Abstract
Objective
The aim of this research was to determine correlates of prevalent cervicovaginal human papillomavirus (HPV) infection in perimenopausal women.
Methods
A total of 178 women, ages 40–60, were recruited from four clinics in the metropolitan area of Baltimore, Maryland. A self-collected cervicovaginal specimen and questionnaire were completed following enrollment and consent. HPV was detected by L1 consensus polymerase chain reaction (PCR) and genotyped using a prototype line blot assay. Adjusted prevalence ratios (aPR) and 95% confidence intervals (CIs) from Poisson regression models with robust variance identified correlates of prevalent HPV infection.
Results
Prevalence of any HPV genotype at baseline among 172 women with complete data was 20% (6% for high-risk HPV). HPV prevalence was higher among single compared to married women (aPR = 4.3 [95% CI: 2.0, 9.5]), and among women with ≥2 sex partners in the last six months compared to women who reported none (aPR = 4.9 [1.7, 13.9]) after adjustment for confounders. Menopausal stage was also associated with HPV detection, with increased prevalence among perimenopausal compared to premenopausal women (aPR 2.3 [1.1, 5.1]), after adjustment for confounders. Age was moderately correlated with menopausal staging (r = 0.57).
Conclusions
Our observations suggest the independent associations of sexual behavior and hormones on prevalent HPV in perimenopausal women. Age was not a good surrogate for menopausal stage, as it was only moderately correlated with menopausal stage.
Introduction
Cervicovaginal infection with human papillomavirus (HPV) is the most common sexually transmitted disease.1 HPV infects an estimated 291 million women worldwide, with variations in prevalence by geographic location and age.2 HPV is considered a necessary causal factor for the development of cervical cancer.3,4,5
Differences in age-specific HPV prevalence have been reported in several international studies.2,6 While prevalence is generally highest around the age of sexual debut and decreases with increasing age, a recent meta-analysis showed a second increase in HPV prevalence among cytologically normal women over age 40 in Europe and North and South America, but not Asia.2 The reason for this second peak in prevalence among women 40–60 years old remains unclear, but could conceivably reflect new sexual exposures, reactivation of latent infection due to perimenopausal hormone fluctuations, or both. Inconsistencies across geographical regions in the presence and average age of the second peak have been used to argue against hormonal influences in the later-in-life increase in HPV prevalence.2 However, these inferences are based on using the average age of menopause (50–51 years) as the sole marker of menopause. Perhaps more biologically relevant is capturing measures of the menopausal transition, where hormones tend to fluctuate unpredictably in the years preceding the final menstrual period.7 Certain hormonal imbalances have been associated with altered immune function8 and are responsible for the movement of the cervical transformation zone during puberty through menopause. If hormonal changes are indeed influencing HPV detection in adult women by any of these hypothesized mechanisms, measures more specific than chronologic age may be required to accurately classify women according to stage of the menopausal transition.
The objectives of our study were three-fold. First, we estimated the prevalence of HPV detection in a group of 40–60-year-old women. Second, we determined the correlation of age and menopausal stage classified using self-reported information of last menstrual date and cycle irregularities. Finally, we investigated the independent associations of sexual exposure, and markers of endogenous and exogenous hormone fluctuations with detection of prevalent HPV detection in women in the menopausal transition.
Methods
A convenience sample of women aged 40–60 who were attending one of four outpatient clinics affiliated with the Johns Hopkins Medical Institutions in the greater Baltimore area were recruited between May and November 2006. The majority of patients are seen for routine medical examinations, though we did not collect individual information on reason for clinic attendance. Women were excluded from the study if they reported having a hysterectomy or were currently pregnant.
All willing and eligible women provided written informed consent and a self-collected vaginal swab, and completed a self-administered questionnaire at the baseline visit. The questionnaire collected exposure information, including demographics, exogenous hormone use (including contraceptives and hormone replacement therapy), non-hormonal medication use, menstrual and reproductive history, history of an abnormal Pap test and treatment, lifetime and recent sexual behavior, and presence of menopausal symptoms.
Menopausal stage (post-, peri-, or premenopause) was classified based on menstrual history according to the executive summary of the Stages of Reproductive Aging Workshop (STRAW),9 and used as an indirect measure of endogenous hormone fluctuations. Specifically, postmenopause was defined as ≥12 months since last reported menstrual period; perimenopause was defined as self-reported changes in menstrual cycle length; and premenopause was defined as current menstruation with no change in menstrual cycle length.
To collect the cervicovaginal specimen, participants were provided with a pre-labeled Digene HPV Sampler (Qiagen, Gaithersburg, MD) collection kit that contained a cervical brush and 1 ml of standard transport medium (STM). Participants were instructed verbally on how to collect the vaginal swab and provided with illustrated and written instructions. This cross-sectional study was approved by the Committee on Human Research at Johns Hopkins Bloomberg School of Public Health.
HPV genotyping was performed on all specimens. Specimens were stored at 4°C before being vortexed and transferred into cryovials for storage at −20°C. DNA was extracted by taking 90 μl of the STM specimen and digesting at 65°C in a digestion buffer (20 mM Tris-HCl, 1 mM EDTA pH 8.5) containing 200 μg/ml proteinase K and 0.1% of Laureth-12 for 1 hour. Following heat inactivation of proteinase K (95°C for 10 minutes), DNA was precipitated with an ammonium acetate and ethanol solution and resuspended in a final volume of 75 μl of TE buffer. For PCR, 5 μl of DNA was amplified using PGMY 09/11 and beta-globin consensus primers and AmpliTaq Gold in a final volume of 100 μl. Amplification was performed using GeneAmp® PCR System 9600 thermal cycler. The PCR product was denatured in 100 μl of 0.4 N sodium hydroxide, and 75 μl was used for HPV genotyping by the Roche prototype PCR-Line blot assay (kind gift of Roche Molecular Systems, Pleasanton, CA) as previously described,10,11 which detects 37 HPV genotypes (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 41, 51–59, 61, 62, 64, 66–73, 81–84, 82 subtype[IS39], and 89[CP6108]). HPV prevalence was calculated both for any HPV genotype and any high-risk genotype.
Statistical analysis
Fisher's exact chi-squared test was used to test for a difference in proportions. Pearson's correlation coefficient was used to determine correlation. To avoid overestimation of association due to frequent outcomes, Poisson regression models with robust variance were used to determine univariate (PR) and adjusted prevalence ratios (aPR) and 95% confidence intervals (CI).12 A two-tailed p-value of <0.05 was considered significant. All analyses were conducted using STATA version 9.0 (STATACorp., College Station, TX).
Results
Of the 178 women enrolled, three participants were dropped due to incomplete demographic information, and an additional three participants were dropped due to a lack of beta-globin amplification on the specimen. This resulted in 172 women (median age = 49.0 years) with complete information who contributed to the analysis. We parsimoniously combined women who were widowed (n = 7), divorced (n = 25), separated (n = 6), or never married (n = 30) into a single category of “not married” since the HPV prevalence was not significantly different across the groups. The majority of women with complete information were non-Hispanic white, with fewer than five lifetime sexual partners, and did not use HRT or other hormones (Table 1). Overall, the prevalence of any HPV was 20% (34/172), with 6% (11/172) having a high-risk HPV detection.
Table 1.
Characteristics of Participants and Correlates of Prevalent HPV Infection (N = 172), Baltimore, Maryland, 2006
| Characteristics, number (%)a | HPV prevalence, number (%)b | Unadjusted prevalence ratio (95% CI)c | Adjusteddprevalence ratio (95% CI)c | |
|---|---|---|---|---|
| Age (in years) | ||||
| ≤45 | 48 (28) | 16 (33) | 1.0 | 1.0 |
| 46–50 | 50 (29) | 6 (12) | 0.4 (0.2–0.8) | 0.3 (0.1–0.7) |
| 51–55 | 36 (21) | 7 (19) | 0.6 (0.3–1.3) | 0.7 (0.3–1.7) |
| ≥56 | 36 (21) | 5 (14) | 0.4 (0.2–1.0) | 0.2 (0.1–0.7) |
| Race/ethnicity | ||||
| Non-Hispanic White | 125 (73) | 24 (19) | 1.0 | |
| Other | 46 (27) | 10 (22) | 1.1 (0.6–2.2) | |
| Education | ||||
| <College graduate | 81 (47) | 19 (24) | 1.0 | |
| ≥College graduate | 90 (52) | 15 (17) | 0.7 (0.4–1.3) | |
| Marital status | ||||
| Married | 103 (60) | 10 (10) | 1.0 | 1.0 |
| Not marriede | 68 (40) | 24 (35) | 3.6 (1.9–7.1) | 4.3 (2.0–9.5) |
| Smoking status | ||||
| Not current | 148 (86) | 27 (18) | 1.0 | 1.0 |
| Current | 24 (14) | 7 (29) | 1.6 (0.8–3.3) | 1.4 (0.6–3.7) |
| Medical history | ||||
| Current hormone replacement therapy (HRT) use | ||||
| No | 152 (88) | 27 (18) | 1.0 | 1.0 |
| Yes | 11 (6) | 6 (55) | 3.1 (1.6–5.8) | 2.3 (1.0–5.4) |
| Other hormone use (not HRT) | ||||
| No | 141 (82) | 26 (18) | 1.0 | |
| Yes | 31 (18) | 8 (26) | 1.4 (0.7–2.8) | |
| History of Pap | ||||
| Normal | 84 (48) | 15 (18) | 1.0 | |
| Abnormal | 68 (40) | 16 (24) | 1.3 (0.7–2.5) | |
| Never screenedf | 14 (8) | 3 (21) | ||
| Don't knowf | 5 (3) | 0 (0) | ||
| Treatment of abnormal Papg | ||||
| No | 22 (32) | 3 (14) | 1.0 | |
| Yes | 43 (63) | 12 (28) | 2.0 (0.6–6.6) | |
| Don't knowf | 3 (4) | 1 (33) | ||
| Sexual and reproductive history | ||||
| Parity | ||||
| 0 | 43 (25) | 11 (26) | 1.0 | |
| 1 | 25 (15) | 8 (32) | 1.3 (0.6–2.7) | |
| 2 | 61 (36) | 9 (15) | 0.6 (0.3–1.3) | |
| ≥3 | 42 (24) | 6 (14) | 0.6 (0.2–1.4) | |
| Menopausal stageh | ||||
| Premenopause | 50 (29) | 8 (16) | 1.0 | 1.0 |
| Perimenopause | 56 (33) | 15 (27) | 1.7 (0.8–3.6) | 2.3 (1.1–5.1) |
| Postmenopause | 51 (30) | 8 (16) | 1.0 (0.4–2.4) | 2.1 (0.9–5.1) |
| Lifetime number of partners | ||||
| ≤5 | 108 (63) | 17 (16) | 1.0 | |
| >5 | 46 (27) | 13 (28) | 1.8 (1.0–3.4) | |
| Decline to answer | 18 (11) | 4 (22) | 1.4 (0.5–3.7) | |
| Number of sexual partners in last 6 months | ||||
| 0 | 39 (23) | 8 (21) | 1.0 | 1.0 |
| 1 | 123 (72) | 21 (17) | 0.8 (0.4–1.7) | 2.0 (0.9–4.8) |
| ≥2 | 6 (4) | 4 (67) | 3.3 (1.4–7.5) | 4.9 (1.7–13.9) |
| Decline to answer | 4 (2) | 1 (25) | 1.2 (0.2–7.5) | 1.4 (0.4–4.4) |
Due to missing data, percentages may not add to 100%. Missing data was largely the result of omission in the self-administered questionnaire.
Percentage calculated from strata-specific total.
95% CI is the 95% confidence interval.
Adjusted for age, marital status, smoking status, current hormone replacement therapy (HRT) use, menopausal stage, and number of sex partners in the last 6 months.
Not married includes all women not currently married, including women who are widowed (n = 7), divorced (n = 25), separated (n = 6), or single women who have never been married (n = 30).
Excluded from unadjusted prevalence ratio analysis.
Percentage calculated out of 68 women reporting abnormal Pap.
Premenopause was defined as not reporting irregular menstrual periods. Perimenopause was defined as reporting irregular menstrual periods. Postmenopause was defined as ≥12 months since last reported menstrual period. Fifteen women were not classified into any menopausal stage due to missing data of menstrual periods.
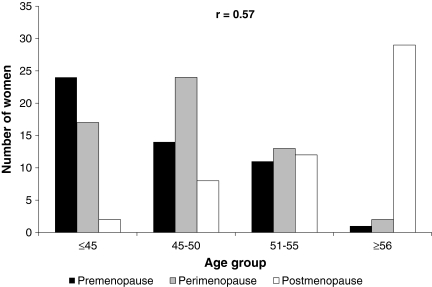
Age (measured in 5-year age categories) and menopausal stage were not strongly correlated (r = 0.57). Age was a poor surrogate when attempting to differentiate pre- from perimenopausal women (Fig. 1).
FIG. 1.
Age group and menopausal stage of participants (N = 172), Baltimore, Maryland, 2006.
After multivariate adjustment for potential confounding effects, HPV prevalence was significantly different across the 5-year age groups; no clear trend with increasing age was observed (Table 1). Measures of sexual exposure opportunity (i.e., single marital status and recent sexual partners) were positively associated with HPV prevalence. The univariate association of HRT use and increased HPV prevalence was attenuated, but marginally statistically significant, after adjustment for age and sexual behavior (aPR = 2.3 [95% CI of 1.0, 5.4]). Interestingly, the association between peri- versus premenopausal stage increased in both strength and significance (aPR = 2.3 [1.1, 5.1] for perimenopausal compared to premenopausal stage) after adjustment for age and sexual behavior.
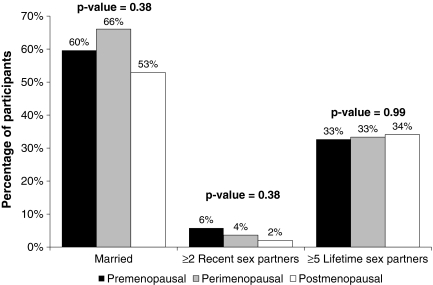
The association of HPV with perimenopausal stage did not appear to be confounded by sexual behavior, as perimenopausal women were more likely to be married, have similar number of lifetime sex partners compared to pre- and postmenopausal women, and were less likely to have ≥2 recent sex partners compared to premenopausal women (Fig. 2).
FIG. 2.
Sexual behavior characteristics of pre-, peri-, and postmenopausal participants (N = 172), Baltimore, Maryland.
Discussion
The suggestion of independent effects of hormones and sexual behavior on HPV detection observed in this study is intriguing. We observed an association between surrogates for sexual exposure opportunity (i.e., likelihood of recent contact with infected partner) and HPV prevalence. This observation supports the notion that women at all ages may be at risk of new HPV infections, given exposure. Even among the sexually active women, however, reactivation cannot be excluded as a source of the HPV detection, as sexual exposure may have been coincidental.
Markers of exogenous and endogenous hormones were also associated with HPV detection in this population. Previous studies have reported conflicting associations between exogenous hormone use (i.e., HRT) and HPV detection,13,14,15,16 with one study suggesting that duration of use may be important.14 We only collected information on current HRT use in this pilot study, and only 11 women reported this exposure. Despite these small numbers, 55% of HRT users had prevalent HPV detected versus 18% of non-users (p = 0.01). While this association was attenuated after adjustment for age, menopausal stage, and sexual behavior, the statistically significant point estimate supports a possible association between prevalent HPV detection and HRT use which should be resolved in larger studies.
To our knowledge, this is the first study investigating the correlates of HPV detection to specifically measure the stage of the menopausal transition. The STRAW menopausal transition staging criteria categorizes menstruating women reporting changes in their menstrual cycle length as being in the “early transition” and women reporting 60 days of amenorrhea as being in the “late transition.” Postmenopausal stage is retrospectively defined as 12 months since the last menstrual period. Using information on self-reported regular versus irregular menstrual cycles and date of last menstrual period, we were able to categorize our study participants as premenopausal, perimenopausal (combines both early and late transition), and postmenopausal. Using this more refined measure of staging of the menopausal transition, we observed a significant increase in HPV detection only among the perimenopausal women. Most studies dichotomize menopausal stage as pre- versus postmenopause, failing to quantify perimenopause. Indeed, in this study, we found no association with HPV detection using a binary pre/post menopause categorization.
There is biological relevance to differentiating premenopausal women with predictable, cyclical hormone levels, from perimenopausal women who experience periods of severe hormonal fluctuations during the menopausal transition,7 since such hormonal imbalances can result in significant physiologic and immunologic dysregulation. Importantly, due to the substantial variation in the frequency and severity of hormonal changes associated with the menopausal transition, use of individual-level data to define menopausal staging, rather than population averages, will be critical to assess the effects of the transition on disease risk. For example, the Study of Women Across the Nation (SWAN) study is a multi-center study of perimenopause in the United States that used stratified sampling to ensure adequate representation of several ethnic groups including Caucasians, African Americans, Hispanics, Asians, and Native Americans.17 SWAN data demonstrated a strong effect of body mass index, age, and ethnicity on hormonal patterns (including total cycle length) in pre- and early perimenopausal women.18 Caucasians, African Americans, and Hispanic women were all significantly more likely to report estrogen surges in excess of premenopausal levels, progesterone declines, and menopausal symptoms compared to their Asian-American counterparts.18 It is intriguing that these ethnic differences show an ecological correlation with the geographical differences in the age-specific HPV prevalence trends.2
Our observation that menopausal staging was more strongly associated with HPV prevalence than chronologic age has important implications when considering the influence of sex hormones on HPV detection among older women. Specifically, in our study, age (measured in 5-year age groups) was not strongly correlated with menopausal stage. Therefore, the misclassification of menopausal stage using age as a surrogate can bias the estimated effect of the menopausal transition on HPV natural history, and may explain the lack of association in previous studies which used age as a surrogate marker for menopause.
We considered whether direct measures of hormonal biomarkers could increase the precision of estimated associations of menopausal stage and prevalent HPV detection. Biomarkers, including a monitor of the gradual increase in follicle stimulating hormone levels throughout the transition period, have been investigated to more precisely define menopausal stage.7 However, intra-individual variability is high using a single measure, and setting meaningful cut-offs has proven difficult.9 Future studies of HPV in perimenopausal women can increase specimen sampling densities to best capture temporal changes in both HPV and hormones, and thereby more accurately estimate the effect of menopause transitioning and hormone fluctuations on HPV detection.
Given the observational design of the study, there are limitations to be considered when interpreting these results. Our study population was comprised of a convenience sample of women attending Johns Hopkins Medical Institutions outpatient clinics; therefore, we cannot completely rule out selection bias as a potential explanation for the observed effects. However, since we have collected information on the most common risk factors for prevalent HPV detection, we do not believe that this is a strong possibility. We acknowledge the findings are limited in generalizability as some racial and ethnic groups, namely Hispanic and Asian, are under-represented in our study population.
We used cervicovaginal self-collected samples to measure HPV outcomes rather than direct cervical sampling. The self-collection methodology, however, has been shown to be a reliable surrogate for clinician-collection.19,20,21,22,23 The agreement of clinician-collected and self-collected specimens has been shown to be high, with the most comprehensive study reporting kappa = 0.73.19 It is noted that while many studies have observed the prevalence of high-risk HPV genotypes to be similar in both cervical and vaginal epithelium, some studies suggest a higher prevalence of low-risk or any HPV genotypes in the vagina compared to the cervix.19,21,24 We are underpowered to investigate the risk-stratified associations of HPV with our exposure variables, therefore future studies are needed to determine whether the effects of menopausal staging on HPV detection are different between the oncogenic risk groups.
Finally, we were limited in power to investigate interactions of menopause-associated changes and sexual behavior, such as decreased sexual activity due to vaginal atrophy or increased sexual activity due to HRT in postmenopausal women. However, perimenopausal women in our study were of similar (or even slightly lower) risk of sexual exposure compared to the pre- and postmenopausal groups. Specifically, the perimenopausal women were slightly less likely to report ≥2 recent sex partners, slightly more likely to be married, and reported no differences in lifetime number of sex partners compared to the pre- and postmenopausal groups (Fig. 2). It is unlikely, therefore, that the association of menopausal stage was confounded by sexual behavior.
Conclusions
The findings in this study highlight some of the shortcomings of conventional methods of measuring menopause stage. Most clinically relevant, age was not a good surrogate of menopausal stage as it was only moderately correlated with menopausal stage defined using date of last menstrual cycle and cycle irregularities similar to the STRAW recommendations.9 To fully understand the roles of sexual behavior and sex hormones in the increased HPV prevalence observed during the menopausal transition, comprehensive information on sexual behavior, endogenous hormone fluctuations, and exogenous hormone use/duration is needed. A larger study with more frequent monitoring is necessary in order to better understand the driving forces behind the increase in prevalent HPV infection among women aged 40–60.
Acknowledgments
This study was funded by NCI SPORE in Cervical Cancer (P50 CA098252). Roche Molecular Systems (Pleasanton, CA) kindly provided the Roche prototype PCR-Line blot assays. K.N.A. was supported by the Johns Hopkins Training Program in Sexually Transmitted Infections (T32-AI050056) from the National Institute of Allergy and Infectious Disease. Findings of this study were presented in part at the American College of Obstetricians and Gynecologists (ACOG) Annual Clinical Meeting by A.E.B., May 2008.
Disclosure Statement
No competing financial interests exist.
References
- 1.Clifford GM. Gallus S. Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991–998. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 2.de Sanjose S. Diaz M. Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–459. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX. Lorincz A. Munoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walboomers JM. Jacobs MV. Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 6.Schiffman M. Kjaer SK. Chapter 2: Natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr. 2003;31:14–19. doi: 10.1093/oxfordjournals.jncimonographs.a003476. [DOI] [PubMed] [Google Scholar]
- 7.Hale GE. Burger HG. Hormonal changes and biomarkers in late reproductive age, menopausal transition and menopause. Best Pract Res Clin Obstet Gynaecol. 2009;23:7–23. doi: 10.1016/j.bpobgyn.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Straub RH. The complex role of estrogens in inflammation. Endocrinol Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 9.Soules MR. Sherman S. Parrott E, et al. Stages of Reproductive Aging Workshop (STRAW) J Womens Health Gend Based Med. 2001;10:843–848. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]
- 10.Gravitt PE. Peyton CL. Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gravitt PE. Peyton CL. Apple RJ, et al. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–3027. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behrens T. Taeger D. Wellmann J, et al. Different methods to calculate effect estimates in cross-sectional studies. A comparison between prevalence odds ratio and prevalence ratio. Methods Inf Med. 2004;43:505–509. [PubMed] [Google Scholar]
- 13.Ferenczy A. Gelfand MM. Franco E, et al. Human papillomavirus infection in postmenopausal women with and without hormone therapy. Obstet Gynecol. 1997;90:7–11. doi: 10.1016/S0029-7844(97)00217-2. [DOI] [PubMed] [Google Scholar]
- 14.Smith EM. Ritchie JM. Levy BT, et al. Prevalence and persistence of human papillomavirus in postmenopausal age women. Cancer Detect Prev. 2003;27:472–480. doi: 10.1016/s0361-090x(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 15.Smith EM. Johnson SR. Figuerres EJ, et al. The frequency of human papillomavirus detection in postmenopausal women on hormone replacement therapy. Gynecol Oncol. 1997;65:441–446. doi: 10.1006/gyno.1997.4703. [DOI] [PubMed] [Google Scholar]
- 16.Smith EM. Levy BT. Ritchie JM, et al. Is use of hormone replacement therapy associated with increased detection of human papillomavirus and potential risk of HPV-related genital cancers? Eur J Cancer Prev. 2002;11:295–305. doi: 10.1097/00008469-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Sowers MF. Crawford SL. Sternfeld B, et al. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, editor; Kelsey J, editor; Marcus R, editor. Menopause: biology and pathobiology. San Diego: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 18.Santoro N. Lasley B. McConnell D, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women's Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab. 2004;89:2622–2631. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 19.Gravitt PE. Lacey JV., Jr. Brinton LA, et al. Evaluation of self-collected cervicovaginal cell samples for human papillomavirus testing by polymerase chain reaction. Cancer Epidemiol Biomarkers Prev. 2001;10:95–100. [PubMed] [Google Scholar]
- 20.Herrero R. Hildesheim A. Rodriguez AC, et al. Rationale and design of a community-based double–blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26:4795–4808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karwalajtys T. Howard M. Sellors JW, et al. Vaginal self sampling versus physician cervical sampling for HPV among younger and older women. Sex Transm Infect. 2006;82:337–339. doi: 10.1136/sti.2005.019430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safaeian M. Kiddugavu M. Gravitt PE, et al. Comparability of self-collected vaginal swabs and physician-collected cervical swabs for detection of human papillomavirus infections in Rakai, Uganda. Sex Transm Dis. 2007;34:429–436. doi: 10.1097/01.olq.0000243623.67673.22. [DOI] [PubMed] [Google Scholar]
- 23.Petignat P. Faltin DL. Bruchim I, et al. Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecol Oncol. 2007;105:530–535. doi: 10.1016/j.ygyno.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Castle PE. Rodriguez AC. Porras C, et al. A comparison of cervical and vaginal human papillomavirus. Sex Transm Dis. 2007;34:849–855. doi: 10.1097/OLQ.0b013e318064c8c5. [DOI] [PMC free article] [PubMed] [Google Scholar]