing of pancreatic xenografts in combination with gemcitabine (39). However, to our knowledge this is the first report demonstrating that deletion of both E1ACR2 and E1B19K interacted synergistically with docetaxel, a drug whose main mechanism of action is the inhibition of cell division rather than DNA-damage. A similar double-deleted virus was previously reported to efficiently kill melanoma cells (19). However, this mutant had viral gene-expression and replication regulated by a tyrosinase promoter/enhancer element resulting in higher levels of expression than with the viral E1A-promoter/enhancer in this study and combinations with other cytotoxic factors were not reported. Even the PC3 cells, insensitive to both virus and docetaxel alone, could be greatly sensitised to docetaxel by the AdΔΔ mutant. Enhancement of cell death was less potent in the LNCaP and 22Rv cells perhaps reflecting the greater sensitivity to mutants and docetaxel when administered alone. These cells might already have reached a “maximal” level of death-induction due to the presence of more functional death and cell-damage response pathways. However, results from sensitisation assays clearly demonstrated that low doses of the AdΔΔ mutant could improve cell killing by docetaxel (and to a lesser extent the Δ19K and ΔCR2 mutants), in effect decreasing the drug concentrations required to kill LNCaP and 22Rv cells analogous to the results in PC3 and DU145 cells.
A contributing factor to the greater increase in cell death with the AdΔΔ mutant was the absence of a functional anti-apoptotic E1B19K-gene (30, 31, 34, 39). We demonstrated that the mutants without E1B19K could activate apoptotic pathways in the 22Rv cells in contrast to Ad5tg and the AdΔCR2 viruses. In addition, the AdΔΔ mutant but not E1B19K expressing viruses induced caspase 3 activation when combined with docetaxel, likely contributing to the greater enhancement of cell killing with AdΔΔ. Although it is known that adenovirus-induced cell death occur through non-apoptotic pathways (47, 48) we demonstrated that viruses deleted in the E1B19K-gene released virus earlier than a virus with this gene intact, suggesting a more rapid induction of cell death mechanisms (30, 31). We also observed caspase 3 activation after infection with the E1B19K-deleted viruses at later time points even in the DU145 cells (>72h; not shown) indicating a more general activation of death programmes due to viral replication and not through direct activation of apoptotic factors by viral proteins. Further studies are necessary to distinguish what viral mechanisms are responsible for the early activation of apoptosis-like events in the 22Rv cells. In addition, we recently determined that the AdΔ19K virus in combination with suboptimal doses of gemcitabine increased drug-induced apoptosis while the virus alone had no effect on either caspase activation or mitochondrial depolarisation in pancreatic cancers (39). This difference in responses to Δ19K mutants alone in prostate and pancreatic cells is probably the result of specific functional inactivating/activating mutations in each cell line for example, intact p53 function in 22Rv but not in PT45 cells (41, 42). Our data also indicate that other mechanisms are equally important to enhance cell-killing activity since both efficient cell death and synergy was achieved with wild type virus and the AdΔCR2 mutant.
Greatly improved anti-tumor efficacy was also seen when the novel AdΔΔ was combined with docetaxel in PC3 and DU145 tumor xenografts. In fact, at doses where no effects were detected when either the virus or drug was administered alone, significantly prolonged survival was observed when combined. At a higher dose of 1×1010vp, tumor growth inhibition was achieved with all mutants in the absence of drug with the greatest effects for the AdΔΔ in DU145 xenografts. Using the murine immunocompetent TRAMPC model we also demonstrated that mutants with intact E3-genes attenuated CD68-positive macrophage infiltration. As expected, the corresponding E3B-deleted mutants dl309, dl922 and dl337 did not prevent this infiltration. Due to high basal levels of CD68 cell infiltration in the TRAMPC xenografts only qualitative assessments were possible. While efficacy could not be optimised due to the rapid proliferation of TRAMPC cells significantly prolonged survival was achieved in the slower growing CMT-93 model with the AdΔΔ but not the corresponding E3B-deleted mutants. A similar trend was also seen in the CMT64 model, the only murine syngeneic model that supports Ad5-replication, albeit at low levels (28). Nevertheless, a trend towards higher efficacy with AdΔΔ was observed in all three immunocompetent models with the lowest responses in the more aggressive and rapidly proliferating TRAMPC and CMT64 models in combination with docetaxel or alone respectively. The greater responses to the AdΔΔ mutant were likely caused by potent E1A-expression in the absence of the E1B19K anti-apoptotic function and presumably prolonged retention of virus compared to E3B-deleted mutants. These findings verified our previous results that quantitatively assessed viral gene expression and macrophage infiltration in animals with intact immune system demonstrating high viral gene expression accompanied by low levels of macrophage infiltration for E3B-intact mutants and lower viral gene expression in the presence of higher levels of macrophage infiltration for E3B-deleted viruses (10). Additional contributions to the higher efficacy in the CMT-93 model are likely due to the more immunogenic nature of these tumors in the presence of virus, as previously indicated (10).
From the data described here, the novel AdΔΔ virus has great potential for future clinical evaluation demonstrating improved therapeutic index and oncolytic potency that was further enhanced in combination with chemotherapeutics.
Statement of Translational Relevance The current standard of care for most patients with solid cancers is rarely curative. The development of replication-selective oncolytic adenoviruses is a new alternative strategy with promising outcomes in numerous clinical trials. Here we show that a novel complementation-mutant with the viral anti-apoptotic E1B19K-gene deleted in combination with the replication-selective E1ACR2-deletion and retained E3B-gene expression has improved efficacy in prostate and pancreatic carcinomas. Combined treatments with docetaxel can further increase the anti-tumor efficacy in vivo.
These findings are important for future design of combination therapies, particularly the potential of oncolytic mutants to target chemotherapy-insensitive cancers such as prostatic and pancreatic and are applicable to the majority of solid cancers.
Supplementary Material
Relative sensitivity to viral mutants in NHBE and PrEC cells.
ACKNOWLEDGEMENTS
This work was supported by grants from the Barts & The London Charity, CORE Digestive Cancer Campaign and Cancer Research UK (C633-A6253/A6251 programme grant). We want to thank Gary Martin and colleagues at Clare Hall (CRUK) for excellent experimental assistance, Keyur Trivedi, Mohammed Ikram and Vipul Bhakta (Centre for Molecular Oncology and Imaging) for immuno-histochemistry and QPCR expertise. We also wish to thank Yaohe Wang for insightful discussions.
REFERENCES
- 1.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–76. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 2.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573–87. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24:7802–16. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 4.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–17. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 5.Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther. 2002;9:979–86. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khuri FR, Nemunaitis J, Ganly I, et al. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–85. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 7.O’Shea CC, Johnson L, Bagus B, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–23. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Garber K. China approves world’s first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 9.Hecht JR, Bedford R, Abbruzzese JL, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–61. [PubMed] [Google Scholar]
- 10.Wang Y, Hallden G, Hill R, et al. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat Biotechnol. 2003;21:1328–35. doi: 10.1038/nbt887. [DOI] [PubMed] [Google Scholar]
- 11.Heise C, Hermiston T, Johnson L, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–9. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 12.Page JG, Tian B, Schweikart K, et al. Identifying the safety profile of a novel infectivity-enhanced conditionally replicative adenovirus, Ad5-delta24-RGD, in anticipation of a phase I trial for recurrent ovarian cancer. Am J Obstet Gynecol. 2007;196:389, e1–9. doi: 10.1016/j.ajog.2006.12.016. discussion e9-10. [DOI] [PubMed] [Google Scholar]
- 13.Stolarek R, Gomez-Manzano C, Jiang H, Suttle G, Lemoine MG, Fueyo J. Robust infectivity and replication of Delta-24 adenovirus induce cell death in human medulloblastoma. Cancer Gene Ther. 2004;11:713–20. doi: 10.1038/sj.cgt.7700731. [DOI] [PubMed] [Google Scholar]
- 14.Small EJ, Carducci MA, Burke JM, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14:107–17. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 15.DeWeese TL, van der Poel H, Li S, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–72. [PubMed] [Google Scholar]
- 16.Schepelmann S, Hallenbeck P, Ogilvie LM, et al. Systemic gene-directed enzyme prodrug therapy of hepatocellular carcinoma using a targeted adenovirus armed with carboxypeptidase G2. Cancer Res. 2005;65:5003–8. doi: 10.1158/0008-5472.CAN-05-0393. [DOI] [PubMed] [Google Scholar]
- 17.Johnson L, Shen A, Boyle L, et al. Selectively replicating adenoviruses targeting deregulated E2F activity are potent, systemic antitumor agents. Cancer Cell. 2002;1:325–37. doi: 10.1016/s1535-6108(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 18.Lei N, Shen FB, Chang JH, et al. An oncolytic adenovirus expressing granulocyte macrophage colony-stimulating factor shows improved specificity and efficacy for treating human solid tumors. Cancer Gene Ther. 2009;16:33–43. doi: 10.1038/cgt.2008.46. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz M, Graf C, Gut T, et al. Melanoma cultures show different susceptibility towards E1A-, E1B-19 kDa- and fiber-modified replication-competent adenoviruses. Gene Ther. 2006;13:893–905. doi: 10.1038/sj.gt.3302739. [DOI] [PubMed] [Google Scholar]
- 20.Freytag SO, Stricker H, Peabody J, et al. Five-year follow-up of trial of replication-competent adenovirus-mediated suicide gene therapy for treatment of prostate cancer. Mol Ther. 2007;15:636–42. doi: 10.1038/sj.mt.6300068. [DOI] [PubMed] [Google Scholar]
- 21.Freytag SO, Movsas B, Aref I, et al. Phase I trial of replication-competent adenovirus-mediated suicide gene therapy combined with IMRT for prostate cancer. Mol Ther. 2007;15:1016–23. doi: 10.1038/mt.sj.6300120. [DOI] [PubMed] [Google Scholar]
- 22.Lamfers ML, Grill J, Dirven CM, et al. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62:5736–42. [PubMed] [Google Scholar]
- 23.Raki M, Sarkioja M, Desmond RA, et al. Oncolytic adenovirus Ad5/3-delta24 and chemotherapy for treatment of orthotopic ovarian cancer. Gynecol Oncol. 2008;108:166–72. doi: 10.1016/j.ygyno.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki K, Alemany R, Yamamoto M, Curiel DT. The presence of the adenovirus E3 region improves the oncolytic potency of conditionally replicative adenoviruses. Clin Cancer Res. 2002;8:3348–59. [PubMed] [Google Scholar]
- 25.Bazan-Peregrino M, Carlisle RC, Hernandez-Alcoceba R, et al. Comparison of molecular strategies for breast cancer virotherapy using oncolytic adenovirus. Hum Gene Ther. 2008;19:873–86. doi: 10.1089/hum.2008.047. [DOI] [PubMed] [Google Scholar]
- 26.Libertini S, Iacuzzo I, Perruolo G, et al. Bevacizumab increases viral distribution in human anaplastic thyroid carcinoma xenografts and enhances the effects of E1A-defective adenovirus dl922-947. Clin Cancer Res. 2008;14:6505–14. doi: 10.1158/1078-0432.CCR-08-0200. [DOI] [PubMed] [Google Scholar]
- 27.Fulci G, Dmitrieva N, Gianni D, et al. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer Res. 2007;67:9398–406. doi: 10.1158/0008-5472.CAN-07-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallden G, Hill R, Wang Y, et al. Novel immunocompetent murine tumor models for the assessment of replication-competent oncolytic adenovirus efficacy. Mol Ther. 2003;8:412–24. doi: 10.1016/s1525-0016(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 29.Cheong SC, Wang Y, Meng JH, et al. E1A-expressing adenoviral E3B mutants act synergistically with chemotherapeutics in immunocompetent tumor models. Cancer Gene Ther. 2008;15:40–50. doi: 10.1038/sj.cgt.7701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu TC, Hallden G, Wang Y, et al. An E1B-19 kDa gene deletion mutant adenovirus demonstrates tumor necrosis factor-enhanced cancer selectivity and enhanced oncolytic potency. Mol Ther. 2004;9:786–803. doi: 10.1016/j.ymthe.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Liu TC, Wang Y, Hallden G, et al. Functional interactions of antiapoptotic proteins and tumor necrosis factor in the context of a replication-competent adenovirus. Gene Ther. 2005;12:1333–46. doi: 10.1038/sj.gt.3302555. [DOI] [PubMed] [Google Scholar]
- 32.White E. Regulation of the cell cycle and apoptosis by the oncogenes of adenovirus. Oncogene. 2001;20:7836–46. doi: 10.1038/sj.onc.1204861. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian T, Vijayalingam S, Chinnadurai G. Genetic identification of adenovirus type 5 genes that influence viral spread. J Virol. 2006;80:2000–12. doi: 10.1128/JVI.80.4.2000-2012.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White E. Mechanisms of apoptosis regulation by viral oncogenes in infection and tumorigenesis. Cell Death Differ. 2006;13:1371–7. doi: 10.1038/sj.cdd.4401941. [DOI] [PubMed] [Google Scholar]
- 35.Cross JR, Postigo A, Blight K, Downward J. Viral pro-survival proteins block separate stages in Bax activation but changes in mitochondrial ultrastructure still occur. Cell Death Differ. 2008 doi: 10.1038/cdd.2008.14. [DOI] [PubMed] [Google Scholar]
- 36.Sauthoff H, Heitner S, Rom WN, Hay JG. Deletion of the adenoviral E1b-19kD gene enhances tumor cell killing of a replicating adenoviral vector. Hum Gene Ther. 2000;11:379–88. doi: 10.1089/10430340050015851. [DOI] [PubMed] [Google Scholar]
- 37.Yoon AR, Kim JH, Lee YS, et al. Markedly enhanced cytolysis by E1B-19kD-deleted oncolytic adenovirus in combination with cisplatin. Hum Gene Ther. 2006;17:379–90. doi: 10.1089/hum.2006.17.379. [DOI] [PubMed] [Google Scholar]
- 38.Lomonosova E, Subramanian T, Chinnadurai G. Mitochondrial localization of p53 during adenovirus infection and regulation of its activity by E1B-19K. Oncogene. 2005;24:6796–808. doi: 10.1038/sj.onc.1208836. [DOI] [PubMed] [Google Scholar]
- 39.Leitner S, Sweeney K, Oberg D, et al. Oncolytic Adenoviral Mutants with E1B19K Gene Deletions Enhance Gemcitabine-induced Apoptosis in Pancreatic Carcinoma Cells and Anti-Tumor Efficacy In vivo. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A, Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol. 1996;70:4805–10. doi: 10.1128/jvi.70.7.4805-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore PS, Sipos B, Orlandini S, et al. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001;439:798–802. doi: 10.1007/s004280100474. [DOI] [PubMed] [Google Scholar]
- 42.Skjoth IH, Issinger OG. Profiling of signaling molecules in four different human prostate carcinoma cell lines before and after induction of apoptosis. Int J Oncol. 2006;28:217–29. [PubMed] [Google Scholar]
- 43.Flinterman MB, Mymryk JS, Klanrit P, et al. p400 function is required for the adenovirus E1A-mediated suppression of EGFR and tumour cell killing. Oncogene. 2007;26:6863–74. doi: 10.1038/sj.onc.1210497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Z, Jia SF, Hung MC, Kleinerman ES. E1A sensitizes HER2/neu-overexpressing Ewing’s sarcoma cells to topoisomerase II-targeting anticancer drugs. Cancer Res. 2001;61:3394–8. [PubMed] [Google Scholar]
- 45.Ueno NT, Bartholomeusz C, Herrmann JL, et al. E1A-mediated paclitaxel sensitization in HER-2/neu-overexpressing ovarian cancer SKOV3.ip1 through apoptosis involving the caspase-3 pathway. Clin Cancer Res. 2000;6:250–9. [PubMed] [Google Scholar]
- 46.Gomez-Manzano C, Alonso MM, Yung WK, et al. Delta-24 increases the expression and activity of topoisomerase I and enhances the antiglioma effect of irinotecan. Clin Cancer Res. 2006;12:556–62. doi: 10.1158/1078-0432.CCR-05-1892. [DOI] [PubMed] [Google Scholar]
- 47.Abou El, Hassan MA, van der Meulen-Muileman I, Abbas S, Kruyt FA. Conditionally replicating adenoviruses kill tumor cells via a basic apoptotic machinery-independent mechanism that resembles necrosis-like programmed cell death. J Virol. 2004;78:12243–51. doi: 10.1128/JVI.78.22.12243-12251.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baird SK, Aerts JL, Eddaoudi A, Lockley M, Lemoine NR, McNeish IA. Oncolytic adenoviral mutants induce a novel mode of programmed cell death in ovarian cancer. Oncogene. 2007 doi: 10.1038/sj.onc.1210977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative sensitivity to viral mutants in NHBE and PrEC cells.