Abstract
Human α-synuclein (α-Syn) is instrumental in maintaining homeostasis of monoamine neurotransmitters in brain, through its trafficking, and regulation of the cell surface expression and, thereby, activity of dopamine, serotonin and norepinephrine transporters. Here we have investigated whether other members of the synuclein family of proteins, γ-synuclein (γ-Syn) and β-synuclein (β-Syn) can similarly modulate the serotonin transporter (SERT). In Ltk− cells co-transfected with SERT and γ-Syn, γ-Syn reduced [3H]5-HT uptake, in a manner dependent on its expression levels. The decrease in SERT activity was via decreased Vmax of the transporter, without change in Km, compared to cells expressing only SERT. By contrast, β-Syn co-expression failed to alter SERT uptake activity, and neither the Vmax nor the Km was changed in the presence of β-Syn. γ-Syn modulation of SERT was only partial, with a maximal ~27% decrease in SERT activity seen even at high expression levels of γ-Syn. By contrast, α-Syn attenuated SERT activity by ~65% at identical expression levels as γ-Syn. Co-immunoprecipitation studies showed the presence of heteromeric protein:protein complexes between γ-Syn or α-Syn and SERT, while β-Syn failed to physically interact with SERT. Both α-Syn and γ-Syn colocalized with SERT in rat primary raphae nuclei neurons. These studies document a novel physiological role for γ-Syn in regulating 5-HT synaptic availability and homeostasis, and may be of relevance in depression and mood disorders, where SERT function is dysregulated.
Keywords: depression, mood disorders, monoamine transporters, γ-synuclein, serotonin transporter, raphe nuclei neurons
Introduction
The serotonin (5-hydroxytryptamine; 5-HT)1 transporter (SERT) is central in the control of 5-HT homeostasis and synaptic tone, since it is the primary mechanism for terminating signal by removing released 5-HT from the synapse, to replenish 5-HT stores in presynaptic neurons [1, 2]. Dysfunctions of SERT activity have been implicated in several mental diseases and mood disorders characterized by abnormal synaptic levels of 5-HT, including depression, schizophrenia, various cognitive diseases, sleeping, mood/anxiety disorders, alcoholism and drug addiction [3–5].
We have recently shown that the activity of SERT is negatively modulated by α-synuclein (α-Syn), through formation of a heteromeric complex by protein:protein interactions [6]. Wild-type α-Syn is a 140 residue acidic protein of ~19 kDa, highly enriched in presynaptic nerve terminals in various brain regions [7, 8], and is increasingly believed to have a central role in the regulation of monoamines transporters at synapses [6, 9–19]. We found α-Syn to decrease SERT activity by removing the transporter from the cell surface through increased trafficking into intracellular compartments, via the microtubular network [6]. In addition to SERT, we have found that α-Syn can also negatively regulate the functional reuptake activities of the dopamine transporter (DAT) [9–15], and the norepinephrine transporter (NET) [16–19], in a manner similar to SERT, suggesting that a primary normative physiological function of α-Syn is the regulation of monoamine homeostasis in brain.
Recently we found that a second member of the synuclein family of proteins, γ-synuclein (γ-Syn) can negatively regulate NET function and that it is overexpressed in the frontal cortex of the Wistar-Kyoto (WKY) rat, a common rodent model of depression [19]. Such overexpression leads to imbalances in γ-Syn/α-Syn protein ratios, resulting in dysfunctional trafficking of the transporter along with reduced cell surface expression of NET and its activity. Chronic treatment (14 days) of the WKY rat with a NET reuptake inhibitor, desipramine, reduced γ-Syn expression, normalizing γ-Syn/α-Syn protein ratios, while restoring appropriate trafficking, cell surface expression and functional response of NET in the frontal cortex. These studies suggested that alterations of γ-Syn may have an important role in the genesis and maintenance of depression.
In this paper, we wanted to ascertain whether γ-Syn is also able to negatively modulate the functional activity of SERT. Our results showing the ability of γ-Syn, but not β-Syn, to regulate SERT activity suggests a novel role for γ-Syn in the physiological regulation and maintenance of 5-HT homeostasis and clearance, while simultaneously further expanding the participation of this protein in mood disorders and depression.
Experimental procedures
Materials
All materials were obtained as described before [6, 19] and all chemicals were of analytical grade.
DNAs, Cell culture and transfections
Ltk− fibroblasts were grown as monolayer cultures in DMEM supplemented with 5 % (v/v) decomplemented and selected FBS at 37°C and 5% CO2. Mycoplasma contamination was routinely checked using Hoechst 33258. Twenty four hours after seeding, 70% confluent cells were transiently transfected using Lipofectamin™ 2000 and Opti-MEM, as described before [6] and by the manufacturer (Invitrogen), using the pcDNA3.1 (Invitrogen) mammalian expression vector carrying human SERT or human α-Syn, β-Syn or γ-Syn cDNAs. The final concentration of DNA during transfections was kept constant by adjusting with a pcDNA3.1 control vector. After transfection, cells were grown for further 2 days in DMEM + 10% FBS, to allow expression of the transgenes.
Primary neuronal cultures from rat raphe nuclei
Raphe nuclei were dissected out of the pons of 18 days-old rat embryos under a binocular microscope in ice-cold HBSS. Tissue was dissociated mechanically in ice cold HBSS. After centrifugation (4°C; 8 min; 175 g), neuronal cells were resuspended in culture medium (Neurobasal with 2 % (v/v) B27 Supplement, 0.5 mM glutamax I and 25 µM ß-mercaptoethanol). Neuronal cells were seeded in 24 well plates (Nunc) containing glass coverslips precoated with poly-L-ornithine (15% w/v; Sigma) and laminin (3 µg/ml; Sigma), and grown for 8 days as described before for mesencephalic dopaminergic neuronal cultures [10]. Neuronal cultures were grown for further 2 days in culture medium supplemented with 1% (v/v) heat inactivated horse serum. Neuronal cells were characterized at day 10 with an anti-SERT monoclonal antibody (Mab Technologies, Lemon Grove, CA; ST51-2), used as a marker of differentiation of serotonergic neurons.
Immunocytochemistry on cultured primary neurons
Neurons grown on coverslips were fixed at RT for 15 min with paraformaldehyde 3% (w/v) and non-specific fuorescence of paraformaldehyde was quenched with 50 mM NH4Cl for 30 min at RT, as described before [10]. Fixed cells were then placed into cryoprotectant medium and stored at 4°C until used for immunocytochemistry. For immunofluorescent double-staining, the nonspecific binding was blocked in D-PBS containing 2 % BSA and 0.3 % triton X-100 for 2 hours. Primary neurons were incubated (16 h; 4°C) with anti-SERT monoclonal (1:500; Mab Technologies; Lemon Grove, CA; ST51-2) and either rabbit anti-α-Syn polyclonal (1:500; sc-7011-R, Santa Cruz Biotechnology) or anti-γ-Syn polyclonal (AB6169-100, Abcam) antibodies. After incubation in the dark for 2 h at RT with 1:1,000 dilutions of Alexa 594- and Alexa 488-conjugated secondary antibodies (Molecular Probes), coverslips were mounted on slides with Prolong Gold antifade reagent and visualized under a Nikon Eclipse E800 fluorescent microscope equiped with a Nikon DXM1200 digital camera.
[3H]5-HT uptake
Uptake was determined as described previously [6] and by Ramamoorthy et al. [20]. Briefly, growth medium was removed, cells washed once with modified Krebs-Ringer Hepes uptake buffer (KRH; 10 mM HEPES, 130 mM NaCl, 1.3 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 1.88 g/l glucose, 100 µM pargyline and 200 µM ascorbic acid pH 7.4). After preincubation for 10 min at 37°C in KRH, [3H]5-HT (20 nM final concentration; Perkin Elmer; 30 Ci/mmol) was added for 10 min at 37°C. Uptake was terminated by three rapid washes with KRH. For kinetic analysis, cells were preincubated in quadruplicate with cold 5-HT [10-11−1.5x10−5 M] for 5 min prior to incubation for 10 min with [3H]5-HT. 10 µM imipramine (IMP) was used to define non-specific uptake. Cells were counted by trypan blue with a Neubauer cell as previously described [11]. After cells were lysed by frreezing/thawing in NaOH 0.1 N, accumulated [3H]5-HT was measured by liquid scintillation spectrometry. [3H]5-HT uptakes were expressed as pmol/min/105 cells, means ± SEM.
Immunoprecipitations and Western blots
Co-transfected cells were lysed, solubilized and co-immunoprecipitations conducted as described before [6]. Solubilized lysates [500–700 µl/assay; 250–500 µg/ml protein] were precleared for 30 min with protein A/G agarose beads [sc-2003; Santa Cruz Biotechnology], and either anti-SERT polyclonal [1:100; Chemicon AB1594P], anti-α-Syn polyclonal (1:100; Santa Cruz Biotechnology N-19) or anti-γ-Syn polyclonal (1:50; AB6169-100 Ab Cam) or non-immune sera [2–3 µg protein] were added for 8 h with gentle shaking at 4°C. After precipitation with protein A/G agarose beads (washed with solubilization buffer) for 90 min, immunopellets were washed 5 times with solubilization buffer, dissociated in Laemmli buffer for 2 h at room temperature followed by heating in Laemmli buffer for 30 min at 65°C, and samples were characterized by immunoblot analysis as previously described [6, 19].
Data analysis
Each experimental [3H]5-HT uptake measurement was performed in quadruplicate and is the means ± SEM of 4 experiments (in pmol/min/105 cells). Kinetic parameters of 5-HT uptake were calculated by linear regressions of the Eadie-Hoffstee plots and confirmed by a non-linear regression program on Kaleidagraph (version 3.0.8 D, Abelbeck Software). Statistical significance of the experimental results was obtained by Variance Analysis with a Fisher’s test. P< 0.05 was considered to denote statistical significance.
Results and discussion
α-Synuclein decreases SERT-mediated uptake of 5-HT in a manner dependent on its expression levels
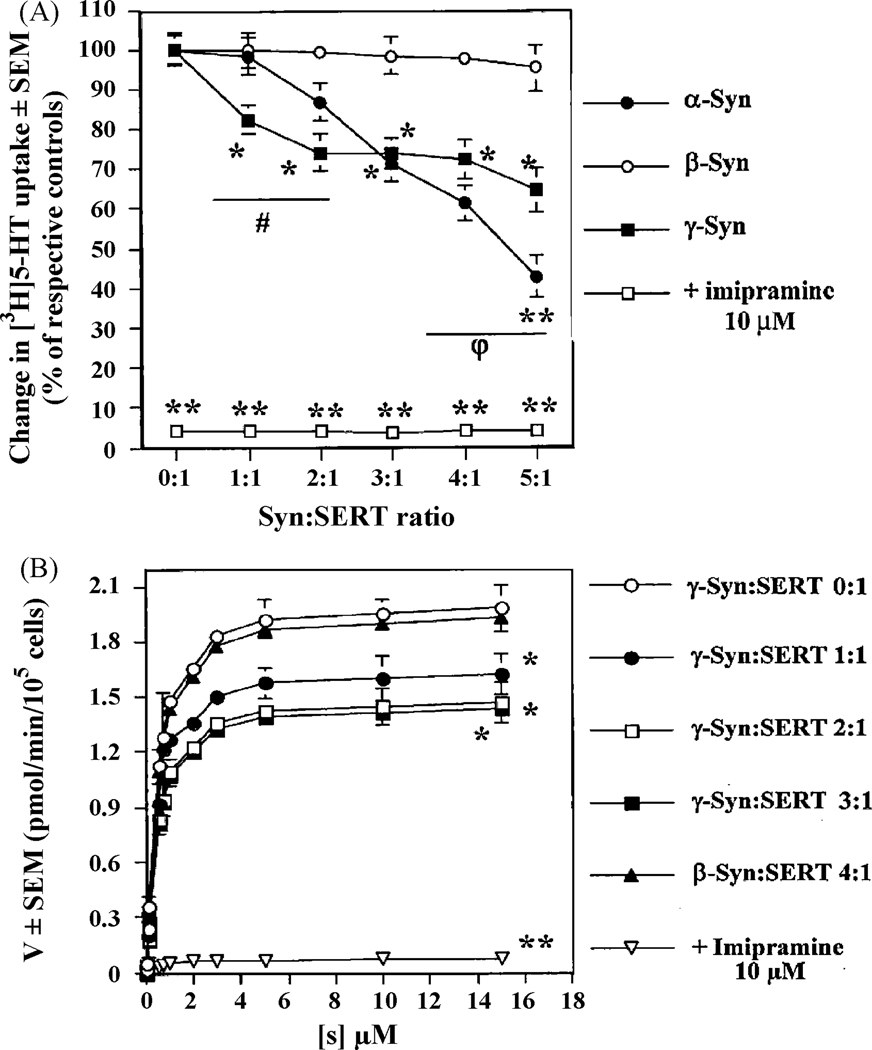
Increasing amounts of DNA (1–5 µg/105 cells) encoding human α-Syn or γ-Syn or β-Syn were co-expressed with 1 µg/105 cells of human SERT DNA in Ltk− cells, to allow for the expression of increasing concentrations of the various synucleins in presence of a defined concentration of SERT, followed by [3H]5-HT uptake studies [Methods]. The final quantity of DNA was kept constant by adjusting with pcDNA3.1 control vector DNA. Increasing the ratios of co-transfection synucleins:SERT DNAs yielded gradual increased protein expression levels of α-, β-, and γ-Syn, without affecting the expression levels of SERT (data not shown). In presence of 1 µg of α-Syn DNA/105 cells (ratio of co-transfection of α-Syn:SERT DNAs of 1:1), α-Syn did not significantly affect [3H]5-HT uptake from that of control cells, expressing only SERT. However, at higher ratios of co-transfection of α-Syn:SERT DNAs (2:1–5:1), there was a progressive and significant (**, p<0.01; *, p<0.05) decrease of [3H]5-HT uptake, compared to cells expressing SERT alone (Fig. 1A). By contrast, increasing expression levels of γ-Syn resulted in an immediate and significant decrease of [3H]5-HT uptake at a ratio of co-transfection of γ-Syn:SERT DNAs of 1:1 (18%), which was further decreased to a maximal value of 26% at ratios of co-transfection of 2:1 or higher (Fig. 1A). Increasing the levels of γ-Syn (up to a 5:1 ratio γ-Syn:SERT DNAs) did not produce any additional decrease in SERT uptake activity. When increasing levels of β-Syn DNA were used in similar assays, there was no appreciable decrease observed in SERT activity, even at a high ratio of co-transfection β-Syn:SERT DNAs of 5:1 (Fig. 1A).
Fig. 1.
(A) Co-expression of SERT with increasing amounts of synucleins differentially attenuates [3H]5-HT uptake in Ltk− cells; (B) Saturation curves of [3H]5-HT uptake in co-transfected Ltk− cells expressing increasing amounts of β- or γ-Syn. Ltk− cells were co-transfected with SERT [1 µg DNA/1.0×105 cells] and increasing amounts of the indicated synuclein DNAs [0–5 µg DNA/1.0×105 cells] to allow for various ratios of co-transfection syncleins:SERT DNAs (0:1, 1:1, 2:1, 3:1, 4:1 or 5:1), and SERT activity was measured in quadruplicate, as described in Methods. The final quantity of DNA used during transfections was kept constant by adjusting with a pcDNA3.1 control vector. In panel A, data is expressed in % of [3H]5-HT uptake of cells expressing only SERT, whereas in panel B [3H]5-HT uptake is expressed in pmol/min/105 cells. The averages of n = 4 experiments are shown. **, p<0.01 and *, p<0.05, significantly different from cells expressing only SERT; # and φ, p<0.05, significant differences between γ- and α-Syn at low and high ratios of co-transfection synucleins:SERT DNAs, respectively.
Kinetic analysis of [3H]5-HT uptake showed that the Vmax was gradually diminished when the ratio of co-transfection of γ-Syn:SERT was increased, without any changes in the Km (a measure of the affinity of the transporter) of SERT for 5HT (Fig. 1B). Whereas a ratio of co-transfection of γ-Syn:SERT of 4:1 induced a decrease of 27% (**, p<0.01, n = 4) of the Vmax of [3H]5-HT uptake, the Vmax in β-Syn expressing cells (β-Syn:SERT DNA ratio of 4:1) was not significantly different from that of cells expressing only SERT (1.94 ± 0.15 and 1.98 ± 0.13 pmol/min/105 cells, respectively, n = 4), in accordance with Fig. 1A. In all instances, the Km of [3H]5-HT uptake was ~0.46 nM.
Together, these results indicate that γ-Syn inhibition of SERT is partial, occurring at lower expression levels of this protein as compared to α-Syn, but that α-Syn causes a larger decrease in SERT reuptake activity. β-Syn, on the other hand, does not appear to modulate SERT activity regardless of the levels at which it was expressed.
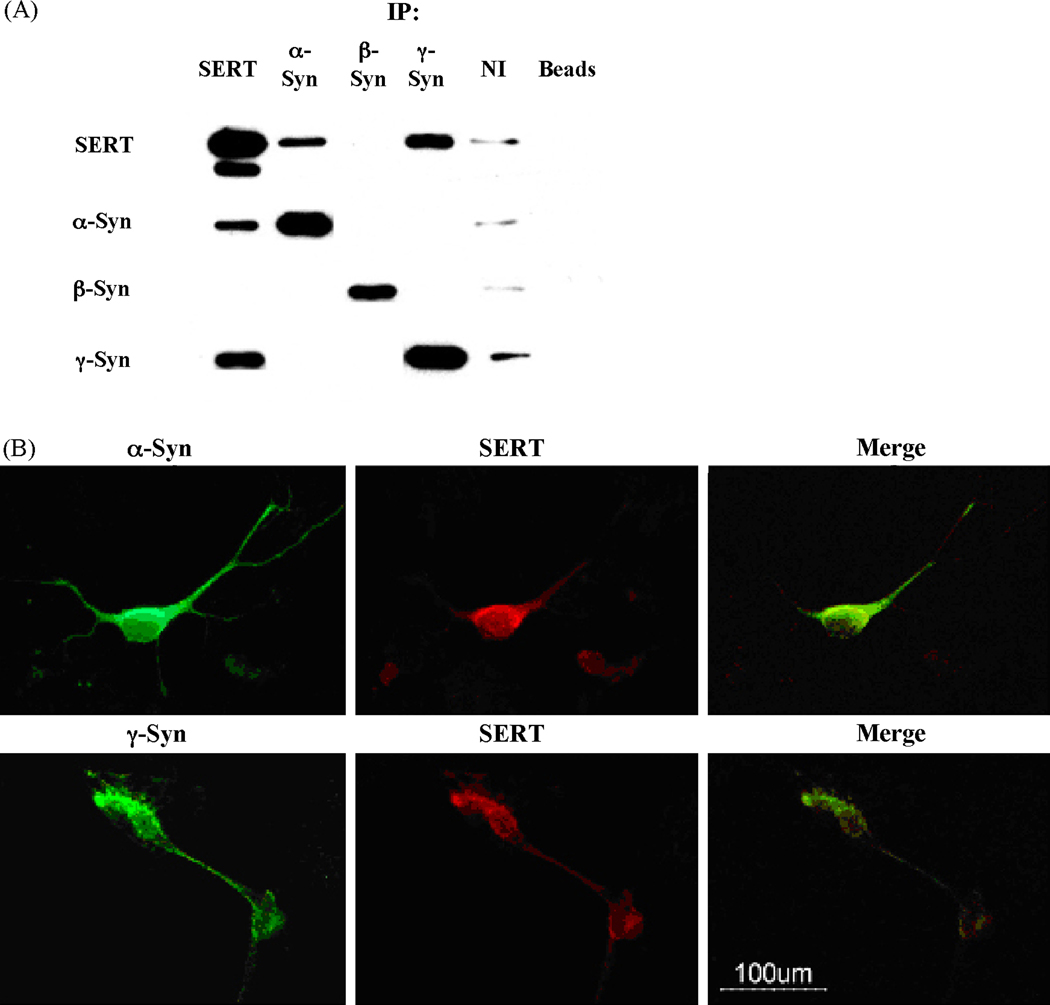
γ-Syn and α-Syn, but not β-Syn, interact with SERT to form protein:protein complexes in co-transfected Ltk− cells
α-Syn regulates both DAT and SERT through formation of heteromeric protein:protein complexes [6, 9–11]. To test whether similar complexes were formed between γ-Syn and SERT, a series of co-immunoprecipitation (co-IP) studies were conducted, using detergent-solubilized lysates isolated from co-transfected cells (Fig. 2A). Thus, anti-SERT antibodies co-immunoprecipitated a single protein band of ~17 and ~14 kDa corresponding to α-Syn and γ-Syn, respectively, in addition to immunoprecipitating their own antigenic protein, SERT. Anti-SERT antibodies failed to co-immunoprecipitate any β-Syn protein. Anti-α-Syn or -γ-Syn antibodies were able to co-immunoprecipitate SERT (seen as a major band at ~ 85 kDa, and a minor band at ~ 65 kDa), as well as their own antigenic proteins. However, α-Syn antibodies did not co-immunoprecipitate any γ-Syn or β-Syn, and conversely, antibodies against these proteins did not co-immunoprecipitate any α-Syn, suggesting lack of protein:protein interactions between the various members of the synuclein family of proteins, while attesting to the specificity of the anti-sera used.
Fig. 2.
Co-immunoprecipitation (Co-IP) and immunostaining studies show a direct physical interaction and colocalization between SERT and either α- or γ-synuclein. (A) Detergent-solubilized lysates from co-transfected Ltk− cells were prepared, and co-immunoprecipitation studies were performed as described in Methods. Negative controls were performed either by replacing antibodies used during immunoprecipitations by their corresponding non-immune sera (NI), or in the absence of any added antiserum (non-specific binding to protein A/G agarose beads; Beads). Immunopellets were probed by western blots using the appropriate monoclonal antibodies. Data shown is representative of 3 independent studies. (B) Immunocytochemistry analysis shows co-localization of α–Syn or γ-Syn with SERT in rat primary raphe nuclei neurons. Double immunofluorescence images show the subcellular distribution of the immunoreactivities of synucleins (green) and SERT (red), and the merger on the third panel [Merge] shows co-localization images. Bar in the panel = 100 µm. Data shown is representative of 4 independent studies.
Anti-β-Syn antibodies immunoprecipitated a single protein corresponding to β-Syn, whereas SERT protein was noticeably absent from the co-immunopellets. In control studies, where IP was done with non-immune sera (NI) or in the absence of any added antisera [protein A/G agarose beads alone], there were no significant levels of synucleins or human SERT immunoreactivities present in the immunopellets, attesting to the specificity of the assays (Fig. 2A). These data suggest that whereas both α-Syn and γ-Syn can form protein:protein complexes with SERT, such interactions are absent between β-Syn and SERT, which is consistent with the absence of any effect of β-Syn on SERT activity.
Co-localization of SERT and either α-Syn or γ-Syn in rat primary raphe nuclei neurons
For these studies, raphe nuclei neurons were grown on glass coverslips, fixed and permeabilized as described under Methods. After an overnight incubation with either mouse anti-SERT monoclonal, or anti-α-Syn- or anti-γ-Syn- polyclonal primary antibodies, immune complexes were revealed with secondary antibodies coupled to Alexa-488 (green) for the synucleins, or coupled to Alexa-594 (red) for SERT.
In 10 day-old rat primary raphe nuclei neurons (Fig. 2B), robust immunoreactivity of both SERT and γ-Syn was seen in the cytoplasm of cell bodies, as well as in the neuritic processes, with substantial labeling at the plasma membrane of soma and axonal processes, and in varicosities along axonal processes. Moreover, there was substantial overlap in the fluorescence of both proteins, suggesting extensive co-localization of the proteins in raphe nuclei neurons (Fig. 2B merge). Similar results were also obtained with α-Syn and SERT, where substantial co-expression and overlap in expression was seen, indicating co-localization of these two proteins (Fig. 2B).
These combined data show that γ-Syn binds to SERT to form protein:protein complexes, and down-regulates SERT activity, in a manner similar to that seen with α-Syn. By contrast, β-Syn was completely unable to negatively modulate the activity of SERT. These results are consistent with the inability of β-Syn to form protein:protein complexes. This inability to form complexes with SERT is probably related to the absence of 11 residues in the hydrophobic non-amyloid β-component (NAC) domain region of β-Syn [11, 21], a region that we have previously shown to be essential for both binding to, and inhibition of the activity of monoamine transporters, including SERT [6, 11, 16]. By contrast, the NAC domain, while somewhat dissimilar, is present in γ-Syn [11, 21]. Our findings also suggest that γ-Syn may have greater efficacy than α-Syn in inhibiting SERT activity at low expression levels. This is especially important in light of the fact that γ-Syn expression in brain occurs in limited areas, in particular in monoaminergic neurons, such as serotonergic neurons of the raphe nuclei, where α-Syn is co-expressed and where β-Syn expression is low [22]. Thus, in these regions, SERT may be primarily regulated by γ-Syn and not α-Syn.
The ability of γ-Syn to only partially modulate SERT reuptake activity contrasts with our earlier findings with NET [19], where we show that γ-Syn-mediated attenuation of NET occurs to the same extent as with α-Syn (~40% maximal inhibition) and at the same expression levels (3:1 ratios of co-transfection) as α-Syn. Interestingly, however, inhibition of NET by γ-Syn also seemed to occur at lower expression levels of γ-Syn than α-Syn (at ratios of co-transfection synucleins:NET of 0.5:1 and 1:1], although differences between the two proteins were not significant at ratios >2:1 [17, 19]. These data, along with the results of the current paper, suggest that at lower expression levels, both NET and SERT may primarily be regulated by γ-Syn, rather than by α-Syn.
The inability of β-Syn to modulate SERT is in contrast to our findings obtained with NET, where β-Syn was found to attenuate NET in a manner virtually identical to that seen with γ-Syn [19]. Thus, both γ-Syn and β-Syn attenuated NET to the same extent when expressed at the same ratios of expression. Together, the combined data with NET and SERT suggest that different synucleins exert different effects on different monoamine transporters, although the molecular basis for such differences remains to be elucidated.
The ability of γ-Syn to negatively regulate both NET and SERT function suggests that this protein, which was previously described to be a key player in certain cancers, ocular biology and to have a limited role in neurodegeneration [21], may be an important player in maintaining brain homeostasis of norepinephrine and serotonin, and in the genesis of mental diseases and mood disorders associated with alterations of these neurotransmitters. In this regard, we have shown that γ-Syn is overexpressed in the Wistar-Kyoto rat, a rodent model of depression, resulting in inappropriate subcellular trafficking and function of NET [19]. Treatment with desipramine, a NET blocker, diminished γ-Syn protein levels by 20%, while simultaneously increasing α-Syn levels, such that α-Syn/γ-Syn expression was similar to control, non-depressed rats. This resulted in restoration of appropriate trafficking and function of NET, along with relief of depressive behavior in the Wistar-Kyoto rats [19]. Thus, γ-Syn may provide for a novel target for controlling and managing certain mental diseases and mood disorders.
Acknowledgements
This work was supported in part by a grant from the National Institute of Mental Health [R01MH-075020]
We thank Dr. Milan Rusnak for his assistance in isolating neuronal cultures and for conducting the immunocytochemistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations : 5-HT, serotonin; SERT, serotonin transporter; α-Syn, α-synuclein; γ-Syn, γ-synuclein; β-Syn, β-synuclein; co-IP, co-immunoprecipitation; IMP, imipramine.
References
- 1.Nelson N. The family of Na+/Cl- neurotransmitter transporters. J. Neurochem. 1998;71:1785–1803. doi: 10.1046/j.1471-4159.1998.71051785.x. [DOI] [PubMed] [Google Scholar]
- 2.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat. Rev. Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 3.Murphy DL, Li Q, Engel S, Wichems C, Andrews A, Lesch KP, Uhl G. Genetic perspectives on the serotonin transporter. Brain Res. Bull. 2001;56:487–494. doi: 10.1016/s0361-9230(01)00622-0. [DOI] [PubMed] [Google Scholar]
- 4.Adell A, Celada P, Abellan MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res. Rev. 2002;39:154–180. doi: 10.1016/s0165-0173(02)00182-0. [DOI] [PubMed] [Google Scholar]
- 5.Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 6.Wersinger C, Rusnak M, Sidhu A. Modulation of the trafficking of the human serotonin transporter by human alpha-synuclein. Eur. J. Neurosci. 2006;24:55–64. doi: 10.1111/j.1460-9568.2006.04900.x. [DOI] [PubMed] [Google Scholar]
- 7.Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249–254. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- 8.Wersinger C, Banta M, Sidhu A. Comparative analyses of alpha-synuclein expression levels in rat brain tissues and transfected cells. Neurosci. Lett. 2004;358:95–98. doi: 10.1016/j.neulet.2003.12.118. [DOI] [PubMed] [Google Scholar]
- 9.Wersinger C, Sidhu A. Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci. Lett. 2003;340:189–192. doi: 10.1016/s0304-3940(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 10.Wersinger C, Prou D, P. Vernier P, Sidhu A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. FASEB J. 2003;17:2151–2153. doi: 10.1096/fj.03-0152fje. [DOI] [PubMed] [Google Scholar]
- 11.Wersinger C, Prou D, Vernier P, Niznik HB, Sidhu A. Mutations in the lipid-binding domain of α-synuclein confer overlapping, yet distinct, functional properties in the regulation of dopamine transporter activity. Mol. Cell. Neurosci. 2003;24:91–105. doi: 10.1016/s1044-7431(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 12.Sidhu A, Wersinger C, Vernier P. Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J. 2004;18:637–647. doi: 10.1096/fj.03-1112rev. [DOI] [PubMed] [Google Scholar]
- 13.Sidhu A, Wersinger C, Vernier P. Alpha-synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson’s disease. FEBS Lett. 2004;565:1–5. doi: 10.1016/j.febslet.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 14.Wersinger C, Vernier P, Sidhu A. Trypsin disrupts the trafficking of the human dopamine transporter by alpha-synuclein and its A30P mutant. Biochemistry. 2004;43:1242–1253. doi: 10.1021/bi035308s. [DOI] [PubMed] [Google Scholar]
- 15.Wersinger C, Sidhu A. Disruption of α-synuclein interaction with the microtubular network accelerates dopamine-induced oxidative stress and cell death. Biochemistry. 2005;18:13612–13624. [Google Scholar]
- 16.Wersinger C, Jeannotte A, Sidhu A. Attenuation of the norepinephrine transporter activity and trafficking via interactions with alpha-synuclein. Eur. J. Neurosci. 2006;24:3141–3152. doi: 10.1111/j.1460-9568.2006.05181.x. [DOI] [PubMed] [Google Scholar]
- 17.Jeannotte AM, Sidhu A. Regulation of the norepinephrine transporter by alpha-synuclein-mediated interactions with microtubules. Eur. J. Neurosci. 2007;26:1509–1520. doi: 10.1111/j.1460-9568.2007.05757.x. [DOI] [PubMed] [Google Scholar]
- 18.Jeannotte AM, Sidhu A. Regulated interactions of the norepineprhine transporter by the actin and microtubule cytoskeletons. J. Neurochem. 2008;105:1668–1682. doi: 10.1111/j.1471-4159.2008.05258.x. [DOI] [PubMed] [Google Scholar]
- 19.Jeannotte AM, McCarthy JG, Redei EE, Sidhu A. Desipramine Modulation of alpha, gamma-Synuclein, and the Norepinephrine Transporter in an Animal Model of Depression. Neuropsychopharmacology. 2008 Sep 17; doi: 10.1038/npp.2008.146. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Ramamoorthy S, Giovanetti E, Qian Y, Blakely RD. Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J. Biol. Chem. 1998;273:2458–2466. doi: 10.1074/jbc.273.4.2458. [DOI] [PubMed] [Google Scholar]
- 21.Surguchov A. Molecular and cellular biology of synucleins. Int. Rev. Cell. Mol. Biol. 2008;270:225–317. doi: 10.1016/S1937-6448(08)01406-8. [DOI] [PubMed] [Google Scholar]
- 22.Li JY, Henning Jensen P, Dahlström A. Differential localization of α-, β- and γ- synucleins in the rat CNS. Neuroscience. 2002;113:463–478. doi: 10.1016/s0306-4522(02)00143-4. [DOI] [PubMed] [Google Scholar]