Abstract
Radiotherapy represents the most effective nonsurgical treatments for gliomas. Yet, gliomas are highly radioresistant and recurrence is nearly universal. Results from our laboratory and other groups suggest that cancer stem cells contribute to radioresistance in gliomas and breast cancers. The Notch pathway is critically implicated in stem cell fate determination and cancer. In this study, we showed that inhibition of Notch pathway with gamma-secretase inhibitors (GSIs) rendered the glioma stem cells more sensitive to radiation at clinically relevant doses. GSIs enhanced radiation-induced cell death and impaired clonogenic survival of glioma stem cells, but not non-stem glioma cells. Similarly, knockdown of Notch1 or Notch2 increased radiosensitivity of glioma stem cells. The specificity of the radiosensitizing effects of GSIs was confirmed by expression of the constitutively active intracellular domains of Notch1 or Notch2 that protected glioma stem cells against radiation. Notch inhibition with GSIs did not alter the DNA damage response of glioma stem cells following radiation, but rather impaired radiation-induced Akt activation and upregulated levels of the truncated apoptotic isoform of Mcl-1 (Mcl-1s). Taken together, our results suggest a critical role of Notch to promote radioresistance of glioma stem cells. Inhibition of Notch signaling holds promise to improve the efficiency of current radiotherapy in glioma treatment.
Introduction
Malignant gliomas, including anaplastic astrocytoma (World Health Organization (WHO) grade III) and glioblastoma multiforme (WHO grade IV) are among the most devastating malignancies. Despite recent advances in therapy, treatment of malignant gliomas remains palliative. Median post-diagnosis survival for anaplastic astrocytoma is less than 3 years and for glioblastoma multiforme is 12–14 months [1,2,3]. Maximal surgical resection of the tumor mass followed by radiotherapy and chemotherapy is the standard of care. Gliomas often respond to radiotherapy, however, subsequent recurrence is almost inevitable, suggesting insufficient killing of tumorigenic cells [4]. Recently, cancer cells with stem cell-like properties have been described in a wide range of human tumors. The cancer stem cells model suggests a hierarchical organization of tumors that a subpopulation of tumor cells at the apex drives and maintains human tumors [5]. Cancer stem cells of glioma and other neoplasms of human central nervous system can be prospectively enriched by selection of the CD133 (prominin-1) cell surface marker [6,7,8,9,10,11], although some tumors may not express CD133 and hence CD133-negative cells may still have characteristics of cancer stem cells [12,13]. Despite expression of certain neural and other stem cell markers (such as CD133, Musashi-1, Nestin, Sox2 and Olig2) by brain tumor stem cells, the definition of cancer stem cells remains functional, requiring sustained self renewal and tumor propagation. Recent reports have proposed that some established cancer cell lines contain cells that resemble cancer stem cells, but strong evidence suggests that cells maintained in pro-differentiation serum conditions acquire genetic differences that do not reflect the original tumor [14]. Our laboratory previously demonstrated that the glioma stem cells were more resistant to radiation compared with the matched non-stem glioma cells due to preferential activation of the DNA damage response pathway [7]. Other groups also reported that the cancer stem cells of breast cancers were relatively resistant to radiation, potentially due to lower levels of reactive oxygen species found in cancer stem cells [15].
The emerging role of cancer stem cells in tumor response to radiotherapy urges investigation on molecular mechanisms underlying radioresistance of these cells. One of these candidates is the Notch signaling pathway. Notch mediates short-range cellular communication through interaction with ligands presented on neighboring cells [16]. The instrumental roles of Notch in regulation of self renewal and cell fate determination in normal stem cells have been well established [17]. In mammals, Notch signals through four Notch receptors (Notch 1–4) and five ligands (Jagged-1, -2, and Delta-like-1, -3, and -4), which are all type I transmembrane proteins [18]. Activation of Notch involves sequential proteolytic cleavages that eventually lead to release and nuclear translocation of the intracellular domains of Notch receptors (NICDs), and subsequent activation of Notch-dependent transcription. The γ-secretase complex, which mediates the last proteolytic step for release of the NICDs, is essentially required for Notch activation [19,20]. Inhibitors of γ-secretase (GSIs) have been used to block Notch signaling in vitro and in vivo.
The Notch signaling pathway is essentially involved in the maintenance of a variety of adult stem cells, such as breast [21,22], intestinal [23,24] and neural stem cells [25,26,27], through promoting self-renewal and repressing differentiation. Aberrant Notch activity is found in a wide range of human tumors, such as leukemia, breast cancer, and glioma [28,29,30,31]. Taking into account the instrumental functions of Notch in both stem cells and cancer biology, it is not surprising to find that Notch plays an important role in cancer stem cells [32]. Notch inhibition impairs the self-renewal capacity of mammosphere initiating cells derived from ductal carcinoma in situ [22], and reduces colony formation by leukemic stem cells [33]. Activation of Notch through expression of NICD1 promotes growth and neurosphere formation of the SHG-44 glioma cell line [34]. Notch inhibition by a γ-secretase inhibitor (GSI-18) induces apoptosis and differentiation in CD133+ cells enriched from medulloblastoma cell lines, and impairs the tumorigenic capacity of these cells. Of particular interest, apoptosis induced by GSI-18 is 10 fold higher in the primitive cells expressing the neural stem cell marker nestin in comparison to nestin-negative cells, suggesting a preferential Notch dependence of cancer stem cells [35].
It was recently reported that Notch pathway was transiently activated in endothelial cells following radiation, as evidenced by upregulation of Notch pathway components, Jag1 and Hey1 [36]. We interrogated the potential role of Notch in regulating glioma stem cell radioresistance using pharmacologic and genetic targeting strategies with an additional focus on potential molecular mechanisms. Our results suggest that Notch inhibition can be developed as an adjuvant approach to improve current radiation treatment for gliomas.
Results
γ-secretase inhibitors impair cell growth and clonogenic survival of glioma stem cells in combination with radiation
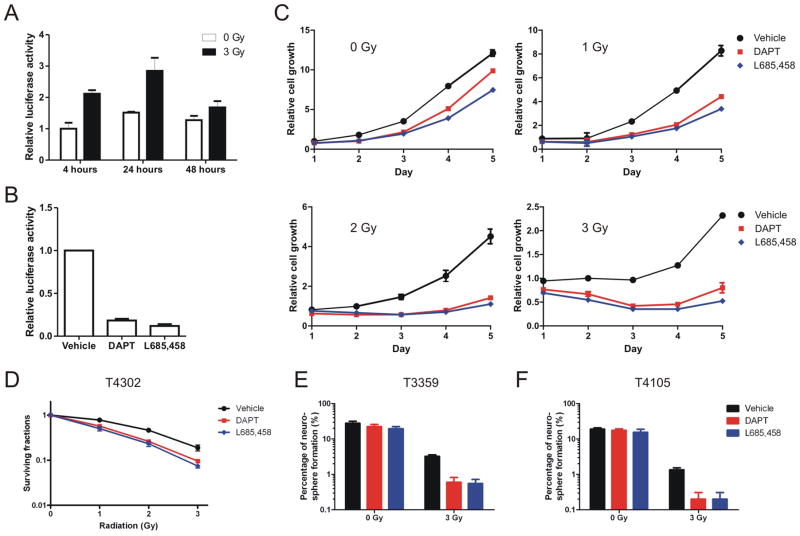
Accumulating evidence suggests that the Notch pathway plays important roles in a wide range of human tumors as well as cancer stem cells [32,37]. As a recent study reported that radiation induced transient upregulation of Notch pathway components in endothelial cells [36], we investigated if Notch activity was involved in the radioresponse of glioma stem cells. Short-term cultures enriched for the glioma stem cells were isolated from surgical biopsy specimens amplified as direct xenografts which have been demonstrated to maintain a cancer stem cell phenotype [38]. The stem cell-like properties of the CD133+ glioma cells specifically used in this study, including sustained self-renewal, expression of stem cell markers, multi-lineage differentiation, and the ability to recapitulate the original tumors in serial xenotransplantation assays, have been demonstrated in our previous publications (Supplemental figure 1 and [6,7,39,40,41,42]). We observed upregulation of Notch-dependent transcription activation in glioma stem cells following radiation using a Notch-responsive luciferase reporter (Figure 1A), although changes of expression of representative Notch targets, such as Hes and Hey family genes, were not significant as measured by quantitative real-time PCR (data not shown). Inhibition of Notch in glioma stem cells by γ-secretase inhibitors, DAPT or L685,458, was validated by Notch-responsive luciferase reporter assays (Figure 1B). Growth of the CD133+ glioma cells was assessed following different radiation doses with or without GSI treatment. GSIs moderately reduced growth of the T4302 CD133+ glioma cells in the absence of radiation (Figure 1C, graph 1), but severely impaired growth of cells treated with 1, 2 or 3 Gy of radiation (Figure 1C, graph 2–4). On the fifth day after 2-Gy radiation, total cell numbers in the sham-treated groups were approximately 4 fold more than in GSI-treated groups. In contrast, without radiation treatment, sham-treated cells were only 25% (vs. DAPT-treated) or 60% (vs. L685,458-treated) more than GSI-treated cells five days after radiation.
Figure 1. γ-secretase inhibitors reduce cell growth and clonogenic survival of glioma stem cells following radiation.
(A) T4302 CD133+ glioma stem cells were transfected with a Notch-responsive luciferase reporter pre-mixed with the vector directing constitutive expression of Renilla luciferase. Cells were left unirradiated or irradiated at 3 Gy, and were then collected to measure luciferase activities at time points as indicated. In this and subsequent luciferase assays, firefly luciferase activity was normalized to the internal control Renilla luciferase activity and the normalized firefly luciferase activity from the control group was assigned a value of 1. Data are presented as the mean ± standard error. (B) T4302 CD133+ cells were transfected with the Notch-responsive luciferase reporter and treated with DMSO (vehicle), 2 μM DAPT or 0.5 μM L685,458 over night, and were then collected for luciferase assays. (C) T4302 CD133+ cells were pre-treated with 2 μM DAPT or 0.5 μM L685,458 for 4 hours, irradiated as indicated, and were aliquoted in 96-well plates in triplicate at 3000 cells per well. Total viable cell numbers were then determined by the CellTiter-Glo Luminescent Cell Viability Assay (Promega) for the following five days. The averaged cell titers on day 1 of the control groups were assigned a value of 1. (D) T4302 CD133+ cells were pre-treated and irradiated as described in Figure 1C. Cells were then plated to form neurospheres, and the surviving fractions were determined as described in methods. The mean ± standard error was derived from 8 replicates within a representative experiment. (E, F) Neurosphere formation of T3359 and T4105 CD133+ cells treat with GSIs alone, 3-Gy radiation alone, or the combination was determined as described in figure 1D.
Clonogenic survival assay is the standard assay to assess radiation responsiveness. In this study, we employed neurosphere formation as a surrogate marker of the clonogenic survival of glioma stem cells. The T4302 CD133+ glioma cells were pretreated with DMSO, DAPT or L685,458, and were permitted to grow or irradiated at multiple dose levels. Cells treated with GSIs in combination with radiation demonstrated significantly reduced clonogenic survival compared with control groups for all radiation doses examined (Figure 1D, p=0.012 at 1 Gy, p<0.001 at 2 and 3 Gy, by one-way ANOVA). The effects of GSIs on clonogenic survival of CD133+ cells derived from the T3359 glioma xenograft were also examined. Consistently, GSIs inhibited neurosphere formation in T3359 CD133+ cells irradiated at 3 Gy (p<0.0001, by one-way ANOVA), but not in unirradiated cells (p>0.05, by one-way ANOVA) (Figure 1E). Similar results were demonstrated using CD133+ cells derived from other glioma sources, such as T4105 and T4597 (Figure 1F and data not shown). Taken together, these results strongly suggest that Notch inhibition with γ-secretase inhibitors compromises radioresistance of glioma stem cells.
γ-secretase inhibitors enhance radiation-induced cell death in glioma stem cells
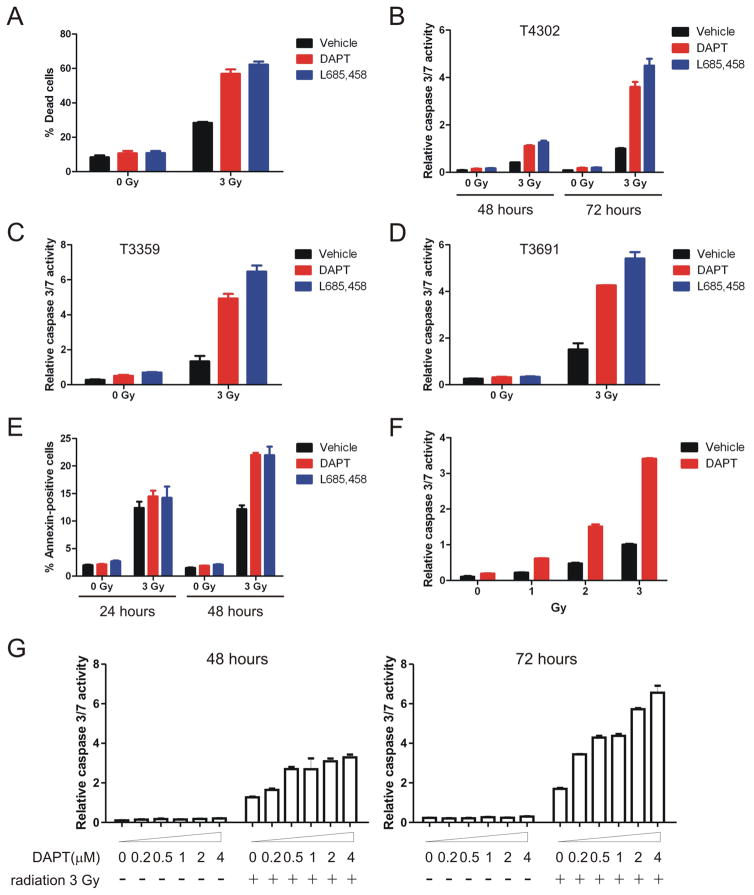
Significantly greater radiation-induced cell death was observed in GSI-treated cells compared with sham-treated cells. Two days after radiation treatment, vehicle-treated T4302 CD133+ cells contained approximately 30% dead cells, whereas 60% of cells were dead in the presence of DAPT or L685,458 (Figure 2A, p=0.0002, by one-way ANOVA). Conversely, GSIs did not have an obvious impact on the ratio of dead cells without radiation treatment (Figure 2A, p>0.05, by one-way ANOVA). The overwhelming majority of GSI-treated cells died within 72 hours after 3 Gy of irradiation. However, excessive amount of cell debris interfered with the trypan blue exclusion assay that was used to count cell numbers. Activation of caspase is a hallmark of apoptotic cell death and is critically involved in radioresponse [43,44,45,46]. Using luminescence-based caspase-3/7 assays, we examined how Notch inhibition affected radiation induced activation of caspase as a surrogate marker of cell death. GSIs alone did not significantly alter caspase activities in the sham-irradiated cells (Figure 2B, p>0.05, by one-way ANOVA). In contrast, combining GSIs with 3-Gy radiation increased caspase activities for approximately 2 folds at 48 hours and 4 folds at 72 hours (Figure 2B, p<0.01, by one-way ANOVA). Similar results were detected using CD133+ glioma cells derived from several glioma sources, including T3359, T3691, T4105 and T4597 (Figure 2C, 2D and data not shown). In validation studies, apoptotic cell death was assessed by flow cytometry analysis of Annexin V, a cell surface marker for cells undergoing apoptosis. Both DAPT and L685,458 significantly increased Annexin V positively labeled cells at 48 hours following radiation (Figure 2E, p<0.01, by one-way ANOVA), although the effects were not obvious at 24 hours following radiation (Figure 2E, p>0.05, by one-way ANOVA). Additionally, DAPT enhanced radiation-induced cell death at different radiation doses (approximately 2-fold increase at 1 Gy, and 3-fold at 2 and 3 Gy, 72 hours after radiation exposure) as determined by caspase assays (Figure 2F). The radiosensitizing effects of DAPT showed a concentration-dependent manner, with 200 nM DAPT (IC50 as suggested by the manufacturer) still being effective (Figure 2G, p<0.01 at 72 hours after radiation, by Student’s t-test). Collectively, these data demonstrate that Notch inhibition using γ-secretase inhibitors sensitize glioma stem cells to radiation-induced cell death.
Figure 2. γ-secretase inhibitors enhance radiation-induced cell death in glioma stem cells.
(A) T4302 CD133+ cells were pre-treated with DAPT or L685,458 for 4 hours, and irradiated at 3 Gy. Percentage of dead cells was determined by trypan blue exclusion assay at 48 hours after radiation exposure. (B) T4302 CD133+ cells were treated with GSIs ± radiation as indicated. Cells were then plated at 3000 cells per well in 96-well plates. Relative caspase 3/7 activities were determined by normalizing caspase activities to the corresponding cell titers. (C, D) Relative caspase 3/7 activities of T3359 and T3691 CD133+ cells at 72 hours after radiation were determined as described above. (E) T4302 CD133+ cells were treated as described in figure 2A. 24 or 48 hours after radiation exposure, cells were labeled with FITC-conjugated Annexin V antibody (BD Sciences) according to manufacturer’s instructions and analyzed by flow cytometry. (F) T4302 CD133+ cells were pre-treated with 2 μM DAPT for 4 hours, and were left unirradiated or irradiated at 1, 2 or 3 Gy. Relative caspase 3/7 activities were determined at 72 hours after radiation. (G) T4302 CD133+ cells were pre-treated with DAPT at indicated concentrations for 4 hours, and irradiated at 3 Gy. Relative caspase activities were determined at 48 or 72 hours after radiation.
CD133-negative glioma cells do not respond to γ-secretase inhibitors
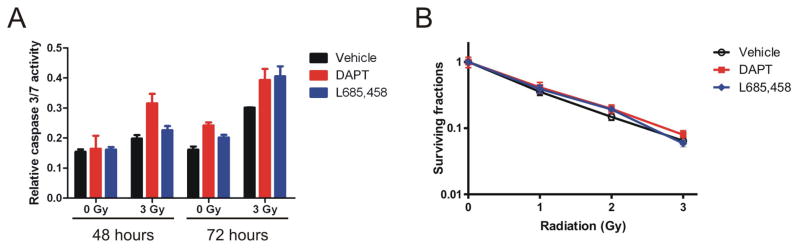
We further determined if Notch inhibition affected radioresponse of the CD133-negative fraction of gliomas. Following similar procedures, cell death and clonogenic survival after radiation exposure were determined in the T4302 CD133-negative cells with or without GSI treatment. Interestingly, in CD133-negative glioma cells, GSI treatment did not significantly promote caspase activation or impair clonogenic survival regardless of radiation exposure (Figure 3A and 3B, p>0.05, by one-way ANOVA). For clonogenic survival assays, the CD133-negative cells were incubated over night and pre-treated with GSIs in stem cell media prior to radiation, and were changed back to DMEM media supplemented with serum two days after radiation. In contrast, GSI treatment still repressed the clonogenic survival of the CD133+ glioma cells if they were plated in serum-containing DMEM media after radiation and formed colonies in monolayer (data not shown). Therefore, the resistance of CD133-negative glioma cells toward Notch inhibition in combination with radiation was not due to difference in culture media. Taken together, our data suggest that the radiosensitizing effects of Notch inhibition are specific to the cancer stem cell fraction of gliomas.
Figure 3. Notch inhibition by GSIs does not significantly alter cell death or clonogenic survival of irradiated T4302 CD133-negative cells.
T4302 CD133-negative cells were pretreated with 2 μM GSI or 0.5 μM L685,458 for 4 hours, and irradiated as indicated. (A) Relative caspase 3/7 activities were determined at 48 or 72 hour after radiation as described in figure 2B. (B) After radiation, T4302 CD133- cells in 6-well plates were cultured for three weeks, fixed and stained to count colonies. Surviving fractions were determined as described in methods.
Expression of the constitutively active intracellular domains of Notch1 or Notch2 attenuates the radiosensitizing effects of GSIs
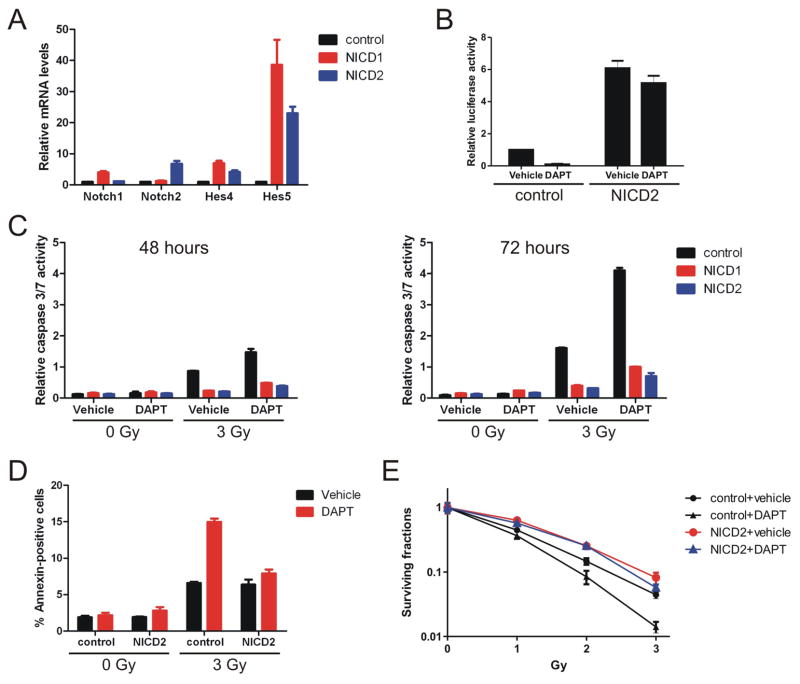
Substrates of GSIs include more than fifty type I membrane proteins in addition to the Notch receptors [47]. The radiosensitizing effects of GSIs may not be necessarily mediated through inhibition of Notch. We therefore validated the role of Notch in radioresponse of glioma stem cells by expression of the constitutively active intracellular domains of Notch1 or Notch2 (NICD1 or NICD2) that function downstream of GSIs. Activation of the Notch pathway by lentivirus-mediated expression of NICD1 or NICD2 was shown by upregulation of Notch target genes, Hes4 and Hes5, using quantitative real-time PCR (Figure 4A), as well as increased luciferase activities generated from the Notch-responsive reporter (Figure 4B). Similar to the parental cells, cells infected with the control lentivirus were sensitive to DAPT when irradiated (Figure 4C). In contrast, cells expressing either NICD1 or NICD2 demonstrated significantly weaker radiation-induced cell death in comparison to control cells regardless of DAPT treatment (Figure 4C, p=0.001 at 48 hours, and p<0.001 at 72 hours, control vs. NICD1/NICD2, by one-way ANOVA). Additionally, DAPT treatment failed to increase the percentage of Annexin V-positive cells in the presence of NICD2 expression (Figure 4D, p>0.05, NICD2 vs. NICD2+DAPT, by Student’s t-test). Finally, expression of NICD2 improved clonogenic survival of the T4302 glioma stem cells following radiation regardless of DAPT treatment (Figure 4E, p=0.004, NICD2 vs. control±DAPT; p<0.01, NICD2+DAPT vs. control±DAPT, at 3-Gy radiation, by one-way ANOVA), whereas DAPT treatment did not significantly alter clonogenic survival of irradiated NICD2-expressing cells (Figure 4E, NICD2 vs. NICD2+DAPT, p>0.05, by Student’s t-test). Collectively, our data demonstrate that Notch activation by expression of NICDs attenuates the radiosensitizing effects of GSIs, suggesting that these activities of GSIs are primarily mediated through inhibition of Notch.
Figure 4. Expression of the intracellular domains of Notch1 or Notch2 attenuates the radiosensitizing effects of GSIs.
(A) T4302 CD133+ cells were infected with control lentivirus or lentivirus directing expression of NICD1 or NICD2, and selected by puromycin. Expression of Notch1, Notch2 and Notch target genes, Hes4 and Hes5 were determined by quantitative real-time PCR. (B) Control T4302 cells or cells expressing NICD2 were transfected with Notch-dependent luciferase reporter, and treated with DMSO or 2 μM DAPT over night. Notch-dependent transcription activities were determined by luciferase reporter assays as described in figure 1A. (C) Control cells or cells expressing NICDs were treated with DAPT ± radiation as indicated. Relative caspase 3/7 activities were determined at 48 or 72 hours after radiation, (D) apoptotic cell death was determined by Annexin V-staining as described in figure 2E, and (E) surviving fractions were determined as described in figure 1D.
Knockdown of Notch1 or Notch2 sensitizes glioma stem cells to radiation
Individual Notch receptors may have distinct biological functions in certain tumor types [48]. In human medulloblastoma, it has been demonstrated that expression of the constitutively intracellular regions of Notch1 or Notch2 appears to exert opposite effects, with Notch1 acting as tumor suppressor and Notch2 acting as tumor promoter [49]. In gliomas, knockdown of Notch1 impairs cell growth and survival [50]. By expression of NICD1 or NICD2, our results suggest that both Notch receptors promote radioresistance of glioma stem cells. To gain a better understanding of how individual Notch receptors are involved in radioresponse, we determined the effects of knockdown of Notch1 or Notch2 on radioresistance through lentivirus-mediated expression of shRNA targeting Notch1 or Notch2. The efficiency of downregulation of the corresponding Notch receptors and Notch target genes were determined by quantitative real-time PCR (Figure 5A). Consistent with previous report, knockdown of Notch1 alone increased apoptotic cell death in T4302 CD133+ cells. Interestingly, similar effects were achieved by knockdown of Notch2 (Figure 5B). Cells with reduced Notch1 or Notch2 expression were highly sensitive to radiation-induced cell death (Figure 5B, p<0.001, non-targeting shRNA vs. Notch1/2 shRNAs, by one-way ANOVA). Consistently, the clonogenic survival of glioma stem cells was severely impaired by knockdown of either Notch1 or Notch2 (Figure 5C). When exposed to 3-Gy radiation, glioma stem cells expressing shRNA specific to Notch1 or Notch2 rarely formed neurospheres. These results further validate the specific role of Notch pathway in regulation of radioresistance, and suggest that multiple Notch receptors may be required for radioresistance of glioma stem cells.
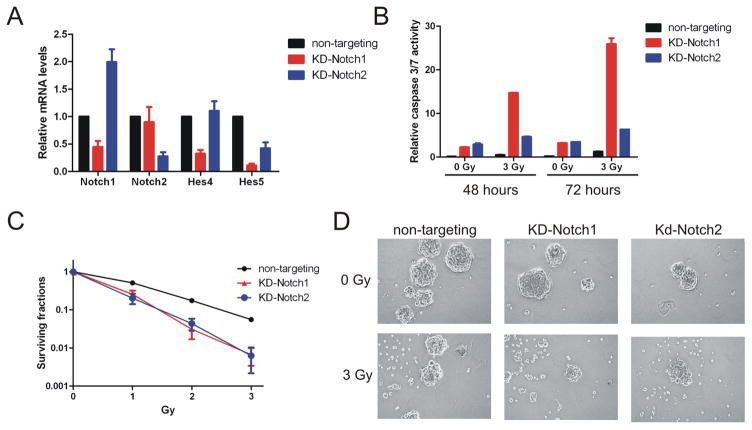
Figure 5. Knockdown of Notch1 or Notch2 increases radiosensitivity of glioma stem cells.
T4302 CD133+ cells were infected with lentivirus directing expression of non-targeting shRNA or shRNAs specific to Notch1 or Notch2 (KD-Notch1 or KD-Notch2). (A) Relative mRNA levels of Notch1, Notch2, Hes4 or Hes5 were determined by quantitative real-time PCR. (B) Cells were irradiated and aliquoted to determine relative caspase 3/7 activities as described in figure 2B. (C) Clonogenic survival was compared between the control group and cells with Notch1 or Notch2 knockdown as described in figure 1D. (D) Representative images (100×) of neurospheres formed by T4302 CD133+ cells with or without Notch knockdown two weeks after radiation.
Notch pathway does not alter DNA damage response of glioma stem cells
The DNA damage checkpoint response plays a critical role in cellular response toward radiation [51,52]. Our previous study shows that increased activation of the DNA damage response is implicated in radioresistance of the glioma stem cells [7]. To determine the mechanisms through which Notch promotes radioresistance of glioma stem cells, we first assessed if the Notch pathway affected activation of the checkpoint kinases following radiation. Neither of the two GSIs examined significantly altered activating phosphorylation of Chk1 or Chk2 following radiation (Figure 6A). Additionally, DAPT increased radiation-induced cell death even when it was added 24 hours after radiation (Figure 6B, p=0.0045, untreated vs. treated at 24 hours, by Student’s t-test), whereas the bulk DNA damage repair process is usually completed within a few hours after radiation exposure [53]. These results suggest that the DNA damage response is not involved in the radioprotective function of Notch pathway.
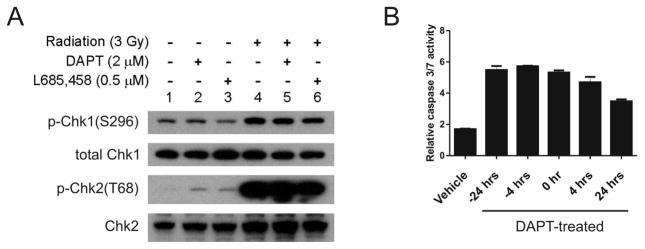
Figure 6. Notch pathway does not alter DNA damage response of glioma stem cells.
(A) T4302 CD133+ cells were pretreated with DPAT or L685,458 for 24 hours prior radiation. One hour after radiation exposure, cells were harvested. The levels of activating phosphorylation of Chk1 and Chk2 were assessed by immunoblotting. (B) T4302 CD133+ cells were treated with 2 μM DAPT at 4, 24 hours prior to radiation (−24 hrs, or −4 hrs), or at 0, 4, 24 hours after radiation. Relative caspase 3/7 activities were measured 3 days after radiation exposure.
Notch promotes radioresistance through regulation of the PI3K/Akt pathway and levels of Mcl-1 proteins
Inhibition of Notch renders glioma stem cells more sensitive to radiation-induced cell death. In a context-dependent manner, Notch can promote cellular survival through different mechanisms, such as activation of the PI3K/Akt pathway [54,55], and upregulation of the prosurvival proteins, Bcl-2 and Mcl-1 [56]. We compared alterations of these prosurvival factors in the NICD2-expressing T4302 glioma stem cells and the control cells treated with DPAT, radiation, or both. Activating phosphorylation at serine 473 of Akt was used as a surrogate marker of Akt activity. In the absence of radiation, neither Notch inhibition by DAPT nor Notch activation by NICD2 expression apparently affected Akt activity. The levels of phospho-S473 Akt gradually increased at 24 hours and 48 hours after radiation (Figure 7A), which were attenuated by DAPT treatment (Figure 7A). In contrast, expression of NICD2 enhanced radiation-induced Akt activation, which was partially attenuated but not abolished by DAPT at 48 hours after radiation (Figure 7A). Our laboratory recently reported that Akt activity was essential for survival of glioma stem cells [40]. In accordance with this finding, treatment of a PI3K inhibitor, LY294002, or the Akt inhibitor III, increased cell death of T4302 glioma stem cells with or without radiation (Figure 7B). Expression of NICD2 did not overcome the apoptotic effects of either LY294002 or the Akt inhibitor III, suggesting that the PI3K/Akt pathway functions downstream of Notch in support of cellular survival (Figure 7B). Interestingly, expression of NICD2 did not reduce the levels of PTEN, a major negative regulator of the PI3K/Akt pathway. Instead, expression of NICD2 increased levels of PTEN independent of radiation treatment. Whether PTEN in the examined glioma stem cells is functional remains to be determined, our results suggest that Notch does not activate the PI3K/Akt pathway by repressing PTEN.
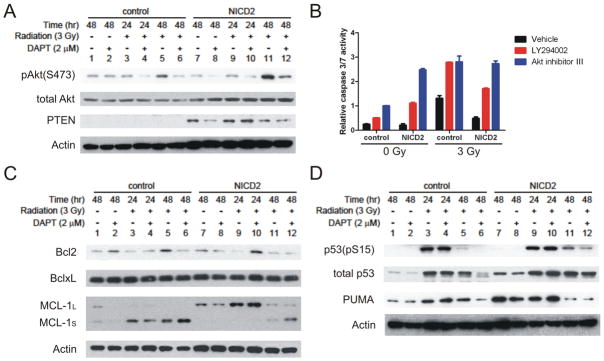
Figure 7. Notch pathway regulates radioresistance of glioma stem cells via the Akt pathway and Mcl-1.
T4302 CD133+ cells were infected with control lentivirus or lentivirus directing expression of NICD2. Cells were pre-treated with 2 μM DPAT for 4 hours and exposed to 3-Gy radiation. Irradiated cells were collected at 24 or 48 hours after radiation, and unirradiated cells were collected at 48 hours after radiation. (A C, D) Protein levels of phospho-Akt (S473), total Akt, PTEN, Bcl-2, Bcl-xL, Mcl-1, phospho-p53 (S15), total p53 and PUMA were assessed by immunoblotting. Actin was used as the loading control. (B) Control cells or NICD2-expressing cells were pre-treated with 10 μM PI3K inhibitor, LY294002, or 50 μM Akt inhibitor III (SH-6), for 4 hours prior to radiation exposure. Relative caspase 3/7 activities were determined at 48 hours after radiation exposure.
The anti-apoptotic Bcl-2 proteins, including Bcl-2, Bcl-xL and Mcl-1, are important promoters for cell survival [57]. The levels of Bcl-2 and Bcl-xL were not apparently altered by either radiation or Notch alteration (Figure 7C). Interestingly, radiation decreased the levels of full-length anti-apoptotic Mcl-1(Mcl-1L), and simultaneously increased the levels of a truncated isoform of Mcl-1 (Mcl-1S) (Figure 7C), which is apoptotic due to loss of BH1 and BH2 domain [58]. DAPT treatment reduced Mcl-1L and increased Mcl-1S following radiation. Conversely, NICD2 expression switched the balance toward Mcl-1L with or without radiation treatment (Figure 7C). These results suggest that the balance between Mcl-1L and Mcl-1S is implicated in post-radiation survival of glioma stem cells, and this balance is regulated by the Notch pathway.
The functional interaction between Notch and p53 is intriguing, as Notch can activate p53 [59,60] or inhibit p53 [61] in a cell type-specific manner. As a crucial regulator of cellular radioresponse, p53 activity may be involved in the radioprotective function of Notch. Our results showed that expression of NICD2 increased p53 levels in the absence of radiation, and enhanced p53 activation induced by radiation (Figure 7D). Additionally, expression of p53 target gene, PUMA, was upregulated by NICD2. These results are consistent with previous study showing that Notch1 upregulates p53 in gliomas [60], suggesting that the radioprotective functions of Notch were not mediated by inhibition of p53.
Discussion
Fundamental improvements in brain cancer treatment will require the development of new therapeutic paradigms. Temozolomide, an oral methylating chemotherapeutic agent, became standard of care for newly diagnosed glioblastoma when used concurrently with external beam radiation followed by adjuvant therapy [3], suggesting that combining therapies with radiation may shift the survival curve. Despite the benefit of temozolomide, glioblastomas continue to be highly resistant to radiation [4]. We recently demonstrated that the cancer stem cell fraction of gliomas was more resistant to radiation than non-stem glioma cells [7]. Given the high tumorigenic capacity of glioma stem cells, this paradigm suggests that disrupting radioresistance of glioma stem cells may augment the efficacy of radiotherapy. We have already shown that inhibitors of the checkpoint kinases, Chk1/2, sensitized glioma stem cells to radiation [7]. However, DNA damage checkpoint inhibitors may have limited therapeutic index due to shared dependence of normal cells on these molecules in radioresponse [62]. In current study, we described a novel radioprotective role of Notch signaling in glioma stem cells. Our results show that Notch inhibition using GSIs or Notch1/2-specific shRNA render glioma stem cells sensitive to radiation. In further support of these observations, activation of Notch via expression of NICD1 or NICD2 promotes radioresistance of glioma stem cells. Cells expressing NICD1 or NICD2 are resistant to the radiosensitizing functions of GSIs, because NICDs act downstream of GSI-mediated Notch inhibition. Taken together, these pharmacological and genetic approaches univocally show that Notch activity is critically implicated in radioresistance of glioma stem cells, suggesting that the Notch pathway may serve as a potential therapeutic target for improvement of radiotherapy against gliomas.
The oncogenic role of Notch is highlighted by the presence of mutations of the Notch pathway components in a variety of human tumors, in particular leukemia and breast cancer [63]. Activating mutations of Notch1 are reported in approximately 50% of human T-cell acute lymphoblastic leukemia (T-ALL), making it the most frequent oncogenic event in this disease [31]. Loss of the Notch inhibitor Numb is detected in ~50% of human mammary carcinomas [29,30]. Upregulated expression of Notch pathway components also present in glioblastoma multiforme cell lines and surgical biopsy specimens [28]. A critical role of the Notch pathway in the maintenance of brain tumor stem cells has recently emerged [37]. Our results suggest that the radioprotective functions of Notch are specific to the CD133+ glioma stem cells. Neither cell death nor clonogenic survival of irradiated non-stem glioma cells are apparently altered by GSI treatment. These studies suggest that stem cell-specific pathways, such as Notch, may serve as potential therapeutic targets that may not be readily appreciated by studies using whole tumors.
The Notch pathway can promote or repress tumorigenesis in a context-dependent manner. For example, loss of Notch1 or expression of a dominant negative MAML1 mutant in mouse epidermis induces hyperplasia and subsequent skin tumors [64,65]. In medulloblastoma, expression of NICD2 promotes tumor growth while NICD1 is tumor suppressive [49]. Conversely, knockdown Notch1 impaired proliferation and survival of glioma cell lines [50]. Using lentivirus-mediated expression of NICD1/2 or Notch1/2-specific shRNAs, our data suggest that both Notch1 and Notch2 promote radioresistance in glioma stem cells, although the relative contribution of each Notch receptor is undefined. It remains to be elucidated why knockdown of individual Notch receptors demonstrated higher toxicity than comprehensive inhibition of Notch using GSIs in glioma stem cells. The toxicity may involve off-target effects of the shRNAs or oversaturation of the microRNA/short hairpin RNA pathways [66]. Alternatively, these results suggest that membrane-bound Notch receptors may have important biological functions in gliomas that have not been recognized. The specific role of each individual Notch receptor in regulation of radioresistance of glioma stem cells is beyond the scope of this study, but it will be important to define the radioprotective functions of each Notch receptor, because specific Notch neutralizing antibodies are under clinical development for systemic cancer treatment and may have distinct adverse effects in a therapeutic setting. Notch1 regulates intestinal stem cell fate determination and gut homeostasis [23,24]. Clinical trials using GSIs for treatment of Alzheimer’s disease are primarily associated with gastrointestinal toxicity [67,68], which is likely due to inhibition of Notch1 by GSIs. If Notch receptors other than Notch1 are also essentially required for radioresistance of glioma stem cells, small molecules or antibodies that specifically target these receptors while spare Notch1 may achieve similar radiosensitizing effects in gliomas with reduced gut toxicity.
Our results demonstrated that the most significant biological consequence of Notch inhibition in irradiated glioma stem cells is the dramatically increased cell death occurring within 3 days after radiation, suggesting that Notch is crucially involved in post-radiation survival of glioma stem cells. In accordance with these observations, Notch activity alters activation of Akt and levels of Mcl-1 in irradiated glioma stem cells. Activation of the PI3K/Akt pathway plays a central role in the radioprotective function of Notch, as shown by our results that inhibitors of PI3K or Akt abolish the radioprotective activities of NICD2. The PI3K/Akt pathway has profound functions in many aspects of cell growth, proliferation and survival [69]. We previously described an important link between the PI3K/Akt pathway with growth and survival of glioma stem cells [40]. Confirmation of these findings came from studies of a mouse medulloblastoma model in which cancer stem cells were defined by stem cell markers and a perivascular location, but without functional validation. In these studies, the PI3K/Akt pathway is activated in medulloblastoma stem cells following radiation and is essentially required for post-radiation cell survival [70]. Consistent with our findings, inhibition of Akt sensitizes the medulloblastoma stem cells to radiation [70]. It has been recently reported that Mcl-1 is a critical regulator of survival in neural precursors during development and after DNA damage [56,71]. Akt activity promotes expression of the anti-apoptotic isoform of Mcl-1L[72]. Therefore, the regulation of post-radiation balance between Mcl-1L and Mcl-1s by Notch is potentially mediated through Akt. Expression of NICD2 increases levels of PTEN, which negatively regulates Akt activity, and p53 independent of radiation exposure, suggesting that the activities of these two proteins may not be directly involved in radioprotective functions of Notch. However, medulloblastoma stem cells survive radiation and undergo p53-dependent cell cycle arrest, where PTEN is required for radiation-induced p53 activation [70]. Therefore, it is possible that in glioma stem cells, Notch-induced PTEN and p53 indirectly contribute to reduced cell death after radiation by promoting cell cycle arrest. However, due to the tumor suppressive functions of these two proteins, their specific roles in radioresponse of glioma stem cells remain to be elucidated. Taken together, our study and results from other groups illustrate a signaling pathway that is critically involved in regulation of radioresponse of glioma stem cells and potentially other types of cancer stem cells. This pathway provides multiple potential therapeutic targets at several levels, including the upstream Notch receptors, the downstream kinases (PI3K, Akt), and the pro-/anti-apoptotic molecules (Mcl-1L and Mcl-1S).
The last proteolytic step that generates the active intracellular domains of Notch receptors is dependent on the γ-secretase complex [19,20]. A substantial number of γ-secretase inhibitors have been developed for treatment of Alzheimer’s disease, because the γ-secretase complex mediates production of β-amyloid peptides, the precursor of amyloid plaques found in Alzheimer’s disease [73]. Given their activities of inhibiting Notch, GSIs have also been actively examined for their anti-cancer efficacy in a variety of tumor types, such as T-ALL [74], breast cancer [75], Kaposi’s sarcoma [76], medulloblastoma [77], intestinal adenoma [24], etc. Clinical trials with MK0752, a γ-secretase inhibitor made by Merck, are underway in patients with breast cancer and pediatric central nervous system malignancies (clinicaltrials.gov). Our study suggest that GSIs alone only display limited growth inhibitory effects in gliomas, while in combination with radiotherapy, GSIs may provide significant therapeutic benefits by promoting the radiosensitivity of glioma stem cells. Therefore, additional efforts will be required to evaluate the clinical benefits of GSIs in combination with radiation as the monotherapy trials of GSIs may not show significant anti-tumor efficacy. Taking account into the prevalent role of Notch in regulation of stem cell behavior and cancer biology, it is reasonable to speculate that the radiosensitizing effects of Notch inhibition are not limited in gliomas and may be a broadly useful treatment paradigm.
Materials and Methods
Enrichment of glioma stem cells and cell culture
Matched cultures enriched or depleted for glioma stem cells were isolated from human surgical specimens (T3359, T3691, T4105, T4302, and T4597) that were immediately implanted in immunocompromised mice (athymic BALB/c nude) according to a method that has been described in our previous studies and those of others to preserve cancer stem cells in glioma models [7,38,39,40,41,42]. Briefly, tumors were dissected, washed in Earle’s balanced salt solution, digested with papain (Worthington Biochemical, Lakewood, NJ) and filtered through 70 μm cell strainer to remove tissue pieces. Red blood cells were lysed in diluted phosphate buffered saline solution (0.25×). Dissociated cells were then cultured overnight in stem cell media (neurobasal media supplemented with B27, epidermal growth factor and basic fibroblast growth factor at 20 ng/ml, Invitrogen, Carlsbad, CA) prior to cell sorting for recovery of cellular surface antigens. The CD133− and CD133+ fractions were separated by magnetic sorting using the CD133 Microbead kit (Miltenyi Biotec, Auburn, CA). CD133− cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (Invitrogen), but were cultured in stem cell media at least 24 hours prior to experiments to control differences in cell media. The cancer stem cell properties of the (DMEM) with 10% fetal bovine serum (Invitrogen), but were cultured in stem cell media at least 24 hours prior to experiments to control differences in cell media. The cancer stem cell properties of the CD133 positive cells was confirmed by fluorescent in situ hybridization, serial neurosphere assays, and xenotransplantation assays, but cultures depleted of cancer stem cells did not initiate tumors ([7,38,39,40,41,42] and data not shown).
Antibodies, DNA constructs, lentiviruses and other reagents
The antibodies purchased from Cell Signaling Technology (Danvers, MA) include phosphor-S296-Chk1 (2349), total Chk1 (2345), phosphor-T68-Chk2 (2661), total Chk2 (2662), phospho-S473 Akt (#9271), total Akt (#9272), PTEN (#9556), phospho-S15-p53 (#9286), Bcl-2 (#2870), Bcl-xL (#2764), Mcl-1 (#4572), Puma (#4976). Other antibodies used in this study are p53 antibody (#sc-126, Santa Cruz Biotechnology, Santa Cruz, CA), and actin antibody (Millipore, Billerica, MA). The pcDNA3-Notch1 construct was kindly provided by Dr. Spyros Artavanis-Tsakonas, Harvard University. The coding sequences of intracellular domain of Notch1 (amino acid residues 1744–2556) was amplified by PCR and subcloned into the lentiviral vector pCDH-CMV-EF1-puro (System Biosciences, Mountain View, CA) through the EcoRI and NotI sites. The pcDNA3-NICD2 is a kind gift of Dr. Charles Eberhart, John Hopkins University. The coding sequence of NICD2 was subcloned into pCDH-CMV-EF1-puro through the BamHI and NotI sites. The pLKO-shNotch1 construct was a kind gift of Dr. Adolfo Ferrando, Columbia University. The pLKO-shNotch2 construct was purchased from Open Biosystems (Huntsville, AL, clone ID, TRCN0000056426). Lentiviruses were produced in 293FT cells as previously described with the packaging plasmids psPAX2 and pCI-VSVG [42]. LY294002, Akt inhibitor III (SH-6), and the γ-secretase inhibitors, DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-Sphenylglycine t-butyl ester) or L685,458, (5S)-(t-Butoxycarbonylamino)-6-phenyl-(4R)hydroxy-(2R)benzylhexanoyl)-L-leu-L-phe-amide) were purchased from Calbiochem (Gibbstown, NJ).
Radiation
Cells were irradiated as single cell suspension for CD133+ cells or as monolayer culture for CD133-negative cells using an AGFA X-RAD 320 irradiation system at 0.85 Gy/min.
Growth Curve, caspase 3/7 assay, and clonogenic survival assay
Cells were infected with lentivirus or treated as described in figure legends. Following radiation, cells were aliquoted into 96-well plate at 3000 cells per well in triplicates. Cell number was measured for 5 days using the CellTiter-Glo assay kit (Promega, Madison, MI). Averaged cell titer of the control group on day 1 was assigned a value of 1. All other relative cell titers were normalized accordingly.
The caspase activities at 48 or 72 hours after radiation were measured using the Caspase-Glo 3/7 assay (Promega) according to the manufacturer’s instructions. The caspase activities were normalized to the corresponding cell titers to obtain the relative caspase 3/7 activities.
To measure clonogenic survival, CD133+ glioma cells were plated in 24-well plates at 50 cells per well for the sham-irradiated groups, 100 cells per well for cells irradiated at 1 Gy, or 500 cells per well for cells irradiated at 2 or 3 Gy. Eight wells were plated for each group. Fourteen days after plating, neurospheres containing more than 50 cells were scored. Alternatively, CD133-negative glioma cells were plated in 6-well tissue culture plates in triplicates at 100 cells per well for the sham-irradiated groups, 200 cells per well for cells irradiated at 1 Gy, or 500 cells per well for cells irradiated at 2 or 3 Gy. Twenty-one days after radiation, cells were fixed and stained with 0.5% crystal violet. Colonies consisting of more than 50 cells were scored. To determine the surviving fractions, the number of neurosphere at each radiation dose was normalized to that of the corresponding unirradiated control group.
Luciferase assay
The Notch-responsive luciferase reporter was purchased from SABiosciences (Frederick, MD), which was pre-mixed with the internal control construct directing constitutive expression of the Renilla luciferase. Cells were transfected with the luciferase reporter by FuGene 6 (Roche). The luciferase activities were determined by the Dual-luciferase assay system (Promega).
Real-time PCR
Total RNA was prepared using the RNeasy kit (Qiagen, Valencia, CA), and reverse transcribed into cDNA by iScript cDNA synthesis kit (BioRad, Hercules, CA). Real-time PCR was performed on a Bio-Rad iCycler system using SYBR-Green Mastermix (SABiosciences, Frederick, MD). PCR products were verified by melting curves. The threshold cycle (CT) values for each gene were normalized to CT of β-Actin and HPRT1 (hypoxanthine phosphoribosyltransferase 1). The primers used were as follows: Notch1, forward 5′-CGC ACA AGG TGT CTT CCA G, reverse 5′-AGG ATC AGT GGC GTC GTG; Notch 2, forward 5′-TGG TGG CAG AAC TGA TCA AC, reverse 5′-CTG CCC AGT GAA GAG CAG AT; Hes4, forward 5′-TGG ACG CCC TCA GAA AAG, reverse 5′-GCT CCG CAG GTG TCT CAC; Hes5, forward 5′-TGG AGA AGG CCG ACA TCC T, reverse 5′-GGC GAC GAA GGC TTT GC.
Acknowledgments
We like to thank Spyros Artavanis-Tsakonas, Charles Eberhart, Adolfo Ferrando, John Alberta for reagents. We are also grateful to Mark Dewhirst for helpful discussion.
References
- 1.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 2.Grossman SA, Batara JF. Current management of glioblastoma multiforme. Semin Oncol. 2004;31:635–644. doi: 10.1053/j.seminoncol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 5.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 6.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 7.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 8.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 9.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 10.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 11.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 13.Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 17.Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Bresnick EH. Bare rudiments of notch signaling: how receptor levels are regulated. Trends Biochem Sci. 2007;32:477–485. doi: 10.1016/j.tibs.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, et al. Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature. 2000;405:966–970. doi: 10.1038/35016111. [DOI] [PubMed] [Google Scholar]
- 20.Armogida M, Petit A, Vincent B, Scarzello S, da Costa CA, et al. Endogenous beta-amyloid production in presenilin-deficient embryonic mouse fibroblasts. Nat Cell Biol. 2001;3:1030–1033. doi: 10.1038/ncb1101-1030. [DOI] [PubMed] [Google Scholar]
- 21.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, et al. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farnie G, Clarke RB. Mammary stem cells and breast cancer--role of Notch signalling. Stem Cell Rev. 2007;3:169–175. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- 23.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 24.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 25.Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, et al. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hitoshi S, Seaberg RM, Koscik C, Alexson T, Kusunoki S, et al. Primitive neural stem cells from the mammalian epiblast differentiate to definitive neural stem cells under the control of Notch signaling. Genes Dev. 2004;18:1806–1811. doi: 10.1101/gad.1208404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura Y, Sakakibara S, Miyata T, Ogawa M, Shimazaki T, et al. The bHLH gene hes1 as a repressor of the neuronal commitment of CNS stem cells. J Neurosci. 2000;20:283–293. doi: 10.1523/JNEUROSCI.20-01-00283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanamori M, Kawaguchi T, Nigro JM, Feuerstein BG, Berger MS, et al. Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg. 2007;106:417–427. doi: 10.3171/jns.2007.106.3.417. [DOI] [PubMed] [Google Scholar]
- 29.Pece S, Serresi M, Santolini E, Capra M, Hulleman E, et al. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J Cell Biol. 2004;167:215–221. doi: 10.1083/jcb.200406140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Politi K, Feirt N, Kitajewski J. Notch in mammary gland development and breast cancer. Semin Cancer Biol. 2004;14:341–347. doi: 10.1016/j.semcancer.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 32.Bolos V, Blanco M, Medina V, Aparicio G, Diaz-Prado S, et al. Notch signalling in cancer stem cells. Clin Transl Oncol. 2009;11:11–19. doi: 10.1007/s12094-009-0305-2. [DOI] [PubMed] [Google Scholar]
- 33.Gal H, Amariglio N, Trakhtenbrot L, Jacob-Hirsh J, Margalit O, et al. Gene expression profiles of AML derived stem cells; similarity to hematopoietic stem cells. Leukemia. 2006;20:2147–2154. doi: 10.1038/sj.leu.2404401. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XP, Zheng G, Zou L, Liu HL, Hou LH, et al. Notch activation promotes cell proliferation and the formation of neural stem cell-like colonies in human glioma cells. Mol Cell Biochem. 2008;307:101–108. doi: 10.1007/s11010-007-9589-0. [DOI] [PubMed] [Google Scholar]
- 35.Fan X, Matsui W, Khaki L, Stearns D, Chun J, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 36.Scharpfenecker M, Kruse JJ, Sprong D, Russell NS, Ten Dijke P, et al. Ionizing radiation shifts the PAI-1/ID-1 balance and activates notch signaling in endothelial cells. Int J Radiat Oncol Biol Phys. 2009;73:506–513. doi: 10.1016/j.ijrobp.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Li Y, Banerjee S, Sarkar FH. Emerging role of Notch in stem cells and cancer. Cancer Lett. 2009;279:8–12. doi: 10.1016/j.canlet.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shu Q, Wong KK, Su JM, Adesina AM, Yu LT, et al. Direct orthotopic transplantation of fresh surgical specimen preserves CD133+ tumor cells in clinically relevant mouse models of medulloblastoma and glioma. Stem Cells. 2008;26:1414–1424. doi: 10.1634/stemcells.2007-1009. [DOI] [PubMed] [Google Scholar]
- 39.Bao S, Wu Q, Li Z, Sathornsumetee S, Wang H, et al. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008;68:6043–6048. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eyler CE, Foo WC, LaFiura KM, McLendon RE, Hjelmeland AB, et al. Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells. 2008;26:3027–3036. doi: 10.1634/stemcells.2007-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z, Bao S, Wu Q, Wang H, Eyler C, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Wang H, Li Z, Wu Q, Lathia JD, et al. c-Myc is required for maintenance of glioma cancer stem cells. PLoS ONE. 2008;3:e3769. doi: 10.1371/journal.pone.0003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coelho D, Holl V, Weltin D, Lacornerie T, Magnenet P, et al. Caspase-3-like activity determines the type of cell death following ionizing radiation in MOLT-4 human leukaemia cells. Br J Cancer. 2000;83:642–649. doi: 10.1054/bjoc.2000.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Essmann F, Engels IH, Totzke G, Schulze-Osthoff K, Janicke RU. Apoptosis resistance of MCF-7 breast carcinoma cells to ionizing radiation is independent of p53 and cell cycle control but caused by the lack of caspase-3 and a caffeine-inhibitable event. Cancer Res. 2004;64:7065–7072. doi: 10.1158/0008-5472.CAN-04-1082. [DOI] [PubMed] [Google Scholar]
- 45.Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, et al. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999;274:21155–21161. doi: 10.1074/jbc.274.30.21155. [DOI] [PubMed] [Google Scholar]
- 46.Michelin S, del Rosario Perez M, Dubner D, Gisone P. Increased activity and involvement of caspase-3 in radiation-induced apoptosis in neural cells precursors from developing rat brain. Neurotoxicology. 2004;25:387–398. doi: 10.1016/j.neuro.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Beel AJ, Sanders CR. Substrate specificity of gamma-secretase and other intramembrane proteases. Cell Mol Life Sci. 2008;65:1311–1334. doi: 10.1007/s00018-008-7462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dotto GP. Notch tumor suppressor function. Oncogene. 2008;27:5115–5123. doi: 10.1038/onc.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 50.Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 51.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 52.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 53.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 54.Palomero T, Sulis ML, Cortina M, Real PJ, Barnes K, et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007;13:1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sade H, Krishna S, Sarin A. The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J Biol Chem. 2004;279:2937–2944. doi: 10.1074/jbc.M309924200. [DOI] [PubMed] [Google Scholar]
- 56.Oishi K, Kamakura S, Isazawa Y, Yoshimatsu T, Kuida K, et al. Notch promotes survival of neural precursor cells via mechanisms distinct from those regulating neurogenesis. Dev Biol. 2004;276:172–184. doi: 10.1016/j.ydbio.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 57.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 58.Bae J, Leo CP, Hsu SY, Hsueh AJ. MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J Biol Chem. 2000;275:25255–25261. doi: 10.1074/jbc.M909826199. [DOI] [PubMed] [Google Scholar]
- 59.Ban J, Bennani-Baiti IM, Kauer M, Schaefer KL, Poremba C, et al. EWS-FLI1 suppresses NOTCH-activated p53 in Ewing’s sarcoma. Cancer Res. 2008;68:7100–7109. doi: 10.1158/0008-5472.CAN-07-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Purow BW, Sundaresan TK, Burdick MJ, Kefas BA, Comeau LD, et al. Notch-1 regulates transcription of the epidermal growth factor receptor through p53. Carcinogenesis. 2008;29:918–925. doi: 10.1093/carcin/bgn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mungamuri SK, Yang X, Thor AD, Somasundaram K. Survival signaling by Notch1: mammalian target of rapamycin (mTOR)-dependent inhibition of p53. Cancer Res. 2006;66:4715–4724. doi: 10.1158/0008-5472.CAN-05-3830. [DOI] [PubMed] [Google Scholar]
- 62.Willers H, Dahm-Daphi J, Powell SN. Repair of radiation damage to DNA. Br J Cancer. 2004;90:1297–1301. doi: 10.1038/sj.bjc.6601729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 64.Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 65.Proweller A, Tu L, Lepore JJ, Cheng L, Lu MM, et al. Impaired notch signaling promotes de novo squamous cell carcinoma formation. Cancer Res. 2006;66:7438–7444. doi: 10.1158/0008-5472.CAN-06-0793. [DOI] [PubMed] [Google Scholar]
- 66.Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 67.Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, et al. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch Neurol. 2008;65:1031–1038. doi: 10.1001/archneur.65.8.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, et al. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 69.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 70.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, et al. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arbour N, Vanderluit JL, Le Grand JN, Jahani-Asl A, Ruzhynsky VA, et al. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J Neurosci. 2008;28:6068–6078. doi: 10.1523/JNEUROSCI.4940-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang JM, Chao JR, Chen W, Kuo ML, Yen JJ, et al. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19:6195–6206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Imbimbo BP. Alzheimer’s disease: [gamma]-secretase inhibitors. Drug Discovery Today: Therapeutic Strategies. 2008;5:169–175. [Google Scholar]
- 74.O’Neil J, Calvo J, McKenna K, Krishnamoorthy V, Aster JC, et al. Activating Notch1 mutations in mouse models of T-ALL. Blood. 2006;107:781–785. doi: 10.1182/blood-2005-06-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rasul S, Balasubramanian R, Filipovic A, Slade MJ, Yague E, et al. Inhibition of gamma-secretase induces G2/M arrest and triggers apoptosis in breast cancer cells. Br J Cancer. 2009;100:1879–1888. doi: 10.1038/sj.bjc.6605034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Curry CL, Reed LL, Golde TE, Miele L, Nickoloff BJ, et al. Gamma secretase inhibitor blocks Notch activation and induces apoptosis in Kaposi’s sarcoma tumor cells. Oncogene. 2005;24:6333–6344. doi: 10.1038/sj.onc.1208783. [DOI] [PubMed] [Google Scholar]
- 77.Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004;64:7794–7800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]