SUMMARY
In studies employing functional magnetic resonance imaging (fMRI), reactivity of the amygdala to threat-related sensory cues (viz., facial displays of negative emotion) has been found to correlate positively with interindividual variability in testosterone levels of women and young men and to increase on acute administration of exogenous testosterone. Many of the biological actions of testosterone are mediated by intracellular androgen receptors (ARs), which exert transcriptional control of androgen-dependent genes and are expressed in various regions of the brain, including the amygdala. Transactivation potential of the AR decreases (yielding relative androgen insensitivity) with expansion a polyglutamine stretch in the N-terminal domain of the AR protein, as encoded by a trinucleotide (CAG) repeat polymorphism in exon 1 of the X-chromosome AR gene. Here we examined whether amygdala reactivity to threat-related facial expressions (fear, anger) differs as a function of AR CAG length variation and endogenous (salivary) testosterone in a mid-life sample of 41 healthy men (mean age = 45.6 yr, range: 34–54 yr; CAG repeats, range: 19–29). Testosterone correlated inversely with participant age (r = −0.39, p = 0.012) and positively with number of CAG repeats (r = 0.45, p = 0.003). In partial correlations adjusted for testosterone level, reactivity in the ventral amygdala was lowest among men with largest number of CAG repeats. This inverse association was seen in both the right (rp = −0.34, p<0.05) and left (rp = −0.32, p<0.05) hemisphere. Activation of dorsal amygdala, correlated positively with individual differences in salivary testosterone, also in right (r = 0.40, p<0.02) and left (r = 0.32, p<0.05) hemisphere, but was not affected by number of CAG repeats. Hence, androgenic influences on threat-related reactivity in the ventral amygdala may be moderated partially by CAG length variation in the AR gene. Because individual differences in salivary testosterone also predicted dorsal amygdala reactivity and did so independently of CAG repeats, it is suggested that androgenic influences within this anatomically distinct region may be mediated, in part, by non-genomic or AR-independent mechanisms.
Keywords: testosterone, androgen receptor, CAG repeat polymorphism, fMRI, amygdala, facial expressions of emotion
INTRODUCTION
In both human and animal studies, elevated levels of the gonadal steroid, testosterone, are associated with social dominance and indices of aggressive conduct, especially in situations of competitive challenge or contested status (Mazur and Booth, 1998; Archer et al., 2005; Archer, 2006). It has been argued that, in part, these relationships reflect affective processes that promote dominance behavior and, abetted by vigilant attention to signals of potential challenge, may incline individuals to social aggression (van Honk et al., 2001). Among healthy young adults, for instance, individuals with high basal levels of testosterone show heightened sensitivity, or attentional bias, to pictures portraying facial expressions of anger and are more likely than others to self-report tense or angry mood (van Honk et al., 1999; Wirth and Schultheiss, 2007). In the canonical brain circuitry of emotional processing, the amygdala plays a key role in detecting stimuli of biological significance, including threat-related cues, and is readily engaged by facial displays of negative affect (Davis and Whalen, 2001). Elevating testosterone by exogenous administration, moreover, enhances reactivity of the amygdala to presentations of angry (vs. happy) faces, as measured by blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) (Hermans et al., 2008). Also, the amygdala response to fearful or angry faces is reduced among middle-aged women in association with an age-related decline in androgen levels and, by acute administration of testosterone, may be increased to levels of amygdala activation seen in young women under the same task conditions (van Wingen et al., 2008). Among healthy young men, moreover, amygdala responses to stimuli depicting similarly fearful and angry faces covary significantly with individual differences in serum testosterone concentrations (Derntl et al., 2009).
Many of the biological actions of testosterone and other androgens are mediated by androgen receptors (ARs), which are expressed in diverse areas of the brain, including the amygdala (Rubinow and Schmidt, 1996). When activated by androgens, ARs translocate to the cell nucleus, where they exert transcriptional control of androgen-dependent genes by binding to androgen response elements within gene regulatory sequences. In this way, androgens (like other steroid hormones) promote or repress the expression of genes specifying an array of cellular proteins (Mangelsdorf et al., 1995). Transactivation of target genes by the AR, however, varies with relative expansion of a polyglutamine stretch in the N-terminal domain of the AR protein, as encoded by a trinucleotide (CAG) repeat polymorphism in exon 1 of the X-chromosome AR gene (Chamberlain et al., 1994; Zitzmann and Nieschlag, 2003). In particular, transactivation potential of the AR appears to decline in graded relation to an increasing number of CAG repeats, which are distributed over a normative range of 11–37 and, in Caucasian populations, commonly average 21–22 repeats (Edwards et al., 1992; Tut et al., 1997; Platz et al., 2000).
CAG length variation has been evaluated in association with numerous conditions affected by high or low androgenicity, including male infertility and male pattern baldness, prostate cancer, bone loss, adiposity and insulin resistance, vascular reactivity, and other cardiovascular disease risk factors (e.g., (Stanford et al., 1997; Tut et al., 1997; Zmuda et al., 1997; Choong and Wilson, 1998; Hsing et al., 2000; Ellis et al., 2001; Mifsud et al., 2001; Van Pottelbergh et al., 2001; Wallerand et al., 2001; Zitzmann et al., 2001a; Zitzmann et al., 2001b; Van Golde et al., 2002; Zitzmann et al., 2002; Zitzmann et al., 2003a; Zitzmann et al., 2003b; Walsh et al., 2005; Campbell et al., 2007; Lapauw et al., 2007)). Little is yet known with respect to behavioral phenotypes, although some evidence suggests a high number of CAG repeats may be associated with cognitive aging (Yaffe et al., 2003) and a low number of repeats with criminal violence, verbal aggressiveness and, in interaction with basal testosterone, depressive symptomatology (Jonsson et al., 2001; Seidman et al., 2001; Cheng et al., 2006; Rajender et al., 2008). To the extent that variation in testosterone, either as individual differences or experimentally manipulated, modulate amygdala responses to affective stimuli, it is possible that such responses also vary along a gradient AR sensitivity, such as that encoded by the CAG length polymorphism of the AR gene.
The purpose of the present study was to determine if amygdala reactivity to threat-related facial expressions can be predicted, in part, by interindividual variation in endogenous (salivary) testosterone and by alleles of the AR CAG repeat polymorphism in a nonpatient sample of middle-aged men. Due to structural and functional heterogeneity of amygdala nuclei implicated in the processing of threat-related cues (LeDoux, 1996; Kim et al., 2003; Whalen, 2007), we independently examined the ventral and dorsal amygdala, which encompass the amygdala's principal input and output regions, respectively. This approach is further suggested by regional differences in the expression of steroid hormones (e.g., the distribution of AR and estrogen receptors and level of aromatase activity) and in related neuromodulatory receptor systems across the amygdala's subnuclei (e.g., Simerly et al., 1990; Roselli et al., 2001; Perez et al., 2003; Perlman et al., 2004; Huber et al., 2005).
METHOD
Subjects
Salivary testosterone, genetic, and fMRI data were available on 41 European American men (mean age, 45.6 years ±6.7 SD; range: 34–54 years). Participants were recruited from a parent study, the Adult Health and Behavior (AHAB) project, which measured diverse behavioral and biological traits among mid-life community volunteers. All subjects provided informed consent in accord with guidelines of the University of Pittsburgh Institutional Review Board. Participants were in good general health and free of the following study exclusions: (1) diagnosed cancer, stroke, diabetes, chronic kidney or liver disease, or lifetime history of psychotic symptoms; (2) use of psychotropic, glucocorticoid, or cardiovascular (antihypertensive, antiarrythmic) medications; (3) conditions affecting cerebral blood flow and metabolism (e.g., hypertension); and (4) any current DSM IV Axis I disorder (as determined by the Structured Clinical Interview for DSM-IV (SCID), non-patient edition (First et al., 1996). Blood sampling for genetic analysis and collection of saliva to determine salivary testosterone level were conducted at AHAB testing sessions preceding subjects' enrollment in the neuroimaging protocol.
Genotyping
Genomic DNA was isolated from peripheral white blood cells using the PureGene kit (Gentra Systems, Minneaopolis, MN). The AR CAG(n) repeat was typed by polymerase chain reaction amplification of genomic DNA in the presence of a VIC labeled forward primer, ARF: 5′–TCCAGAATCTGTTCCAGAGCGTGC-3′. Fragments were resolved on an ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA) and analyzed using GenMapper software v3.7 (ABI). Fragment sizes were assigned by comparison to a sequence-verified fragment ladder by two independent readers. CAG repeat number varied between 18 and 29 repeats in this sample (M = 22, SD: 2.7, which is consistent with allele distributions reported in other studies of European American men (Edwards et al., 1992; Tut et al., 1997; Platz et al., 2000).
Salivary testosterone
Measurement of salivary testosterone provides an index of the free (i.e., unbound, or biologically available) portion of testosterone in circulation (Arregger et al., 2007). Due to diurnal variation in testosterone levels, saliva was collected in the laboratory between 10 00 and 11 00 h. Unstimulated saliva was deposited by straw (passive drool) into a collection vial (1.8 mL cryo-vial, Salimetrics, Inc., State College, PA) and stored at −80° C until the time of assay. Salivary testosterone was determined using a colormetric enzyme immunoassay kit (Salimetrics, Inc., State College, PA), run according to manufacturer's directions. Standards and samples were bound to rabbit antibody to human testosterone, coated on microtiter plates. Plates were incubated with horseradish peroxidase and tetramethylbenzidine substrate, followed by treatment with 2M sulfuric acid. The intensity of the resultant color was measured using a microplate reader (Molecular Devises Corporation, Sunnyvale, CA) at 450 nm. To enhance reliability of measurement, testosterone assays were performed in duplicate, with the mean of the two determinations used in statistical analyses. The lower limit of detection of this assay is 14.6 ±3 pg/mL, and accuracy of determination was found to average ±5.8% of predicted values for samples of known concentration across varying levels of testosterone (40 – 430 pg/mL). The mean intra- and inter-assay CVs are 2.5% and 5.6%, respectively. Testosterone determinations by this assay also correlate highly (r = .96) with serum free testosterone concentrations (Salimetric Inc., State College, PA), which is in accord with prior literature validating the salivary measurement of this hormone (Vittek et al., 1985; Navarro et al., 1986; Arregger et al., 2007).
Amygdala reactivity protocol
The experimental fMRI paradigm consisted of four blocks of a face processing task interleaved with five blocks of a sensorimotor control task (Brown et al., 2005; Brown et al., 2006; Neumann et al., 2006; Manuck et al., 2007). Subject performance (accuracy and reaction time) was monitored during all scans. During the face processing task, subjects viewed a trio of faces (expressing either anger or fear) and selected one of two faces (bottom) identical to a target face (top). Within this context, we interpret the amygdale activation elicited by our task as being threat-related. Each face processing block consisted of six images, balanced for sex and target affect (angry or fearful), all derived from a standard set of pictures of facial affect (Ekman and Friesen, 1976). During the sensorimotor control blocks, subjects viewed a trio of simple geometric shapes (circles, vertical and horizontal ellipses) and selected one of two shapes (bottom) identical to a target shape (top). Each sensorimotor control block consisted of six different shape trios. All blocks were preceded by a brief instruction (“Match Faces” or “Match Shapes”) lasting 2 second. In the face processing blocks, each of the six face trios was presented for 4 seconds with a variable inter-stimulus interval of 2–6 seconds (mean = 4 seconds) for a total block length of 48 seconds. In the sensorimotor control blocks, each of the six shape trios was presented for 4 seconds with a fixed inter-stimulus interval of 2 seconds for a total block length of 36 seconds. Total task time was 390 seconds. As we were not interested in neural networks associated with face-specific processing per se, but instead in eliciting a maximal amygdala response that we could then interrogate for genetic influence, we chose not to use neutral faces as control stimuli. Additionally, neutral faces can be experienced as affectively laden or ambiguous and thus variably engage the amygdala (Schwartz et al., 2003; Wright et al., 2003).
BOLD fMRI acquisition parameters
Each subject was scanned with a Siemens (Berlin, Germany) 3T MAGNETOM Allegra scanner developed specifically for advanced brain imaging applications and characterized by increased T2* sensitivity and fast gradients (slew rate = 400 T/m/s), which minimized echo-spacing and thereby reduce EPI (echo planar imaging) gometric distortions and improve image quality. BOLD functional images were acquired with a gradient echo EPI sequence (TR/TE = 2000/25 msec, FOOV = 20 cm, matrix = 64 × 64), which covered 34 interleaved axial slices (3 mm slice thickness) aligned with the AC-PC plane and encompassing the entire cerebrum and the majority of the cerebellum. All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before the collection of fMRI data for each participant, a reference EPI scan was acquired and visually inspected for artifacts (e.g., ghosting), as well as for good signal across the entire volume of acquisition, including the amygdala and ventral striatum. Additionally, an autoshimming procedure was conducted before the acquisition of BOLD data in each subject to minimize field inhomogeneities. The fMR data of all 41 participants included in the present analyses were free of the foregoing problems.
Image processing and analysis
Whole brain image analysis was completed using SPM2 (http://www.fil.ion.ucl.ac.uk/spm). For each scan, images for each participant were realigned to the first volume in the time series to correct for head motion. Data sets were then selected for their high quality (scan stability), as demonstrated by small (<2 mm translational and <2° rotational) motion correction. Based on these criteria, data from all participants were included in subsequent analyses. Realigned images were spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model. These normalized images were then smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter, set at 6 mm full-width at half-maximum. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean.
Following preprocessing, linear contrasts employing canonical hemodynamic response functions were used to estimate condition-specific (i.e., Faces > Shapes) BOLD activation for each individual and scan. These individual contrast images (i.e., weighted sum of the beta images) were then used in second-level random effects models that account for both scan-to-scan and participant-to-participant variability to determine mean condition-specific regional responses using one-sample t-tests. All analyses were thresholded at a voxel level of p < 0.05, FDR corrected for multiple comparisons across the entire brain volume, and an extent threshold of at least 10 contiguous voxels.
Regions of Interest
BOLD contrast estimates were extracted from functional clusters exhibiting a main effect of task using the above threshold within anatomically defined regions of interest (ROIs). We constructed separate ROIs containing the amygdala's basolateral complex (ventral amygdala) and central nuclei (dorsal amygdala) using Marsbar (v 0.41). The ventral amygdala ROI was anchored by MNI coordinates x = +/−21, y = −3, z = −23, with widths of 14mm, 6mm, and 6mm along the x-, y-, and z-axes, respectively. The total volume of the ventral amygdala was 1024 mm3 in each hemisphere. The dorsal amygdala ROI was anchored by the MNI coordinates x = +/−21, y = −4, z = −13, with widths of 14mm, 8mm and 10mm along the x-, y-, and z-axes, respectively. The total volume of the dorsal amygdala was 1920 mm3 in each hemisphere. The reported widths reflect the total for the ROI along each axis and are centered on the MNI coordinate anchoring each axis (i.e., with x = 21 and width = 14mm, the range of coordinates included along that axis of the ROI are from x = 14 to x = 28). We defined a larger volume for the dorsal amygdala to encompass the extended projection of the central nucleus to the substantia inominata and nucleus basalis of Meynert, cholinergic projection fields providing glutamateric regulation of the prefrontal cortex (P.J. Whalen, personal communication). The posterior extent of both the dorsal and ventral amygdala was carefully defined to exclude the hippocampus. We also constructed a whole-amygdala ROI using the Talairach Daemon option of the WFU PickAtlas Tool, version 1.04 (Wake Forest University School of Medicine, Winston-Salem, North Carolina) with an additional 1x dilation to encompass the dorsal extended amygdala. To examine the specificity of any observed relationships between salivary testosterone, the AR CAG repeat polymorphism and amygdala activation within these anatomically defined regions of interest, we also extracted BOLD contrast estimates from task-related (i.e., functional) activation clusters in the right posterior fusiform gyrus (BA37; x = 42, y = −46; z = −20), orbitofrontal cortex (BA11; x = 2, y = 58, z = −14), and ventrolateral prefrontal cortex (BA47; right x = 32, y = 34, z = −18, left x = −24, y = 10, z = −20), using a sphere of 5 mm radius centered on the MNI coordinates of the maximally activated voxel within each activation cluster.
Statistical analysis
Primary dependent variables were the standardized BOLD contrast estimates extracted bilaterally from clusters of maximal activation in the ventral and dorsal amygdala. Associations of salivary testosterone and CAG(n) with indices of amygdala reactivity were examined by Pearson correlation. Because testosterone was found to correlate inversely with CAG repeat number in preliminary analyses, partial correlations were also computed to adjust for covariation among predictor variables. Finally, parallel analyses were conducted on an exploratory basis to examine whether task-related activation of posterior fusiform gyrus (BA37), orbitofrontal cortex (BA11), or ventrolateral prefrontal cortex (BA47) were predicted by testosterone or CAG repeat variation. Statistical analyses were performed in SPSS Version 15.0, and tests of significance were conducted at conventional alpha (p<0.05, 2-tailed).
RESULTS
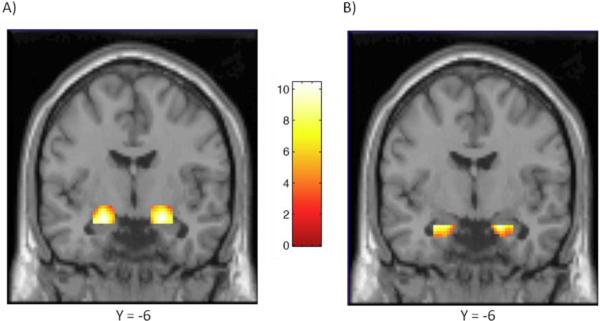
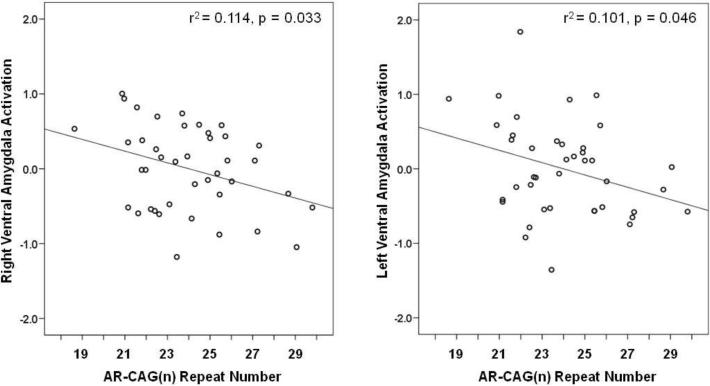
As shown in Figure 1, task-related activation was significant bilaterally within the anatomically defined ventral and dorsal amygdala ROIs. Bivariate correlations, listed in Table 1, show that participant age correlated inversely with level of salivary testosterone in this sample (r = −0.39, p = 0.012) and that testosterone correlated positively with number of CAG repeats (r = 0.45, p = 0.003). Neither testosterone nor CAG repeat length alone predicted relative activation in the ventral amygdala, but controlling for correlated variation in testosterone (by partial correlation) revealed a significant inverse association of CAG(n) with ventral amygdala reactivity in both the right and left hemisphere (rp's = −0.34, p = 0.033 and −0.32, p = 0.045, respectively) (Figure 2). Alternatively, controlling for CAG repeats in partial correlation showed testosterone to covary positively with right ventral amygdala reactivity at a trend level of significance (rp = 0.29, p = 0.070).
Figure 1.
Coronal overlays on canonical structural images illustrating mean bilateral threat-related amygdala reactivity. A) Bilateral dorsal amygdala reactivity: right hemisphere maximal voxel MNI coordinates x = 22, y =−6, z = −14; 233 voxels; z = 7.29, p < 0.001; left hemisphere maximal voxel MNI coordinates x = −22, y =−6, z = −16; 212 voxels; z = 7.40, p < 0.001. B) Bilateral ventral amygdala reactivity: right hemisphere maximal voxel MNI coordinates x = 20, y = −6, z = −20; 106 voxels; z = 6.53, p < 0.001; left hemisphere maximal voxel MNI coordinates x = −26, y = −2, z = −20; 82 voxels; z = 6.87, p < 0.001. Color bar represents approximate z-values.
Table 1.
Correlations and partial correlations between predictor variables (age, salivary testosterone, CAG repeat number) and bilateral ventral and dorsal amygdala reactivity.
| Ventral Amygdala |
Dorsal Amygdala |
||||||
|---|---|---|---|---|---|---|---|
| df | T | CAGn | Right | Left | Right | Left | |
| Age | 39 | −0.39** | −0.17 | −0.11 | −0.12 | −0.27 | −0.23 |
| Testosterone (T) | 39 | — | 0.45*** | 0.14 | 0.06 | 0.40** | 0.32* |
| partial r: adjusted for CAGn | 38 | — | — | 0.29+ | 0.20 | 0.38** | 0.29+ |
| CAGn | 39 | — | — | −0.23 | −0.26 | 0.12 | 0.13 |
| partial r: adjusted for T | 38 | — | — | −0.34* | −0.32* | −0.07 | −0.01 |
p=0.07
p<0.05
p<0.02
p<0.01
Figure 2.
Right and left ventral amygdala reactivity (z-scores) as a function of number of CAG repeats, when adjusted in partial correlation for concomitant variation in salivary testosterone concentration.
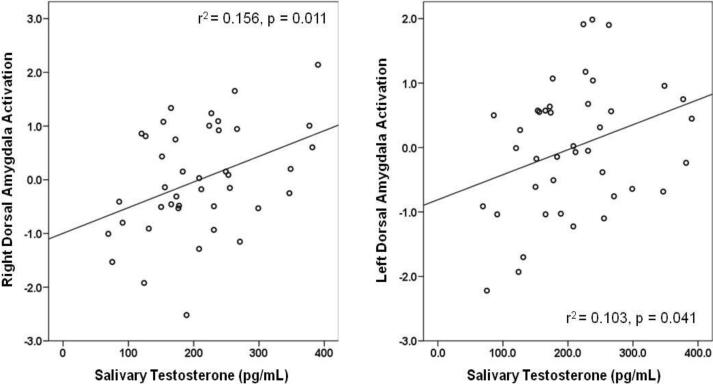
In contrast, testosterone level correlated bilaterally with activation extent in the dorsal amygdala (r = 0.40, p = 0.011 and r = 0.32, p = 0.041) (Table 1; Figure 3). These associations were minimally affected by adjustment for number of CAG repeats, and CAG(n) was itself unrelated to magnitude of response in the dorsal amygdala. The correlation of salivary testosterone with dorsal amygdala reactivity here partly reflects the sample's age-related decline in testosterone, as partial correlation controlling for age slightly attenuates these relationships (right: rp = 0.33, p = 0.040; left: rp = 0.26, p = 0.106). Finally, contrast estimates extracted from whole-amygdala ROIs also showed reactivity in both the right and left hemisphere predicted by testosterone concentration (x = 20, y = −3, z = −12; 482 voxels; z = 7.08, p < 0.0005; r = 0.40, p = 0.010, and x = −20, y = −6, z = −13; 432 voxels; z = 6.88, p < 0.0005; r = 0.34, p = 0.034), but not by CAG repeat length variation (r's = 0.06, n.s.). That testosterone was similarly associated with activation extent in dorsal and whole-amygdala ROIs likely reflects a greater extent of overlapping voxels between these regions, compared to that between the ventral and whole-amygdala ROI. This is due to the fact that the dorsal amygdala ROI is larger than the ventral to accommodate anatomically and functionally linked regions such as the sub-lenticular extended amygdala and nucleus basalis of Meynert.
Figure 3.
Right and left dorsal amygdala reactivity (z-scores) as a function of salivary testosterone concentration.
Exploratory analyses were conducted on BOLD contrast estimates extracted for functional clusters showing a main effect of task in the right posterior fusiform gyrus (BA37; 47 voxels, z = 7.81, p < 0.001), orbitofrontal cortex (BA11; 39 voxels, z = 4.69, p < 0.001), and ventrolateral prefrontal cortex (BA47; right hemisphere: 48 voxels, z = 5.83, p < 0.001; left hemisphere: 45 voxels, t = 6.21, p < 0.001). Testosterone was found to correlate significantly with task-elicited activation in orbitofrontal cortex (BA11) (r = 0.45, p= 0.003), and this association remained significant on adjustment for both CAG(n) (rp = 0.40, p = 0.010) and age (rp = 0.36, p = 0.02). There was no relationship of activation in orbitofrontal cortex with number of CAG repeats, and neither CAG(n) nor testosterone predicted activation in ventrolateral prefrontal cortex (BA47) or posterior fusiform gyrus (BA37).
It was noted earlier that saliva was collected for determination of testosterone level before enrollment in the imaging protocol, as a component of subjects' participation in the parent (AHAB) study from which the present sample was recruited. The interval between saliva collection and fMRI assessment averaged 5.4 ±2.1 months. There was no correlation between testosterone concentration and the interval between study sessions (r = 0.02), and adjusting for this interval in correlations between testosterone level and amygdala and orbitofrontal activation yielded partial coefficients identical to the bivariate correlations reported above and in Table 1.
DISCUSSION
Analysis of the present data revealed several significant associations. First, salivary testosterone correlated negatively with participant age and positively with CAG length variation in the AR gene. Second, when adjusted for correlated variation in testosterone, CAG repeat number varied inversely with reactivity of the ventral amygdala to facial expressions of negative affect. And third, individual differences in salivary testosterone predicted activation of the dorsal amygdala, but independently of AR genotype.
The first of these associations, the negative correlation of testosterone with age, is well-documented in both cross-sectional and longitudinal studies and for both serum and salivary measures of testosterone (e.g., (Gray et al., 1991; Morley et al., 1997; Zmuda et al., 1997; Ferrini and Barrett-Connor, 1998; Leifke et al., 2000; Harman et al., 2001; Ellison et al., 2002; Uchida et al., 2006; Chu et al., 2008)). In men, testosterone declines linearly across the adult life course, with an average drop of about 1–2% per year of age. The cross-sectional rate of decline in salivary testosterone over the 20-year range of ages represented here was 1.8% per year, which is consistent with the steeper decline typically seen with measures of free testosterone, relative to total testosterone – an effect that may be attributable, in part, to an age-related rise in sex hormone binding globulin (SHBG) (Gray et al., 1991; Morley et al., 1997; Ferrini and Barrett-Connor, 1998; Leifke et al., 2000; Harman et al., 2001; Chu et al., 2008).
That higher salivary testosterone was likewise associated with a greater number of AR CAG repeats corroborates findings reported by other investigators (Mifsud et al., 2001; Walsh et al., 2005; Crabbe et al., 2007). AR-mediated transactivation of androgen-responsive genes may be diminished by altered affinity of co-regulatory proteins to ARs that encode a longer polyglutamine stretch, yielding relative androgen insensitivity in ARs with a larger number of CAG repeats (Irvine et al., 2000; Zitzmann and Nieschlag, 2003). Because circulating testosterone is regulated via negative feedback through the hypothalamic-pituitary-gonadal axis, diminished androgen sensitivity at higher CAG repeat lengths may reduce feedback suppression of luteinizing hormone (LH). LH would then be maintained at higher levels, in turn promoting higher testosterone production (Crabbe et al., 2007). Consistent with this hypothesis, Crabbe et al (2007) report that LH covaried positively with free testosterone in two populations of eugonadal men, an association the authors note would not be predicted if differences in testosterone resulted instead largely from variation in testicular Leydig cell secretory capacity or from differential rates of testosterone catabolism. Nonetheless, basal levels of testosterone have not been found to correlate with AR CAG repeat length in all studies and reasons for these discrepancies remain unclear (Krithivas et al., 1999; Van Pottelbergh et al., 2001; Harkonen et al., 2003; Canale et al., 2005).
Whatever the mechanism or its generality, a higher testosterone level among men with a greater number of CAG repeats may obscure (suppress) effects of CAG length variation (and its putative inverse association with androgen sensitivity) on AR-dependent phenotypes. Here, for instance, we hypothesized a negative relationship between CAG repeat number and the magnitude of subjects' amygdala reactivity to threat-related facial expressions of emotion. Bivariate correlations did not support the predicted association, although coefficients were in the expected (inverse) direction with regard to task-induced reactivity in the ventral (but not dorsal) amygdala. When adjusted for correlated variation in testosterone, however, these associations were significant in both right and left hemisphere (Figure 2). And conversely, adjusting for number of CAG repeats revealed a marginally significant association of testosterone level with right ventral amygdala reactivity. These results suggest that while a functional polymorphism of the AR gene may modulate certain androgen-sensitive neural phenotypes, these associations in part may be mitigated by counter-regulatory effects of the same AR gene variation on testosterone production. Here, for instance, relative AR insensitivity at high CAG repeat numbers may be offset by heightened availability of the endogenous ligand.
Two aspects of our findings in the dorsal amygdala are noteworthy. First, higher levels of salivary testosterone were associated bilaterally with heightened amygdala reactivity. These observations emerged also on analysis of whole-amygdala ROIs, thus extending observations reported recently on young adult males (Derntl et al., 2009) to midlife men studied under a similar protocol. Second, the distribution of CAG repeats among study participants was unrelated to reactivity in the dorsal amygdala, either directly or when adjusted for variation in testosterone concentration. Regarding the latter observation, it is at least conceivable that testosterone affects dorsal amygdala activity, in part, through increasingly recognized nongenomic actions of androgens (Foradori et al., 2008). These include rapid stimulation of second messenger cascades, as mediated by the AR and SHBG receptor, and modulation of intracellular calcium levels through an androgen-binding membrane receptor (Heinlein and Chang, 2002). However, little is yet known about the relative roles of nongenomic and classical AR-mediated androgen effects or their potential interactions within discrete regions of the brain.
It is also possible that testosterone affects stimulus-dependent activation of the dorsal amygdala indirectly. For instance, neurons expressing argenine vasopressin (AVP) in this region -- which corresponds to the central nucleus, or principal “output” center, of the amygdala – activate emotion-related physiological responses peripherally via efferent projections to structures governing autonomic activity (e.g., hypothalamus) (Huber et al., 2005). Testosterone up-regulates AVP expression in the amygdala and, at least in rodents, appears to do so partly through activation of estrogen receptors on AVP neurons (Pak et al., 2007). This is made possible by the aromatized conversion of testosterone's metabolite, dihydrotestosterone (DHT), to estradiol (Plumari et al., 2002). Both estrogen receptors and aromatase are prevalent in the primate amygdala (Osterlund et al., 2000; Roselli and Resko, 2001), and deletion of the aromatase enzyme in a mouse “knock out” model reduces (but does not fully suppress) AVP mRNA expression in the medial amygdala (Plumari et al., 2002). Thus, activation of estrogen receptors by the aromatized metabolite of testosterone may augment amygdala reactivity through transcriptional control of AVP expression. Interestingly, though, the natural increase in circulating estrogen experienced in mid-menstrual cycle in premenopausal women has been associated with reduced (not increased) responses in dorsal amygdala to facial expressions of negative affect (Goldstein et al., 2005) and reward-related stimuli (Dreher et al., 2007). Although speculative, the rapid rise in circulating estrogen at mid-cycle might diminish amygdala reactivity in women through preferential engagement of the sexually dimorphic oxytocin system (Carter, 2007). Oxytocin exerts an inhibitory influence on AVP expression in the central amygdala, and the synthesis of oxytocin is mediated by estrogen and estrogen receptors (Huber et al., 2005). Moreover, acute administration of oxytocin has been shown to attenuate reactivity of the amygdala to facial expressions of fear and anger (Kirsch et al., 2005).
As noted previously, the amygdala plays a sentinel role in emotional processing, harnessing attentional resources and registering the behavioral salience of stimuli that, in the case of faces expressing negative emotion, might convey signals of deference, threat, challenge, or competitive opportunity. The basolateral amygdala (corresponding to the ventral amygdala ROI defined in the present study) effects convergent processing of multimodal sensory inputs received via thalamic and cortical relays, while the central nucleus of the amygdala (dorsal amygdala ROI here) initiates autonomic, neuroendocrine, and behavioral responses through efferent “downstream” (e.g., hypothalamic, brainstem) projections and also provides input to cortical regions implicated in higher order processing and emotional regulation (Davis and Whalen, 2001). Because people with high levels of endogenous testosterone exhibit an attentional bias toward facial expressions of anger (van Honk et al., 2001; Wirth and Schultheiss, 2007) and because administration of exogenous testosterone enhances amygdala activation and autonomic reactions to faces displaying negative affect (van Honk et al., 1999; Hermans et al., 2008; van Wingen et al., 2008), we hypothesized that individual differences in salivary testosterone or genetic modulation of androgen sensitivity would moderate similarly elicited amygdala reactivity in men. Our findings are largely consistent with this hypothesis, in as much as: a) higher testosterone predicted greater activation of the dorsal amygdala; and b) with testosterone held constant, CAG length variation was associated inversely with ventral amygdala activation. Finally, our findings appear to be largely, but not entirely, specific to amygdala reactivity. Thus, concomitant activation of orbitofrontal cortex (BA11), which is reciprocally interconnected with the amygdala and functions synchronously with the amygdala to evaluate the relative salience (amygdala) and valence (orbitofrontal cortex) of environmental stimuli (e.g. Small et al., 2003), correlated positively with testosterone level, as it did also in the recent study of young adult men reported by Derntl et al (2009). Yet neither testosterone nor CAG repeat number predicted activation in posterior fusiform gyrus (BA37) or ventrolateral PFC (BA47), the functions of which are more distal (and typically subsequent) to the response of the amygdala (Hariri et al., 2000; Vuilleumier and Pourtois, 2007).
One limitation of this study is its reliance on a single measure of salivary testosterone and assessment of subjects' amygdala reactivity to threat-related facial expressions on a separate and single occasion. With respect to testosterone, longitudinal studies show a robust stability of rank ordered relationships among individuals (Dabbs, 1990; Vermeulen and Verdonck, 1992; Granger et al., 2004). A single measurement among men, for instance, has been found to correlate highly (r = 0.845) with the average of seven measurements obtained over a year's interval (Vermeulen and Verdonck, 1992). Measurements of salivary testosterone recorded at two points in time also covary appreciably, at least in males, with re-test correlations as high at 0.91 across two days and 0.79 over a year (Granger et al., 2004). At present, it is unclear whether neural responses to behavioral stimuli (and associated interindividual variability) are comparably reproducible over time, although the few studies that have employed similar fMRI protocols and assessed amygdala reactivity on multiple occasions yield moderate re-test correlations (Johnstone et al., 2005; Manuck et al., 2007). Nonetheless, reliability of measurement is constrained in any investigation relying on single assessments, which necessarily also limits the magnitude of association detectable among variables of interest (Manuck et al., 1989). Equally though, when significant relationships are observed between variables measured only once -- such as those reported here among salivary testosterone and amygdala reactivity – findings may conceivably underestimate, but not inflate, the magnitude of true associations. Finally, because we limited our study to Caucasian men of European ancestry in order to mitigate confounding of genetic associations by population substructure, it remains unclear how well these findings may generalize to more diverse study samples, to populations that differ normatively in testosterone production (e.g., young adults, elderly men) or in distribution of AR CAG allele frequencies (Edwards et al., 1992; Platz et al., 2000; van Houten and Gooren, 2000; Zitzmann and Nieschlag, 2003), or to women.
Acknowledgements
None.
Role of Funding Source Funding for this work and preparation of the manuscript were supported by NIH grants HL040962 and HL065137 to SBM and MH072837 to ARH, as well as a NARSAD Young Investigator Award to ARH. Study sponsors had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure The authors have no conflicts of interest or competing financial interests to disclose.
REFERENCES
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci. Biobehav. R. 2006;30(3):319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Archer J, Graham-Kevan N, Davies M. Testosterone and aggression: A reanalysis of Book, Starzyk, and Quinsey's (2001) study. Aggress. Violent Beh. 2005:10241–261. [Google Scholar]
- Arregger AL, Contreras LN, Tumilasci OR, Aquilano DR, Cardoso EM. Salivary testosterone: a reliable approach to the diagnosis of male hypogonadism. Clin. Endocrinol. (Oxf) 2007;67(5):656–662. doi: 10.1111/j.1365-2265.2007.02937.x. [DOI] [PubMed] [Google Scholar]
- Brown SM, Manuck SB, Flory JD, Hariri AR. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6(2):239–245. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, Ferrell RE, Hariri AR. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol. Psychiatr. 2005;10(9):884–888. doi: 10.1038/sj.mp.4001716. [DOI] [PubMed] [Google Scholar]
- Campbell BC, Gray PB, Eisenberg DT, Ellison P, Sorenson MD. Androgen receptor CAG repeats and body composition among Ariaal men. Int. J. Androl. 2007 Nov. 26 doi: 10.1111/j.1365-2605.2007.00825.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Canale D, Caglieresi C, Moschini C, Liberati CD, Macchia E, Pinchera A, Martino E. Androgen receptor polymorphism (CAG repeats) and androgenicity. Clin. Endocrinol. (Oxf) 2005;63(3):356–361. doi: 10.1111/j.1365-2265.2005.02354.x. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav. Brain Res. 2007;176(1):170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22(15):3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Hong CJ, Liao DL, Tsai SJ. Association study of androgen receptor CAG repeat polymorphism and male violent criminal activity. Psychoneuroendocrino. 2006;31(4):548–552. doi: 10.1016/j.psyneuen.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Choong CS, Wilson EM. Trinucleotide repeats in the human androgen receptor: a molecular basis for disease. J. Mol. Endocrinol. 1998;21(3):235–257. doi: 10.1677/jme.0.0210235. [DOI] [PubMed] [Google Scholar]
- Chu LW, Tam S, Kung AW, Lo S, Fan S, Wong RL, Morley JE, Lam KS. Serum total and bioavailable testosterone levels, central obesity, and muscle strength changes with aging in healthy Chinese men. J. Am. Geriatr. Soc. 2008;56(7):1286–1291. doi: 10.1111/j.1532-5415.2008.01746.x. [DOI] [PubMed] [Google Scholar]
- Crabbe P, Bogaert V, De Bacquer D, Goemaere S, Zmierczak H, Kaufman JM. Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: contribution of the androgen receptor polyglutamine tract polymorphism. J. Clin. Endocr. Metab. 2007;92(9):3604–3610. doi: 10.1210/jc.2007-0117. [DOI] [PubMed] [Google Scholar]
- Dabbs JM., Jr. Salivary testosterone measurements: reliability across hours, days, and weeks. Physiol. Behav. 1990;48(1):83–86. doi: 10.1016/0031-9384(90)90265-6. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol. Psychiatr. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, Moser E, Habel U. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrino. 2009 doi: 10.1016/j.psyneuen.2008.11.007. In press. [DOI] [PubMed] [Google Scholar]
- Dreher J-C, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. P. Natl. Acad. Sci. USA. 2007;104(7):2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A, Hammond HA, Jin L, Caskey CT, Chakraborty R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics. 1992;12(2):241–253. doi: 10.1016/0888-7543(92)90371-x. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Ellis JA, Stebbing M, Harrap SB. Polymorphism of the androgen receptor gene is associated with male pattern baldness. J. Invest. Dermatol. 2001;116(3):452–455. doi: 10.1046/j.1523-1747.2001.01261.x. [DOI] [PubMed] [Google Scholar]
- Ellison PT, Bribiescas RG, Bentley GR, Campbell BC, Lipson SF, Panter-Brick C, Hill K. Population variation in age-related decline in male salivary testosterone. Hum. Reprod. 2002;17(12):3251–3253. doi: 10.1093/humrep/17.12.3251. [DOI] [PubMed] [Google Scholar]
- Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am. J. Epidemiol. 1998;147(8):750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition. New York Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front. Neuroendocrin. 2008;29(2):169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J. Neurosci. 2005;25(40):9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. The “trouble” with salivary testosterone. Psychoneuroendocrino. 2004;29(10):1229–1240. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J. Clin. Endocr. Metab. 1991;73(5):1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Harkonen K, Huhtaniemi I, Makinen J, Hubler D, Irjala K, Koskenvuo M, Oettel M, Raitakari O, Saad F, Pollanen P. The polymorphic androgen receptor gene CAG repeat, pituitary-testicular function and andropausal symptoms in ageing men. Int. J. Androl. 2003;26(3):187–194. doi: 10.1046/j.1365-2605.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J. Clin. Endocr. Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol. Endocrinol. 2002;16(10):2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Ramsey NF, van Honk J. Exogenous testosterone enhances responsiveness to social threat in the neural circuitry of social aggression in humans. Biol. Psychiat. 2008;63(3):263–270. doi: 10.1016/j.biopsych.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Hsing AW, Gao YT, Wu G, Wang X, Deng J, Chen YL, Sesterhenn IA, Mostofi FK, Benichou J, Chang C. Polymorphic CAG and GGN repeat lengths in the androgen receptor gene and prostate cancer risk: a population-based case-control study in China. Cancer Res. 2000;60(18):5111–5116. [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Irvine RA, Ma H, Yu MC, Ross RK, Stallcup MR, Coetzee GA. Inhibition of p160-mediated coactivation with increasing androgen receptor polyglutamine length. Hum. Mol. Genet. 2000;9(2):267–274. doi: 10.1093/hmg/9.2.267. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, Whalen PJ. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage. 2005;25(4):1112–1123. doi: 10.1016/j.neuroimage.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, von Gertten C, Gustavsson JP, Yuan QP, Lindblad-Toh K, Forslund K, Rylander G, Mattila-Evenden M, Asberg M, Schalling M. Androgen receptor trinucleotide repeat polymorphism and personality traits. Psychiatr. Genet. 2001;11(1):19–23. doi: 10.1097/00041444-200103000-00004. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14(18):2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krithivas K, Yurgalevitch SM, Mohr BA, Wilcox CJ, Batter SJ, Brown M, Longcope C, McKinlay JB, Kantoff PW. Evidence that the CAG repeat in the androgen receptor gene is associated with the age-related decline in serum androgen levels in men. J. Endocrinol. 1999;162(1):137–142. doi: 10.1677/joe.0.1620137. [DOI] [PubMed] [Google Scholar]
- Lapauw B, Goemaere S, Crabbe P, Kaufman JM, Ruige JB. Is the effect of testosterone on body composition modulated by the androgen receptor gene CAG repeat polymorphism in elderly men? Eur. J. Endocrinol. 2007;156(3):395–401. doi: 10.1530/EJE-06-0607. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Emotional networks and motor control: a fearful view. Prog. Brain. Res. 1996:107437–446. doi: 10.1016/s0079-6123(08)61880-4. [DOI] [PubMed] [Google Scholar]
- Leifke E, Gorenoi V, Wichers C, Von Zur Muhlen A, Von Buren E, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin. Endocrinol. (Oxf) 2000;53(6):689–695. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Brown SM, Forbes EE, Hariri AR. Temporal stability of individual differences in amygdala reactivity. Am. J. Psychiat. 2007;164(10):1613–1614. doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Kasprowicz AL, Monroe SM, Larkin KT, Kaplan JR. Psychophysiologic reactivity as a dimension of individual differences. In: Schneiderman N, Weiss SM, Kaufmann PG, editors. Handbook of Research Methods in Cardiovascular Behavioral Medicine. Plenum Publishing Corporation; New York: 1989. pp. 365–382. [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behav. Brain Sci. 1998;21(3):353–363. discussion 363–397. [PubMed] [Google Scholar]
- Mifsud A, Sim CK, Boettger-Tong H, Moreira S, Lamb DJ, Lipshultz LI, Yong EL. Trinucleotide (CAG) repeat polymorphisms in the androgen receptor gene: molecular markers of risk for male infertility. Fertil. Steril. 2001;75(2):275–281. doi: 10.1016/s0015-0282(00)01693-9. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- Navarro MA, Juan L, Bonnin MR, Villabona C. Salivary testosterone: relationship to total and free testosterone in serum. Clin. Chem. 1986;32(1 Pt 1):231–232. [PubMed] [Google Scholar]
- Neumann SA, Brown SM, Ferrell RE, Flory JD, Manuck SB, Hariri AR. Human choline transporter gene variation is associated with corticolimbic reactivity and autonomic-cholinergic function. Biol. Psychiat. 2006;60(10):1155–1162. doi: 10.1016/j.biopsych.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000;95(2):333–342. doi: 10.1016/s0306-4522(99)00443-1. [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Hinds LR, Handa RJ. Estrogen receptor-beta mediates dihydrotestosterone-induced stimulation of the arginine vasopressin promoter in neuronal cells. Endocrinology. 2007;148(7):3371–3382. doi: 10.1210/en.2007-0086. [DOI] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Dev. Brain Res. 2003;145(1):117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biol. Psychiat. 2004;56(11):844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Platz EA, Rimm EB, Willett WC, Kantoff PW, Giovannucci E. Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals. J. Natl. Cancer I. 2000;92(24):2009–2017. doi: 10.1093/jnci/92.24.2009. [DOI] [PubMed] [Google Scholar]
- Plumari L, Viglietti-Panzica C, Allieri F, Honda S, Harada N, Absil P, Balthazart J, Panzica GC. Changes in the arginine-vasopressin immunoreactive systems in male mice lacking a functional aromatase gene. J. Neuroendocrinol. 2002;14(12):971–978. doi: 10.1046/j.1365-2826.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- Rajender S, Pandu G, Sharma JD, Gandhi KP, Singh L, Thangaraj K. Reduced CAG repeats length in androgen receptor gene is associated with violent criminal behavior. Int. J. Legal Med. 2008;122(5):367–372. doi: 10.1007/s00414-008-0225-7. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Klosterman S, Resko JA. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J. Comp. Neurol. 2001;439(2):208–223. doi: 10.1002/cne.1343. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. Cytochrome P450 aromatase (CYP19) in the non-human primate brain: distribution, regulation, and functional significance. J. Steroid Biochem. 2001;79(1–5):247–253. doi: 10.1016/s0960-0760(01)00141-8. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. Androgens, brain, and behavior. Am. J. Psychiat. 1996;153(8):974–984. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, Rauch SL. Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biol. Psychiat. 2003;53(10):854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Seidman SN, Araujo AB, Roose SP, McKinlay JB. Testosterone level, androgen receptor polymorphism, and depressive symptoms in middle-aged men. Biol. Psychiat. 2001;50(5):371–376. doi: 10.1016/s0006-3223(01)01148-9. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J. Comp. Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39(4):701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Stanford JL, Just JJ, Gibbs M, Wicklund KG, Neal CL, Blumenstein BA, Ostrander EA. Polymorphic repeats in the androgen receptor gene: molecular markers of prostate cancer risk. Cancer Res. 1997;57(6):1194–1198. [PubMed] [Google Scholar]
- Tut TG, Ghadessy FJ, Trifiro MA, Pinsky L, Yong EL. Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility. J. Clin. Endocr. Metab. 1997;82(11):3777–3782. doi: 10.1210/jcem.82.11.4385. [DOI] [PubMed] [Google Scholar]
- Uchida A, Bribiescas RG, Ellison PT, Kanamori M, Ando J, Hirose N, Ono Y. Age related variation of salivary testosterone values in healthy Japanese males. Aging Male. 2006;9(4):207–213. doi: 10.1080/13685530601060461. [DOI] [PubMed] [Google Scholar]
- Van Golde R, Van Houwelingen K, Kiemeney L, Kremer J, Tuerlings J, Schalken J, Meuleman E. Is increased CAG repeat length in the androgen receptor gene a risk factor for male subfertility? J. Urology. 2002;167(2 Pt 1):621–623. doi: 10.1016/S0022-5347(01)69098-0. [DOI] [PubMed] [Google Scholar]
- van Honk J, Tuiten A, Hermans E, Putman P, Koppeschaar H, Thijssen J, Verbaten R, van Doornen L. A single administration of testosterone induces cardiac accelerative responses to angry faces in healthy young women. Behav. Neurosci. 2001;115(1):238–242. doi: 10.1037/0735-7044.115.1.238. [DOI] [PubMed] [Google Scholar]
- van Honk J, Tuiten A, Verbaten R, van den Hout M, Koppeschaar H, Thijssen J, de Haan E. Correlations among salivary testosterone, mood, and selective attention to threat in humans. Horm. Behav. 1999;36(1):17–24. doi: 10.1006/hbeh.1999.1521. [DOI] [PubMed] [Google Scholar]
- van Houten ME, Gooren LJ. Differences in reproductive endocrinology between Asian men and Caucasian men--a literature review. Asian J. Androl. 2000;2(1):13–20. [PubMed] [Google Scholar]
- Van Pottelbergh I, Lumbroso S, Goemaere S, Sultan C, Kaufman JM. Lack of influence of the androgen receptor gene CAG-repeat polymorphism on sex steroid status and bone metabolism in elderly men. Clin. Endocrinol. (Oxf) 2001;55(5):659–666. doi: 10.1046/j.1365-2265.2001.01403.x. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Zylicz SA, Pieters S, Mattern C, Verkes RJ, Buitelaar JK, Fernandez G. Testosterone increases amygdala reactivity in middle-aged women to a young adulthood level. Neuropsychopharmacol. 2008 Jan. 30 doi: 10.1038/npp.2008.2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck G. Representativeness of a single point plasma testosterone level for the long term hormonal milieu in men. J. Clin. Endocr. Metab. 1992;74(4):939–942. doi: 10.1210/jcem.74.4.1548361. [DOI] [PubMed] [Google Scholar]
- Vittek J, L'Hommedieu DG, Gordon GG, Rappaport SC, Southren AL. Direct radioimmunoassay (RIA) of salivary testosterone: correlation with free and total serum testosterone. Life Sci. 1985;37(8):711–716. doi: 10.1016/0024-3205(85)90540-5. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45(1):174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Wallerand H, Remy-Martin A, Chabannes E, Bermont L, Adessi GL, Bittard H. Relationship between expansion of the CAG repeat in exon 1 of the androgen receptor gene and idiopathic male infertility. Fertil. Steril. 2001;76(4):769–774. doi: 10.1016/s0015-0282(01)01987-2. [DOI] [PubMed] [Google Scholar]
- Walsh S, Zmuda JM, Cauley JA, Shea PR, Metter EJ, Hurley BF, Ferrell RE, Roth SM. Androgen receptor CAG repeat polymorphism is associated with fat-free mass in men. J. Appl. Physiol. 2005;98(1):132–137. doi: 10.1152/japplphysiol.00537.2004. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends Cogn. Sci. 2007;11(12):499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Schultheiss OC. Basal testosterone moderates responses to anger faces in humans. Physiol. Behav. 2007;90(2–3):496–505. doi: 10.1016/j.physbeh.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, Shin LM, Fischer HH, McMullin K, Rauch SL. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage. 2003;18(3):660–669. doi: 10.1016/s1053-8119(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Edwards ER, Lui LY, Zmuda JM, Ferrell RE, Cauley JA. Androgen receptor CAG repeat polymorphism is associated with cognitive function in older men. Biol. Psychiat. 2003;54(9):943–946. doi: 10.1016/s0006-3223(03)00115-x. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Brune M, Kornmann B, Gromoll J, Junker R, Nieschlag E. The CAG repeat polymorphism in the androgen receptor gene affects bone density and bone metabolism in healthy males. Clin. Endocrinol. (Oxf) 2001a;55(5):649–657. doi: 10.1046/j.1365-2265.2001.01391.x. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Brune M, Kornmann B, Gromoll J, von Eckardstein S, von Eckardstein A, Nieschlag E. The CAG repeat polymorphism in the AR gene affects high density lipoprotein cholesterol and arterial vasoreactivity. J. Clin. Endocr. Metab. 2001b;86(10):4867–4873. doi: 10.1210/jcem.86.10.7889. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Brune M, Nieschlag E. Vascular reactivity in hypogonadal men is reduced by androgen substitution. J. Clin. Endocr. Metab. 2002;87(11):5030–5037. doi: 10.1210/jc.2002-020504. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Depenbusch M, Gromoll J, Nieschlag E. Prostate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: a longitudinal pharmacogenetic study. J. Clin. Endocr. Metab. 2003a;88(5):2049–2054. doi: 10.1210/jc.2002-021947. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Gromoll J, von Eckardstein A, Nieschlag E. The CAG repeat polymorphism in the androgen receptor gene modulates body fat mass and serum concentrations of leptin and insulin in men. Diabetologia. 2003b;46(1):31–39. doi: 10.1007/s00125-002-0980-9. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Nieschlag E. The CAG repeat polymorphism within the androgen receptor gene and maleness. Int. J. Androl. 2003;26(2):76–83. doi: 10.1046/j.1365-2605.2003.00393.x. [DOI] [PubMed] [Google Scholar]
- Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. Am. J. Epidemiol. 1997;146(8):609–617. doi: 10.1093/oxfordjournals.aje.a009326. [DOI] [PubMed] [Google Scholar]