Abstract
Leg crossing increases arterial pressure and combats symptomatic orthostatic hypotension in patients with sympathetic failure. This study compared the central and cerebrovascular effects of leg crossing in patients with sympathetic failure and healthy controls. We addressed the relationship between MCA Vmean (middle cerebral artery blood velocity; using transcranial Doppler ultrasound), frontal lobe oxygenation [O2Hb (oxyhaemoglobin)] and MAP (mean arterial pressure), CO (cardiac output) and TPR (total peripheral resistance) in six patients (aged 37–67 years; three women) and age- and gender-matched controls during leg crossing. In the patients, leg crossing increased MAP from 58 (42–79) to 72 (52–89) compared with 84 (70–95) to 90 (74–94) mmHg in the controls. MCA Vmean increased from 55 (38–77) to 63 (45–80) and from 56 (46–77) to 64 (46–80) cm/s respectively (P<0.05), with a larger rise in O2Hb [1.12 (0.52–3.27)] in the patients compared with the controls [0.83 (−0.11 to 2.04) μmol/l]. In the control subjects, CO increased 11% (P<0.05) with no change in TPR. By contrast, in the patients, CO increased 9% (P<0.05), but also TPR increased by 13% (P<0.05). In conclusion, leg crossing improves cerebral perfusion and oxygenation both in patients with sympathetic failure and in healthy subjects. However, in healthy subjects, cerebral perfusion and oxygenation were improved by a rise in CO without significant changes in TPR or MAP, whereas in patients with sympathetic failure, cerebral perfusion and oxygenation were improved through a rise in MAP due to increments in both CO and TPR.
Keywords: autonomic disease, blood flow velocity, cardiac output, leg crossing, near-IR spectroscopy (NIRS), sympathetic nervous system
Abbreviations: BP, blood pressure; CA, cerebral autoregulation; CBF, cerebral blood flow; CO, cardiac output; HHb, deoxyhaemoglobin; HR, heart rate; MAP, mean arterial pressure; MAPheart MAP at the heart level; MCA, middle cerebral artery; MAPmca; MAP at the brain level; MCA Vmean, MCA flow velocity; NIRS, near-infrared spectroscopy; O2Hb, oxyhaemoglobin; PETCO2, end-tidal CO2; SV, stroke volume; TI, thoracic electrical impedance; TPR, total peripheral resistance
INTRODUCTION
In humans, the upright position challenges the cardiovascular system by gravitational displacement of blood to lower parts of the body with a decline in venous return and CO (cardiac output), and a reduction in cerebral perfusion pressure [1]. Adjustment to the postural decrease in central blood volume involves increased systemic vascular resistance through autonomic reflex activity, but patients with sympathetic dysfunction lack this ability to increase vasomotor tone resulting in a decrease in MAP (mean arterial pressure) [2,3]. In these patients, the transcranial Doppler-determined MCA Vmean [MCA (middle cerebral artery) flow velocity] and the frontal lobe oxygenation [O2Hb (oxyhaemoglobin)] determined by NIRS (near-IR spectroscopy) decrease markedly, and they develop symptoms of cerebral hypoperfusion when standing [4].
Both patients with sympathetic dysfunction [5] and recurrent vasovagal syncope [6] can combat symptomatic orthostatic hypotension by crossing one leg against the other (leg crossing). Leg crossing in the upright position in healthy subjects increases central venous pressure and CO with a reduction in low-frequency arterial pressure variability, signifying reduced sympathetic activity and suggesting that the central blood volume is restored [7]. Although leg crossing has no effect on MAP in healthy subjects, both MCA Vmean and O2Hb increase signifying enhanced cerebral perfusion [7]. However, the effects of leg crossing on cerebral perfusion have not been evaluated in patients with orthostatic hypotension due to sympathetic failure. We therefore tested the hypothesis that in patients with autonomic failure, leg crossing enhances cerebral perfusion, by evaluating the cerebrovascular and central cardiovascular adaptation to orthostatic stress in patients with orthostatic hypotension due to sympathetic failure, and in age- and gender-matched control subjects.
MATERIALS AND METHODS
Patients
Eight patients (range, 37–67 years; three females) with severe orthostatic hypotension related to sympathetic failure classified as PAF (pure autonomic failure; n=7) and MSA (multiple system atrophy; n=1), but with no symptoms or signs of heart disease participated in the present study (Table 1). Oral and written informed consent was obtained, and the study was approved by the Ethics Committee of the Academic Medical Center. Age- and gender-matched healthy subjects with intact autonomic circulatory control and normal orthostatic tolerance served as controls.
Table 1. Patient characteristics.
PAF, pure autonomic failure; MSA, multiple system atrophy; BPsup, supine BP; BPstd, standing BP; HUT, sleeping 12 ° head-up-tilt; NaCl, dietary salt supplementation; Flu, fludrocortisone.
| Patient | Gender | Disease | Treatment | BPsup (mmHg) | BPstd (mmHg) | Age (years) | Weight (kg) | Height (cm) |
|---|---|---|---|---|---|---|---|---|
| S1 | Male | PAF | None | 156/68 | 97/51 | 65 | 78 | 172 |
| S2 | Male | PAF | HUT | 175/101 | 76/47 | 51 | 83 | 176 |
| S3 | Female | PAF | None | 175/99 | 85/48 | 40 | 65 | 176 |
| S4 | Female | PAF | NaCl/Flu | 112/71 | 73/49 | 37 | 56 | 164 |
| S5 | Male | PAF | Flu | 159/81 | 60/42 | 65 | 80 | 175 |
| S6 | Female | PAF | NaCl/Flu | 163/98 | 75/55 | 61 | 65 | 160 |
| S7 | Male | MSA | Flu/HUT | 135/84 | 76/45 | 54 | 96 | 196 |
| S8 | Male | PAF | NaCl/Flu/HUT | 192/102 | 68/47 | 67 | 98 | 189 |
Protocol
At 08:00 h, after an overnight fast, all subjects reported to the laboratory that was maintained at 22 °C. The subjects were supine when instrumented at least 2 h after a light breakfast without caffeine-containing beverages. After instrumentation, a test run was performed to familiarize the subjects with the protocol. After 10 min of supine rest, the subjects stood up, and after 2 min upright, they crossed their legs and maintained that position for 1 min, then they uncrossed the legs and maintained upright for another minute (Figure 1). The duration of orthostatic stress was set so as to enable the patients to fulfil the protocol without symptoms of cerebral hypoperfusion, and the protocol was repeated twice.
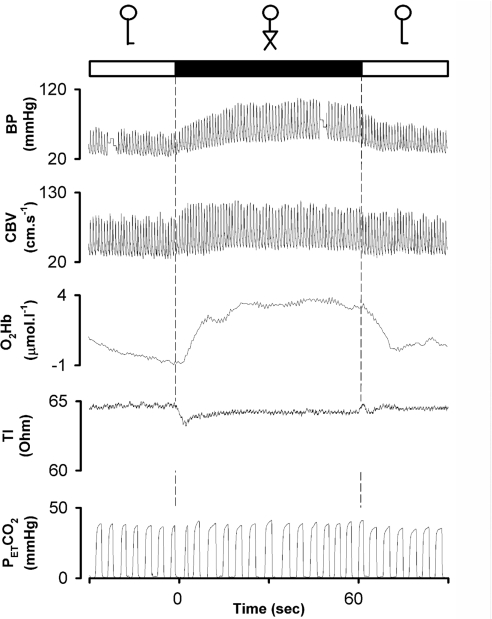
Figure 1. Leg crossing in a patient with pure autonomic failure.
Solid box, leg crossing; open boxes, standing legs uncrossed. Leg crossing induced an instantaneous increase in arterial pressure (BP), and CBV and oxygenation (O2Hb) with a reduction in TI, indicating an acute increase in thoracic blood volume.
Measurements
Arterial pressure was measured with a Finapres™ model 5 (Netherlands Organization for Applied Scientific Research, Biomedical Instrumentation) from the middle finger of the non-dominant arm fixed in the anterior axillary line at heart level. Finger arterial pressure tracks beat-to-beat changes in arterial pressure during hypotension. Cerebral oxygenation was monitored using NIRS based on the transparency of tissue to light in the near-infrared region with the O2 status dependent changes on absorption caused by chromophores dominated by O2Hb and HHb (deoxyhaemoglobin) [8–10]. To estimate the concentration changes in O2Hb and HHb, a differential path length factor of 6.0 was applied to account for the scattering of light in the tissue. A continuous wave NIRS instrument with three wavelengths (901, 848 and 770 nm) and 10-Hz sampling time was used (Oxymon; Artinis Medical Systems). The NIRS optodes were attached high on the forehead avoiding the temporalis muscle but sufficiently lateral to avoid the superior sagittal sinus with the transmitting and receiving optodes placed 5.5 cm apart [10]. Changes in O2Hb and HHb (μmol/l) were reported with steady-state standing values set at 0 μmol/l.
The MCA Vmean was measured in the proximal segment and insonated (DWL Multidop X4) through the posterior temporal ultrasound window. Once the optimal signal-to-noise ratio was obtained, the probe was secured with a head band. Both at rest and during exercise, determination of the MCA Vmean has a coefficient of variation of ~5% [11]. The MCA Vmean was obtained from the maximal TCD (transcranial Doppler ultrasound) frequency shifts over one beat divided by the corresponding beat interval and changes in MCA Vmean were taken to represent those in CBF (cerebral blood flow).
PETCO2 (end-tidal CO2) was measured using an IR analyser (Hewlett Packard 78345A) as a reflection of partial arterial CO2 pressure [12]. TI (thoracic electrical impedance) [13] (Kardio-Dynagraph) was used as an index of changes in central blood volume, whereas SV (stroke volume) was obtained from the BP (blood pressure) waveform using the Model flow method incorporating age, gender, height and weight (BeatScope 1.0 software) [14]. Model flow tracks fast changes in SV during postural stress as well as static exercise of relevance for leg crossing [15].
Data acquisition and analysis
The signals of arterial pressure, the spectral envelope of MCA velocity, TI, PETCO2 and a marker signal were A/D converted at 100 Hz with NIRS data sampled at 10 Hz. Signals were routed through an interface providing electrical isolation with offset and sensitivity adjustments when appropriate. Variables were also recorded on a thermo-writer (Graphtec WR7700™; Western Graphtec) for online inspection. The MCA Vmean was expressed as the integral of the maximal frequency shifts over one beat divided by the corresponding beat interval. MAP was the integral of pressure over one beat divided by the beat interval and expressed both at heart and brain level (MAPheart and MAPmca). HR (heart rate) was the inverse of the interbeat pressure interval in beats/min, CO was SV×HR, and TPR (total peripheral resistance) was the ratio of MAP to CO.
Beat-to-beat data were transformed to equidistantly re-sampled data at 2 Hz by polynomial interpolation, and averages for both groups are shown in Figure 2. BP, HR, MCA Vmean, PETCO2 and TI were expressed in absolute values, whereas resting supine values for SV, CO and TPR were set at 100% (control), and changes were expressed as percentages. Within each subject, the average of all supine values measured over a period of 30 s prior to standing up was used as the baseline value and compared with the average value of 55 to 65 s of standing. Similarly, the average of all values measured over a period of 30 s of quiet standing before leg crossing was used as the baseline value and compared with the average of the last 10 s of leg crossing. The average of the 30 s of quiet standing before leg crossing was then used as the baseline for evaluating the effect of leg crossing, the data of the three runs being averaged. The effect of leg crossing on cerebral and systemic circulatory variables were evaluated by the Wilcoxon signed rank test, whereas differences between patients and controls were analysed by the Mann–Whitney rank sum test. Correlation between variables were evaluated by non-linear regression analysis. A P value <0.05 was considered to indicate a statistically significant difference.
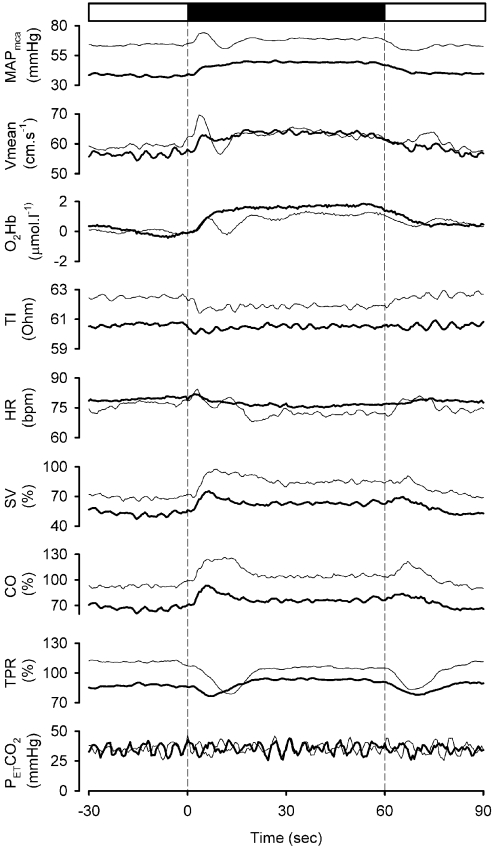
Figure 2. Cardiovascular and cerebral perfusion and oxygenation responses to leg crossing.
Solid box, leg crossing; open box, standing. Bold lines, patients; thin lines, controls (averaged responses).
RESULTS
In one patient (S2), the quality of the Doppler velocity spectrum in the upright posture was insufficient, and in the standing position, one patient (S5) developed serious orthostatic complaints and could, therefore, not complete three runs, and these data were excluded from the analysis. As a result, responses from six patients and their matched controls are reported of whom two patients (S7 and S8) had minor orthostatic complaints during standing which, however, disappeared with leg crossing.
Posture
In the supine position, MAP and MCA Vmean were high in the patients (Table 2). Standing induced a large fall in MAP in the patients [−46 (−11 to 78) mmHg] but not in the controls [−4 (−8 to 8) mmHg] (P<0.05); accompanied by a larger fall in SV [46 (41–55)%] compared with 32 (15-46)%; P<0.05)] and CO [34 (21–42)% compared with 7 (−9 to 25)%; P<0.05)] without the trend of an increase in TPR seen in the controls, such that TPR was significantly different in patients and controls during standing. Resting HR did not differ between the two groups of subjects when supine; during standing, HR increased to a comparable level in the two groups, whereas MCA Vmean decreased only in the patients [from 77 (64–123) to 55 (38–77) cm/s], resulting in a comparable MCA Vmean for patients and controls. Upon standing, the decrease in O2Hb was also larger in the patients [−13.1 (−16.9 to −4.0) compared with −4.9 (−14.6 to −2.8) μmol/l; P<0.05)], whereas the HHb increased in both groups of subjects, the larger increase being in the patients [7.5 (3.7–10.9) compared with 2.2 (1.7–9.1) μmol/l; P<0.05)] (Table 3). The PETCO2 was comparable for patients and control subjects and independent of body position and resting TI, and the trend for TI to increase on standing were also similar in the patients and control subjects (Table 2).
Table 2. Postural cardiovascular and cerebral blood velocity responses.
Values are medians (range). *P<0.05 compared with supine; †P<0.05 compared with controls.
| Measurement | Supine | Standing |
|---|---|---|
| MAPheart (mmHg) | ||
| Controls | 84 (65–94) | 84 (70–95) |
| Patients | 106 (85–134)† | 57 (42–79)*† |
| MAPmca (mmHg) | ||
| Controls | 84 (65–94) | 62 (56–73)* |
| Patients | 106 (85–134)† | 36 (19–58)*† |
| MCA Vmean (cm/s) | ||
| Controls | 58 (47–83) | 56 (46–77) |
| Patients | 77 (64–123)† | 55 (38–77)* |
| PETCO2 (mmHg) | ||
| Controls | 38 (30–40) | 35 (31–38) |
| Patients | 37 (26–46) | 36 (28–39) |
| HR (beats/min) | ||
| Controls | 58 (46–68) | 74 (72–86)* |
| Patients | 66 (49–78) | 75 (64–97)*† |
| SV (%) | ||
| Controls | 100 | 68 (54–85)* |
| Patients | 100 | 54 (45–59)*† |
| CO (%) | ||
| Controls | 100 | 93 (75–109) |
| Patients | 100 | 66 (58–79)*† |
| TPR (%) | ||
| Controls | 100 | 113 (93–126) |
| Patients | 100 | 84 (58–115)† |
| TI (Ohm) | ||
| Controls | 57 (47–80) | 60 (54–80) |
| Patients | 56 (47–66) | 60 (55–64) |
Table 3. Cardiovascular and cerebral responses to leg crossing in standing position.
Values are medians (range). *P<0.05 in comparison to standing before leg cross, †P<0.05 compared with controls.
| Measurement | Standing | Leg cross | Standing |
|---|---|---|---|
| MAPheart (mmHg) | |||
| Controls | 84 (70–95) | 90 (74–94) | 86 (70–94) |
| Patients | 58 (42–79)† | 72 (52–89)* | 60 (41–82)† |
| MAPmca (mmHg) | |||
| Controls | 62 (56–73) | 71 (59–72) | 64 (55–72) |
| Patients | 36 (19–58)† | 50 (28–73)* | 39 (17–62)† |
| MCA Vmean (cm/s) | |||
| Controls | 56 (46–77) | 64 (46–80)* | 57 (47–72) |
| Patients | 55 (38–77) | 63 (45–80)* | 57 (38–78) |
| ΔO2Hb (μmol/l) | |||
| Controls | 0 | 0.83 (−0.11 to 2.04) | 0.69 (−0.11 to 0.93) |
| Patients | 0 | 1.12 (0.52–3.27)* | 0.46 (−0.12 to 0.89) |
| ΔHHb (μmol/l) | |||
| Controls | 0 | −0.33 (−0.92 to 0.01) | −0.05 (−0.80 to 0.45) |
| Patients | 0 | −0.53 (−2.33 to 0.16) | 0.07 (−0.23 to 0.66) |
| PETCO2 (mmHg) | |||
| Controls | 35 (31–38) | 35 (34–42) | 35 (33–40) |
| Patients | 36 (28–39) | 36 (29–40) | 35 (29–39) |
| HR (beats/min) | |||
| Controls | 74 (72–86) | 71 (58–79)† | 73 (65–83) |
| Patients | 75 (64–97) | 75 (59–92) | 74 (63–95) |
| SV (%) | |||
| Controls | 68 (54–85) | 84 (73–102)* | 69 (61–92) |
| Patients | 54 (45–59)† | 62 (56–69)*† | 53 (43–61)† |
| CO (%) | |||
| Controls | 93 (75–109) | 103 (91–119)* | 88 (82–114) |
| Patients | 66 (58–79)† | 72 (66–95)*† | 67 (51–83)† |
| TPR (%) | |||
| Controls | 113 (93–126) | 105 (82–117) | 112 (81–124) |
| Patients | 84 (58–115)† | 95 (68–118)* | 89 (65–113) |
| TI (Ohm) | |||
| Controls | 60 (54–80) | 60 (54–78) | 61 (54–80) |
| Patients | 60 (55–64) | 60 (55–64) | 60 (55–64) |
Cardiovascular effects of leg crossing
At the onset of leg crossing, in each group, CO and MAP increased (Figure 2), whereas TPR decreased. When analysed in the final 10 s of leg crossing, in the controls, there was an increase in SV (16%) and CO (10%), a trend for a lower HR, but no significant change in TPR or MAP (Table 3 and Figure 2). Pulse pressure increased from 48 (38–55) to 55 (50–58) mmHg (P<0.05). By contrast, in patients, leg crossing induced an increase in MAP and in pulse pressure [from 33 (23–48) to 40 (28–55) mmHg; P<0.05)]. The underlying haemodynamic change was an increase in SV (8%), CO (6%) and TPR (11%). HR did not change (Table 3 and Figure 2).
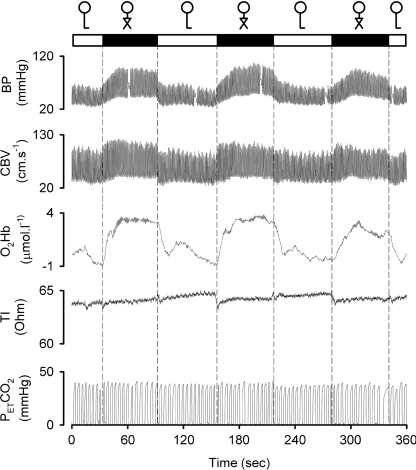
Representative examples of the reproducibility of the responses to leg crossing manoeuvre in patients are provided in Figures 3 and 4.
Figure 3. Effects of repeated leg crossing on brain perfusion and oxygenation in a patient with pure autonomic failure.
Original tracings in a patient with pure autonomic failure. Solid box, leg crossing; open box, standing.
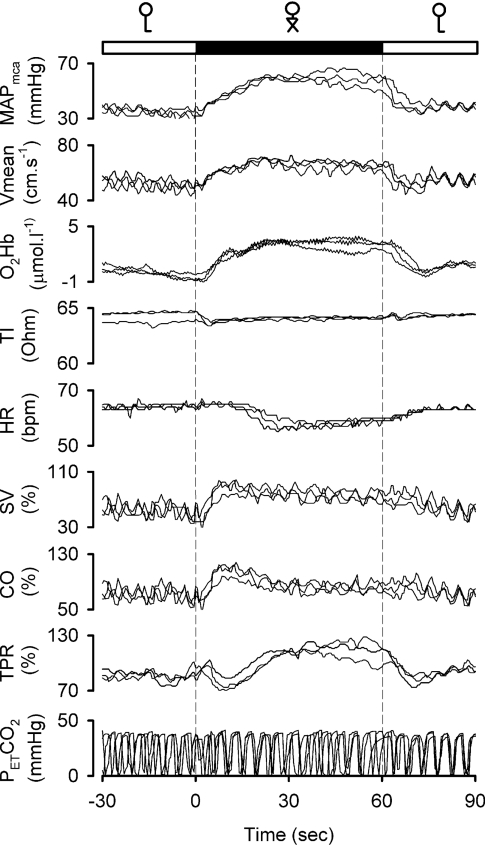
Figure 4. Reproducibility of systemic and cerebral responses to leg crossing in a patient with pure autonomic failure.
Recordings obtained in three separate experimental runs are superimposed.
In both patients and healthy subjects, a marked transient decrease in TI (increase in central blood volume) in response to leg crossing was observed in 13 out of 18 of each group (sign test P<0.05). After the initial fall, TI increased again, and there was no significant change over 1 min of leg crossing.
Cerebrovascular effects of leg crossing
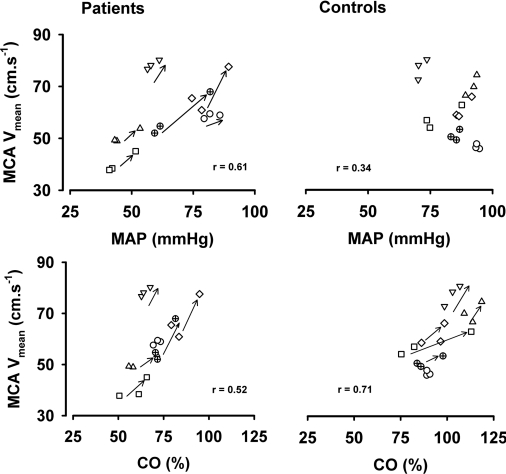
In both patients and controls, leg crossing induced a similar increase in MCA Vmean with a concomitant rise in O2Hb in the patients and a trend towards lower values for HHb (Figures 2–5 and Table 3). The PETCO2 was comparable for patients and controls and did not change during leg crossing. Within 1 min after leg crossing, all values returned to baseline. The relationship between MCA Vmean and MAP and CO during free standing, leg crossing and again free standing with three data points plotted for each subject is shown in Figure 5. In the patients, the regression coefficient for MAP and MCA Vmean was 0.61 (P<0.02), and for CO and MCA Vmean, it was 0.52 (P<0.05). In the control subjects, this value for MAP and MCA Vmean was 0.34 (not significant), whereas this was 0.71 for CO and MCA Vmean (P<0.05).
Figure 5. Relationship between MAP, CO and MCA Vmean during leg crossing.
Standing–leg crossing–standing as three data points plotted for each subject.
DISCUSSION
In the present study, leg crossing consistently improved cerebral perfusion and oxygenation in patients with orthostatic hypotension related to sympathetic failure as in controls. However, the mechanisms of enhanced cerebral perfusion and oxygenation induced by leg crossing appeared different for healthy subjects in whom leg crossing increased CO without significantly affecting TPR or MAP. In the patients, MAP was increased, and this was supported by an increase in TPR as well as in CO. The immediate drop in TI at the onset of leg crossing in both patients and healthy subjects suggests translocation of blood to the chest with less pooling due to sustained muscle tensing. However, given the defective vasomotor control in the patients, the increase in TPR induced by leg crossing is likely to be related to compression of venous and arterial vascular beds.
In healthy subjects, the postural fall in venous return as a consequence of pooling of blood reduces SV and CO [4]. However, an increase in vasomotor tone in response to cardiopulmonary and arterial baroreflex-mediated sympathoexcitation, local myogenic responses and venoarteriolar axon reflex activity, elevates mean and diastolic arterial pressure [3,16]. By contrast, patients with sympathetic failure cannot modulate vascular tone and thus develop orthostatic hypotension [2,3] because of an excessive fall in SV and CO, attributable to both increased pooling of venous blood and impaired inotropic and chronotropic cardiac responses [2]. The tendency for TI to be higher upon standing is consistent with a reduction in central blood volume in both groups (Table 2). In addition to the marked reduction in MAP and CO, a decrease in PETCO2 as an index of the arterial value might have contributed to the observed reduction in MCA Vmean that occurred in the patients on standing. However, the contribution of PETCO2 to the postural reduction in MCA Vmean is transient [17], and in the present study, any such influence must have been small, if any. In addition, leg crossing did not change PETCO2 rendering a contribution of partial arterial CO2 pressure to the increase in cerebral perfusion during leg crossing unlikely. In healthy subjects, autonomic neural control of the cerebral circulation is tonically active [18–20], but in patients with autonomic failure, a reduced spillover of noradrenaline from the brain renders such an influence unlikely [21].
Under normal circumstances, CBF is maintained across a range of MAP of ~60 to 150 mmHg by CA (cerebral autoregulation). Furthermore, a reduction in MAP below the lower limit of CA challenges CBF and a reduction in central blood volume, and therefore in CO, adversely influences the lower limit of CA [20,22–24]. The significant relationship between MAP and MCA Vmean in the upright position in the patients (Figure 5) suggests that leg crossing in the patients raised MAP from below to within the accepted autoregulatory range [25]. Patients with autonomic failure are remarkably tolerant of postural hypotension [26] suggesting they have well-maintained cerebral autoregulatory capacity. On the other hand, a parallel postural reduction in MAP and MCA Vmean does occur in these patients suggesting a reduced autoregulatory capacity [27]. Thus whether CA is maintained in autonomic dysfunction remains debatable.
In the present study, MCA Vmean was used as a measure of cerebral perfusion, assuming that in patients with sympathetic failure changes in MCA Vmean are representative of those in CBF. However, critical for the interpretation of the data is the extent to which MCA Vmean reflects volume flow in cerebral circulation. Changes in resistance to flow occur mainly in the cerebral arteriolar bed; the major cerebral conductance arteries are non-compliant and act as a conduit for the pulsatile arterial flow. This is important because MCA Vmean calculated from the frequency distribution of the Doppler shifts is related to CBF only if the insonated vessel diameter remains constant. This has been shown to be the case for the MCA over a ~30-mmHg range of BP such that changes with MCA Vmean do reflect changes in CBF [28]. Indeed, direct observations made during craniotomy have revealed that lowering BP by sodium nitroprusside does not affect MCA diameter [29]. Moreover, orthostatic stress, as simulated by lower body negative pressure, does not alter the MCA diameter as assessed with MR imaging [30]. Nevertheless, the low BP levels developed in the patients with sympathetic failure do not rule out the possibility that a considerable fall in BP might have reduced the diameter of the MCA, such that the measurement of MCA Vmean led to underestimation of the postural reduction in CBF [31].
An attempt was therefore made to overcome this possibility by combining the use of the transcranial Doppler technique with the use of NIRS, which is based on different physical principles, as an alternative means of assessing changes in CBF. The NIRS signal for the frontal lobe, which depends on oxygenation at the level of the capillaries, has been shown to reflect the brain capillary O2 saturation over a 2-fold variation in CBF [32]. Furthermore, with postural stress, cerebrovascular oxygenation as determinated by NIRS has been shown to be directly related to cerebral perfusion as determined by the transcranial Doppler technique [9,33]. Thus, when MCA Vmean and O2Hb increase in parallel, we believe it is reasonable to conclude that CBF changed in the same direction, as has been confirmed by clinical evaluation [9,32,34–36].
It must be acknowledged that there are potential complications when interpreting changes in cerebral perfusion in the upright position. Although the brain is elevated above the heart, the contention that the cerebral perfusion pressure falls accordingly [37–39] is not generally accepted [40]. For the brain, not only the inflow pressure but also the venous and cerebral spinal fluid pressures decline in proportion to the vertical distance above the heart [1,41–44], and veins above the heart collapse because the surrounding tissue pressure is greater than the pressure inside the veins, so creating a Starling resistor [45]. Within the brain, a potential siphon exists at the level of the sinus sagittalis which, in contrast to the jugular veins, does not collapse upon standing up [46]. However, because of the collapsible veins, gravitational pressure gradients are not matched on the arterial and venous sides of the circulation at all levels above the heart as would be necessary for a siphon to operate [37,47]. Indeed, the effects of posture on spinal fluid pressure [48] and internal jugular vein cross-sectional area [48,49] have been evaluated in healthy subjects, and it has been shown that when humans are upright, the prevailing pressure for both the venous outflow and for the spinal fluid approaches zero at the base of the brain, so negating the idea that a siphon supports CBF. Thus, it seems that in upright humans, the magnitude of cerebrovascular resistance breaks the continuity requirement for a siphon, implying that the heart has to work against gravity to perfuse the brain. As far as the present study is concerned, we have no reason to suppose that these principles apply differently to patients with sympathetic failure.
In the present study on healthy subjects, both the MCA Vmean and O2Hb increased in response to leg crossing without significant changes in MAP confirming earlier findings [7] and suggesting an increase in cerebral perfusion. There was a direct relationship between CO and MCA Vmean that cannot be explained by changes in MAP (Figure 5) [50,51]. In the patients, the increase in cerebral perfusion as indicated by the increase in MCA Vmean was comparable, but in contrast, MCA Vmean was related to both CO and MAP. In healthy subjects, the arterial baroreflex instantaneously maintains MAP by buffering changes in preload. However, patients with sympathetic failure lack this buffering capacity, and MAP is largely dependent on cardiac preload and CO. Thus, on this basis alone, it is not clear whether cerebral perfusion is determined by MAP as well as by CO (Figure 5). However, if we consider our results in more detail, it is clear that leg crossing increased MAP in each individual patient, and there was an associated increase in MCA Vmean. Indeed, it seems that leg crossing increased MAP in the patients from below to within in the accepted cerebral autoregulatory range. In other words, the direct relationship between MCA Vmean and MAP may be accounted for because standing allowed MAP to fall below the autoregulatory range. When MAP is within the autoregulatory range, there is no reason to suppose that the factors that regulate cerebral perfusion are different in healthy subjects and patients with sympathetic failure.
In conclusion, leg crossing consistently improves cerebral perfusion in patients with sympathetic failure as reflected by a rise of cerebral blood velocity and in cerebral oxygenation, and this is associated with a relief of symptoms. In healthy subjects, CO increases during leg crossing without significant changes in MAP, whereas in the patients, there is an increase in MAP that is supported by an increase in TPR. There is no definite explanation for this increase in TPR in the patients with sympathetic failure, but we hypothesize that the positive mechanical effect of leg crossing on TPR overcomes the vasodilator effect caused by the absence of sympathetic vascular control. Patients with orthostatic intolerance should, therefore, be advised, in addition to increasing their salt intake and to sleep head-up tilted, also to apply leg crossing when they stand up as a simple means of maintaining cerebral perfusion and avoiding postural syncope.
FUNDING
This study was supported by the Netherlands Heart Foundation (grant number 94.132).
References
- 1.Rosner M. J., Coley I. B. Cerebral perfusion pressure, intracranial pressure, and head elevation. J. Neurosurg. 1986;65:636–641. doi: 10.3171/jns.1986.65.5.0636. [DOI] [PubMed] [Google Scholar]
- 2.Bevegård B. S., Jonsson B., Karlöf I. Circulatory response to recumbent exercise and head-up tilting in patients with disturbed sympathetic cardiovascular control (postural hypotension): observations on the effect of norepinephrine infusion and antigravity suit inflation in the head-up tilted position. Acta Med. Scand. 1962;172:623–636. [Google Scholar]
- 3.Smit A. A. J., Halliwill J. R., Low P. A., Wieling W. Pathophysiological basis of orthostatic hypotension in autonomic failure. J. Physiol. 1999;519:1–10. doi: 10.1111/j.1469-7793.1999.0001o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harms M. P. M., Colier W. N. J. M., Wieling W., Lenders J. W., Secher N. H., Van Lieshout J. J. Orthostatic tolerance, cerebral oxygenation, and blood velocity in humans with sympathetic failure. Stroke. 2000;31:1608–1614. doi: 10.1161/01.str.31.7.1608. [DOI] [PubMed] [Google Scholar]
- 5.Van Lieshout J. J., Ten Harkel A. D. J., Wieling W. Physical manoeuvres for combating orthostatic dizziness in autonomic failure. Lancet. 1992;339:897–898. doi: 10.1016/0140-6736(92)90932-s. [DOI] [PubMed] [Google Scholar]
- 6.Krediet C. T., Van Lieshout J. J., Bogert L. W., Immink R. V., Kim Y. S., Wieling W. Leg crossing improves orthostatic tolerance in healthy subjects: a placebo-controlled crossover study. Am J. Physiol. Heart Circ. Physiol. 2006;291:H1768–H1772. doi: 10.1152/ajpheart.00287.2006. [DOI] [PubMed] [Google Scholar]
- 7.Van Lieshout J. J., Pott F., Madsen P. L., Van Goudoever J., Secher N. H. Muscle tensing during standing: effects on cerebral tissue oxygenation and cerebral artery blood velocity. Stroke. 2001;32:1546–1551. doi: 10.1161/01.str.32.7.1546. [DOI] [PubMed] [Google Scholar]
- 8.Colier W. N. J. M., Binkhorst R. A., Hopman M. T., Oeseburg B. Cerebral and circulatory haemodynamics before vasovagal syncope induced by orthostatic stress. Clin. Physiol. 1997;17:83–94. doi: 10.1046/j.1365-2281.1997.01414.x. [DOI] [PubMed] [Google Scholar]
- 9.Madsen P. L., Secher N. H. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58:541–560. doi: 10.1016/s0301-0082(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 10.Al-Rawi P. G., Smielewski P., Kirkpatrick P. J. Evaluation of a near-infrared spectrometer (NIRO 300) for the detection of intracranial oxygenation changes in the adult head. Stroke. 2001;32:2492–2500. doi: 10.1161/hs1101.098356. [DOI] [PubMed] [Google Scholar]
- 11.Pott F., Jensen K., Hansen H., Christensen N. J., Lassen N. A., Secher N. H. Middle cerebral artery blood velocity and plasma catecholamines during exercise. Acta Physiol. Scand. 1996;158:349–356. doi: 10.1046/j.1365-201X.1996.564325000.x. [DOI] [PubMed] [Google Scholar]
- 12.Immink R. V., Secher N. H., Roos C. M., Pott F., Madsen P. L., Van Lieshout J. J. The postural reduction in middle cerebral artery blood velocity is not explained by PaCO2. Eur. J. Appl. Physiol. 2006;96:609–614. doi: 10.1007/s00421-006-0136-6. [DOI] [PubMed] [Google Scholar]
- 13.Jonsson F., Madsen P., Jørgensen L. G., Lunding M., Secher N. H. Thoracic electrical impedance and fluid balance during aortic surgery. Acta Anaesthesiol. Scand. 1995;39:513–517. doi: 10.1111/j.1399-6576.1995.tb04110.x. [DOI] [PubMed] [Google Scholar]
- 14.Harms M. P. M., Wesseling K. H., Pott F., Jenstrup M., Van Goudoever J., Secher N. H., Van Lieshout J. J. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin. Sci. 1999;97:291–301. [PubMed] [Google Scholar]
- 15.Van Dijk N., de Bruin I. G., Gisolf J., de Bruin-Bon H. A., Linzer M., van Lieshout J. J., Wieling W. Hemodynamic effects of legcrossing and skeletal muscle tensing during free standing in patients with vasovagal syncope. J. Appl. Physiol. 2005;98:584–590. doi: 10.1152/japplphysiol.00738.2004. [DOI] [PubMed] [Google Scholar]
- 16.Wieling W., Van Lieshout J. J. Maintenance of postural normotension in humans. In: Low P. A., Benarroch E. F., editors. Clinical Autonomic Disorders. Lippincott: Williams & Wilkins; 2008. pp. 69–78. [Google Scholar]
- 17.Immink R. V., Truijen J., Secher N. H., Van Lieshout J. J. Transient influence of end-tidal carbon dioxide tension on the postural restraint in cerebral perfusion. J. Appl. Physiol. 2009;107:816–823. doi: 10.1152/japplphysiol.91198.2008. [DOI] [PubMed] [Google Scholar]
- 18.Zhang R., Zuckerman J. H., Iwasaki K., Wilson T. E., Crandall C. G., Levine B. D. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002;106:1814–1820. doi: 10.1161/01.cir.0000031798.07790.fe. [DOI] [PubMed] [Google Scholar]
- 19.Ogoh S., Brothers R. M., Eubank W. L., Raven P. B. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke. 2008;39:1979–1987. doi: 10.1161/STROKEAHA.107.510008. [DOI] [PubMed] [Google Scholar]
- 20.Van Lieshout J. J., Secher N. H. Sympathetic activity does/does not influence cerebral blood flow. J. Appl. Physiol. 2008;105:1364–1366. doi: 10.1152/japplphysiol.90597.2008. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell D. A., Lambert G., Secher N. H., Raven P. B., van Lieshout J. J., Esler M. D. Jugular venous overflow of noradrenaline from the brain: a neurochemical indicator of cerebrovascular sympathetic nerve activity in humans. J. Physiol. 2009;587:2589–2597. doi: 10.1113/jphysiol.2008.167999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldemar G., Vorstrup S., Andersen A. R., Pedersen H., Paulson O. B. Angiotensin-converting enzyme inhibition and regional cerebral blood flow in acute stroke. J. Cardiovasc. Pharmacol. 1989;14:722–729. doi: 10.1097/00005344-198911000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Jørgensen L. G., Perko M., Perko G., Secher N. H. Middle cerebral artery velocity during head-up tilt induced hypovolaemic shock in humans. Clin. Physiol. 1993;13:323–336. doi: 10.1111/j.1475-097x.1993.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 24.Truijen J., Bundgaard-Nielsen M., van Lieshout J. J. A definition of normovolaemia and consequences for cardiovascular control during orthostatic and environmental stress. Eur. J. Appl. Physiol. 2009 doi: 10.1007/s00421-009-1346-5. doi: 10.1007/s00421-009-1346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panerai R. B. Cerebral autoregulation: from models to clinical applications. Cardiovasc. Eng. 2008;8:42–59. doi: 10.1007/s10558-007-9044-6. [DOI] [PubMed] [Google Scholar]
- 26.Thomas D. J., Bannister R. Preservation of autoregulation of cerebral blood flow in autonomic failure. J. Neurol. Sci. 1980;44:205–212. doi: 10.1016/0022-510x(80)90127-6. [DOI] [PubMed] [Google Scholar]
- 27.Lagi A., Bacalli S., Cencetti S., Paggetti C., Colzi L. Cerebral autoregulation in orthostatic hypotension. A transcranial Doppler study. Stroke. 1994;25:1771–1775. doi: 10.1161/01.str.25.9.1771. [DOI] [PubMed] [Google Scholar]
- 28.Giller C. A., Bowman G., Dyer H., Mootz L., Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–741. [PubMed] [Google Scholar]
- 29.Qureshi A. I., Wilson D. A., Hanley D. F., Traystman R. J. Pharmacologic reduction of mean arterial pressure does not adversely affect regional cerebral blood flow and intracranial pressure in experimental intracerebral hemorrhage. Crit. Care Med. 1999;27:965–971. doi: 10.1097/00003246-199905000-00036. [DOI] [PubMed] [Google Scholar]
- 30.Serrador J. M., Picot P. A., Rutt B. K., Shoemaker J. K., Bondar R. L. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- 31.Thoresen M., Haaland K., Steen A. Cerebral Doppler and misinterpretation of flow changes. Arch. Dis. Child. 1994;71:F103–F106. doi: 10.1136/fn.71.2.f103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasmussen P., Dawson E. A., Nybo L., Van Lieshout J. J., Secher N. H., Gjedde A. Capillary-oxygenation-level-dependent near-infrared spectrometry in frontal lobe of humans. J. Cereb. Blood Flow Metab. 2007;27:1082–1093. doi: 10.1038/sj.jcbfm.9600416. [DOI] [PubMed] [Google Scholar]
- 33.Madsen P. L., Pott F., Olsen S. B., Nielsen H. B., Burcev I., Secher N. H. Near-infrared spectrophotometry determined brain oxygenation during fainting. Acta Physiol. Scand. 1998;162:501–507. doi: 10.1046/j.1365-201X.1998.0308f.x. [DOI] [PubMed] [Google Scholar]
- 34.Van Lieshout J. J., Wieling W., Karemaker J. M., Secher N. H. Syncope, cerebral perfusion, and oxygenation. J. Appl. Physiol. 2003;94:833–848. doi: 10.1152/japplphysiol.00260.2002. [DOI] [PubMed] [Google Scholar]
- 35.Pott F., Van Lieshout J. J., Ide K., Madsen P., Secher N. H. Middle cerebral artery blood velocity during intense static exercise is dominated by a Valsalva maneuver. J. Appl. Physiol. 2003;94:1335–1344. doi: 10.1152/japplphysiol.00457.2002. [DOI] [PubMed] [Google Scholar]
- 36.Kim Y. S., Bogert L. W., Immink R. V., Harms M. P., Colier W. N., Van Lieshout J. J. Effects of aging on the cerebrovascular orthostatic response. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.02.019. doi:10.1016/j.neurobiolaging.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Hill L., Barnard H. The influence of the force of gravity on the circulation. Part II. J. Physiol. 1897;22:323–352. doi: 10.1113/jphysiol.1897.sp000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosner M. J., Coley I. B. Cerebral perfusion pressure, intracranial pressure, and head elevation. J. Neurosurg. 1986;65:636–641. doi: 10.3171/jns.1986.65.5.0636. [DOI] [PubMed] [Google Scholar]
- 39.Gisolf J., Gisolf A., Van Lieshout J. J., Karemaker J. M. The siphon controversy: an integration of concepts and the brain as baffle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R627–R629. doi: 10.1152/ajpregu.00709.2004. [DOI] [PubMed] [Google Scholar]
- 40.Hicks J. W., Munis J. R. The siphon controversy counterpoint: the brain need not be ‘baffling’. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R629–R632. doi: 10.1152/ajpregu.00810.2004. [DOI] [PubMed] [Google Scholar]
- 41.Ng I., Lim J., Wong H. B. Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery. 2004;54:593–597. doi: 10.1227/01.neu.0000108639.16783.39. [DOI] [PubMed] [Google Scholar]
- 42.Asgeirsson B., Grände P. O. Local vascular responses to elevation of an organ above the heart. Acta Physiol. Scand. 1996;156:9–18. doi: 10.1046/j.1365-201X.1996.444165000.x. [DOI] [PubMed] [Google Scholar]
- 43.Kongstad L., Grände P. O. The role of arterial and venous pressure for volume regulation of an organ enclosed in a rigid compartment with application to the injured brain. Acta Anaesthesiol. Scand. 1999;43:501–508. doi: 10.1034/j.1399-6576.1999.430503.x. [DOI] [PubMed] [Google Scholar]
- 44.Dawson S. L., Panerai R. B., Potter J. F. Critical closing pressure explains cerebral hemodynamics during the Valsalva maneuver. J. Appl. Physiol. 1999;86:675–680. doi: 10.1152/jappl.1999.86.2.675. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Muniz R., Stephens N. L., Bromberger-Barnea B., Permutt S., Riley R. L. Critical closure of pulmonary vessels analyzed in terms of Starling resistor model. J. Appl. Physiol. 1968;24:625–635. doi: 10.1152/jappl.1968.24.5.625. [DOI] [PubMed] [Google Scholar]
- 46.Mosso M., Schmid-Priscoveanu A., Straumann D., Baumgartner R. W. Absence of gravity-dependent modulation of straight sinus flow velocity in healthy humans. Ultrasound Med. Biol. 2008;34:726–729. doi: 10.1016/j.ultrasmedbio.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Seymour R. S., Johansen K. Blood flow uphill and downhill: does a siphon facilitate circulation above the heart? Comp. Biochem. Physiol. A. 1987;88:167–170. doi: 10.1016/0300-9629(87)90465-8. [DOI] [PubMed] [Google Scholar]
- 48.Dawson E. A., Secher N. H., Dalsgaard M. K., Ogoh S., Yoshiga C. C., González-Alonso J., Steensberg A., Raven P. B. Standing up to the challenge of standing: a siphon does not support cerebral blood flow in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R911–R914. doi: 10.1152/ajpregu.00196.2004. [DOI] [PubMed] [Google Scholar]
- 49.Gisolf J., Van Lieshout J. J., Van Heusden K., Pott F., Stok W. J., Karemaker J. M. Human cerebral venous outflow pathway depends on posture and central venous pressure. J. Physiol. 2004;560:317–327. doi: 10.1113/jphysiol.2004.070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogoh S., Brothers R. M., Barnes Q., Eubank W. L., Hawkins M. N., Purkayastha S., O-Yurvati A., Raven P. B. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J. Physiol. 2005;569:697–704. doi: 10.1113/jphysiol.2005.095836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Secher N. H., Seifert T., Van Lieshout J. J. Cerebral blood flow and metabolism during exercise, implications for fatigue. J. Appl. Physiol. 2008;104:306–314. doi: 10.1152/japplphysiol.00853.2007. [DOI] [PubMed] [Google Scholar]