Abstract
Tissue engineering of the small intestine remains experimental despite worldwide attempts to develop a functional substitute for short bowel syndrome. Most published studies have reported predominant use of PLLA (poly-L-lactide acid)/PGA (polyglycolic acid) copolymer as the scaffold material, and studies have been limited by in vivo experiments. This lack of progress has inspired a fresh perspective and provoked further investigation and development in this field of tissue engineering. In the present paper, we exploit a relatively new nanocomposite of POSS (polyhedral oligomeric silsesquioxane) and PCL [poly(caprolactone-urea)urethane] as a material to develop porous scaffolds using a solvent casting/particulate leaching technique to fabricate porous scaffolds in different pore sizes and porosities. Scaffolds were characterized for pore morphology and porosity using scanning electron microscopy and micro-computed tomography. Rat intestinal epithelial cells were then seeded on to the polymer scaffolds for an in vitro study of cell compatibility and proliferation, which was assessed by Alamar Blue™ and lactate dehydrogenase assays performed for 21 days post-seeding. The results obtained demonstrate that POSS–PCL nanocomposite was produced as a macroporous scaffold with porosity over the range of 40–80% and pore size over the range of 150–250 μm. This scaffold was shown to support epithelial cell proliferation and growth. In conclusion, as a further step in investigating small intestinal tissue engineering, the nanocomposite employed in this study may prove to be a useful alternative to poly(lactic-co-glycolic acid) in the future.
Keywords: intestinal epithelial cell (IEC), nanocomposite, poly(caprolactone-urea)urethane (PCL), scaffold, tissue engineering
Abbreviations: DMAC, dimethylacetamide; FTIR, Fourier-transform infrared; IEC, intestinal epithelial cell; LDH, lactate dehydrogenase; micro-CT, micro-computed tomography; PCL, poly(caprolactone-urea)urethane; PCU, poly(carbonate-urea)urethane; PGA, polyglycolic acid; PLGA, poly(lactic-co-glycolic acid); PN, parenteral; POSS, polyhedral oligomeric silsesquioxane; SBS, short bowel syndrome; SEM, scanning electron microscopy
Introduction
SBS (short bowel syndrome) is a dreaded condition of nutritional malabsorption related to the surgical removal or disease of a large portion of the small intestine (loss of more than 70%) [1]. Improving the nutritional status of the patients by EN (enteral) and PN (parenteral) feeding has been used as a life-saving treatment for patients with intestinal failure. The high costs and high mortality [2,3] associated with PN feeding further emphasize the need for a more definitive treatment for SBS. Attempts have been made to treat SBS by surgical procedures like sequential intestinal loop lengthening, but with little success [4,5]. For patients dependent on PN feeding, intestinal transplantation remains the most promising treatment in the present medical field. However, patient survival rates are poor [6,7]; there are high incidences of rejection and limited availability of donor organs.
There have been attempts to engineer the small intestine since 1998 and it has the potential to be a clinically viable option for those suffering from SBS in the future [8]. We have highlighted the major advances made so far in this field [8]. The development of a tissue-engineered intestine requires overcoming obstacles in developing epithelial growth and differentiation on top of a vascularized subepithelial matrix as well as developing an innervated muscular layer. Choi and Vacanti [9] have employed rat intestine epithelial organoid units [10] as a cell source to be seeded on to porous synthetic biodegradable polymer scaffolds made of PGA (polyglycolic acid) or copolymer PLGA [poly(lactic-co-glycolic acid)] and have achieved considerable success in regenerating neointestinal tissue.
Despite the demonstrated success of Choi and Vacanti [9] in rat models, there does not appear to be a concrete way forward for the employment of this technique in humans. There are many questions that remain unanswered and several difficulties to overcome. First, it is unclear whether the absorptive capacity is improved through the anastomosed segment of a tissue-engineered small intestine because of added mucosal surface area or because of slow transit. Secondly, the entire intestinal length of 40 neonatal rats was required to make organoid unit-polymer constructs sufficient to implant in 10 adult rats. The question of availability of human neonatal intestine in such large amounts is likely to be one of the major limiting factors in developing this procedure clinically. Finally, the immunocompatibility is not going to be any better than the transplant procedure.
It would appear that the entire concept of small intestinal tissue engineering needs a fresh impetus to progress. One field currently of great interest in tissue engineering is the potential use of human stem cells [11,12]. Stem cells from peripheral blood, if they can be cued towards intestinal lineage, could prove to be a holy grail in the development of a successful procedure. A better understanding of cell–cell and cell–ECM (extracellular matrix) interactions, specific stem cell markers and trophic growth factors is required [13]. So far, only PLGA or PGA alone has been predominantly experimented for small intestinal tissue engineering [8]. One area in which a fresh perspective may be achieved is by looking at other potential scaffolds such as a relatively new biodegradable nanocomposite developed by incorporating the biostable POSS (polyhedral oligomeric silsesquioxane) nanocages into a poly(caprolactone/carbonate)urethane urea [14]. Nanocomposites can be defined as multiphase solid materials where one of the phases has a dimension of less than 100 nm [15]. POSS molecules are 6 nm in size. Studies on the biodegradation of this polymer have been carried out previously [14]. These studies showed that the incorporation of POSS nanocomposites into the poly(caprolactone/carbonate)urethane/urea resulted in the development of a degradable polymer that preserves its mechanical properties as it undergoes oxidation, hydrolysis or degradation by plasma protein fractions [16]. The nanocages provide a ‘shielding effect’ on the soft phase of the polymer. PCL (polycaprolactone) is a degradable aliphatic polyester that has been extensively reported to demonstrate biocompatibility in vivo. Its degradation by hydrolysis and enzymes is well documented. PCL has been used in several copolymers and polymer blends as the foundation for a tailor-made polymer with specific properties. The in vivo host response of POSS–PCL has not been studied; however, POSS–PCU [poly(carbonate-urea)urethane], the non-biodegradable counterpart lacking PCL, made by the same group, was implanted in sheep for 36 months and showed only minimal inflammation as compared with silicone implants [17]. It is proposed that caprolactone will provide controlled degradation, whereas the POSS nanocages will contribute to the mechanical strength required for a tissue engineering scaffold for soft tissue such as the small intestine. Our laboratory is investigating individually the two main components involved in small intestinal tissue engineering: scaffolds using nanocomposite (POSS–PCL) developed and patented by our group [18], and in another simultaneous study, trying to extract stem cells from peripheral blood and co-culturing them with intestinal myofibroblasts in order to differentiate them into IECs (intestinal epithelial cells). The present paper describes our studies on the nanocomposite biodegradable polymer scaffold fabricated by us, examining its preparation, characterization, mechanical testing and in vitro cell culture results using a rat IEC cell line in order to optimize the scaffold conditions most suitable for small intestinal tissue engineering.
Materials and methods
Preparation of the polymer (POSS–PCL)
Dry polyol blend [80% (w/w) polycaprolactone diol and 20% (w/w) polycarbonate diol; Mr 2000] and trans-cyclohexanediolisobutyl-POSS were placed in a reaction flask equipped with a mechanical stirrer and a gas inlet. The mixture was heated to dissolve the POSS cage into the polyol and then cooled. Subsequently, flake MDI (methylene diphenyl isocyanate) was added to the polyol blend and then made to react, under nitrogen, to form a prepolymer. Dry DMAC (dimethylacetamide) was added slowly to the prepolymer to form a solution, which was cooled to 40 °C. Chain extension of the prepolymer was carried out by dropwise addition of a mixture of ethylenediamine and diethylamine to dry DMAC. After completion of the chain extension, 1-butanol in DMAC was added to the polymer solution to form a 20% POSS–PCL solution. All chemicals and reagents were purchased from Sigma–Aldrich.
Characterization of the polymer
The surface tension, viscosity and electrical conductivity of the polymer were measured. Surface tension was measured using a Krüss Tensiometer K9 (Du Novy's ring 6 method). Viscosity was estimated using a Bohlin CVO rheometer (Malvern Instruments). Electrical conductivity was assessed using a Hach SensION Tm 156 probe. All measurements were performed at the ambient temperature and all instruments were calibrated before use.
Preparation of scaffolds using particulate leaching and solvent casting
Two pore size ranges of 150–250 μm (macroporous) and <100 μm (microporous) were used in the present study. Dried NaCl was sieved in the desired pore size range using stainless steel sieves (Fisher Scientific). In total, 10% (w/v) POSS–PCL in DMAC was used in all experiments. Two different salt concentrations, namely 40 and 80% (w/v), in polymer were used for both macroporous and microporous salt sizes. Both salt sizes were mixed in one of the samples to obtain a mixed-pore-sized sample. A control sample was prepared without any salt in it. Salt was mixed in the polymer solution using a homogenizer (Ultra-Turrax T25; IKA Labortechnik) and then poured over the stainless steel plates to spread. The mixture was left in the oven at 50 °C for 20 h. The sheets of polymer were peeled off the plates and kept in deionized water for 72 h with a regular change of water at 4 h intervals. After 72 h the polymer sheets were air-dried and specimens from the samples were studied for their physical and chemical properties.
Porosity
The porosity of the scaffolds was calculated by using simple formulae as given below (eqn 1, eqn 2) and was compared for its accuracy with the porosity measured by X-ray microtopography [micro-CT (micro-computed tomography)] in one of the samples:
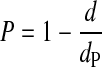 |
where P is the porosity of the scaffold sample, d is the density of the scaffolds and dp is the density of the nonporous polymer. The density of the scaffold, d, can be calculated from:
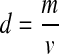 |
where m is the mass and v is the volume of the scaffold (see Table 1).
Table 1. Physical properties of the polymer and the organic solvent.
The values are means±S.D. (n=3).
| Sample | Surface tension (mN/m) | Electrical conductivity (10−4 S/m) | Viscosity (mPa·s) |
|---|---|---|---|
| DMAC | 36.0±0.4 | 0.20±0.01 | 2.20±0.12 |
| POSS–PCL | 48.0±0.3 | 1.70±0.10 | 9720±120 |
SEM (scanning electron microscopy)
The samples were attached to aluminium stubs with double-sided sticky tabs and then coated with gold using an SC500 (EMScope) sputter coater before being examined and photographed using a Philips 501 scanning electron microscope at 15 kV. SEM was also carried out after 21 days of in vitro cell culture.
Micro-CT (X-ray microtomography)
In the present study, the pore morphology was mainly studied by SEM and the porosity was calculated by using the formula mentioned above; however, for one sample (sample 1), the porosity and pore size of the scaffolds were also investigated by X-ray microtomography, in order to validate the results. The porous scaffold sample was examined using a SkyScan-1072 high-resolution desktop X-ray microtomography system (Skyscan). The X-ray radiographs were collected at 20 kV/120 μA with a 0.5 mm Al filter, an 8 μm pixel size, 0.23 ° angle step (0–180 ° rotation) and 3 frame averages per acquired radiograph. A cone-beam accusation was selected and cone-beam volumetric reconstruction (Feldkamp algorithm) was employed for image reconstruction. During the image reconstruction process, the beam hardening correction parameter was set to 20–30%, depending on the individual sample. Each original reconstructed image contained 1024×1024 pixels. The porosity and pore size were calculated and analysed using CTAn software (SkyScan) as the mean value of 100 sections studied.
Mechanical properties
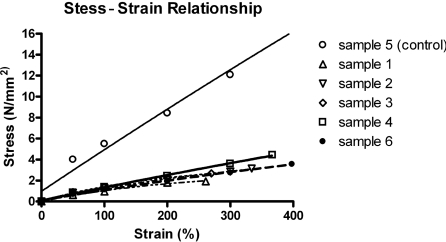
Mechanical tests were performed in uniaxial tension on a ZWICK BDO-FB.5TS tester (Ulm) unit at room temperature (20±1 °C). Specimens in the form of flat dumbbells with a 40-mm-long working part were loaded at a constant tension rate of 100 mm/min. The thickness of samples was measured using a digital electronic outside micrometer (UKAS Calibration) at three places of the dumbbell and averaged. Stress–strain relationships were obtained for the samples and graphs were plotted (Figure 1).
Figure 1. Graph presenting the stress–strain relationships for POSS–PCL scaffold samples 1–6.
Sample 5 is the non-porous sample (control). The results are mean values (n=3) for the salt concentrations of 40 and 80% (w/v).
FTIR (Fourier-transform infrared) spectroscopy
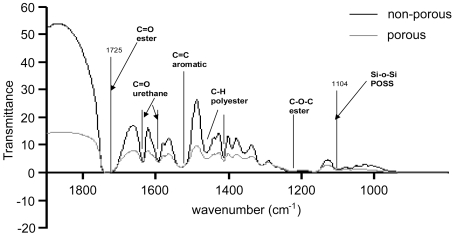
Infrared spectra of porous (sample 1) and non-porous (sample 5) scaffolds were recorded on a PerkinElmer 1750 FTIR spectrometer equipped with a triglycine sulfate detector. Spectral results were acquired from a 10 μl volume gas-tight CaF2 cell (path length 6 μm) and the temperature of recorded spectra was 25 °C. A sample shuttle was employed to permit the sample to be signal-averaged with the background. For each sample, 200 scans were signal-averaged at a resolution of 4 cm−1.
Polymer preparation for cell seeding
Polymer sheets of samples 1–6 were cast as above and thoroughly washed in distilled water. Eight circular discs of 15 mm diameter per sample were then cut using a metal die. Discs were then autoclaved to sterilize them.
Cell seeding of polymer samples
Discs of polymer samples 1–6 (n=6) prepared as above were placed in a 24-well plate (BD Biosciences) and seeded with rat small intestine epithelial cells [IEC 6 cell line; European Collection of Animal Cell Cultures] at a concentration of 2.5×106 cells per well in 1 ml of cell culture medium [Dulbecco's modified Eagle's medium supplemented with 0.1 IU/ml insulin and 5% (v/v) fetal bovine serum plus penicillin at 100 units/ml and streptomycin at 10 μg/ml (Invitrogen)]. Tissue culture plastic wells with no polymer were seeded with an identical amount of cells as a positive control. Wells with polymer but no cells were employed as a negative control. Cells were allowed to attach for 24 h after which the medium containing unattached cells was removed for LDH (lactate dehydrogenase) analysis to assess initial cell damage. The seeded polymer discs were then transferred to a fresh 24-well plate (to avoid the possibility of measuring cells seeded on to the initial well bottom during the seeding process) and cell metabolism was assessed using an Alamar Blue™ assay. Cell metabolism was then further measured on days 3, 6, 10, 14 and 21 post initial seeding. Samples of seeded polymer were also taken for analysis by SEM on day 21 as above.
Assessment of initial cell damage by LDH analysis
LDH was measured using a CytoTox 96® Non-Radioactive Cytotoxicity Assay kit (Promega). LDH is a stable cytosolic enzyme released on cell lysis into the cell culture medium. The amount of LDH released is measured using a 30 min coupled enzymatic assay based on the conversion of a tetrazolium salt INT (2-p-iodophenyl-3-p-nitrophenyl-5-phenyltetrazolium chloride) into a red formazin product, with the amount of colour formed being proportional to the number of lysed cells. A 50 μl portion of the cell culture medium from each sample was transferred to a 96-well plate (Helena Biosciences). A 50 μl portion of Substrate Mix (1 vial of substrate plus 12 ml of assay buffer; Promega) was added to each well and the plate was covered in a foil to prevent exposure to light. Samples were then incubated at room temperature for 30 min after which the reaction was stopped by the addition of 50 μl of stop solution (1 M acetic acid). Attenuance (D) was then read at 450 nm using a Multiscan MS UV–visible spectrophotometer (Labsystems).
Assessment of cell growth and metabolism by the Alamar Blue™ assay
Alamar Blue™ (Serotec) is a commercially available assay that aims to measure quantitatively cell proliferation, cytotoxicity and viability. This is achieved by incorporating resazurin and resarfurin as colorimetric oxidation reduction indicators. These indicators respond to chemical reduction resulting from cell metabolism by changing colour. This colour change may be measured by monitoring fluorescence (λex=530 nm and λem=620 nm). The advantages of this assay are that it is soluble in media, stable in solution, minimally toxic to cells and produces changes that are easily monitored.
Alamar Blue™ was added to the cell culture medium at a concentration of 10% (v/v). At each time point, polymer samples were washed with 1 ml of PBS and transferred to a fresh 24-well plate to prevent the possibility of measuring cells growing on the bottom of the plate. A 0.5 ml portion of the Alamar Blue™/cell culture medium mixture was then added to each sample and the positive control wells. A 0.5 ml portion of the mixture was added into each of the negative control samples. After 4 h, a 100 μl sample of the mixture was removed and the attenuance at fluorescence (λex=530 nm and λem=620 nm) was measured in a 96-well plate (Helena Biosciences) using a Fluoroscan Ascent FL spectrophotometer (Thermo Labsystems). The remaining mixture was then removed and replaced with fresh cell culture medium to continue the culture of the cells.
Statistical analysis
Statistical analysis of the results was performed using GraphPad Prism version 5 software. The groups were analysed for statistical significance by one- or two-way ANOVA tests. P<0.05 was considered statistically significant.
Results
Scaffold fabrication
Table 1 presents the physical properties of the POSS–PCL polymer used in this study. Table 2 shows the varying porosities obtained when salt was used in different concentrations and different particle sizes. The porosity obtained, shown in Table 2, was calculated using the simple formulae given in the Materials and methods section (eqn 1, eqn 2).
Table 2. Preparation of macroporous and microporous POSS–PCL scaffolds using varying salt concentrations.
Sample 5 (control) is the sample with no salt. Porosity values are means±S.D. (n=3). –, not determined.
| Sample | Concentration of NaCl (w/v) in the polymer (%) | NaCl particle size (μm) | Porosity obtained (%) |
|---|---|---|---|
| 1 | 80 | 150–250 | 83.9±0.5 |
| 2 | 40 | 150–250 | 59.5±0.9 |
| 3 | 80 | <100 | 74.6±0.4 |
| 4 | 40 | <100 | 61.9±0.3 |
| 5 | – | – | 5.4±6.0 |
| 6 | 80 | <100 and 150–250 | 75.3±0.3 |
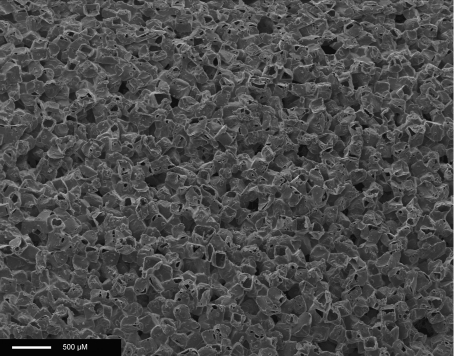
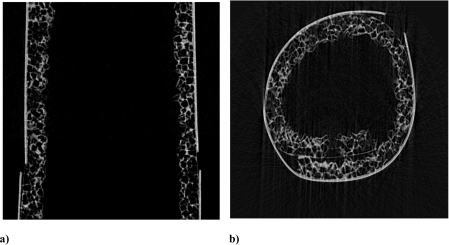
Figure 2 shows the SEM image of an 80% porous scaffold (sample 1) made of POSS–PCL, showing pore size over the range of 150–250 μm and pore interconnectivity. The results of micro-CT (Figure 3) confirmed the porosity calculated by using simple formulae. As a mean value from 100 sections of the sample, the porosity for sample 1 was found to be 80%±2%, and the pore size varied from 140 to 250 μm, as expected from the polymer/salt ratios. Good pore interconnectivity was seen in the sample studied.
Figure 2. SEM of an 80% porous scaffold (sample 1) made of POSS–PCL, showing pore sizes in the range 150–250 μm and pore interconnectivity.
Scale bar, 500 μM.
Figure 3. Micro-CT images of the tubular scaffold of sample 1.
(a) Sagittal section and (b) cross section. Pore interconnectivity can be seen in both sections. The outer thick white line is an artefact from the tape used to secure the sample while processing for micro-CT. Pores are shown in black, whereas the polymer and a small amount of the salt remaining are shown in white.
Characterization of scaffold samples
Stress–strain patterns of scaffold samples are shown in Figure 1. Porous scaffolds (samples 1, 2, 3, 4 and 6) are significantly less stiff as compared with the non-porous scaffold (sample 5); P<0.05. As expected, the porous scaffolds were less strong than the non-porous scaffold; however, among the porous scaffolds with different porosities and pore sizes, there was no significant difference observed (P>0.05). FTIR was performed as a characterization tool on a porous (sample 1) and a non-porous (sample 5) scaffold in order to confirm the preservation of the original structure of the polymer after the processing was performed in the form of solvent casting/particulate leaching (Figure 4).
Figure 4. FTIR of the porous sample (sample 1) and the non-porous sample (control), essentially showing the preservation of the basic structure of the scaffold after particulate leaching.
Assessment of initial cell damage by LDH analysis
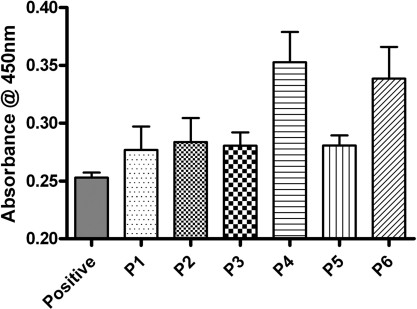
LDH release was examined to look at potential cell damage in the initial seeding period. The differences in LDH levels are actually quite small compared with the levels from the tissue culture plastic seeded cells, indicating that little initial cell damage has occurred. When statistical analysis was carried out on the levels for the scaffold samples, there was in fact no significant difference (P>0.05) between any of the scaffold samples (Figure 5).
Figure 5. LDH assay test performed on IECs seeded on POSS–PCL samples for 24 h.
Attenuance was measured in arbitrary units at 450 nm wavelength. Results are means±S.E.M. (n=3). P1–P6 are the seeded discs of polymer samples 1–6. ‘Positive’ indicates the sample with an equal number of cells seeded on the tissue culture plate without the polymer discs, serving as a positive control.
Assessment of cell growth and metabolism by the Alamar Blue™ assay
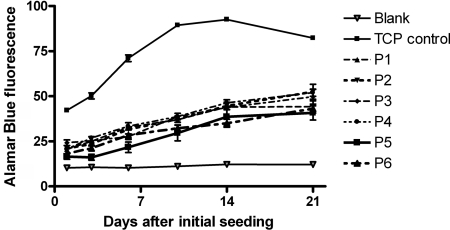
The results obtained from the investigation of cell growth and metabolism of IECs seeded on the various polymer samples show an increase in cell growth and metabolism over the course of the 21-day study (Figure 6). When seeded on tissue culture plastic, cells reached confluence by day 10 (Figure 6, TCP control). All polymer samples showed a significant reduction in cell growth and metabolism when compared with the positive control; P<0.05 (as anticipated, tissue culture plastic being optimal for cell growth). However, cell growth occurred on each polymer sample over the whole 21 day period, suggesting a lower growth rate when compared with tissue culture plastic. There was no significant difference between any of the polymers examined over the time period investigated (P>0.05).
Figure 6. Alamar Blue™ cell viability assay test on IECs seeded on POSS–PCL samples over 21 days.
Results are means±S.E.M. (n=6). P1–P6 are discs of polymer samples 1–6. ‘TCP control’ indicates the tissue culture plate control.
Assessment of cell seeding by SEM
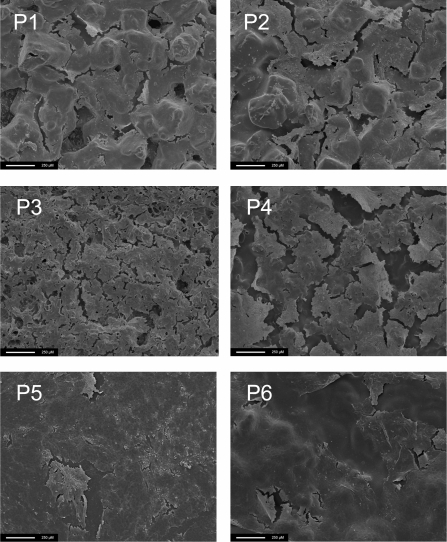
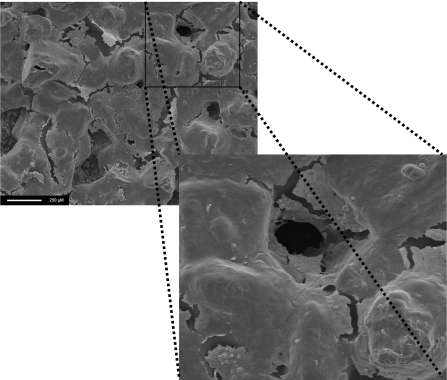
The results obtained from SEM examination of seeded polymer samples at day 21 show that cells were present on all the polymers examined and confirm the findings of the cell growth and metabolism study (Figure 7). The cell layers are also seen to grow within the pores, suggesting cell in-growth (Figure 8).
Figure 7. IECs seeded on the nanocomposite (POSS–PCL) on day 21.
Scale bar, 250 μm. P1–P6 are polymer samples 1–6.
Figure 8. The cell layer is seen to encroach into the pore from the surface, suggesting cell in-growth.
Scale bar, 250 μm.
Discussion
Tissue engineering is based on the utilization of cells and biomaterials. An ideal scaffold should be highly porous, and biocompatible with a controlled degradation rate; should have an appropriate surface for cell adhesion, proliferation and differentiation; and should maintain proper mechanical properties.
Our laboratory has already demonstrated the advantages and the successful applications of silica nanocomposite-based polyurethanes in producing vascular tissue-engineered grafts, such as improved cell adhesion characteristics using a silicon pendant nanocage [19]. In the present study, we assessed whether similar, although biodegradable, nanocomposite POSS–PCL was safe and compatible with in vitro cell cultures when IECs were employed. As this was intended as a study of cytocompatibility, it was felt that it was reasonable to use a rat intestinal cell line rather than employ primary rat intestinal cells for which a large number of rats would have been required to obtain an adequate number of cells for the study. Another aim of this study was to investigate the suitability of alternative biodegradable polymers like POSS–PCL for soft tissue engineering, as opposed to the widely accepted sole use of PGA/PLLA (poly-L-lactide acid) copolymers in small intestinal tissue engineering. PCL has the potential advantages of being less expensive and dissolves more readily in commonly available organic solvents when compared with PGA (which is very hard in consistency and requires the use of highly fluorinated solvents such as hexafluoroisopropanol).
The nanocomposite fabricated by us maintains mechanical stability due to the presence of nanocages while simultaneously allowing controlled degradation. The present study employed two different pore size ranges (150–250 μm and <100 μm) and investigated two porosities (one over the range of 80% and the other over the range of 40%). By utilizing solvent casting and salt leaching, production of scaffolds with pore size and porosity in the desired range was successfully achieved. The pores were interconnected as can be seen from SEM and micro-CT. The mechanical properties as well as the suitability of cell growth on the scaffolds produced were then examined. When tested for stress–strain relationships the results obtained show that the strength of the scaffold decreases with an increase in porosity.
In the present study, the approximate cell size of the IECs was much smaller than the organoid unit employed in the previously mentioned rat studies. IEC proliferation was followed for 21 days on scaffolds made of high and low porosities and large and small pore sizes. All the samples examined were able to support both initial cell seeding and prolonged cell growth over the course of the 21 days investigated. In our preliminary experiments, as the scaffold had not been washed adequately, they failed to show any cell attachment or growth (results not shown). The washing regime was initially to just rinse the scaffolds in deionized water. This was then made more stringent by rinsing the scaffolds for 72 h on a shaker with changes of water every 4 h. It is believed that DMAC or the residual salt levels used in scaffold preparation could be cytotoxic if not removed completely. Studies of LDH levels showed that there was little initial cell damage. There was no significant difference in cell seeding or growth between any of the samples investigated. These findings were confirmed by the SEM studies, which showed that cells were present in all samples on day 21. The potential of the POSS–PCL scaffold to support cell seeding and growth was therefore confirmed.
Conclusion
In the present study, a simple and inexpensive method of solvent casting and particulate leaching was employed to fabricate porous scaffolds using a newly developed nanocomposite of silsesquixane-based PCL (POSS–PCL). We showed that these scaffolds carry physical and chemical properties for intestinal cells to remain viable within the culture medium exposed to POSS–PCL, adhere to the polymer surface, remain viable and achieve confluence on the scaffold. This in vitro study for tissue engineering of the small intestine provides the option of using an alternative polymer to the conventionally used PGA or polylactide. The pore size and porosity of scaffolds should be tailor-made depending on the type and size of the cell source.
Acknowledgements
We thank Dr Jie Huang (Department of Mechanical Engineering, University College London, London, U.K.) for micro-CT and Mr Innes Clatworthy (Electron Microscopy Unit, Royal Free Hampstead NHS Trust Hospital, London, U.K.) for SEM.
Funding
This work was supported by the U.K. Engineering and Physical Sciences Research Council [grant number EP/D064872 to A.S.]. All the authors declare that there is no conflict of interest to disclose.
References
- 1.Messing B., Crenn P., Beau P., Boutron-Ruault M. C., Rambaud J. C., Matuchansky C. Gastroenterology. 1999;117:1043–1050. doi: 10.1016/s0016-5085(99)70388-4. [DOI] [PubMed] [Google Scholar]
- 2.Moreno J. M., Planas M., Lecha M., Virgili N., Gomez-Enterria P., Ordonez J., de la Cuerda C., Apezetxea A., Marti E., Garcia Luna P. P., et al. Nutr. Hosp. 2005;20:249–253. [PubMed] [Google Scholar]
- 3.Wales P. W., de Silva N., Kim J. H., Lecce L., Sandhu A., Moore A. M. J. Pediatr. Surg. 2005;40:755–762. doi: 10.1016/j.jpedsurg.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 4.Georgeson K., Halpin D., Figueroa R., Vincente Y., Hardin W., Jr J. Pediatr. Surg. 1994;29:316–320. doi: 10.1016/0022-3468(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 5.Safford S. D., Freemerman A. J., Safford K. M., Bentley R., Skinner M. A. Gut. 2005;54:1085–1090. doi: 10.1136/gut.2004.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brook G. Nutrition. 1998;14:813–816. doi: 10.1016/s0899-9007(98)00091-4. [DOI] [PubMed] [Google Scholar]
- 7.Kato T., Ruiz P., Thompson J. F., Eskind L. B., Weppler D., Khan F. A., Pinna A. D., Nery J. R., Tzakis A. G. World J. Surg. 2002;26:226–237. doi: 10.1007/s00268-001-0210-5. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A., Dixit A., Sales K. M., Winslet M. C., Seifalian A. M. Biomacromolecules. 2006;7:2701–2709. doi: 10.1021/bm060383e. [DOI] [PubMed] [Google Scholar]
- 9.Choi R. S., Vacanti J. P. Transplant. Proc. 1997;29:848–851. doi: 10.1016/s0041-1345(96)00164-9. [DOI] [PubMed] [Google Scholar]
- 10.Takezawa T., Ozaki K., Nitani A., Takabayashi C., Shimo-Oka T. Cell Transplant. 2004;13:463–473. doi: 10.3727/000000004783983882. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi N. Cell Transplant. 2008;17:3–9. doi: 10.3727/000000008783907099. [DOI] [PubMed] [Google Scholar]
- 12.Vara D. S., Punshon G., Sales K. M., Hamilton G., Seifalian A. M. Biotechnol. Appl. Biochem. 2006;45:119–130. doi: 10.1042/BA20060051. [DOI] [PubMed] [Google Scholar]
- 13.Heckmann L., Fiedler J., Mattes T., Dauner M., Brenner R. E. Biotechnol. Appl. Biochem. 2008;49:185–194. doi: 10.1042/BA20070071. [DOI] [PubMed] [Google Scholar]
- 14.Raghunath J., Zhang H., Edirisinghe M. J., Darbyshire A., Butler P. E., Seifalian A. M. Biotechnol. Appl. Biochem. 2009;52:1–8. doi: 10.1042/BA20070256. [DOI] [PubMed] [Google Scholar]
- 15.Kannan R. Y., Salacinski H. J., Butler P. E., Seifalian A. M. Acc. Chem. Res. 2005;38:879–884. doi: 10.1021/ar050055b. [DOI] [PubMed] [Google Scholar]
- 16.Raghunath J., Georgiou G., Armitage D., Nazhat S. N., Sales K. M., Butler P. E., Seifalian A. M. J. Biomed. Mater. Res. A. 2009;91:833–844. doi: 10.1002/jbm.a.32335. [DOI] [PubMed] [Google Scholar]
- 17.Kannan R. Y., Salacinski H. J., Ghanavi J. E., Narula A., Odlyha M., Peirovi H., Butler P. E., Seifalian A. M. Plast. Reconstr. Surg. 2007;119:1653–1662. doi: 10.1097/01.prs.0000246404.53831.4c. [DOI] [PubMed] [Google Scholar]
- 18.Seifalian A. M., Handcock S., Salacinski H. J. Polymer for use in conduits, medical devices and biomedical surface modification. 2005 World Patent Number WO2005070998.
- 19.Kannan R. Y., Salacinski H. J., De G. J., Clatworthy I., Bozec L., Horton M., Butler P. E., Seifalian A. M. Biomacromolecules. 2006;7:215–223. doi: 10.1021/bm050590z. [DOI] [PubMed] [Google Scholar]