Abstract
Purpose
To evaluate the expression of βB2-crystallin mRNA and protein in rat, bovine, and human nonlens and nonocular tissues.
Methods
βB2-crystallin mRNA levels were detected by RT-PCR. βB2-crystallin protein was purified from rat and bovine tissues by FPLC chromatography. FPLC fractions were analyzed by immunoblotting. The identity of βB2-crystallin protein, isolated from the retina, was confirmed by protein microsequencing.
Results
βB2-crystallin transcript was detected in rat brain, rat testis, and human retina by RT-PCR. βB2-crystallin transcript was not found in rat lung, heart, ovary, spleen, thymus, kidney, and liver or in human brain and testis. βB2-crystallin protein was partially purified from and its identity confirmed in rat brain, rat testis, and bovine retina. The bovine retinal protein was further confirmed to be authentic βB2-crystallin by protein microsequencing.
Conclusions
These results establish that βB2-crystallin mRNA and protein are expressed in tissues outside of the lens and outside of the eye including retina, brain, and testis. Extralenticular and extraocular expression of βB2-crystallin, coupled with its participation in phosphorylation pathways, suggests that it has nonrefractive functions in these tissues.
The crystallin proteins that make up the transparent eye lens were originally thought to be restricted to the lens and to have entirely refractive functions. It is now established that several crystallins, including members of the α-, β-, and γ-crystallin gene families, are expressed outside of the lens where they may have nonrefractive functions.1–11 Indeed, both αA- and αB-crystallins are expressed in nonlens tissues1,2,4,5 and are functional chaperones that protect other proteins against thermal insult.12,13 α-Crystallins are also phosphorylated by cAMP-dependent14,15 and cAMP-independent16–18 pathways, suggesting that they may participate in signal transduction pathways.18
Like α-crystallin, βB2-crystallin has also been detected outside of the lens.3,7,9,10 βB2-crystallin protein has been reported in chicken retina,3 and βB2-crystallin mRNA has been reported in rat brain, testis, lung, and other tissues.9,10 βB2-crystallin is also involved in cAMP-dependent19 and cAMP-independent phosphorylation pathways.9 Extralenticular expression of βB2-crystallin, coupled with its role in phosphorylation pathways, suggests that it may have an important nonrefractive function. To date, βB2-crystallin protein has not been reported outside of the eye, and no information about its human nonlens expression is available. In the present report, we investigated the mRNA and protein expression patterns of βB2-crystallin in rat, bovine, and human tissues. We show that both βB2-crystallin mRNA and protein are present in nonlens and nonocular tissues.
The results support the possibility that βB2-crystallin has one or more nonrefractive functions in nonocular tissues.
Methods
Reverse Transcriptase PCR of βB2-Crystallin
Three different procedures were used to amplify βB2-crystallin mRNA. Rat Procedure: Total RNA from 3-day-old rat brain, testis, and lens was prepared using RNAzol (Tel-Test, Friendsworth, TX) as directed by the manufacturer. Ten or 2.0 μg of rat brain or testis and 2.0 μg of rat lens RNA were reverse-transcribed using mouse Moloney tumor virus reverse transcriptase (100 U/rxn; Gibco-BRL, Bethesda, MD) according to the manufacturer. Identical samples were prepared in the absence of reverse transcriptase as controls. After first-strand synthesis, PCR was performed for 30 cycles using Taq polymerase (Perkin-Elmer Cetus, Foster City, CA) as recommended by the manufacturer. The oligonucleotide sequences used in this procedure are shown in Table 1 as primer sets 3 and 4, annealing to exons 3 and 6, and 4 and 6, respectively. The resulting RT-PCR products were separated by electrophoresis on 1.0% gels and visualized by ethidium staining. In addition to the above, total RNA was also analyzed by isolation from 60- to 90-day-old rat tissues using RNAzol. Total RNA from rat testis was also obtained from Ambion, Inc. (Austin, TX). Reverse transcription and cDNA amplification (35 and 40 cycles) were then conducted with the One-Step RT-PCR System according to the manufacturer (Gibco-BRL), and identical reactions were performed with heat-inactivated reverse transcriptase as control. The oligonucleotides used in this procedure are shown in Table 1 as primer sets 1 and 2 and anneal to βB2-crystallin exons 2 and 6. As control, β-actin was coamplified in identical reactions. The sequence of the β-actin primers were 5′OH-TCATGAAGTGTGACGTTGACATCCGT-3′ + 5′OH-CCTAGAAGCATTTGCGGTGCACGATG-3′. Products were separated by electrophoresis on 0.8% gels and visualized by ethidium staining. To confirm the identity of the RT-PCR fragments, the lens and brain products were cloned and sequenced to ensure they represented authentic βB2-crystallin. Human Procedure: βB2-crystallin was reverse-transcribed and PCR-amplified for 40 cycles from human lens RNA (prepared as indicated above) or PCR-amplified for 40 cycles using purified total cDNA prepared from retina, brain, and testis. The retina, brain, and testis total cDNAs were a gift from Ignacio Rodriquez of the National Eye Institute. Total cDNA was amplified with Amplitaq polymerase according to the manufacturer (BRL). The oligonucleotide sequences used for this procedure are shown in Table 1 as primer pair 5. These primers anneal to exons 2 and 6 of the βB2-crystallin mRNA. Control β-actin transcripts were coamplified in identical reactions.
Table 1.
Oligonucleotide Sets Used for RT-PCR Amplification
| Species | Oligonucleotide Sequence | Position | Temperature (°C) | No. Cycles |
|---|---|---|---|---|
| Rat | (1) F: 5′GTCACCTCGACACCAGAGAG3′ | 1–20 | 52 | 35 |
| R: 5′CACTCTGGGTGTCAGGAGGA3′ | 710–691 | |||
| (2) F: 5′CCACCATGGCCTCAGACCACCAGACAC3′ | 22–48 | 68 | 40 | |
| R: 5′GTCCTTGTAATCCCCCTTCTCCAGCAG3′ | 545–518 | |||
| (3) F: 5′CTCCGTCCTGGTGCAGGCTGG3′ | 176–196 | 54 | 30 | |
| R: 5′GTAATCCCCCTTCTCCAGCAG3′ | 539–519 | |||
| (4) F: 5′CCCATCAAAGTGGACAGCCAG3′ | 321–341 | 54 | 30 | |
| R: 5′GTAATCCCCCTTCTCCAGCAG3′ | 539–519 | |||
| Human | (5) F: 5′CCACCATGGCCTCAGATCACCAGAC3′ | 8–32 | 68 | 40 |
| R: 5′GTCCTTGTAGTCTCCCTTCTCCAGCAG3′ | 531–505 |
F, forward; R, reverse.
Purification of βB2-Crystallin Protein from Nonlens Tissues
Proteins were prepared and separated by FPLC chromatography on a Pharmacia HR-6 column (Uppsala, Sweden) as previously described.20 Fractions coeluting with purified bovine βB2-crystallin were collected, and protein concentrations were determined by standard methods.
Immunoblotting of Purified βB2-Crystallin Fractions
One to 2 μg of each FPLC fraction (above) was denatured by boiling in 10% SDS buffer [10% (w/v) SDS, 0.5 M Tris-HCl (pH 6.8), 5% (v/v) 2-mercaptoethanol, and 5% (v/v) glycerol], loaded onto 12.5% polyacrylamide SDS gels, and electrophoresed. After electrophoresis, the proteins were transferred (30 V for 1 hour in 12 mM Tris-HCl, 96 mM glycine, 15% methanol) to nitrocellulose filters. The resulting blot was fixed in 25% isopropanol/10% acetic acid for 1 hour, washed with PBS for 30 minutes, and blocked with 3% bovine serum albumin in PBS for 1 hour. The blot was then washed three times in TBS (10 mM Tris-HCl, 50 mM NaCl, pH 7.5) over 15 minutes and incubated for 1 hour at room temperature with 1:1000 βB2-crystallin antibody in 1% bovine serum albumin in TBS. The blot was subsequently washed five times in TBS over a period of 1 hour. Immunoreactive βB2-crystallin was visualized by using the ABC kit (Pierce Biochemicals, Rockford, IL) as described by the manufacturer.
Microsequencing
A bovine retinal protein fraction coeluting with purified βB2-crystallin was separated on a 12.5% SDS-PAGE gel, electrophoretically transferred to nitrocellulose, stained with ponceau S, and excised. The resulting sample was digested with trypsin; peptides were separated by HPLC, and a single peptide was microsequenced.
William Lane at the Harvard Microchemistry Facility conducted the HPLC and microsequencing procedures. The resulting protein sequence was aligned with the reported rat, bovine, and human βB2-crystallin sequences using Lasergene (ver. 5.1) software (DNAStar, Madison, WI) and further analyzed using the BLAST algorithm, National Library of Medicine (NIH, Bethesda, MD).
Results
Detection of βB2-Crystallin Transcript in Rat Testis and Brain by RT-PCR
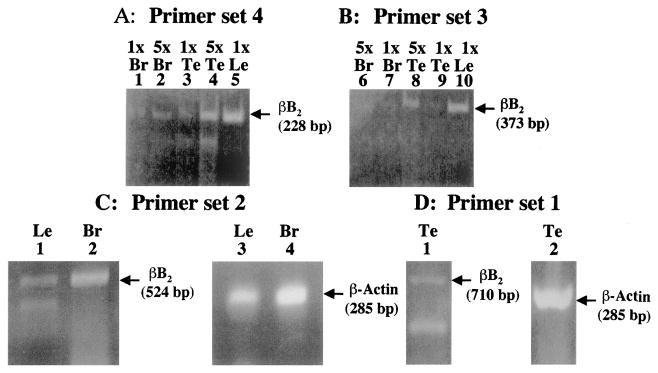
Figure 1A shows the 228-bp and Figure 1B the 373-bp βB2-crystallin products generated with primer pairs 4 and 3 (Table 1), respectively. Two and 10 μg total RNA (1× and 5×) were used in the RT-PCR procedure. With primer set 4 (Fig. 1A), βB2-crystallin mRNA was detected in brain (lanes 1 and 2), testis (lanes 3 and 4), and as control lens (lane 5). With primer set 3 (Fig. 1B), very low amounts of βB2-crystallin mRNA were detected in brain (lanes 6 and 7), whereas high levels of βB2-crystallin mRNA were detected in testis (lanes 8 and 9) and lens (lane 10). To further confirm βB2-crystallin expression in these tissues, fresh total RNA samples were prepared from 60-to 90-day-old rat lens, brain, lung, heart, testis, ovary, spleen, thymus, kidney, and liver and analyzed by RT-PCR with additional primer sets (Table 1, oligo sets 1 and 2). Significant amounts of βB2-crystallin were detected in brain, lens, and testis total RNA (Fig. 1C, lanes 1 and 2; Fig. 1D, lane 1). The lens band appeared weaker than that found for brain. This is likely the result of minor degradation of this sample as evidenced by the presence of a lower band in the lens lane (Fig. 1C, lane 1) and reduced levels of the corresponding β-actin band (Fig. 1C, lane 2). All products were reverse transcriptase-specific, and representative control products from brain and lens were sequenced and confirmed to be authentic βB2-crystallin (data not shown). β-Actin transcript was detected at high levels in corresponding reactions (Fig. 1C, lanes 3 and 4; Fig. 1D, lane 2). βB2-crystallin mRNA was not detected by RT-PCR in 1.0 μg (Table 1, oligo set 1) or 1.4 μg (Table 1, oligo set 2) total RNA from lung, heart, ovary, spleen, thymus, kidney, or liver (data not shown).
Figure 1.
RT-PCR of βB2-crystallin mRNA in rat brain (Br), testis (Te), and lens (Le). (A) Ethidium bromide–stained gel showing the levels of βB2-crystallin mRNA (228 bp) in 10 μg (5×) or 2.0 μg (1×) of rat brain (lanes 1 and 2), testis (lanes 3 and 4), and lens (lane 5). Total RNA was reverse transcribed and amplified for 30 PCR cycles using primer set 4 from Table 1. (B) Ethidium bromide–stained gel showing the levels of βB2-crystallin mRNA (373 bp) in 10 μg (5×) or 2.0 μg (1×) of rat brain (lanes 6 and 7), testis (lanes 8 and 9), and lens (lane 10). Total RNA was reverse transcribed and amplified for 30 PCR cycles using primer set 3 from Table 1. (C) Ethidium bromide–stained gel showing the levels of βB2-crystallin mRNA (524 bp) detected by reverse transcription followed by 40 RT-PCR cycles using 1.4 μg of rat lens (lane 1) and brain (lane 2) total RNA with primer set 2 from Table 1. Indicated as control are the corresponding levels of β-actin transcript (285 bp) produced with 0.14 μg of the same lens (lane 3) and brain (lane 4) RNAs amplified at 40 cycles after reverse transcription. (D) Ethidium bromide–stained gel showing the levels of βB2-crystallin mRNA (710 bp) in 1 μg of rat testis (lane 1) at 35 PCR cycles using primer set 1 from Table 1. Also shown as control is the corresponding level of β-actin transcript (285 bp) in 1 μg of the same sample (lane 2) amplified at 35 cycles after reverse transcription.
Detection of βB2-Crystallin Transcript in Human Retina
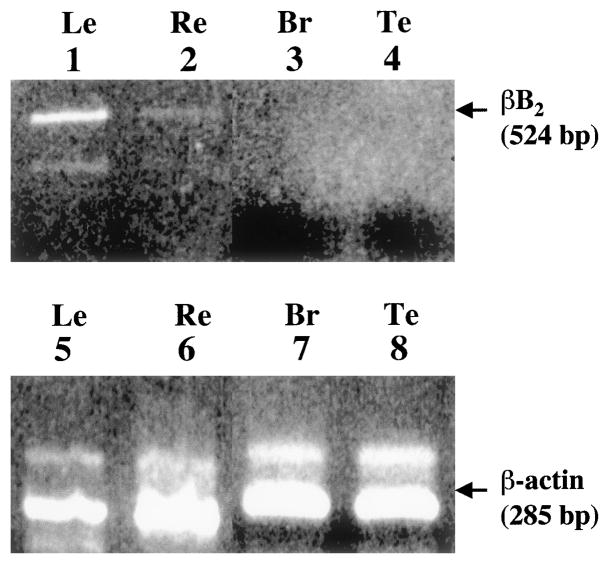
To determine whether parallel patterns of βB2-crystallin expression extended to human tissues, βB2-crystallin mRNA levels were examined in human retina, brain, testis, and lens. Total RNA (1 μg) was used for monitoring βB2-crystallin mRNA in lens, and purified total cDNA was used for monitoring βB2-crystallin transcript in retina (6 ng), brain (3 ng), and testis (3 ng). Because about 3% of total RNA is mRNA and reverse-transcriptase is not 100% efficient, this amount of total cDNA is at least equivalent to the 1.0 to 1.4 μg total RNA used in the rat studies. The primers used for this analysis are shown in Table 1 (oligo set 5). βB2-crystallin was only detected in the retina (Fig. 2, lane 2). Shown as control are βB2-crystallin mRNA in lens (Fig. 2, lane 1) and positive expression of β-actin mRNA in all tissues (Fig. 2, lanes 5–8).
Figure 2.
RT-PCR of βB2-crystallin mRNA in human tissues. Ethidium bromide–stained gel showing the relative levels of βB2-crystallin mRNA (524 bp) after reverse transcription and amplification for 40 PCR cycles in 1 μg human lens RNA (lane 1), 6 ng purified human retina cDNA (lane 2), 3 ng purified human brain cDNA (lane 3), and 3 ng purified human testis cDNA (lane 4). Also indicated are the levels of β-actin transcripts (285 bp) in the same samples amplified under identical conditions (lanes 5 through 8). The primers for human βB2-crystallin are shown in Table 1 as oligo set 5.
FPLC Elution Profile and Immunoblotting of Rat and Bovine Protein Extracts
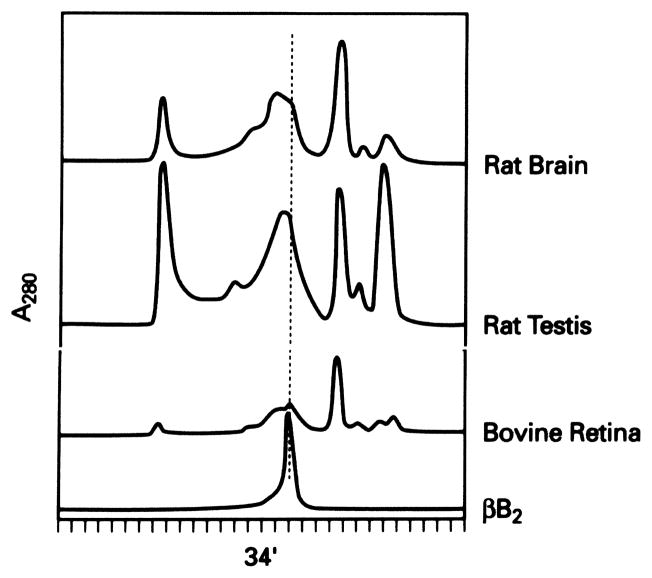
To confirm that βB2-crystallin protein was present in tissues where βB2-crystallin mRNA was detected, protein extracts were prepared from the indicated tissues and partially purified by FPLC chromatography. Fractions coeluting as a dimer with purified bovine lens βB2-crystallin (34 minutes, Fig. 3) were collected and 1 to 2 μg of each fraction analyzed by Western blot analysis with βB2-crystallin–specific antibody. Figure 3 shows the elution profile, and Figure 4 shows the corresponding Western blot. A single 27-kDa immunoreactive band comigrating with purified βB2-crystallin was detected in rat testis, rat brain, and bovine retina (Fig. 4, lanes 3–5, respectively). Shown as control are purified bovine lens βB2-crystallin (Fig. 4, lane 1) and molecular weight standards (Fig. 4, lane 2).
Figure 3.
FPLC elution profile of brain, testis, and retinal tissue extracts. Individual tissues and species are indicated. Fractions coeluting with dimeric purified bovine βB2-crystallin (indicated at 34 minutes) were collected and further analyzed.
Figure 4.
Immunoblotting of purified βB2-crystallin. Lane 1, purified bovine lens control (2 μg); lane 3, testis (2 μg); lane 4, brain (2 μg); and lane 5, retina (2 μg). Indicated are the positions of the 27-kDa reactive bands and the prestained molecular weight standards (lane 2).
The minor bands migrating above or below the purified βB2-crystallin control are most likely due to slight degradation and cross-linking of the overloaded sample.
Microsequencing of βB2-Crystallin from Bovine Retina
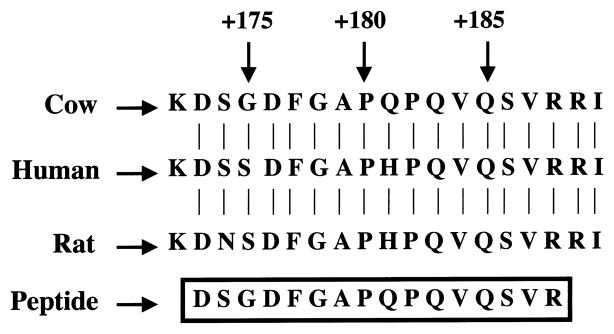
To further confirm the identity of βB2-crystallin protein in bovine retina, bovine retinal protein extract was prepared. FPLC was purified as described above and separated by SDS-PAGE, and the major band comigrating with purified lens βB2-crystallin was excised from the gel. The resulting gel slice was digested with trypsin, and the resulting peptides were separated by HPLC. One peptide was chosen for microsequencing. Its sequence was identical with that reported for the corresponding region (amino acids 172–190) of bovine lens βB2-crystallin. The sequence of this peptide, aligned with bovine,21 human,22 and rat23 βB2-crystallin is shown in Figure 5.
Figure 5.
The amino acid sequence of the HPLC-purified peptide obtained from trypsin digestion of βB2-crystallin purified from bovine retina (boxed). Indicated are the corresponding sequences of cow, human, and rat βB2-crystallin. Numbers located above the sequences indicate the relative amino acid positions.
Discussion
The present report shows that βB2-crystallin protein and mRNA are expressed in the retina, brain, and testis of multiple species. βB2-crystallin mRNA was detected in human retina, rat brain, and rat testis by RT-PCR. βB2-crystallin protein was purified from and its identity confirmed by immunoblotting in bovine retina, rat brain, and rat testis protein extracts. The identity of bovine retinal βB2-crystallin protein was further confirmed by microsequencing.
Differences in βB2-crystallin expression were found between rat and human tissues. In rat, βB2-crystallin transcript was detected in brain and testis, whereas in human, βB2-crystallin transcript was detected only in the retina. We believe that these differences are species-specific; however, we cannot rule out the possibility that developmental or spatial expression differences could also be involved. The rat brain RNA preparation was made from cerebellum and brain stem, whereas the human brain RNA preparation was made from whole brain. Because specific subregions of human brain or testis are not available, further localization of βB2-crystallin in these tissues will have to await the availability of these tissues.
The results of the present study are consistent with previous studies demonstrating expression of βB2-crystallin protein in the retina of several species including rat.3,10,16 However, the results do not coincide with a previous study that failed to detect βB2-crystallin mRNA in rat brain and failed to detect βB2-crystallin protein outside of the eye.10 We do not know the reason for this discrepancy; however, it is likely to be related to the fact that βB2-crystallin protein was not FPLC fractionated in this other study and that a blotting procedure instead of direct PCR monitoring was used to examine the βB2-crystallin transcript. The potential nonrefractive function of βB2-crystallin in the retina, brain, and testis is not known. One possibility is that it is related to signal transduction pathways because βB2-crystallin is known to be phosphorylated by a cAMP-dependent pathway.16 Evidence has also been reported for non-cAMP–dependent phosphorylation of βB2-crystallin.9 Whatever its function, the present data extend our knowledge of βB2-crystallin expression to the retina, brain, and testis, and they suggest a nonrefractive function for βB2-crystallin in these tissues.
Acknowledgments
Supported in part by National Eye Institute Grants EY3897 (JH) and EY13022 (MK) and a Research to Prevent Blindness Senior Investigator Award (JH).
The authors thank Ignacio Rodriguez of the National Eye Institute, Paula Ousley and Rory Dunaway of the Lions Eye Bank of Oregon, John Hawse and Ashley Halstead of the Kantorow laboratory, Quingling Huang and Lin-lin Ding of the Horwitz laboratory, Barbara Norman of the Piatigorsky laboratory, and William Lane of the Harvard Microchemistry Facility.
Footnotes
Commercial relationships policy: N.
References
- 1.Bhat SP, Nagineni CN. αB subunit of lens-specific protein α-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989;58:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- 2.Dubin RA, Wawrousek EF, Piatigorsky J. Expression of the muring αB-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989;9:1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Head MW, Peter A, Clayton RM. Evidence for the extralenticular expression of members of the β-crystallin gene family in the chick and a comparison with δ-crystallin during differentiation and trans-differentiation. Differentiation. 1991;48:147–156. doi: 10.1111/j.1432-0436.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 4.Kato K, Shinohara H, Kurobe N, Goto S, Inaguma Y, Oshima K. Immunoreactive αA-crystallin in rat non-lenticular tissues detected with a sensitive immunoassay method. Biochim Biophys Acta. 1991;1080:173–180. doi: 10.1016/0167-4838(91)90146-q. [DOI] [PubMed] [Google Scholar]
- 5.Sax CM, Piatigorsky J. Expression of the α-crystallin/small heat-shock protein/molecular chaperone genes in the lens and other tissues. Adv Enzymol Related Areas Mol Biol. 1994;69:155–201. doi: 10.1002/9780470123157.ch5. [DOI] [PubMed] [Google Scholar]
- 6.Smolich BD, Tarkington SK, Saha MS, Grainger RM. Xenopus γ-crystallin gene expression: evidence that the γ-crystallin gene family is transcribed in lens and nonlens tissues. Mol Cell Biol. 1994;14:1355–1363. doi: 10.1128/mcb.14.2.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Head MW, Sedowofia K, Clayton RM. βB2-Crystallin in the mammalian retina. Exp Eye Res. 1995;61:423–428. doi: 10.1016/s0014-4835(05)80137-x. [DOI] [PubMed] [Google Scholar]
- 8.Brunekreef GA, van Genesen ST, Destree OHJ, Lubsen NH. Extralenticular expression of Xenopus laevis α-, β-, and γ-crystallin genes. Invest Ophthalmol Vis Sci. 1997;38:2764–2771. [PubMed] [Google Scholar]
- 9.Kantorow M, Horwitz J, Sergeev Y, Hejtmancik JF, Piatigorsky J. Extralenticular expression, cAMP-dependent, and cAMP-independent phosphorylation of βB2-crystallin [ARVO Abstract] Invest Ophthalmol Vis Sci. 1997;38(4):S205. Abstract nr 998. [Google Scholar]
- 10.Dirks RP, Genesen ST, Kruse J, Jorissen L, Lubsen NH. Extralenticular expression of the rodent βB2-crystallin gene [Letter] Exp Eye Res. 1998;66:267–269. doi: 10.1006/exer.1997.0439. [DOI] [PubMed] [Google Scholar]
- 11.Jones SE, Jomary C, Grist J, Makwana J, Neal MJ. Retinal expression of γ-crystallins in the mouse. Invest Ophthalmol Vis Sci. 1999;40:3017–3020. [PubMed] [Google Scholar]
- 12.Horwitz J. α -Crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakob U, Gaestel M, Engel K, Buchner J. Small heat-shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 14.Spector A, Cheisa R, Sredy J, Garner W. cAMP-dependent phosphorylation of bovine lens α-crystallin. Proc Natl Acad Sci USA. 1985;82:4712–4716. doi: 10.1073/pnas.82.14.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voorter CEM, Mulders JWM, Bloemendal H, de Jong WW. Some aspects of the phosphorylation of α-crystallin A. Eur J Biochem. 1986;160:203–210. doi: 10.1111/j.1432-1033.1986.tb09958.x. [DOI] [PubMed] [Google Scholar]
- 16.Kantorow M, Piatigorsky J. α-Crystallin/small heat-shock protein has autokinase activity. Proc Natl Acad Sci USA. 1994;91:3112–3116. doi: 10.1073/pnas.91.8.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantorow M, Horwitz J, van Boekel MAM, de Jong WW, Piatigorsky J. Conversion from oligomers to tetramers enhances autophosphorylation by lens αA-crystallin: specificity between α A and α B-crystallin subunits. J Biol Chem. 1995;270:17215–17220. doi: 10.1074/jbc.270.29.17215. [DOI] [PubMed] [Google Scholar]
- 18.Kantorow M, Piatigorsky J. Phosphorylations of α -crystallin. Int J Biol Macromol. 1998;22:307–314. doi: 10.1016/s0141-8130(98)00028-2. [DOI] [PubMed] [Google Scholar]
- 19.Kleinman NJ, Chiesa R, Kolks MA, Spector A. Phosphorylation of β-crystallin B2 (beta Bp) in the bovine lens. J Biol Chem. 1988;263:14978–14983. [PubMed] [Google Scholar]
- 20.McFall-Ngai M, Horwitz J, Ding L, Lacey L. Age-dependent changes in the heat-stable crystallin, βBp, of the human lens. Curr Eye Res. 1986;5:387–393. doi: 10.3109/02713688609025178. [DOI] [PubMed] [Google Scholar]
- 21.Hogg D, Gorin MB, Heinzmann C, et al. Nucleotide sequence for the cDNA of the bovine beta B2 crystallin and assignment of the orthologous human locus to chromosome 22. Curr Eye Res. 1987;6:1335–1342. doi: 10.3109/02713688708997559. [DOI] [PubMed] [Google Scholar]
- 22.Miesbauer LR, Smith JB, Smith DL. Amino acid sequence of human lens beta B2-crystallin. Protein Sci. 1993;2:290–291. doi: 10.1002/pro.5560020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aarts HJ, Lubsen NH, Schoenmakers JG. Crystallin gene expression during rat lens development. Eur J Biochem. 1989;183:31–36. doi: 10.1111/j.1432-1033.1989.tb14892.x. [DOI] [PubMed] [Google Scholar]