Abstract
Dpl (doppel) is a paralogue of the PrPC (cellular prion protein), whose misfolded conformer (the scrapie prion protein, PrPSc) is responsible for the onset of TSEs (transmissible spongiform encephalopathies) or prion diseases. It has been shown that the ectopic expression of Dpl in the brains of some lines of PrP-knockout mice provokes cerebellar ataxia, which can be rescued by the reintroduction of the PrP gene, suggesting a functional interaction between the two proteins. It is, however, still unclear where, and under which conditions, this event may occur. In the present study we addressed this issue by analysing the intracellular localization and the interaction between Dpl and PrPC in FRT (Fischer rat thyroid) cells stably expressing the two proteins separately or together. We show that both proteins localize prevalently on the basolateral surface of FRT cells, in both singly and doubly transfected clones. Interestingly we found that they associate with DRMs (detergent-resistant membranes) or lipid rafts, from where they can be co-immunoprecipitated in a cholesterol-dependent fashion. Although the interaction between Dpl and PrPC has been suggested before, our results provide the first clear evidence that this interaction occurs in rafts and is dependent on the integrity of these membrane microdomains. Furthermore, both Dpl and PrPC could be immunoprecipitated with flotillin-2, a raft protein involved in endocytosis and cell signalling events, suggesting that they share the same lipid environment.
Keywords: doppel (Dpl), epithelial cell, lipid raft, prion protein (PrP), protein trafficking
Abbreviations: CNS, central nervous system; CNX, calnexin; Dpl, doppel; DRM, detergent-resistant membrane; EEA1, early endosome antigen 1; endo H, endoglycosidase H; ER, endoplasmic reticulum; FBS, fetal bovine serum; FRT, Fischer rat thyroid; GFP, green fluorescent protein; GPI, glycosylphosphatidylinositol; HRP, horseradish peroxidase; hu, human; MDCK, Medin–Darby canine kidney; MEV, mevinolin; MβCD, methyl-β-cyclodextrin; mo, murine; PNGase F, peptide N-glycosidase F; PrPC, cellular prion protein; PrPSc, scrapie prion protein; TRITC, tetramethylrhodamine β-isothiocyanate; TSE, transmissible spongiform encephalopathy
INTRODUCTION
The PrPC (cellular prion protein) is a cell-surface glycoprotein of unknown function expressed in mammalian tissues, particularly in the CNS (central nervous system) [1]. PrPC can be misfolded into the PrPSc (scrapie prion protein) isoform, which is the essential component of the prion agent causing TSE (transmissible spongiform encephalopathie) or prion diseases [2]. Whereas some lines of PrP-knockout mice display a normal phenotype, the Ngsk, Zurich II and Rcm0 lines of PrP-knockout mice develop a late-onset ataxia [3–5]. This phenotype was not associated with the absence of PrPC, but rather with the ectopic brain expression of a PrPC paralogue, named doppel (Dpl) [4,6]. Dpl is present in the CNS during embryogenesis and in the early post-natal life, whereas in adults it is expressed at high levels mainly in the testis, where it plays a key role in spermatogenesis [4,7,8]. Dpl is composed of 179 amino acids encoded by the Prnd [prion protein 2 (dublet)] gene, located approx. 20 kb downstream of the PrP gene [4]. It is homologous with the structured C-terminal end of PrPC, but lacks both the octa-repeat and the hydrophobic domains present in the flexible N-terminal tail of PrPC [4,9]. Examination of post-translational modifications of Dpl and PrPC have shown that the two proteins share several biochemical features: both have two N-linked oligosaccharide groups, are anchored to the external cell surface by a GPI (glycophosphatidylinositol) moiety and form intramolecular disulfide bonds [4,9–11].
Interestingly, the expression of Dpl, or of some PrP-deletion mutants closely resembling Dpl, in transgenic PrP-knockout mice causes cerebellar degeneration that is antagonized by wild-type PrPC. This has led to the suggestion that the two proteins may functionally interact [5,12–16]. More specifically, it has been proposed that Dpl competes for a putative PrPC ligand that is necessary to transduce a cell survival signal [15–17] or that PrPC could block a neurotoxic signal induced by Dpl by competing for its binding to a third molecule, α-2-macroglobulin [8,18]. Contrasting results on the putative interaction of Dpl with PrPC have been reported [18–23]. Indeed, whereas in neuronal cells the results support an interaction between the two proteins [19,21,24], in testis such an interaction was not found [20]. Interestingly, and differently from the testis [20], in cells of neuronal origin both proteins seem to share common membrane microdomains and internalization pathways [24]. Specifically in neuroblastoma cells PrPC and Dpl were shown to associate, independently from each other, to membrane microdomains enriched in cholesterol and sphingolipids, known as DRMs (detergent-resistant membranes) or lipid rafts. DRMs are thought to modulate several cellular events, such as polarized sorting of lipids and proteins, signal transduction and endocytosis [25], and could also be the site of PrPC into PrPSc conversion [26–28].
Intriguingly, Uelhoff et al. [29] have shown that the co-expression of Dpl with PrPC in polarized MDCK (Madin–Darby canine kidney) cells prevented the basolateral sorting of PrPC, which was sorted to the apical membrane together with Dpl. Although a direct interaction between the two proteins was not demonstrated, the authors hypothesized that the apical mis-sorting of PrPC could be caused by the capacity of Dpl to interact with PrPC, masking the basolateral sorting signal possibly present on this protein, but absent in Dpl.
Having characterized previously the intracellular trafficking and raft association of PrPC in FRT (Fischer rat thyroid) cells [30,31], we set out to investigate the behaviour of Dpl either transfected alone or together with PrPC in this cell line. We focussed our attention on their trafficking and possible physical and/or functional interaction. In the present study we report that, in contrast with MDCK cells [29], both proteins localize prevalently on the basolateral domain of the plasma membrane and in the Golgi complex of FRT cells and associate with DRMs. Interestingly we demonstrate that, when expressed together, Dpl and PrPC co-immunoprecipitate in DRMs and that this interaction is impaired by cholesterol depletion. In addition we found that each protein immunoprecipitates with flotillin-2, a scaffold protein involved in both endocytosis and signalling in rafts [32], both independently and together.
EXPERIMENTAL
Reagents and antibodies
The following reagents were used: cell culture reagents from Euroclone; Protein A–Sepharose from GE Healthcare; Lysotracker Red DND-99 from Molecular Probes; biotin and HRP (horseradish peroxidase)-conjugated streptavidin from Pierce Chemicals; FBS (fetal bovine serum) from Hyclone; and MβCD (methyl-β-cyclodextrin) and MEV (mevinolin), as well as all the other reagents, from Sigma–Aldrich. For immunocytochemistry and biochemical assays, the following monoclonal and polyclonal antibodies were used: monoclonal antibody SAF-32 against PrP (Cayman Chemical); monoclonal antibody Dpl 151 against Dpl (a gift from Dr J. Grassi, Commisariat a l'Energie Atomique, Saclay, France); anti-Dpl affinity-purified rabbit polyclonal antibody Q55 [produced in-house and raised against recombinant human Dpl (residues 28–152); all experiments using animals were in accordance with legal requirements] monoclonal antibody against GFP (green fluorescent protein; Molecular Probes); polyclonal antibody against GFP (Clontech); polyclonal antibodies against CNX (calnexin), EEA1 (early endosome antigen 1) and CLR (calreticulin) (StressGene Biotechnologies); polyclonal antibody against giantin (a gift from Dr S. Bonatti, Università degli Studi di Napoli ‘Federico II’, Napoli, Italy); anti-flotillin-2 monoclonal antibody (Signal Transduction); monoclonal anti-p58K antibody (Sigma–Aldrich); and monoclonal anti-antigen 35/40 kDa antibody (a gift from Dr A. Quaroni, Cornell University, New York, NY, U.S.A.).
Constructs and transfections
To obtain singly PrPC-expressing clones, FRT and MDCK cells were stably transfected with a cDNA encoding 3F4-tagged murine (mo) PrPC (a gift from Dr S. Lehmann, Centre National de la Recherche Scientifique, France), with the calcium phosphate procedure as described previously [33]. Stably transfected PrPC clones were selected on the basis of resistance to antibiotic G418. To obtain GFP–Dpl and Dpl clones, the cells were stably transfected with pEGFP-C1 in which the GFP was cloned in the human (hu) Dpl gene, or with a pEGFP in which the human Dpl gene completely substitutes the GFP coding region [24]. To co-express GFP–Dpl, or Dpl, with PrPC, we transfected FRT and MDCK clones stably expressing GFP–Dpl, and carrying the resistance to the antibiotic neomycin, with a plasmid coding for moPrPC, carrying the resistance to zeocin. Alternatively, we transfected FRT clones, stably expressing moPrPC and carrying the resistance to zeocin, with a plasmid coding for huDpl, and carrying neomycin resistance. The two different genes were under the control of the strong cytomegalovirus promoter. Stably transfected clones were selected with zeocin or neomycin and tested for PrPC and Dpl expression by immunofluorescence and Western blotting.
Cell culture and drug treatments
FRT cells were grown in F12 Coon's modified medium containing 5% (v/v) FBS, and MDCK cells were grown in DMEM (Dulbecco's modified Eagle's medium) containing 5% (v/v) FBS, Treatments with MEV and MβCD were carried out as described in [30,31]. Briefly, FRT cells were plated on dishes or filters in F12 Coon's medium supplemented with 5% (v/v) FBS. After 24 h the plated cells were washed and 10 µM or 30 µM MEV was added to the cells in fresh F12 Coon's medium supplemented with 5% (v/v) de-lipidated FBS and 200 µM mevalonate. MβCD (10 mM) dissolved in a fresh medium (20 mM Hepes, pH 7.5, and 0.2% BSA) was then added to the cells for 1 h at 37 °C.
Cholesterol determination
To determine the cholesterol level before and after treatment with MEV/mevalonate and MβCD, we used a colorimetric assay. Briefly, FRT cells grown in the presence, or in the absence, of MEV/mevalonate and MβCD were washed twice with PBS, lysed with appropriate lysis buffer 1 [20 mM Tris/HCl, pH 8.0, containing 1% Triton X-100, 150 mM NaCl, 5 mM EDTA, 0.2% BSA and a protease inhibitor cocktail (leupeptin, antipain, pepstatin)], and were then mixed for 5 min at 37 °C with the Infinity Cholesterol Reagent at a 1:10 ratio (according to the suggested Sigma–Aldrich protocols). Samples were then analysed spectrophotometrically at 550 nm.
Treatment with PNGase F (peptide N-glycosidase F), endo H (endoglycosidase H) and sialidase
Digestion with PNGase F, endo H or sialidase (10 milliunits/sample) was carried out after boiling the immunoprecipitated proteins for 5 min, with antibodies against GFP–Dpl or PrP, in 10 mM EDTA, 0.5% Triton X-100, 0.1% SDS and 1% 2-mercaptoethanol when using PNGase F, and with 0.2 M sodium citrate and 0.5% SDS (pH 5.5) when using the other two enzymes. Each treatment was carried out for 16 h at 37 °C. Before adding endo H and sialidase, additional Triton X-100 was added to give a final concentration of 2.5% (v/v). Samples were then subjected to SDS/PAGE and analysed by Western blotting after addition of reducing Laemmli sample buffer (containing 2-mercaptoethanol; 1:10 dilution).
Fluorescence microscopy
Cells were grown on transwell permeable filter supports or coverslips and were washed with PBS containing 1 mM CaCl2 and 1 mM MgCl2 before being fixed with 2% (w/v) paraformaldehyde for 20 min. They were then either permeabilized with 0.075% saponin or were processed directly, and were used for immunofluorescence analysis. Images were collected using a Zeiss laser scanning confocal microscope (LSCM 510), equipped with a planapo ×63 oil-immersion objective lens (numerical aperture of 1.4).
Immunoprecipitation assays
To immunoprecipitate GFP–Dpl, native Dpl and PrPC, cells were washed three times with PBS containing 1 mM CaCl2 and 1 mM MgCl2 and lysed for 20 min in lysis buffer 1. Lysates were pre-cleared with Protein A–Sepharose beads (5 mg/sample) for 30 min and incubated overnight at 4 °C with 2 mg/ml SAF-32 antibody, to immunoprecipitate PrPC, or with a monoclonal antibody against GFP (1 μg/sample), to immunoprecipitate GFP–Dpl (both coupled to Protein A–Sepharose) overnight at 4 °C. Pellets were washed three times with ice-cold lysis buffer 1, boiled in SDS sample buffer, subjected to SDS/PAGE (12% gels) and immunoblotted on to nitrocellulose membranes. PrPC, GFP–Dpl and native Dpl were revealed by immunoblotting the membranes with the appropriate antibodies.
Biotinylation assay
Cells were grown for 4 days on the transwell filters and were selectively biotinylated and processed as described previously [33]. Cells were directly lysed for 20 min in the transwell chamber, using 50 mM Tris/HCl, pH 7.5, containing 0.5% sodium deoxycholate, 0.5% Triton X-100 and 150 mM NaCl. Biotinylated PrPC and GFP–Dpl were immunoprecipitated with the specific antibodies and were then revealed with HRP-conjugated streptavidin and ECL (enhanced chemiluminescence). Alternatively, biotinylated PrPC and native Dpl were immunoprecipitated with streptavidin beads, and were revealed by Western blotting using specific antibodies.
Assay for DRM association
Analysis of Triton X-100-insoluble materials in OptiPrep™ density gradients was performed using protocols published previously [34]. Cells were grown in 150-mm-diameter dishes, washed in PBS containing 1 mM CaCl2 and 1 mM MgCl2 and lysed for 20 min in lysis buffer 1 on ice. Lysates were scraped from dishes, passed ten times through a 22-gauge needle, then OptiPrep™ was added to give a final concentration of 40%, and the resulting mixture was placed at the bottom of a centrifuge tube. An OptiPrep™ gradient (5–30% OptiPrep™ in 200 mM Tris/HCl, pH 7.5, containing 150 mM NaCl and 1 mM EDTA) was layered on top of the lysates, and samples were ultracentrifuged at 4 °C for 4 h at 20000 rev./min in an ultracentrifuge (Optima™ L90 K Beckman Coulter using a SW41 rotor; Beckman). Fractions (12 of 1 ml each) were harvested from the top of the gradients. GFP–Dpl, native Dpl and PrPC were revealed by immunoblotting after tricloroacetic acid precipitation, or by immunoprecipitation with the specific antibodies, after adjusting the DRM fractions to 1% Triton X-100.
RESULTS
Intracellular and surface distribution of PrPC and GFP–Dpl in singly and doubly transfected FRT clones
FRT clones, constructed to stably express moPrP and GFP–huDpl alone, or together, were selected and tested for the expression of the proteins by immunofluorescence and Western blot analyses (results not shown). The cellular localization of the two proteins, in both singly and doubly transfected clones was first analysed by immunofluorescence performed in permeabilized conditions on cells grown in a polarized monolayer on polycarbonate filters (Figure 1) [33]. We found that, in both cases, PrPC and GFP–Dpl distribute in a polarized fashion, mainly along the basolateral surface of the plasma membrane (Figure 1A), independently of their expression level (results not shown).
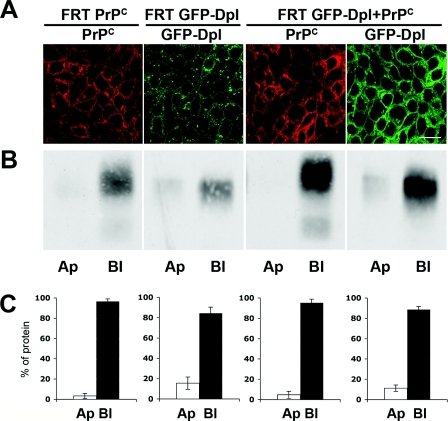
Figure 1. GFP–Dpl and PrPC distribute to the cell surface of singly and doubly transfected FRT clones.
(A) After growth on transwell filters for 4 days, singly (FRT PrPC; FRT GFP-Dpl), or doubly (FRT GFP-Dpl+PrPC), transfected FRT cells were fixed with 2% (w/v) paraformaldehyde and permeabilized with 0.075% saponin, prior to a 20 min incubation with the monoclonal antibody SAF-32 (against PrPC) followed by a 20 min incubation with the secondary TRITC (tetramethylrhodamine β-isothiocyanate)-conjugated antibody (red). Localization of Dpl was visualized by exploiting the fluorescence emitted by the GFP tag (green). Images were acquired with a Zeiss laser confocal microscope (LSCM 510). Scale bar, 10 μm. (B) After selective biotinylation of apical (Ap) or basolateral (Bl) surface proteins, biotinylated GFP–Dpl and PrPC were recovered from cell lysates by immunoprecipitation with the specific antibodies (anti-GFP and SAF-32 antibodies respectively), and were detected by immunoblotting with HRP-conjugated streptavidin. (C) Percentage of apical (Ap), or basolateral (Bl), cell-surface PrPC and GFP–Dpl relative to the total the apical and basolateral signals, which was considered as 100%. Results were quantified from five different independent experiments and represent the means±S.D.
In order to quantify this distribution, filter-grown singly and doubly transfected clones were selectively biotinylated from the apical, or basolateral, surface, and then immunoprecipitated with the anti-GFP or anti-PrP antibodies. As shown in Figures 1(B) and 1(C), we found that when co-expressed PrPC and GFP–Dpl segregated almost completely to the basolateral surface (95±2.5% and 88±4.0% of the total of each protein respectively) which is similar to when they are expressed alone (96±2.5% and 83±6.0% respectively).
These results were in contrast with those observed previously in transfected MDCK cells, in which singly expressed Dpl and PrPC reside in opposite membrane domains (i.e. in the apical and basolateral surface of the plasma membrane respectively), whereas in co-transfected cells both were delivered to the apical membrane [29]. Therefore to try and understand this discrepancy we repeated the same experiments in MDCK cells (see Supplementary Figure S1 available at http://www.BiochemJ.org/bj/425/bj4250341add.htm). As we could reproduce the results from Uelhoff et al. [29] in MDCK cells (Supplementary Figures S1A and S1B), the discrepancy in the results could be explained by the fact that the mechanism of protein sorting in polarized cells is tissue-specific, as already demonstrated for other GPI anchored proteins [35,36].
We then proceeded with the characterization of the intracellular localization of the two proteins in FRT cells by double immunofluorescence using antibodies against different intracellular organelle markers. We found that both PrPC and GFP–Dpl localized to the Golgi apparatus and were not in the ER (endoplasmic reticulum) or in early and late endosomal compartments (Figure 2). As the same distribution was observed in cells expressing each protein alone (results not shown, and [30]), these results support the hypothesis that PrPC and GFP–Dpl do not interfere with each other's intracellular trafficking in doubly transfected FRT cells.
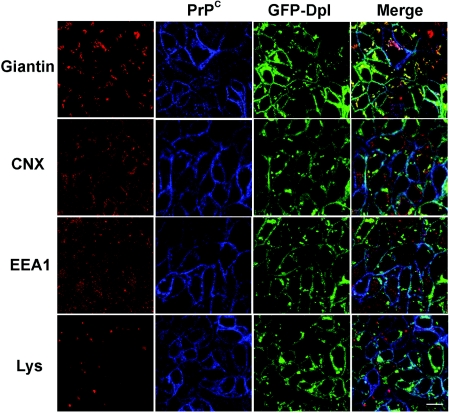
Figure 2. GFP–Dpl and PrPC localize in the Golgi apparatus.
Doubly transfected FRT cells were treated as in Figure 1(A) before being incubated with SAF-32 (against PrPC; blue) and primary polyclonal antibodies against different markers of intracellular compartments (CNX for the ER, giantin for Golgi and EEA1 for early endosomes; red). Secondary antibodies were Cy5-conjugated anti-(mouse Ig) antibody and TRITC-conjugated anti-(rabbit Ig) respectively. To label lysosomes, Lysotracker Red DND-99 (Lys, 1:10000 dilution in cell culture medium) was added to live cells 1 h before fixation and confocal imaging. Dpl was also visualized through the fluorescence of the GFP tag (green). Confocal microscopy was performed as described in Figure 1(A). The localization of GFP–Dpl and PrPC in the Golgi network is clearly evident from the merging of their respective signals with giantin. Scale bar, 10 μm.
Glycosylation pattern of PrPC and GFP–Dpl in singly and doubly transfected clones
It has been reported previously that the glycosylation pattern of Dpl is tissue-specific [20,24,37]. As the glycosylation of proteins has important implications for their trafficking, function and for their interaction with other partners [38], we characterized the glycosylation pattern of PrPC and GFP–Dpl in FRT cells. After immunoprecipitating the proteins (using antibodies against PrPC or GFP), cell lysates were digested with three different de-glycosylating enzymes, namely PNGase F, endo H and sialidase. We found that, differently from PrPC, which was digested by PNGase F and sialidase, but not by endo H, GFP–Dpl was partially sensitive to all of the tested enzymes both in doubly (Figure 3A) and singly (results not shown) transfected cells. The sensitivity of Dpl to endo H suggests that the protein could be in part retained in the ER and/or in the cis-medial Golgi, as modifications of oligosaccharides that render proteins resistant to the enzyme take place later in the secretory pathway (i.e. in medial- or trans-compartments of the Golgi network). Immunofluorescence experiments using specific antibodies against ER- or Golgi-resident proteins (Figure 2) indicated that GFP–Dpl does not reside in the ER. However, because we followed the fluorescence of GFP, it was necessary to discount the possibility that GFP failed to fluoresce properly in the ER [39]. We thus performed double immunofluorescence experiments, using an antibody against Dpl together with an antibody against the ER marker CNX or an antibody against p58K, which labels the cis-Golgi compartment. As shown in Figures 3(B) and 3(C), the fluorescent signal of Dpl co-localized with p58K, but not with CNX, thus confirming that GFP–Dpl resides in the cis- to medial-Golgi and not in the ER.
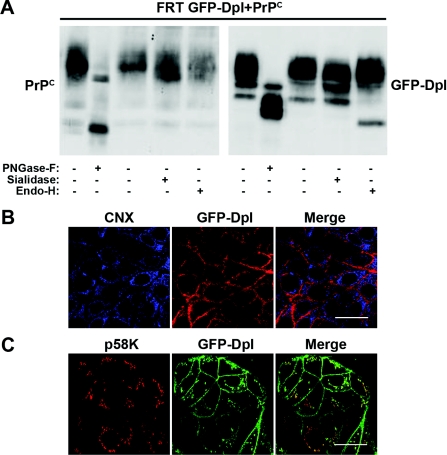
Figure 3. Co-transfected GFP–Dpl and PrPC display different glycosylation patterns, indicating retention of GFP–Dpl in the proximal Golgi network.
(A) Glycan attachment to GFP–Dpl and PrPC was analysed after immunoprecipitation of the proteins (with anti-GFP and the anti-SAF-32 antibodies) from the lysates of doubly transfected cells (FRT GFP-Dpl+PrPC), following a 16 h incubation at 37 °C in the absence (−), or in the presence (+), of the indicated deglycosylating enzymes. Immunoblotting was performed with antibodies against GFP-Dpl and PrPC (SAF-32). (B) The doubly transfected clone, after being fixed and permeabilized (as detailed in Figure 1A), was subjected to a double immunofluorescence assay, using the monoclonal antibody Dpl 151, against Dpl, and a polyclonal antibody against the ER marker CNX, followed by incubation with an anti-mouse TRITC-conjugated secondary antibody and an anti-rabbit Cy5-conjugated secondary antibody [to reveal GFP–Dpl (red) and CNX (blue) respectively]. No co-localization of the two signals was detected. Scale bar, 10 μm. (C) GFP–Dpl-transfected cells grown on coverslips were fixed with paraformaldehyde and incubated under permeabilized conditions with an antibody against the cis-Golgi marker p58K (red). The cells were then treated with a TRITC-conjugated secondary antibody and examined with a Zeiss laser confocal microscope (LSCM 510). Clearly, the merge signal indicates that GFP–Dpl (green), assessed by monitoring the GFP florescence, resides in the cis- to medial-Golgi compartments. Scale bar, 10 μm.
Association of PrPC and GFP–Dpl to DRMs
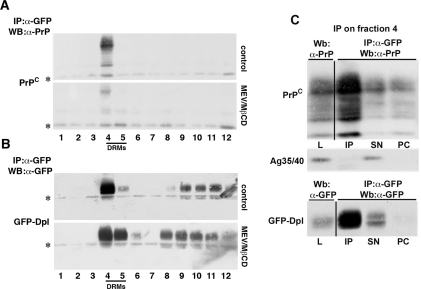
Both Dpl and PrPC have been found to be associated with DRMs in different cells and tissues [20,24,26]. As this association could have a role in the trafficking and function of the two proteins, especially with regards to their interaction, we analysed the association of PrPC and GFP–Dpl with these lipid domains by following their distribution on OptiPrep™ density gradients after extraction of the cells in an non-ionic detergent. We found that, in singly transfected clones, the majority of PrPC (60±2.1% of the total) and GFP–Dpl (65±5.3% of the total) associated with the low-density DRM-rich fractions 4 and 5 of the gradients (Figures 4A and 4C). However, in doubly expressing clones, only GFP–Dpl maintained this distribution (65±5.3 and 62±2.4%); PrPC floated into DRM fractions in minor proportions compared with singly transfected clones (36±1.8% compared with 60±2.1%; Figures 4A and 4C). These results suggested that the expression of Dpl affected the distribution of PrPC in DRMs. A similar observation was reported previously in neuronal cells in which co-transfected Dpl and PrPC partitioned to a greater extent with high-density fractions [24].
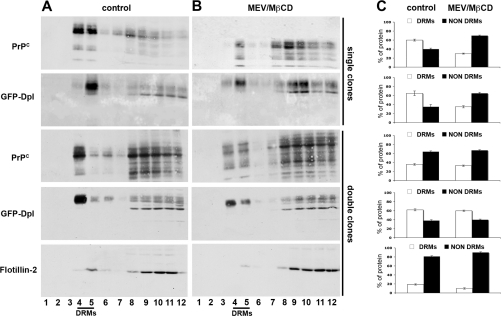
Figure 4. Association of GFP–Dpl and PrPC to DRMs.
(A and B) Singly or doubly transfected clones were grown on 150-mm dishes in the (A) absence (control) or (B) presence (MEV/MβCD) of the cholesterol-depleting agents MEV/mevalonate and MβCD. After cell lysis in 1% Triton X-100, 2 mg of total protein were run through a two-step OptiPrep™ density gradient (5–30%) (see the Experimental section, and [34]). Fractions (12 of 1 ml each) were collected from the top of the tube after centrifugation to equilibrium. Each fraction was acid-precipitated and immunoblotted using a polyclonal antibody against GFP and/or the monoclonal antibody SAF-32 against PrP. As shown in the control samples (A), fractions 4 and 5, which are the richest in DRMs, contained most of GFP–Dpl both in singly and doubly transfected cells, whereas PrPC migrated to these fractions only to a minor extent in doubly transfected cells. In (B) it is shown that, after cell cholesterol depletion in singly transfected cells GFP–Dpl and PrPC floated to heavier density fractions, whereas in doubly transfected cells the distribution of the proteins remained practically unaffected compared with control conditions. (C) Relative percentage of DRM-associated (DRMs) and DRM-unassociated (NON DRMs) GFP–Dpl and PrPC under the different conditions. The amount of each protein was calculated by setting the amount of total protein distributed in the 12 fractions of the gradient as 100%, and represents means±S.D. (n=4). Distribution of flotillin-2 along the fractions of the gradient (bottom panel) was used as control for the procedure.
In order to further characterize the behaviour of the two proteins when expressed together we analysed whether cholesterol, a key structural and functional component of DRMs, was implicated in the association of PrPC and GFP–Dpl with DRMs. To this aim we depleted the cells of approx. 50% of their cholesterol content, using a combined treatment with MEV/mevalonate and MβCD [30,31]. As expected, in singly transfected clones this treatment caused the redistribution of the majority of each protein to the high-density fractions of the gradient (fraction numbers 8–12). This behaviour was in parallel with that of the DRM marker flotillin-2 [32] both in singly (results not shown) and doubly transfected clones (Figure 4). Intriguingly, cholesterol depletion of doubly transfected clones had no gross effect on the raft association of GFP–Dpl and PrPC, in that the proteins migrated to the raft fractions of the gradients to approximately the same extent as that observed under control conditions (60±1.5% compared with 62±2.4% for GFP–Dpl; 33±1.5% compared with 36±1.8% for PrPC; Figure 4). These results indicated that when co-expressed the association of both proteins to membrane microdomains became less sensitive to cholesterol depletion and suggests that they might occupy the same membrane microdomains.
Interaction between PrPC and GFP–Dpl
Double immunofluorescence and flotation experiments indicated that GFP–Dpl and PrPC co-localize to a good extent and that they partition in DRMs with similar characteristics. To understand whether the two proteins entertained physical interactions in DRMs, we subjected them to co-immunoprecipitation after first being separated by OptiPrep™ flotation assays. Specifically, we first immunoprecipitated GFP–Dpl from all OptiPrep™ fractions and then immuno-identified PrPC in the precipitate by Western blot analysis. As shown in the upper panels of Figures 5(A) and 5(B), GFP–Dpl and PrPC could be co-immunoprecipitated only from DRM fraction 4 of the gradients. Importantly, the same held true when using an antibody against PrPC in the precipitation step and one against GFP to visualize GFP–Dpl in the Western blot of the immunoprecipitate (see Supplementary Figure S2 available at http://www.BiochemJ.org/bj/425/bj4250341add.htm). This interaction appeared to be specific as we did not observe co-immunoprecipitation with another basolateral marker, antigen 35/40 kDa [40] (Figure 5C). Furthermore, we observed a significant diminution in the amount of co-immunoprecipitated PrPC when performing the experiment in cells depleted of cholesterol (Figures 5A and 5B, lower panels). Overall, these results indicate that the two proteins interact within DRMs and that this interaction requires the integrity of these membrane microdomains.
Figure 5. GFP–Dpl and PrPC residing in the DRM fractions co-immunoprecipitate.
(A) Total protein (2 mg) of doubly transfected cells grown in control (control) or cholesterol-depleting (MEV/MβCD), conditions were subjected to OptiPrep™ density gradient purification and each gradient fraction was immunoprecipitated (IP) using a monoclonal antibody against GFP (α-GFP). The immunoprecipitate was then immunolabelled with a monoclonal antibody (SAF-32) against PrP. (*) indicates immunoglobulins. (B) To confirm the occurrence of the immunoprecipitation, membranes from (A) were stripped and probed with a polyclonal antibody against GFP. (C) The loading control (L, 60 μg of total FRT GFP-Dpl+PrPC lysate), the anti-GFP antibody-immunoprecipitated DRM fraction 4 (IP), 1/10 of the supernatant (SN) and the pre-clearing (PC, precleared of the immunoprecipitated beads) material were subjected to immunoblotting with the monoclonal antibody SAF-32 against PrP (to indicate co-immunoprecipitation) (top panel). The membrane was stripped and blotted with a monoclonal antibody against the antigen 35/40 kDa (Ag35/40) to confirm the specificity of the co-immunoprecipitation between GFP–Dpl and PrPC (middle panel). To confirm the occurrence of the immunoprecipitation, the membrane was stripped and hybridized with a polyclonal antibody against GFP (bottom panel). WB, Western blot.
Interaction of PrPC and GFP–Dpl with flotillin-2
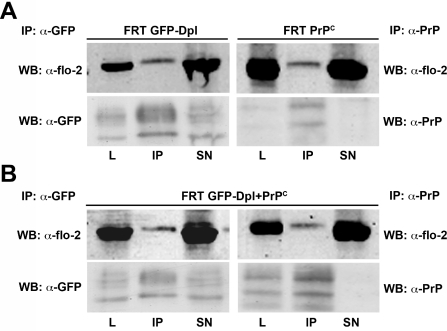
Although little is known about Dpl interactors it has been shown that it can interact with flotillin-2 in testis [20]. Interestingly, PrPC was also found to interact with flotillin-2 and flotillin-1 in T-cells [41], but not in testis [20]. As flotillins have been proposed to have a role both in the endocytosis and function of PrPC [42–44], we then analysed whether flotillin-2 interacted with Dpl and/or PrPC in singly and/or doubly transfected FRT cells. To this end, we performed co-immunoprecipitation experiments using antibodies against GFP–Dpl or PrPC in the precipitation step, and used an antibody against flotillin-2 to reveal this protein in the immunoprecipitate by Western blot analysis. Intriguingly, we found that both PrPC and GFP–Dpl co-immunoprecipitated with flotillin-2 both in singly (Figure 6A) and doubly (Figure 6B) transfected clones. These findings highlight the possible existence of membrane protein complexes in which flotillin-2 associates with PrPC and Dpl, either alone or together.
Figure 6. GFP–Dpl and PrPC co-immunoprecipitate with flotillin-2 in both singly and doubly transfected clones.
Lysates from (A) singly (FRT GFP-Dpl; FRT PrPC) or (B) doubly (FRT GFP-Dpl+PrPC) transfected FRT cells were immunoprecipitated (IP) with a monoclonal antibody against GFP (left-hand panels) or the monoclonal antibody SAF-32 against PrP (right-hand panels), and immunolabelled with a monoclonal antibody against flotillin-2 (α-flo-2) to indicate co-immunoprecipitation. Lysate loading control (L) and 1/10 of the supernatant of the IP (SN) were also analysed. Each membrane was then stripped and further immunolabelled with antibodies against GFP (α-GFP) or PrPC (α-PrP) to confirm the occurrence of the immunoprecipitation. WB, Western blot.
Characterization of clones expressing native Dpl alone or together with PrPC
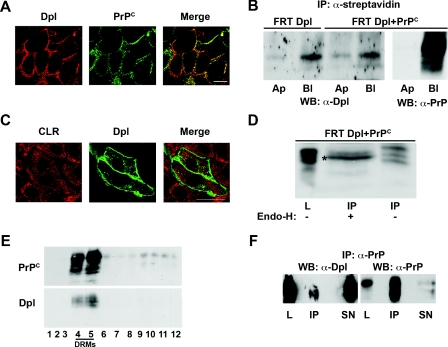
In all of the above experiments, we used a construct of Dpl tagged to GFP. This strategy was chosen for practical reasons, in view of the possibility to directly follow Dpl through the fluorescence of GFP. Although it was previously shown in neuronal cells that GFP–Dpl behaves like the wild-type protein [24], in order to discard a possible interference of the tag with the metabolism of native Dpl in our cell system, we stably transfected FRT clones with native Dpl alone, or together with PrPC, and repeated all the experiments shown for GFP–Dpl in these clones (see the Experimental section). We found that native Dpl behaved like GFP–Dpl. In particular we show that in FRT cells native Dpl: (i) segregated to the basolateral plasma membrane (Figure 7A); (ii) did not interfere with the basolateral sorting of PrPC (Figure 7B); (iii) did not reside in the ER (Figure 7C); (iv) was sensitive to endo H digestion both when expressed alone (results not shown) and together with PrPC (Figure 7D); (v) associated with DRMs both in singly (results not shown) and doubly (Figure 7E) transfected cells; and (vi) co-immunoprecipitates with PrPC in the DRM-rich fractions of OptiPrep™ gradients (Figure 7F). Altogether, these results both confirm our findings and validate the use of GFP–Dpl as an excellent tool in studying the physiology of Dpl.
Figure 7. GFP-tagging does not alter the intracellular trafficking of Dpl in FRT cells and its ability to co-immunoprecipitate with PrPC.
(A) Doubly transfected FRT cells were grown on coverslips and subjected to immunofluorescence analysis under unpermeabilized conditions. After paraformaldehyde fixation, cells were incubated with the anti-Dpl polyclonal Q55 antibody (red) and the monoclonal SAF-32 primary antibody (green), and were then incubated with appropriate secondary antibodies. The images were analysed with a confocal microscope; the merge shows the co-localization of Dpl and PrPC on the basolateral cell surface (an optical section, from a Z-stack, near the basolateral surface is shown). Scale bar, 10 μm. (B) Dpl and Dpl and PrPC expressing cells were grown on transwell filters and selectively biotinylated on the apical (Ap) or basolateral (Bl) surface. Biotinylated Dpl and PrPC were then isolated from cell lysates by immunoprecipitation with streptavidin beads and detected by immunoblotting with the specific antibodies (Dpl 151 or SAF-32 respectively). WB, Western blot. (C) Cells transfected with an untagged Dpl protein were plated on coverslips and fixed with paraformaldehyde. After saponin permeabilization cells were stained with a monoclonal antibody against Dpl (Dpl 151; green) and a polyclonal antibody against the ER marker calreticulin (CLR; red). Scale bar, 10 μm. (D) Sensitivity of Dpl to endo H treatment was analysed after immunoprecipitation (IP) of the protein (with the monoclonal antibody Dpl 151) from the lysates of doubly transfected cells (FRT Dpl+PrPC), after incubation for 16 h at 37 °C in the absence (−), or in the presence (+), of endo H enzyme (see the Experimental section) and immunoblotting with a polyclonal antibody against Dpl (Q55). (*) indicates the band resulting from the enzymatic digestion. L, lysate. (E) Triton X-100 (1%) lysates from doubly expressing clones grown on 150-mm dishes were run through a two-step (5–30%) OptiPrep™ density gradient, as described in the Experimental section. Fractions (12 of 1 ml each) were collected from the top to the bottom of the tube after centrifugation to equilibrium, and Dpl and PrPC were revealed in each fraction by Western blotting with the Dpl 151 or the SAF-32 antibodies respectively. (F) The OptiPrep™ density gradient DRM-rich fractions 4 and 5 of doubly transfected FRT cell lysates were first immunoprecipitated (IP) with an anti-PrP antibody (SAF-32) and then revealed by Western blotting (WB) with an anti-Dpl antibody (Dpl 151; to show co-immunoprecipitation) or an anti-PrP antibody (SAF-32; to confirm immunoprecipitation). The loading control (L, 60 μg of cell lysate) and 1/10 of the supernatants (SN) were also analysed.
DISCUSSION
Dpl and PrPC, although sharing a similar three-dimensional structure and amino acid sequence, appear to have antagonistic functions [8]. In particular it has been shown that Ngsk, ZurichII and Rcm0 lines of PrP-knockout mice develop late-onset ataxia [3–5] due to the ectopic overexpression of Dpl in the brains of these mice [4,5]. Interestingly, the symptoms are rescued by reintroducing the PrP gene, suggesting a functional interaction between PrPC and Dpl [8,13]. Consistent with this hypothesis an interaction has been suggested to occur in neuronal cells, but not in testis [19–21]. Furthermore, Dpl seems to affect the trafficking of PrPC when co-expressed in polarized epithelial MDCK cells [29], supporting further the possibility that the two proteins interact. However, the localization of this interaction and its physiopathological role are still debated [8,18,20–23].
In order to shed light on these issues, we have studied the intracellular trafficking of Dpl both in the presence and absence of PrPC in transfected polarized FRT cells, which have already been extensively characterized for the trafficking of PrPC [30,31]. Specifically, we asked the question of whether the intracellular localization of each protein was affected by the presence of the other, and whether and where the two proteins interacted in doubly transfected cells.
Confocal microscopy and biochemical approaches allowed us to demonstrate that Dpl and PrPC preferentially distribute in the Golgi apparatus and on the basolateral cell surface in both singly and doubly expressing FRT clones, where they partially co-localize (Figures 1, 2, 7A and 7B).
In contrast with what has been shown in MDCK cells [29], we found that Dpl and PrPC do not interfere with each other's localization in FRT cells. The discrepancy between the two cell lines could be explained by the fact that FRT and MDCK cells differ in the polarized sorting of GPI-anchored proteins [35,36]; Dpl therefore represents another model GPI protein which has an opposite localization in the two cell lines.
As glycans are important modifiers of the behaviour of secretory proteins, we also analysed the glycoforms of Dpl expressed alone (results not shown) or together with PrPC (Figure 3). Intriguingly, and differently from PrPC, the unglycosylated band of Dpl could be detected only upon digestion with PNGase F, suggesting that the attachment of glycans to this protein occurs very efficiently in FRT cells. Alternatively the degradation process of unglycosylated Dpl might be very efficient in our model cell system (Figure 3A). Another distinct feature of Dpl is that it displayed a partial sensitivity to digestion by endo H (Figures 3A and 7D). Accordingly, we found that Dpl (most probably its monoglycosylated form) was partially retained in the cis- to medial-Golgi complex (Figure 3C). In addition we found Dpl was also sensitive to endo H digestion in MDCK cells both when expressed alone (results not shown) or together with PrPC (Supplementary Figure S1C) suggesting that differences between the two cell lines in the polarized sorting of Dpl, and the mis-sorting of PrPC, are not regulated by endo H-sensitive glycan modification. Thus, despite the homologous glycosylation sites, a diverse sugar remodelling might occur for the two proteins and this may be linked to their different function and/or to their different susceptibility to conformation transition, which is typical of PrPC, but not of Dpl [9].
As both proteins have been found associated with DRMs in different cells and tissues [20,24,26], we next analysed their raft association in singly and doubly transfected FRT clones (Figures 4 and 7E). Whereas the association of Dpl with DRMs remained approximately the same in the two clones (65±5.3 and 62±2.4%), the amount of PrPC floating to the DRM-rich fractions in the doubly transfected cells was lower compared with the singly transfected ones (36±1.8 and 60±2.1% respectively) (Figure 4). Moreover, in cells expressing both PrPC and Dpl, cholesterol depletion was not able to induce the dissociation of the two proteins from DRMs (from 62±2.4 to 60±1.5% for GFP–Dpl and from 36±1.8 to 33±1.5% for PrPC), which was observed in singly expressing clones (from 65±5.3 to 35±2.4% of GFP–Dpl and from 60±2.1 to 30±1.3% of PrPC; Figure 4). We speculate that the expression of Dpl could modulate the capacity of PrPC to associate with lipid rafts inducing it to move to a less-ordered lipid microenvironment. Nevertheless, we show that the two proteins co-immunoprecipitate exclusively from DRMs (Figure 5). Importantly, the integrity of these domains appeared to be essential for the interaction of the two proteins, as demonstrated by the reduction in the co-immunoprecipitated protein levels after cholesterol depletion (Figures 5A and B). Although the interaction between PrPC and Dpl has been suggested previously [18,19,21], our results provide the first clear evidence that this interaction occurs in specific membrane microdomains (rafts) and is dependent on cholesterol. Our results are consistent with the finding that in neuronal cells the two proteins were found to reside in the same membrane microdomains [24] and with the fact that in testis, where the two proteins do not co-immunoprecipitate, they reside in different membrane environments [20].
The demonstration that PrPC and Dpl interact in rafts could be of importance, considering the relevance of membrane microdomains in the pathogenesis of prion and other neurodegenerative diseases [26,27,45,46]. The association of these proteins to different or the same rafts could account for their different function in different tissues, supporting the hypothesis that PrPC, when interacting with Dpl in rafts of neurons, is able to block its toxicity. Finally, on the basis of co-immunoprecipitation experiments, we show in the present study that PrPC and Dpl form a complex with flotillin-2 in singly and doubly transfected clones (Figure 6). This finding is quite interesting considering that interaction with flotillins has been suggested to have a role in the physiological function of PrPC; it can lead to activation of ERK (extracellular-signal-regulated kinase) 1/2 upon PrPC capping in T-cells [43], and can trigger the formation of Ca2+-independent PrPC-mediated cell adhesions in S2 cells [44]. Moreover, on one hand flotillin-1, which forms a heterocomplex with flotillin-2 in N2a cells, has been reported to interact with neuroglobin, a protein involved in neuroprotective pathways [47], and on the other hand flotillin levels are increased in Alzheimers-disease-affected brains [48]. Interestingly PrPSc was shown to accumulate in flotillin-1-positive vesicles in infected GT1-7 cells [42], suggesting a possible involvement of these raft proteins in neurotoxic processes. Thus considering the antagonistic behaviour of Dpl and PrPC regarding the onset of neurodegeneration, it will be interesting to investigate further whether and how this mutual or exclusive interaction with flotillin is involved in the signalling function of both proteins and/or in the pathways leading to neurodegeneration. Indeed it is tempting to speculate that during embryogenesis, when both proteins are present, PrPC and Dpl together transduce a beneficial signal through a complex containing flotillin-2. Accordingly, one could hypothesize that the complex gets disrupted, and/or malfunctions, if Dpl does not interact with PrPC (i.e. in the absence of PrPC) thus reversing the beneficial effect of a neuroprotective signal.
Online data
AUTHOR CONTRIBUTION
Chiara Zurzolo conceived and co-ordinated the project, wrote the manuscript and helped with the experimental planning and analysis of results. Anna Caputo and Daniela Sarnataro designed and performed most of the experiments, analysed the results and assisted in refining the manuscript text. Vincenza Campana produced stable cell lines and discussed the experiments. Maddalena Costanzo produced stable clones of MDCK cells and performed some of the experiments in these cell lines. Alessandro Negro cloned the Dpl gene in the different expression vectors, produced the anti-Dpl Q55 antibody and discussed the experiments. All of the authors discussed the results and the manuscript text.
ACKNOWLEDGEMENTS
We thank Dr J. Grassi (Commisariat a l'Energie Atomique, Saclay, France) for the antibody Dpl 151.
FUNDING
This work was supported by MURST (Ministero dell'Istruzione, dell'Università e della Ricerca) [grant numbers PRIN 2005, PRIN 2006, FIRB 2003 to C.Z. and M.C.S.]; the Telethon Foundation [grant numbers GGP0414, GTF03001]; and from the European Union [grant number LSHB-CT-2006–019090 to C.Z.]. D.S. is the recipient of a fellowship from the Research Programme FIRB 2003.
References
- 1.Linden R., Martins V. R., Prado M. A., Cammarota M., Izquierdo I., Brentani R. R. Physiology of the prion protein. Physiol. Rev. 2008;88:673–728. doi: 10.1152/physrev.00007.2007. [DOI] [PubMed] [Google Scholar]
- 2.Aguzzi A., Polymenidou M. Mammalian prion biology: one century of evolving concepts. Cell. 2004;116:313–327. doi: 10.1016/s0092-8674(03)01031-6. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S., Katamine S., Nishida N., Moriuchi R., Shigematsu K., Sugimoto T., Nakatani A., Kataoka Y., Houtani T., Shirabe S., et al. Loss of cerebellar Purkinje cells in aged mice homozygous for a disrupted PrP gene. Nature. 1996;380:528–531. doi: 10.1038/380528a0. [DOI] [PubMed] [Google Scholar]
- 4.Moore R. C., Lee I. Y., Silverman G. L., Harrison P. M., Strome R., Heinrich C., Karunaratne A., Pasternak S. H., Chishti M. A., Liang Y., et al. Ataxia in prion protein (PrP)-deficient mice is associated with upregulation of the novel PrP-like protein doppel. J. Mol. Biol. 1999;292:797–817. doi: 10.1006/jmbi.1999.3108. [DOI] [PubMed] [Google Scholar]
- 5.Rossi D., Cozzio A., Flechsig E., Klein M. A., Rulicke T., Aguzzi A., Weissmann C. Onset of ataxia and Purkinje cell loss in PrP null mice inversely correlated with Dpl level in brain. EMBO J. 2001;20:694–702. doi: 10.1093/emboj/20.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li A., Sakaguchi S., Atarashi R., Roy B. C., Nakaoke R., Arima K., Okimura N., Kopacek J., Shigematsu K. Identification of a novel gene encoding a PrP-like protein expressed as chimeric transcripts fused to PrP exon 1/2 in ataxic mouse line with a disrupted PrP gene. Cell. Mol. Neurobiol. 2000;20:553–567. doi: 10.1023/a:1007059827541. [DOI] [PubMed] [Google Scholar]
- 7.Li A., Sakaguchi S., Shigematsu K., Atarashi R., Roy B. C., Nakaoke R., Arima K., Okimura N., Kopacek J., Katamine S. Physiological expression of the gene for PrP-like protein, PrPLP/Dpl, by brain endothelial cells and its ectopic expression in neurons of PrP-deficient mice ataxic due to Purkinje cell degeneration. Am. J. Pathol. 2000;157:1447–1452. doi: 10.1016/S0002-9440(10)64782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watts J. C., Westaway D. The prion protein family: diversity, rivalry, and dysfunction. Biochim. Biophys. Acta. 2007;1772:654–672. doi: 10.1016/j.bbadis.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Mo H., Moore R. C., Cohen F. E., Westaway D., Prusiner S. B., Wright P. E., Dyson H. J. Two different neurodegenerative diseases caused by proteins with similar structures. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2352–2357. doi: 10.1073/pnas.051627998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman G. L., Qin K., Moore R. C., Yang Y., Mastrangelo P., Tremblay P., Prusiner S. B., Cohen F. E., Westaway D. Doppel is an N-glycosylated, glycosylphosphatidylinositol-anchored protein. Expression in testis and ectopic production in the brains of Prnp0/0 mice predisposed to Purkinje cell loss. J. Biol. Chem. 2000;275:26834–26841. doi: 10.1074/jbc.M003888200. [DOI] [PubMed] [Google Scholar]
- 11.Lu K., Wang W., Xie Z., Wong B. S., Li R., Petersen R. B., Sy M. S., Chen S. G. Expression and structural characterization of the recombinant human doppel protein. Biochemistry. 2000;39:13575–13583. doi: 10.1021/bi001523m. [DOI] [PubMed] [Google Scholar]
- 12.Flechsig E., Hegyi I., Leimeroth R., Zuniga A., Rossi D., Cozzio A., Schwarz P., Rulicke T., Gotz J., Aguzzi A., Weissmann C. Expression of truncated PrP targeted to Purkinje cells of PrP knockout mice causes Purkinje cell death and ataxia. EMBO J. 2003;22:3095–3101. doi: 10.1093/emboj/cdg285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishida N., Tremblay P., Sugimoto T., Shigematsu K., Shirabe S., Petromilli C., Erpel S. P., Nakaoke R., Atarashi R., Houtani T., et al. A mouse prion protein transgene rescues mice deficient for the prion protein gene from purkinje cell degeneration and demyelination. Lab. Invest. 1999;79:689–697. [PubMed] [Google Scholar]
- 14.Shmerling D., Hegyi I., Fischer M., Blattler T., Brandner S., Gotz J., Rulicke T., Flechsig E., Cozzio A., von Mering C., et al. Expression of amino-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell. 1998;93:203–214. doi: 10.1016/s0092-8674(00)81572-x. [DOI] [PubMed] [Google Scholar]
- 15.Li A., Christensen H. M., Stewart L. R., Roth K. A., Chiesa R., Harris D. A. Neonatal lethality in transgenic mice expressing prion protein with a deletion of residues 105–125. EMBO J. 2007;26:548–558. doi: 10.1038/sj.emboj.7601507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumann F., Tolnay M., Brabeck C., Pahnke J., Kloz U., Niemann H. H., Heikenwalder M., Rulicke T., Burkle A., Aguzzi A. Lethal recessive myelin toxicity of prion protein lacking its central domain. EMBO J. 2007;26:538–547. doi: 10.1038/sj.emboj.7601510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin S. M., Sy M. S., Yang H. Y., Tien P. Interaction of Doppel with the full-length laminin receptor precursor protein. Arch. Biochem. Biophys. 2004;428:165–169. doi: 10.1016/j.abb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Benvegnu S., Franciotta D., Sussman J., Bachi A., Zardini E., Torreri P., Govaerts C., Pizzo S., Legname G. Prion protein paralog doppel protein interacts with α-2-macroglobulin: a plausible mechanism for doppel-mediated neurodegeneration. PLoS One. 2009;4:e5968. doi: 10.1371/journal.pone.0005968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui T., Holme A., Sassoon J., Brown D. R. Analysis of doppel protein toxicity. Mol. Cell. Neurosci. 2003;23:144–155. doi: 10.1016/s1044-7431(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 20.Shaked Y., Hijazi N., Gabizon R. Doppel and PrPC do not share the same membrane microenvironment. FEBS Lett. 2002;530:85–88. doi: 10.1016/s0014-5793(02)03430-0. [DOI] [PubMed] [Google Scholar]
- 21.Qin K., Zhao L., Tang Y., Bhatta S., Simard J. M., Zhao R. Y. Doppel-induced apoptosis and counteraction by cellular prion protein in neuroblastoma and astrocytes. Neuroscience. 2006;141:1375–1388. doi: 10.1016/j.neuroscience.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 22.Hundt C., Weiss S. The prion-like protein Doppel fails to interact with itself, the prion protein and the 37 kDa/67 kDa laminin receptor in the yeast two-hybrid system. Biochim. Biophys. Acta. 2004;1689:1–5. doi: 10.1016/j.bbadis.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Azzalin A., Del Vecchio I., Chiarelli L. R., Valentini G., Comincini S., Ferretti L. Absence of interaction between doppel and GFAP, Grb2, PrPc proteins in human tumor astrocytic cells. Anticancer Res. 2005;25:4369–4374. [PubMed] [Google Scholar]
- 24.Massimino M. L., Ballarin C., Bertoli A., Casonato S., Genovesi S., Negro A., Sorgato M. C. Human Doppel and prion protein share common membrane microdomains and internalization pathways. Int. J. Biochem. Cell Biol. 2004;36:2016–2031. doi: 10.1016/j.biocel.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Pike L. J. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J. Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Taylor D. R., Hooper N. M. The prion protein and lipid rafts. Mol. Membr. Biol. 2006;23:89–99. doi: 10.1080/09687860500449994. [DOI] [PubMed] [Google Scholar]
- 27.Taylor D. R., Hooper N. M. Role of lipid rafts in the processing of the pathogenic prion and Alzheimer's amyloid-β proteins. Semin. Cell Dev. Biol. 2007;18:638–648. doi: 10.1016/j.semcdb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Campana V., Sarnataro D., Zurzolo C. The highways and byways of prion protein trafficking. Trends Cell Biol. 2005;15:102–111.. doi: 10.1016/j.tcb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Uelhoff A., Tatzelt J., Aguzzi A., Winklhofer K. F., Haass C. A pathogenic PrP mutation and doppel interfere with polarized sorting of the prion protein. J. Biol. Chem. 2005;280:5137–5140. doi: 10.1074/jbc.C400560200. [DOI] [PubMed] [Google Scholar]
- 30.Sarnataro D., Paladino S., Campana V., Grassi J., Nitsch L., Zurzolo C. PrPC is sorted to the basolateral membrane of epithelial cells independently of its association with rafts. Traffic. 2002;3:810–821.. doi: 10.1034/j.1600-0854.2002.31106.x. [DOI] [PubMed] [Google Scholar]
- 31.Sarnataro D., Campana V., Paladino S., Stornaiuolo M., Nitsch L., Zurzolo C. PrPC association with lipid rafts in the early secretory pathway stabilizes its cellular conformation. Mol. Biol. Cell. 2004;15:4031–4042. doi: 10.1091/mbc.E03-05-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babuke T., Tikkanen R. Dissecting the molecular function of reggie/flotillin proteins. Eur. J. Cell Biol. 2007;86:525–532. doi: 10.1016/j.ejcb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Zurzolo C., Le Bivic A. L., Rodriguez-Boulan E. Cell surface biotinylation techniques. In: Celis J. E., editor. Cell Biology: A Laboratory Handbook. San Diego, CA, U.S.A.: Academic Press; 1994. pp. 185–192. [Google Scholar]
- 34.Sarnataro D., Pisanti S., Santoro A., Gazzerro P., Malfitano A. M., Laezza C., Bifulco M. The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits human breast cancer cell proliferation through a lipid raft-mediated mechanism. Mol. Pharmacol. 2006;70:1298–1306. doi: 10.1124/mol.106.025601. [DOI] [PubMed] [Google Scholar]
- 35.Zurzolo C., van't Hof W., van Meer G., Rodriguez-Boulan E. Glycosphingolipid clusters and the sorting of GPI-anchored proteins in epithelial cells. Braz. J. Med. Biol. Res. 1994;27:317–322. [PubMed] [Google Scholar]
- 36.Paladino S., Sarnataro D., Tivodar S., Zurzolo C. Oligomerization is a specific requirement for apical sorting of glycosyl-phosphatidylinositol-anchored proteins but not for non-raft-associated apical proteins. Traffic. 2007;8:251–258. doi: 10.1111/j.1600-0854.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 37.Travaglino E., Comincini S., Benatti C., Azzalin A., Nano R., Rosti V., Ferretti L., Invernizzi R. Overexpression of the Doppel protein in acute myeloid leukaemias and myelodysplastic syndromes. Br. J. Haematol. 2005;128:877–884. doi: 10.1111/j.1365-2141.2005.05386.x. [DOI] [PubMed] [Google Scholar]
- 38.Ohtsubo K., Marth J. D. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Waldo G. S., Standish B. M., Berendzen J., Terwilliger T. C. Rapid protein-folding assay using green fluorescent protein. Nat. Biotechnol. 1999;17:691–695. doi: 10.1038/10904. [DOI] [PubMed] [Google Scholar]
- 40.Amerongen H. M., Mack J. A., Wilson J. M., Neutra M. R. Membrane domains of intestinal epithelial cells: distribution of Na+,K+-ATPase and the membrane skeleton in adult rat intestine during fetal development and after epithelial isolation. J. Cell Biol. 1989;109:2129–2138. doi: 10.1083/jcb.109.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reuter A., Binkle U., Stuermer C. A., Plattner H. PrPc and reggies/flotillins are contained in and released via lipid-rich vesicles in Jurkat T cells. Cell. Mol. Life Sci. 2004;61:2092–2099. doi: 10.1007/s00018-004-4193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pimpinelli F., Lehmann S., Maridonneau-Parini I. The scrapie prion protein is present in flotillin-1-positive vesicles in central- but not peripheral-derived neuronal cell lines. Eur. J. Neurosci. 2005;21:2063–2072. doi: 10.1111/j.1460-9568.2005.04049.x. [DOI] [PubMed] [Google Scholar]
- 43.Stuermer C. A., Langhorst M. F., Wiechers M. F., Legler D. F., Von Hanwehr S. H., Guse A. H., Plattner H. PrPc capping in T cells promotes its association with the lipid raft proteins reggie-1 and reggie-2 and leads to signal transduction. FASEB J. 2004;18:1731–1733. doi: 10.1096/fj.04-2150fje. [DOI] [PubMed] [Google Scholar]
- 44.Malaga-Trillo E., Solis G. P., Schrock Y., Geiss C., Luncz L., Thomanetz V., Stuermer C. A. Regulation of embryonic cell adhesion by the prion protein. PLoS Biol. 2009;7:e55. doi: 10.1371/journal.pbio.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benarroch E. E. Lipid rafts, protein scaffolds, and neurologic disease. Neurology. 2007;69:1635–1639. doi: 10.1212/01.wnl.0000279590.22544.c3. [DOI] [PubMed] [Google Scholar]
- 46.Michel V., Bakovic M. Lipid rafts in health and disease. Biol. Cell. 2007;99:129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- 47.Wakasugi K., Kitatsuji C., Morishima I. Possible neuroprotective mechanism of human neuroglobin. Ann. N.Y. Acad. Sci. 2005;1053:220–230. doi: 10.1196/annals.1344.020. [DOI] [PubMed] [Google Scholar]
- 48.Kokubo H., Lemere C. A., Yamaguchi H. Localization of flotillins in human brain and their accumulation with the progression of Alzheimer's disease pathology. Neurosci. Lett. 2000;290:93–96. doi: 10.1016/s0304-3940(00)01334-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.