Abstract
Background information. Cell motility entails the reorganization of the cytoskeleton and membrane trafficking for effective protrusion. The GIT–PIX protein complexes are involved in the regulation of cell motility and adhesion and in the endocytic traffic of members of the family of G-protein-coupled receptors. We have investigated the function of the endogenous GIT complexes in the regulation of cell motility stimulated by fMLP (formyl-Met-Leu-Phe) peptide, in a rat basophilic leukaemia RBL-2H3 cell line stably expressing an HA (haemagglutinin)-tagged receptor for the fMLP peptide.
Results. Our analysis shows that RBL cells stably transfected with the chemoattractant receptor expressed both GIT1–PIX and GIT2–PIX endogenous complexes. We have used silencing of the different members of the complex by small interfering RNAs to study the effects on a number of events linked to agonist-induced cell migration. We found that cell adhesion was not affected by depletion of any of the proteins of the GIT complex, whereas agonist-enhanced cell spreading was inhibited. Analysis of agonist-stimulated haptotactic cell migration indicated a specific positive effect of GIT1 depletion on trans-well migration. The internalization of the formyl-peptide receptor was also inhibited by depletion of GIT1 and GIT2. The effects of the GIT complexes on trafficking of the receptors was confirmed by an antibody-enhanced agonist-induced internalization assay, showing that depletion of PIX, GIT1 or GIT2 protein caused decreased perinuclear accumulation of internalized receptors.
Conclusions. Our results show that endogenous GIT complexes are involved in the regulation of chemoattractant-induced cell motility and receptor trafficking, and support previous findings indicating an important function of the GIT complexes in the regulation of different G-protein-coupled receptors. Our results also indicate that endogenous GIT1 and GIT2 regulate distinct subsets of agonist-induced responses and suggest a possible functional link between the control of receptor trafficking and the regulation of cell motility by GIT proteins.
Keywords: adhesion, chemotactic response, GIT–PIX complex, migration, spreading, endocytosis
Abbreviations: ARF, ADP-ribosylation factor; Cdc42, cell division cycle 42; Fluo4/AM, Fluo-4 acetoxymethyl ester; fMLP, formyl-Met-Leu-Phe; FN, fibronectin; FPR, fMLP peptide receptor; GAP, GTPase-activating protein; GIT, G-protein-coupled-receptor-kinase-interacting protein; GRK2, G-protein-coupled receptor-kinase 2; HA, haemagglutinin; mAb, monoclonal antibody; PAK, p21-activated kinase; PIX, PAK-interacting exchange factor; siRNA, small interfering RNA
Introduction
The chemotactic responses in leucocytes are mediated through receptors coupled to G-proteins (Moore et al., 2007). The n-fMLP (n-formyl-Met-Leu-Phe) chemoattractant is a bacterial peptide that regulates leucocyte adhesion, trans-endothelial migration and chemotaxis during the inflammatory response (Prossnitz and Ye, 1997). Rat basophilic leukaemia RBL-2H3 cells have been used as a model cell line to study leucocyte adhesion (Molteni et al., 2009) and fMLP-induced chemotactic responses (Ali et al., 1998). Like cell migration in general, chemotaxis is driven by protrusion at the leading edge of cells, where continuous actin and adhesion remodellings are required. Membrane traffic may contribute to the extension of the cell border (Hopkins et al., 1994; Bretscher and Aguado-Velasco, 1998), but the underlying molecular mechanisms are still largely unknown. The GIT (G-protein-coupled-receptor-kinase-interacting protein) family includes the GIT1 and GIT2 proteins, two GAPs (GTPase-activating proteins) for the ARF (ADP-ribosylation factor) family of small GTP-binding proteins. GIT proteins have been implicated in the regulation of cell adhesion and migration (Hoefen and Berk, 2006). One hypothesis is that these proteins co-ordinate ARF-mediated membrane trafficking with cell adhesion and cytoskeletal organization during cell motility (de Curtis, 2001). GIT1 and GIT2 interact with a number of proteins including the PIX [PAK (p21-activated kinase)-interacting exchange factor] exchanging factors for Rac1 and Cdc42 (cell division cycle 42) (Bagrodia et al., 1998; Manser et al., 1998), the focal adhesion protein paxillin (Turner et al., 1999), and the synaptic adaptor proteins Piccolo and liprin-α (Kim et al., 2003; Ko et al., 2003; Totaro et al., 2007). In vitro and in vivo studies suggest that GIT proteins regulate the activity of Arf6 in cells (Vitale et al., 2000; Albertinazzi et al., 2003), a member of the ARF family involved in membrane trafficking between plasma membrane and endosomes (Donaldson, 2003). On the other hand, the interaction of the multidomain GIT proteins with the PIX–PAK complex, including activator and downstream effector kinases for the Rho family proteins Rac and Cdc42, suggests the involvement of the GIT complexes in the regulation of actin-dependent processes regulated by these GTPases at the cell edge (Bagrodia et al., 1998; Manser et al., 1998; Di Cesare et al., 2000).
Internalization of heterologous β2-adrenergic receptors in HEK-293 cells (human embryonic kidney cells) is inhibited by the ARF GAPs GIT1 and GIT2, and promoted by the ARF activator ARNO (ARF nucleotide-binding-site opener) (Premont et al., 1998, 2000; Claing et al., 2000, 2001). Moreover, both the expression of ARF6 mutants and its depletion by siRNA (small interfering RNA) consistently affect the internalization of G-protein-coupled receptors (Claing et al., 2001; Houndolo et al., 2005).
By using different GIT1 and βPIX mutants, we have shown that βPIX is important for the subcellular localization of GIT1, and that the GIT complexes may affect the organization of endocytic compartments and interfere with the cellular response to motogenic stimuli, both in neuronal and non-neuronal cells (Za et al., 2006). In the present study, we have analysed the contribution of the endogenous GIT complexes to the chemotactic response of rat basophilic leukaemia RBL-2H3 cells, which are utilized as a cellular model to study agonist-induced chemotaxis (Richardson et al., 1998). In particular, we have used down-regulation of components of the endogenous GIT complexes to test the effects on agonist-induced cell adhesion and motility, and receptor trafficking. We have analysed the effects of knockdown of GIT1, GIT2 and PIX on a number of cellular events involved in agonist-induced cell migration that include receptor internalization, adhesion, spreading and cell migration. For this, we have used a stably transfected cell line derived from RBL-2H3 cells to express an HA (haemagglutinin)-tagged form of the receptor for fMLP (RBL-FPR), with the aim of addressing some aspects of the signalling underlying the chemotactic responses to fMLP.
Results and discussion
Characterization of the endogenous GIT–PIX complexes in RBL-FPR cells
Others and we have found that GIT and PIX proteins are constitutively associated in complexes in different cell types. We have used the available antibodies directed to GIT and PIX proteins to detect the endogenous complexes expressed in the RBL-FPR cell line obtained in our laboratories. Immunoprecipitation experiments with either anti-GIT1 (serum SI-64) recognizing both GIT1 and GIT2 (Paris et al., 2003) or anti-βPIX recognizing both αPIX and βPIX (Botrugno et al., 2006) showed the presence of both GIT1–PIX and GIT2–PIX complexes in these cells (Figures 1A and 1B). Immunochemical analysis, including the use of GIT1-specific antibodies, showed that GIT1 and GIT2 were about equally expressed in RBL-FPR cells (Figure 1A), whereas the 80 kDa band corresponding to βPIX was more abundant than the higher band expected to be αPIX (Figure 1B).
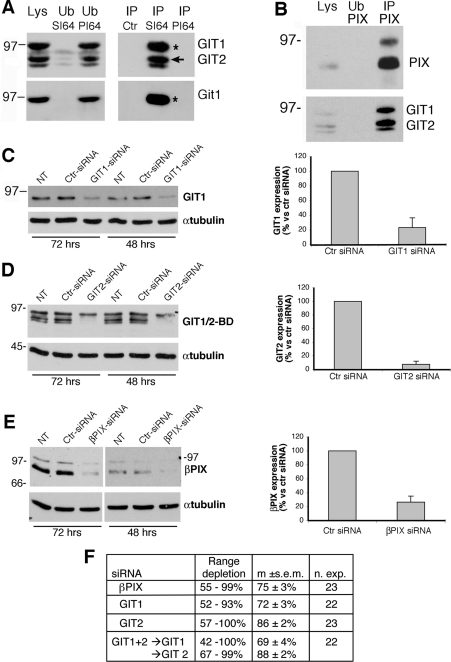
Figure 1. Expression in RBL-FPR cells and down-regulation by siRNAs of endogenous GIT and PIX proteins.
(A) Protein lysates (1 mg) from RBL-FPR cells were incubated with Protein A–Sepharose beads with no antibody (Ctr), coupled with SI-64 immune serum (SI64) or pre-immune serum (PI64). Immunoprecipitates (IP), fractions of unbound material (Ub, 1/5 of total) and lysates (Lys; 200 μg) were subjected to SDS/PAGE, and filters were then incubated for immunoblotting with the antibodies specific for the proteins indicated on the right: a mAb recognizing both GIT1 and GIT2 (upper panels) or a polyclonal antibody specific for GIT1 (lower panels). The upper band is GIT1 (asterisk), whereas the lower bands are different variants of GIT2 (arrow). (B) Immunoprecipitate with anti-PIX pAb, lysate and unbound fraction blotted for PIX and the GIT proteins. (C–E) RBL-FPR cells were transfected with siRNAs specific for rat GIT1 (C), GIT2 (D) or βPIX (E). Transfections with a control siRNA (Ctr, for luciferase) and non-transfected cells (NT) were included in each experiment. At 48 or 72 h after transfection, cells were lysed in SDS/PAGE loading buffer and immunoblotted with antibodies for GIT1 (C), GIT1 and GIT2 (D) and PIX (E). Quantification of protein down-regulation by the specific versus control siRNAs at 48 h after transfection is shown on the right of each panel. Results are are means±S.D. for three independent experiments. (F) Quantification of protein depletion by the different siRNAs used in the present study. Percentages refer to the level of protein expression evaluated 48 h after electroporation and compared with control cells (electroporated with siRNA for luciferase) taken as 100%. m, mean; n. exp., number of experiments.
To address the function of the GIT–PIX complexes in rat RBL-FPR cells we first identified specific siRNAs that were able to down-regulate the expression of the endogenous proteins. We found that each of the specific siRNAs was able to efficiently down-regulate the expression of the specific target, both at 48 or 72 h after transfection (Figures 1C–1E). Quantification of the effects of the siRNAs 48 h after transfection showed efficient reduction of each protein by the specific siRNA even when double transfections with siRNAs for both GIT1 and GIT2 were performed (Figures 1C–1F).
Effects of GIT and PIX depletion on cell adhesion, spreading and motility
fMLP-induced chemotaxis on the extracellular matrix involves integrin receptor engagement in cell adhesion, followed by actin-driven protrusion. To analyse the role of the GIT/PIX complexes in different aspects of fMLP-stimulated adhesion and motility, we have used functional assays to measure adhesion, spreading and migration.
Stimulation by fMLP induced a fast and stronger adhesion of RBL-FPR cells to FN (fibronectin) even during the short period at room temperature (2 min at 25°C) required for the procedures before starting the incubation at 37°C (corresponding to zero time in Figure 2A). The difference in the adhesion of stimulated and non-stimulated cells was evident up to 10 min at 37°C. Therefore we have analysed the effects of the depletion of the components of the GIT–PIX complexes in siRNA-transfected cells incubated after plating on to FN for 0 and 5 min at 37°C. The quantitative analysis did not show any significant difference among cells treated with the different siRNAs, both in the presence or absence of fMLP (Figure 2B), thus showing that GIT or PIX depletion did not interfere with either basal or stimulated adhesion to FN.
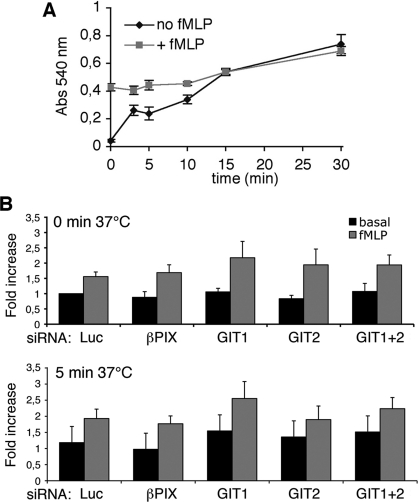
Figure 2. Depletion of GIT and PIX proteins does not affect fMLP-induced cell adhesion to FN.
(A) Quantification of RBL-FPR cell adhesion to FN. Cells were stimulated with 100 nM fMLP and centrifuged for 1 min at room temperature (zero time). Cells were then allowed to adhere at 37°C for the indicated times. Each point is the mean for at least four samples per experiment (analysis from three experiments). Adhesion after 0 min at 37°C corresponded to an approx. 2 min incubation at room temperature (see the Materials and methods section). (B) RBL-FPR cells transfected with the indicated siRNAs were resuspended with or without 100 nM fMLP, centrifuged for 1 min at room temperature on FN-coated wells, and incubated for up to 10 min at 37°C before processing. Adhesion is expressed as fold increase with respect to basal adhesion of cells with control siRNA (luciferase). No significant differences in the adhesion to FN were observed between cells transfected with specific or control siRNAs, both under basal and stimulated conditions. Results are means±S.E.M. for three different experiments (two for GIT1+GIT2), with four wells per condition per experiment.
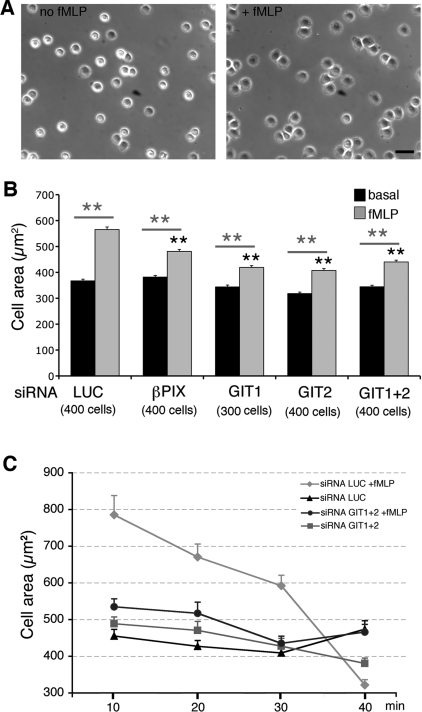
We next examined the effects of the GIT complex on cell spreading on FN, measured as the projected cell area after 10–15 min of incubation at 37°C on FN-coated substrates. Spreading was enhanced by fMLP as compared with non-treated cells (Figure 3A). Down-regulation of GIT1, GIT2 or βPIX inhibited the fMLP-stimulated spreading on FN (15% inhibition for βPIX siRNA; 26% inhibition for GIT1 siRNA; 28% inhibition for GIT2 siRNA), while basal spreading on FN was not significantly affected (Figure 3B). We then performed a kinetic analysis at different time points during spreading on FN of fMLP-stimulated versus non-stimulated cells transfected with siRNAs. Cells transfected with control siRNA showed that fMLP induced a transient increase of spreading on FN, which became negligible after 40 min (Figure 3C). The fMLP-induced transient increase in spreading was inhibited by simultaneous knockdown of GIT1 and GIT2, while differences between cells transfected with control or specific siRNAs were not evident after 40 min, when the differences between fMLP-treated and non-treated cells became negligible also in control cells.
Figure 3. Depletion of either PIX or GIT proteins inhibits fMLP-induced spreading on FN.
(A) Phase-contrast images of RBL-FPR cells incubated with or without 100 nM fMLP and allowed to adhere for 15 min on FN. Scale bar, 40 μm. (B) RBL-FPR cells with or without 100 nM fMLP were centrifuged for 1 min at room temperature and allowed to spread for 10 min on FN-coated coverslips before fixation. Phase-contrast images were analysed with ImageJ software to measure the projected cell areas. Results are means±S.E.M. (300–400 cells per experimental condition, from 3–4 independent experiments). **P<0.005. Black asterisks refer to comparisons of the fMLP-induced cell area between control (LUC) and GIT- or PIX-depleted cells; grey asterisks refer to comparisons between basal and fMLP-induced areas for each specific siRNA. (C) Projected areas of cells transfected with the indicated siRNA (mean±S.E.M.; n=50 cells per condition) were measured after spreading for 10–40 min on FN at 37°C with or without stimulation (100 nM fMLP).
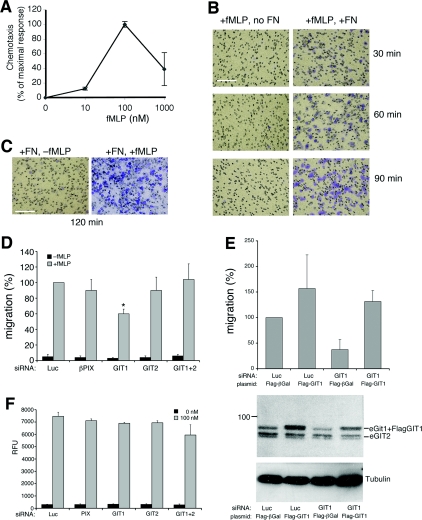
Finally, we investigated whether the endogenous GIT–PIX complexes played a role in fMLP-stimulated cell migration. Addition of increasing concentrations of fMLP to the lower side of Boyden chambers coated with FN was used to determine the optimal concentration to stimulate cell migration of RBL-FPR cells. We found that 100 nM fMLP gave the highest migratory responses in these cells (Figure 4A). Efficient migration of RBL-FPR under these conditions was dependent both on substrate-bound FN and soluble fMLP, since lack of either component on the lower side of the transwells prevented cell migration through the filters (Figures 4B and 4C). We used this experimental setting to test the effects of the depletion of the components of the endogenous GIT–PIX complexes on fMLP-induced, FN-mediated chemotaxis. Interestingly, depletion of GIT1 specifically inhibited migration of RBL-FPR cells, whereas down-regulation of any of the other components had no significant influence on the migration of these cells (Figure 4D). Rescue experiments showed that the inhibitory effects induced by transfection of GIT1 siRNA on cell migration were specific, since co-transfection of avian FLAG–GIT1 with GIT1 siRNA was able to prevent the defect in migration (Figure 4E). Our results indicate that GIT1 plays a specific positive role in fMLP-induced migration of RBL-FPR cells. On the other hand, the lack of evident effects on fMLP-induced, FN-mediated chemotaxis after double knockdown of GIT1 and GIT2 is surprising. These findings suggest that GIT2 is required for the inhibitory effects on migration induced by GIT1 depletion, although the underlying mechanisms remain to be determined. Interestingly, it has been shown that the spreading and migration of other cell types are negatively regulated by endogenous GIT2 (Frank et al., 2006), suggesting that GIT proteins may exert different functions in distinct cellular environments.
Figure 4. Down-regulation of GIT1 affects fMLP-induced chemotaxis.
(A) Directional migration of RBL-FPR cells towards fMLP. Serum-starved RBL-FPR cells were subjected to chemotactic assay towards different concentrations of fMLP for 4 h at 37°C. Cells migrating to the lower side of FN-coated filters were quantified by densitometric analysis. Chemotaxis was calculated as the percentage of maximal chemotactic response (observed at 100 nM fMLP). Means±S.D. for two independent experiments, each performed in duplicate. (B) Coating with FN is required for fMLP-induced migration. Crystal Violet-stained representative fields after transwell migration of RBL-FPR cells for the indicated times are shown. (C) Representative fields after transwell migration towards FN, with or without 100 nM fMLP. Scale bars, 200 μm. (D) RBL-FPR cells transfected with the indicated siRNAs were used for transwell migration assays (2 h at 37°C) in the presence of FN. Directional motility in the presence (grey bars) or absence (black bars) of 100 nM fMLP was expressed as a percentage compared with stimulated control cells (100%=siRNA luciferase+fMLP). Results are mean percentages±S.E.M. of migrated cells per field (n=4 experiments; quantifications from eight fields from two different filters per sample per experiment). siRNA for GIT1 significantly reduced the fMLP-induced migration by 40±6% (S.E.M.; *P<0.05). (E) RBL-FPR cells were co-transfected with the indicated siRNAs and plasmids, and analysed for fMLP-stimulated migration as in (D). Results are mean percentages±S.D. (n=3). The panel under the graph shows an example of a filter from a gel loaded with equal amounts of lysates from the different transfections, incubated with antibodies to detect endogenous and overexpressed GIT proteins (upper panel) or tubulin antibodies (lower panel). (F) Depletion of the components of the endogenous GIT–PIX complexes does not affect calcium response to fMLP. siRNA-transfected RBL-FPR cells were loaded 48 h after electroporation with Fluo4/AM and monitored for changes in intracellular [Ca2+] before and after stimulation with or without fMLP (100 nM). The maximum relative fluorescence unit (RFU) value registered within 3 min after fMLP addition was considered as the response. None of the tested siRNAs caused any significant effect on fMLP-induced calcium response.
No effects of GIT/PIX knockdown on fMLP-induced calcium responses
Leucocytes migrate to sites of inflammation to take part in host defence by activation of chemoattractant receptors. In this process, pro-inflammatory agents such as fMLP, interleukin-8 or platelet-activating factor activate chemoattractant receptors coupled to G-proteins to induce cellular responses. Increase in cytosolic calcium is a measure of signalling after activation of several G-protein-coupled receptors. In this respect, it has been shown that GIT1 is required for angiotensin II-stimulated calcium mobilization, since the knockdown of GIT1 with antisense GIT1 oligonucleotides causes inhibition of calcium mobilization induced by angiotensin II (Haendeler et al., 2003). Here, we have checked for the effects of GIT–PIX depletion on fMLP-induced cytosolic calcium elevation in RBL-FPR cells. We found that the expression of siRNAs to deplete the different components of the endogenous GIT–PIX complexes did not affect the response to 100 nM fMLP in terms of cytosolic calcium elevation (Figure 4F). These results indicate that the effects observed on the motility of cells depleted of specific components of the endogenous GIT complexes were not due to changes in the calcium elevation in response to agonist signalling.
Effect of GIT and PIX depletion on the internalization of fMLP receptors
GIT1 was first identified in a two-hybrid screen for proteins interacting with the GRK2 (G-protein-coupled-receptor kinase 2) (Premont et al., 1998), and recently the interaction between GIT1 and GRK2 was found to be transiently stimulated by sphingosine-1-phosphate in HeLa-GRK2 cells stably transfected with GRK2 (Penela et al., 2008). The interaction between overexpressed GRK2 and GIT1 proteins was confirmed by us in COS7 cells (S. Paris and I. de Curtis, unpublished data). We thus set out to identify the biochemical interaction between endogenous GRK2 and GIT proteins both in non-stimulated and in fMLP-stimulated RBL-FPR cells. No interaction between the endogenous GRK2 and GIT proteins could be detected by co-immunoprecipitation under different conditions, using antibodies specific for proteins of the GIT complex or for GRK2. We were unable to detect the interaction between the endogenous GRK2 and GIT proteins either in resting and fMLP-stimulated adherent RBL-FPR cells, or in RBL-FPR cells kept in suspension during stimulation for different times with fMLP (L. Za and I. de Curtis, unpublished data).
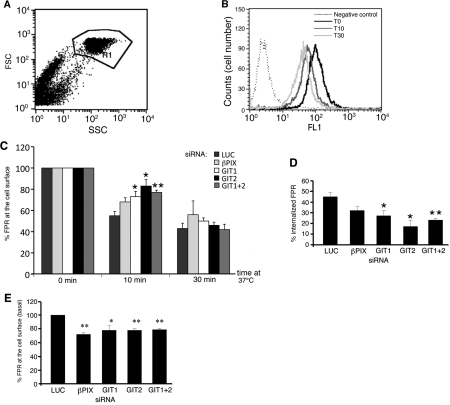
On the other hand, a number of reports have shown that GIT proteins are implicated in the regulation of different endocytic pathways, including the trafficking of G-protein-coupled receptors. In particular, GIT1 overexpression inhibits the endocytosis of the β2-adrenergic receptors and of several other G-protein-coupled receptors (Premont et al., 1998; Claing et al., 2000). We have therefore addressed the role of proteins of the GIT complexes in the internalization of the FPR (fMLP peptide receptor), by using cytofluorimetric measures of the HA-tagged FPR left on the surface of fMLP-stimulated RBL-FPR cells. Incubation at 37°C of serum-starved cells stimulated with fMLP induced a clear decrease in cell surface fluorescence intensity after both 10 and 30 min internalization (Figures 5A and 5B). We tested the effects of the down-regulation of components of the GIT complexes on the internalization of the FPR by quantifying the amount of receptors left on the cell surface after incubation for 10 min at 37°C in the presence of fMLP. A significant inhibition of receptor internalization was detected after 10 min of stimulation when GIT1 and/or GIT2 were down-regulated (Figures 5C and 5D). This effect was not as evident after 30 min with fMLP, where the differences in the amount of receptor internalized in control and GIT-depleted cells became non-significant (Figure 5C). Interestingly, the depletion of the endogenous proteins of the GIT complexes also affected the amount of FPR expressed on the surface of non-stimulated cells, with a 21–28% decrease compared with control cells, suggesting a role of these proteins in the regulation of surface expression of this receptor at steady state (Figure 5E).
Figure 5. Effects of the depletion of components of the GIT–PIX complexes on fMLP-induced FPR internalization.
(A) Flow cytometry after fMLP stimulation to analyse the cell surface expression of HA-tagged FPR in RBL-FPR cells. The setting of the gate was based on cell morphology. FSC, forward scatter; SSC, side angle scatter. (B) Flow cytometry of serum-starved cells stimulated with 100 nM fMLP and incubated at 37°C for the indicated times. Down-regulation of the cell surface receptor was detected as a shift towards the left of the peaks of fluorescence intensity (FL1) after internalization at 37°C. (C) Silencing of GIT1, GIT2 or GIT1+GIT2 inhibited receptor internalization. Cells transfected with the indicated siRNAs were incubated at 37°C with fMLP for the indicated times before quantification of the residual receptors at the cell surface by cytofluorimetry. Results are means±S.E.M. (n=3–4 experiments; normalized for each population of transfected cells with respect to the surface receptors at zero time=100%). (D) Percentage of FPR internalized after incubation with fMLP for 10 min at 37°C. (E) Decreased expression of cell surface FPR 48 h after siRNA for βPIX, GIT1, GIT2 or GIT1+GIT2, compared with control siRNA (means±S.E.M. for 3–4 experiments). *P<0.05, **P<0.005 (Student's t test).
The transient inhibition of ligand-induced receptor internalization in cells depleted of endogenous components of the GIT complexes could cause a sustained signalling by the increased concentration of receptors remaining at the cell surface. In contrast, we observed the inhibition of cell motility upon expression of specific siRNAs. This apparent contradiction may be explained by recent findings showing that endocytosis is relevant to the maintenance of the signalling linked to motogenic stimuli (Byers et al., 2008; Palamidessi et al., 2008).
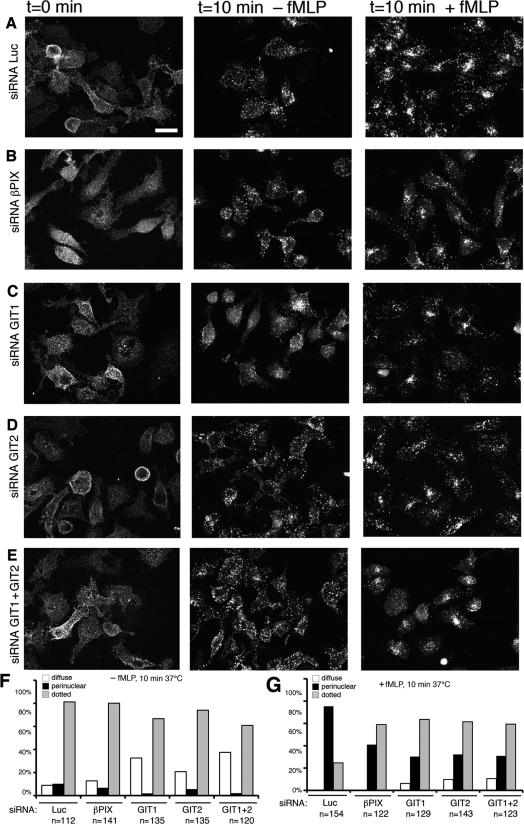
We then utilized an antibody-enhanced FPR internalization assay to further analyse, by immunofluorescence, the effects of the endogenous GIT complexes on fMLP-stimulated FPR endocytosis. First trials aimed at detecting the internalization of the receptors by immunofluorescence after fMLP stimulation indicated that the high background from the intracellular FPR present along the biosynthetic pathway could obscure the pool of internalized receptors (results not shown). We therefore prelabelled the surface receptors by incubating cells at 0°C with anti-HA antibodies as detailed in the Materials and methods section, and then performed the internalization at 37°C, both in the presence and absence of fMLP. Although cross-linking of FPR molecules at the cell surface by the antibodies is likely to enhance receptor internalization, this approach could still allow the detection of clear differences in the internalization between stimulated and non-stimulated cells (Figure 6A). In control cells, the distribution of the surface-labelled FPR was diffuse, while incubation of cells for 10 min in the absence of fMLP induced the formation of clusters of receptors that may include both internalized and clustered receptors at the cell surface (Figure 6A). Very few cells showed a clear accumulation of the internalized receptors in the perinuclear region after incubation at 37°C in the absence of the ligand (100 nM fMLP) (Figures 6A and 6F). In contrast, incubation for 10 min at 37°C in the presence of fMLP induced the accumulation of the internalized receptors in the perinuclear region of most of the cells (Figures 6A and 6G). Interestingly, the analysis of the fMLP-induced internalization of the receptors in cells depleted of any of the components of the GIT complexes analysed showed a clear inhibition of the perinuclear accumulation of the internalized FPR (Figures 6B–6E). The quantitative analysis from three independent experiments showed a decrease in the percentage of cells with perinuclear accumulation of the receptor, and a corresponding increase in cells with punctate staining when compared with control cells (Figure 6G). Moreover, the down-regulation of the GIT proteins induced an increase in the percentage of cells that showed a diffuse surface staining after incubation at 37°C without fMLP ligand (Figure 6F). Overall, these results support a role for the endogenous GIT complexes in the regulation of ligand-stimulated trafficking of fMLP receptors. The results indicate the requirement of the endogenous GIT complexes for the internalization of FPR. Moreover, the decrease in concentration of the internalized receptor in the perinuclear region after stimulation of cells transfected with the specific siRNAs suggests that the GIT complexes may also play a role in the regulation of the transport of the internalized receptors to the perinuclear region.
Figure 6. Inhibition of fMLP-induced FPR internalization by siRNA for GIT and PIX.
(A–E) Immunofluorescence showing three-dimensional projections from stacks of confocal images of cells transfected with the indicated siRNAs and prelabelled at 0°C with anti-HA and Alexa Fluor® 568-conjugated anti-mouse Ig, before incubation at 37°C for the indicated times with or without fMLP. Scale bar, 20 μm. (F, G) Quantification from fluorescence images, shown in (A–E), of the distribution of FPR after incubation of antibody-labelled, siRNA-transfected cells for 10 min at 37°C, either in the absence (F) or in the presence of fMLP (G). Numbers indicate the number of cells examined from three independent experiments.
Conclusions
The internalization of G-protein-coupled receptors is finely regulated by the action of several proteins required to control this complex process. Included among the regulators of this process are the small GTPase ARF6 (Houndolo et al., 2005) and its GAPs belonging to the GIT family (Claing et al., 2000). The function of GIT and PIX proteins has mainly been addressed by overexpression of wild-type and mutant forms of these proteins. On the other hand, recent studies have addressed the function of some of these proteins using cells isolated from knockout mice. For example, it has been shown that neutrophils from either GIT2 or αPIX knockout animals have impaired chemotactic responses (Li et al., 2003; Mazaki et al., 2006). Of the two PIX proteins, αPIX is particularly abundant in cells of the immune system, whereas βPIX is widely expressed. Further analysis of immune cells from αPIX knockout mice has shown that basal migration of αPIX-null T- and B-cells was enhanced, and suggested a redundant role of βPIX in these cells (Missy et al., 2008). Here we have shown that endogenous GIT complexes are involved in the regulation of chemoattractant-induced cell motility and receptor trafficking. In agreement with previous overexpression studies, our results show that the endogenous GIT complexes are important for the regulation of some of the G-protein-coupled receptors-mediated events, and also indicate that endogenous GIT1 and GIT2 regulate distinct subsets of agonist-induced responses, and suggest a functional link between the control of receptor trafficking and the regulation of cell motility by GIT proteins.
Signal transduction events do not occur only at the plasma membrane, but also at different intracellular compartments after internalization (Disanza et al., 2009; Gould and Lippincott-Schwartz, 2009). Endocytic/recycling pathways are not only required to control the timing, but also the polarized compartmentalization of the signalling originating from different types of receptors, including tyrosine kinase receptors (Palamidessi et al., 2008) and chemokine receptors (Minina et al., 2007; Byers et al., 2008). In fact the inhibition of spreading and migration observed following knockdown of the endogenous GIT proteins may be a consequence of the perturbation of FPR trafficking, which could affect membrane recycling to the leading edge of motile cells. It has been shown for other types of receptors that endocytic trafficking is important for the localization of their signalling during cell migration (Polo and Di Fiore, 2006; Palamidessi et al., 2008). The same may apply to signalling by G-protein-coupled receptors, where perturbation of signalling by altering their trafficking after GIT knockdown may at least partially explain some of the effects on cell motility observed.
Materials and methods
Cell lines and transfections
We generated the RBL-FPR cell line by stable transfection of rat RBL-2H3 leukaemia cells (Seldin et al., 1985) with a pcDNA3 plasmid including the cDNA for the N-terminally HA-tagged human FPR (a gift from Dr Silvano Sozzani, Department of Biomedical Sciences and Biotechnology, University of Brescia, Italy). The tagged receptor was detected by using the anti-HA mAb (monoclonal antibody) 12CA5. Plasmids for FLAG–β-galactosidase and FLAG–GIT1 have been described previously (Di Cesare et al., 2000).
For transfection of cDNAs or siRNA heteroduplexes, RBL-FPR cells were electroporated (4×106 cells per electroporation) and cultured in DMEM (Dulbecco's modified Eagle's medium; Gibco) with 20% (v/v) fetal bovine serum (BioWhittaker) with 1 mg/ml G418 (Calbiochem). COS7 cells were transfected with Lipofectamine™ 2000 (Invitrogen).
siRNAs used in the present study were specific for targeting RNA sequences corresponding to the following DNA sequences: 5′-CAACAGGAATGACAATCAC-3′ for rat βPIX and 5′-GCACTCAGCAACCGGCTCT-3′ for rat GIT1 (MWG-Biotech AG) (Zhang et al., 2005); 5′-CTGAGTACTCCTCGACACGAA-3′ for rat GIT2 (Qiagen); 5′-CAUCACGTACGCGGAATAC-3′ for luciferase (Invitrogen). Their ability to silence endogenous proteins was verified 48 and 72 h after electroporation by Western blotting with antibodies specific for GIT1 (goat pAb from Santa Cruz Biotechnology), GIT1 and GIT2 (mAb from BD Transduction Laboratories) and rabbit pAb anti-GIT and anti-PIX prepared in our laboratory (Paris et al., 2003; Botrugno et al., 2006). Protein loading was normalized with respect to α-tubulin (mAb from Sigma). All functional studies were performed 48 h after electroporation unless otherwise indicated.
Cell adhesion
96-well plates (Costar 3590; Corning) were coated with 10 μg/ml FN in PBS and incubated for 2 h at 37°C with 1% BSA. Cells were starved for 4 h in serum-free medium, collected by trypsinization and resuspended in serum-free medium to 106 cells/ml. Then, 5×104 cells were plated on to each well (four wells per condition per experiment), with or without 100 nM fMLP. Seeding of cells to the bottom of wells was synchronized by 1 min centrifugation at 1000 g. Cells were incubated at 37°C between 0 min (≈2 min at room temperature) and 30 min. Adhesion was blocked by washing cells twice with warm medium before fixation with 4% (w/v) paraformaldehyde, staining with Crystal Violet [0.5% in 20% (v/v) methanol] and solubilization with 1% SDS. Adhesion was quantified by measuring the intensity of the attenuance at 540 nm. Adhesion to BSA-coated wells was negligible in all experiments.
Cell spreading
Cells were trypsinized, resuspended in serum-free medium to 106 cells/ml, and plated on to 13 mm coverslips (105 cells per coverslip) coated with 10 μg/ml FN and 1% BSA. Cells were centrifuged for 1 min at 1000 g before incubation at 37°C for the indicated times, with or without 100 nM fMLP. After fixation (3% paraformaldehyde and 2% sucrose in PBS), cells were analysed by phase-contrast microscopy with an Axiovert S100 TV2 microscope (Zeiss) equipped with a Hamamatsu Orca II CCD digital camera (Hamamatsu Photonic). Projected cell areas were measured by the public software ImageJ (NIH). A total of 100 cells were measured per condition in each experiment.
Cell migration
Modified Boyden chamber assays were performed to measure cell migration. The lower sides of polycarbonate filters (8 μM pore, 6.5 mm; Costar) were coated with 10 μg/ml FN. Transfected cells starved for 4 h were loaded on to the upper chambers (105 cells per filter). The bottom chambers were filled with serum-free culture medium with or without 100 nM fMLP. Cells were incubated for 2–4 h at 37°C. Cells were removed from the upper part of the filters with a cotton swab, whereas the lower part was fixed in cold methanol and stained with Crystal Violet. Brightfield images were collected with a Leica DM IRB microscope equipped with a C plan ×20/0.3 and a Leica DC 300FX camera using IMG50 software (Leica Microsystems). We quantified the number of cells in four fields per filter per experiment.
Internalization assays by flow cytometry
The ability of FPRs to be internalized was measured by flow cytometry. At 48 h after electroporation of siRNAs, RBL-FPR cells were trypsinized and washed with Hanks balanced salt solution modified with 0.1% inactivated BSA, 10 mM Hepes, 1 mM CaCl2 and 1 mM MgCl2. 3×105 cells were incubated for 0–30 min at 37°C with or without 100 nM fMLP. Receptor internalization was blocked with a cold washing buffer (1% serum in PBS). Cells were immunostained on ice with the anti-HA 12CA5 mAb. Cells were washed with a cold washing buffer and incubated for 25 min on ice with Alexa Fluor® 488 goat anti-mouse Ig (Molecular Probes). After washing with cold buffer, cells were resuspended in 300 μl of PBS for FACS analysis by FACScan (Becton Dickinson). The results were analysed with Cell Quest software (Becton Dickinson). Cells incubated with secondary antibody only represented negative controls.
Antibody-enhanced internalization assays
RBL-FPR cells electroporated in the presence of siRNAs were trypsinized and plated on to 13 mm coverslips coated with 10 μg/ml FN in serum-free medium. After starvation for 4 h, cells were incubated for 25 min on ice with anti-HA 12CA5, washed twice on ice and incubated for 25 min on ice with Alexa Fluor® 568 goat anti-mouse Ig (Molecular Probes). Prelabelled cells were then incubated for the indicated times at 37°C with or without 100 nM fMLP. Internalization of the antibody-bound FPR was stopped by washing with cold modified Hanks balanced salt solution and by fixation with 3% paraformaldehyde and 2% sucrose. In some experiments, cells were stained with FITC–phalloidin (Sigma) after fixation. Images were captured with an Axiophot microscope equipped with a PLAN-Apochromat ×63/1.4 lens (Carl Zeiss MicroImaging) and a C4742-95-12HR digital camera (Hamamatsu). Images were collected with HiPic 32 software (High Performance Image Control System) and processed with Photoshop (Adobe). Confocal images were collected with a Bio-Rad MRC 1024 confocal microscope (Bio-Rad) equipped with a PLAN-Apochromat ×63/1.4 lens. Images were collected with Lasersharp 2000 software (Bio-Rad) and processed with ImageJ and Photoshop (Adobe). For quantification of integrin internalization, cells were classified into three categories based on the distribution of the immunofluorescence: cells with diffuse staining; cells with dotted, sparse staining; and cells with perinuclear accumulation of the staining.
Calcium imaging
RBL-FPR cells were electroporated with siRNAs and seeded on to 384-well plates (Thermo Fisher Scientific). After 48 h, cells were loaded with 25 μl per well of a dye mixture containing 2 μM Fluo4/AM (Fluo-4 acetoxymethyl ester) (Invitrogen), 0.02% Pluronic F-127 (Invitrogen), 2.5 mM Probenecid (Invitrogen) in Tyrode's buffer (130 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM NaHCO3 and 20 mM Hepes, pH 7.4). After 1 h at 37°C in the dark, plates were gently washed with Tyrode's buffer. Cells were then stimulated with 100 nM fMLP, and relative changes in fluorescence were monitored for 3 min at the FliprTETRA (Molecular Devices). The response was the maximum relative fluorescence unit value registered within 3 min after fMLP addition. Data analysis was done using GraphPad Prism (GraphPad Software).
Acknowledgements
Plasmids with cDNA for the N-terminally HA-tagged human FPR were provided by Dr Silvano Sozzani. We thank the personnel of the Alembic facility for technical support with confocal microscopy.
Funding
This work was supported by the European Union Network of Excellence ‘Targeting Cell Migration in Chronic Inflammation’ [MAIN; grant number FP6-502935].
References
- Albertinazzi C., Za L., Paris S., de Curtis I. ADP-ribosylation factor 6 and a functional PIX/p95-APP1 complex are required for Rac1B-mediated neurite outgrowth. Mol. Biol. Cell. 2003;14:1295–1307. doi: 10.1091/mbc.E02-07-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali H., Sozzani S., Fisher I., Barr A.J., Richardson M., Haribabu B., Snyderman R. Differential regulation of formyl peptide and platelet-activating factor receptors. Role of phospholipase Cbeta3 phosphorylation by protein kinase A. J. Biol. Chem. 1998;273:11012–11016. doi: 10.1074/jbc.273.18.11012. [DOI] [PubMed] [Google Scholar]
- Bagrodia S., Taylor S.J., Jordon K.A., Van Aelst L., Cerione R.A. A novel regulator of p21-activated kinases. J. Biol. Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- Botrugno O.A., Paris S., Za L., Gualdoni S., Cattaneo A., Bachi A., de Curtis I. Characterization of the endogenous GIT1-betaPIX complex, and identification of its association to membranes. Eur. J. Cell Biol. 2006;85:35–46. doi: 10.1016/j.ejcb.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Bretscher M.S., Aguado-Velasco C. EGF induces recycling membrane to form ruffles. Curr. Biol. 1998;8:721–724. doi: 10.1016/s0960-9822(98)70281-7. [DOI] [PubMed] [Google Scholar]
- Byers M.A., Calloway P.A., Shannon L., Cunningham H.D., Smith S., Li F., Fassold B.C., Vines C.M. Arrestin 3 mediates endocytosis of CCR7 following ligation of CCL19 but not CCL21. J. Immunol. 2008;181:4723–4732. doi: 10.4049/jimmunol.181.7.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claing A., Perry S.J., Achiriloaie M., Walker J.K.L., Albanesi J.P., Lefkowitz R.J., Premont R.T. Multiple endocytic pathways of G-protein-coupled receptors delineated by GIT1 sensitivity. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1119–1124. doi: 10.1073/pnas.97.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claing A., Chen W., Miller W.E., Vitale N., Moss J., Premont R.T., Lefkowitz R.J. β-Arrestin-mediated ADP-ribosylation factor 6 activation and β2-adrenergic receptor endocytosis. J. Biol. Chem. 2001;276:42509–42513. doi: 10.1074/jbc.M108399200. [DOI] [PubMed] [Google Scholar]
- de Curtis I. Cell migration: GAPs between membrane traffic and the cytoskeleton. EMBO Rep. 2001;2:277–281. doi: 10.1093/embo-reports/kve072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare A., Paris S., Albertinazzi C., Dariozzi S., Andersen J., Mann M., Longhi R., de Curtis I. p95-APP1 links membrane transport to Rac-mediated reorganization of actin. Nat. Cell Biol. 2000;2:521–530. doi: 10.1038/35019561. [DOI] [PubMed] [Google Scholar]
- Disanza A., Frittoli E., Palamidessi A., Scita G. Endocytosis and spatial restriction of cell signaling. Mol. Oncol. 2009;3:280–296. doi: 10.1016/j.molonc.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J.G. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- Frank S.R., Adelstein M.R., Hansen S.H. GIT2 represses Crk- and Rac1-regulated cell spreading and Cdc42-mediated focal adhesion turnover. EMBO J. 2006;25:1848–1859. doi: 10.1038/sj.emboj.7601092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould G.W., Lippincott-Schwartz J. New roles for endosomes: from vesicular carriers to multi-purpose platforms. Nat. Rev. Mol. Cell Biol. 2009;10:287–292. doi: 10.1038/nrm2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haendeler J., Yin G., Hojo Y., Saito Y., Melaragno M., Yan C., Sharma V.K., Heller M., Aebersold R., Berk B.C. GIT1 mediates Src-dependent activation of phospholipase Cgamma by angiotensin II and epidermal growth factor. J. Biol. Chem. 2003;278:49936–49944. doi: 10.1074/jbc.M307317200. [DOI] [PubMed] [Google Scholar]
- Hoefen R.J., Berk B.C. The multifunctional GIT family of proteins. J. Cell Sci. 2006;119:1469–1475. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- Hopkins C.R., Gibson A., Shipman M., Strickland D.K., Trowbridge I.S. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J. Cell Biol. 1994;125:1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houndolo T., Boulay P.L., Claing A. G-protein-coupled receptor endocytosis in ADP-ribosylation factor 6-depleted cells. J. Biol. Chem. 2005;280:5598–5604. doi: 10.1074/jbc.M411456200. [DOI] [PubMed] [Google Scholar]
- Kim S., Ko J., Shin H., Lee J.R., Lim C., Han J.H., Altrock W.D., Garner C.C., Gundelfinger E.D., Premont R.T., et al. The GIT family of proteins forms multimers and associates with the presynaptic cytomatrix protein Piccolo. J. Biol. Chem. 2003;278:6291–6300. doi: 10.1074/jbc.M212287200. [DOI] [PubMed] [Google Scholar]
- Ko J., Kim S., Valtschanoff J.G., Shin H., Lee J.R., Sheng M., Premont R.T., Weinberg R.J., Kim E. Interaction between liprin-alpha and GIT1 is required for AMPA receptor targeting. J. Neurosci. 2003;23:1667–1677. doi: 10.1523/JNEUROSCI.23-05-01667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Hannigan M., Mo Z., Liu B., Lu W., Wu Y., Smrcka A.V., Wu G., Li L., Liu M., et al. Directional sensing requires G-mediated PAK1 and PIX-dependent activation of Cdc42. Cell. 2003;114:215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- Manser E., Loo T.H., Koh C.G., Zhao Z.S., Chen X.Q., Tan L., Tan I., Leung T., Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- Mazaki Y., Hashimoto S., Tsujimura T., Morishige M., Hashimoto A., Aritake K., Yamada A., Nam J.M., Kiyonari H., Nakao K., Sabe H. Neutrophil direction sensing and superoxide production linked by the GTPase-activating protein GIT2. Nat. Immunol. 2006;7:724–731. doi: 10.1038/ni1349. [DOI] [PubMed] [Google Scholar]
- Minina S., Reichman-Fried M., Raz E. Control of receptor internalization, signaling level, and precise arrival at the target in guided cell migration. Curr. Biol. 2007;17:1164–1172. doi: 10.1016/j.cub.2007.05.073. [DOI] [PubMed] [Google Scholar]
- Missy K., Hu B., Schilling K., Harenberg A., Sakk V., Kuchenbecker K., Kutsche K., Fischer K.D. {alpha}PIX RhoGEF regulates lymphocyte functions and antigen receptor signaling. Mol. Cell. Biol. 2008;28:3776–3789. doi: 10.1128/MCB.00507-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R., Lage Crespo C., Feigelson S., Moser C., Fabbri M., Grabovsky V., Krombach F., Laudanna C., Alon R., Pardi R. β-Arrestin 2 is required for the induction and strengthening of integrin-mediated leukocyte adhesion during CXCR2-driven extravasation. Blood. 2009;114:1073–1082. doi: 10.1182/blood-2008-10-183699. [DOI] [PubMed] [Google Scholar]
- Moore C.A.C., Milano S.K., Benovic J.L. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Palamidessi A., Frittoli E., Garré M., Faretta M., Mione M., Testa I., Diaspro A., Lanzetti L., Scita G., Di Fiore P.P. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Paris S., Longhi R., Santambrogio P., de Curtis I. Leucine-zipper-mediated homo- and hetero-dimerization of GIT family p95-ARF GTPase-activating protein, PIX-, paxillininteracting proteins 1 and 2. Biochem. J. 2003;372:391–398. doi: 10.1042/BJ20030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penela P., Ribas C., Aymerich I., Eijkelkamp N., Barreiro O., Heijnen C.J., Kavelaars A., Sánchez-Madrid F., Mayor F., Jr G-protein-coupled receptor kinase 2 positively regulates epithelial cell migration. EMBO J. 2008;27:1206–1218. doi: 10.1038/emboj.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo S., Di Fiore P.P. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Premont R.T., Claing A., Vitale N., Freeman J.L.R., Pitcher J.A., Patton W.A., Moss J., Vaughan M., Lefkowitz R.J. Beta(2)-adrenergic receptor regulation by GIT1, a G-protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont R.T., Claing A., Vitale N., Perry S.J., Lefkowitz R.J. The GIT family of ADPribosylation factor GTPase-activating proteins: functional diversity of GIT2 through alternative splicing. J. Biol. Chem. 2000;275:22373–22380. doi: 10.1074/jbc.275.29.22373. [DOI] [PubMed] [Google Scholar]
- Prossnitz E.R., Ye R.D. The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol. Ther. 1997;74:73–102. doi: 10.1016/s0163-7258(96)00203-3. [DOI] [PubMed] [Google Scholar]
- Richardson R.M., Pridgen B.C., Haribabu B., Ali H., Snyderman R. Differential cross-regulation of the human chemokine receptors CXCR1 and CXCR2. Evidence for timedependent signal generation. J. Biol. Chem. 1998;273:23830–13836. doi: 10.1074/jbc.273.37.23830. [DOI] [PubMed] [Google Scholar]
- Seldin D.C., Adelman S., Austen K.F., Stevens R.L., Hein A., Caulfield J.P., Woodbury R.G. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc. Natl. Acad. Sci. U.S.A. 1985;82:3871–3875. doi: 10.1073/pnas.82.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totaro A., Paris S., Asperti C., de Curtis I. Identification of an intramolecular interaction important for the regulation of GIT1 functions. Mol. Biol. Cell. 2007;18:5124–5138. doi: 10.1091/mbc.E07-06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C.E., Brown M.C., Perrotta J.A., Riedy M.C., Nikolopoulos S.N., McDonald A.R., Bagrodia S., Thomas S., Leventhal P.S. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J. Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale N., Patton W.A., Moss J., Vaughan M., Lefkowitz R.J., Premont R.T. GIT proteins, A novel family of phosphatidylinositol 3,4, 5-trisphosphate-stimulated GTPase-activating proteins for ARF6. J. Biol. Chem. 2000;275:13901–13906. doi: 10.1074/jbc.275.18.13901. [DOI] [PubMed] [Google Scholar]
- Za L., Albertinazzi C., Paris S., Gagliani M., Tacchetti C., de Curtis I. BetaPIX controls cell motility and neurite extension by regulating the distribution of GIT1. J. Cell Sci. 2006;119:2654–2666. doi: 10.1242/jcs.02996. [DOI] [PubMed] [Google Scholar]
- Zhang H., Webb D.J., Asmussen H., Niu S., Horwitz A.F. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J. Neurosci. 2005;25:3379–3388. doi: 10.1523/JNEUROSCI.3553-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]