Abstract
Dysregulated apoptosis is a critical failure associated with prominent degenerative diseases including osteoporosis. In bone, estrogen deficiency has been associated with accelerated osteoblast apoptosis and susceptibility to osteoporotic fractures. Hormone therapy continues to be an effective option for preventing osteoporosis and bone fractures. Induction of apoptosis in G-292 human osteoblastic cells by exposure to etoposide or the inflammatory cytokine TNFα promoted acute caspase-3/7 activity and this increased activity was inhibited by pretreatment with estradiol. Etoposide also increased the expression of a battery of apoptosis-promoting genes and this expression was also inhibited by estradiol. Among the apoptotic genes whose expression was inhibited by estradiol was ITPR1, which encodes the type 1 InsP3R. InsP3Rs are intracellular calcium channels and key proapoptotic mediators. Estradiol via estrogen receptor β1 suppresses ITPR1 gene transcription in G-292 cells. These analyses suggest that an underlying basis of the beneficial activity of estrogens in combating osteoporosis may involve the prevention of apoptosis in osteoblasts and that a key event in this process is the repression of apoptotic gene expression and inhibition of caspase-3/7.
Keywords: apoptosis, estrogen receptor, inositol trisphosphate receptor, caspase-3/7, osteoporosis
INTRODUCTION
Estrogen is essential in women for healthy bone. When the production of estrogen declines, as occurs normally in postmenopausal women and pathogenically after exposure to radiation or chemotherapeutic drugs, bone density decreases and fractures occur more readily. The mechanisms involved in these processes are not clearly understood; however, an intriguing hypothesis is that the protective effect of estrogen may involve the prevention of apoptosis or programmed cell death in bone-forming osteoblasts.
The significance of the study of estrogen effects in bone has been made clear from large-scale clinical trials demonstrating the efficacy of hormone therapy in combating osteoporosis. Meta-analyses of nearly two dozen randomized trials showed that postmenopausal hormone therapy taken for at least one year reduced the incidence of nonvertebral fractures by 27% in all women and by 33% in women under the age of 60 (1). More recently, results from the Women’s Health Initiative involving over 27,000 women in a prospective, randomized, placebo-controlled study, demonstrated that 5 to 6 years of either combined estrogen-progestin or estrogen-only therapy resulted in a 30 to 40% reduction in hip fractures and a 20 to 30% reduction in total bone fractures (2).
Osteoporosis Therapies
Essential to bone health are behavioral lifestyles incorporating routine weight bearing exercise, the cessation of smoking, curtailment of alcohol consumption, and proper nutrition that includes adequate dietary calcium and vitamin D. In addition to lifestyle changes, pharmacological interventions have proven to be efficacious in limiting the development and pathologic consequences of osteoporosis for a large sector of the susceptible population. Among the FDA-approved pharmacotherapies, hormone therapy with estrogen or estrogen plus progestin continues to be an effective option for the prevention of postmenopausal osteoporosis in many women. This has become especially true in light of recent concern regarding the long-term safety and efficacy of the highly prescribed bisphosphonate drugs as well as the result of positive outcomes that have come from secondary analyses of hormone therapy data from the Women’s Health Initiative (3–6).
In addition to the Women’s Health Initiative, other large multicenter clinical studies have shown hormone therapy to be protective against bone fracture. Both the PEPI trial (Postmenopausal Estrogen/Progestin Interventions) and the CHART study (Continuous Hormones as Replacement Therapy) showed that women taking hormone therapy within 5 years of menopause significantly increased their bone mineral densities (7,8). In one prospective study involving over 500 perimenopausal women aged 50–54, women were grouped into those receiving no hormone therapy and those receiving either short-term (2–4 years) or long-term (9 years) therapy (9,10). Confirming many studies, the women receiving no treatment lost BMD in the hip (−4.2%) and spine (−8.0%) over the 9 year study period while women on long-term hormone therapy had significantly increased BMD at both sites (2.4% and 8.0%). Interestingly, the women on short-term therapy had no significant loss of BMD at the hip or spine at the 9 year time point even though they were on hormone therapy for just the first 2–4 years. These results indicate that short-term hormone therapy in early menopausal women provides significant long-term BMD benefits.
How is estrogen osteoprotective?
In postmenopausal women, bone loss takes place in part as a result of reduced estrogen levels, although other age-related medical and lifestyle changes certainly contribute. Physiological bone remodeling occurs at the cellular level through the concerted actions of osteoblasts and osteoclasts in millions of microscopic basic multicellular units throughout the skeleton (11). Each BMU may function for 6–9 months with the osteoclasts turning over within the unit every couple weeks and the osteoblasts renewing every couple months (12). Under estrogen deficiency, there are more remodeling units and an unbalanced coupling of bone resorption and formation. This promotes increased rates of initiation of remodeling cycles and excess bone resorption, ultimately leading to lower overall bone density.
A key mechanism by which estrogens may affect osteoblast-osteoclast coupling within the BMU is through the regulation of osteoblast longevity by inhibition of cellular apoptosis. By inhibiting apoptosis, estrogens may extend the life span of osteoblasts and thus enable bone formation to keep pace with bone resorption. Osteoblast average survival has been shown to be a critical factor affecting the onset of postmenopausal osteoporosis. Osteoblasts isolated from bone samples from postmenopausal women express higher levels of the inflammatory receptor Fas and they are more sensitive to Fas ligand-induced cell death compared to samples from healthy young women (13). Classical activators of apoptosis including glucocorticoids, TNFα, and bone morphogenetic protein-2 all promote the intracellular release of mitochondrial cytochrome c, activation of caspases-3 and -9, and osteoblast cell death in culture (14).
EXPERIMENTAL OBSERVATIONS
Our laboratory has tested the hypothesis that estrogen treatment of human osteoblasts affects apoptosis by examining the expression of apoptosis-regulating genes and the activity of caspase-3/7 driven by inflammatory and other apoptosis-promoting agents.
G-292 human osteosarcoma cells were used as osteoblastic models and the anticancer drug etoposide and the inflammatory cytokine TNFα were used to promote apoptosis. G-292 cells are a transformed human osteosarcoma cell line initiated from a primary bone tumor of a female Caucasian. Under defined culture conditions, G-292 cells mimic normal osteoblasts. G-292 cells respond to regulators of bone turnover, differentiate into osteoid-secreting cells, and maintain the capacity to undergo cellular apoptosis. G-292 cells respond to estrogens and express approximately equivalent amounts of estrogen receptor α (ERα) (1.00) and estrogen receptor β1 (ERβ1) (1.34 ± 0.34), both being low to intermediate expression mRNAs. Etoposide is a semisynthetic podophyllotoxin agent and topoisomerase II inhibitor used therapeutically in combination regimens for common malignant diseases (15). TNFα is a physiologic apoptotic stimulus, an important regulator of bone turnover, and one of the principal inflammatory mediators in bone (16).
To assess the effects of estrogens on potential regulatory steps of apoptosis in osteoblasts, G-292 cells were treated with or without estradiol and then specific activities of apoptotic markers were assessed. Preliminary studies showed that etoposide (3µM) treatment caused a significant reduction in cell number after 48h treatment (8,490 ± 240 etoposide-treated vs. 9,570 ± 400 control, p < 0.025 Student’s T-test), although after 24h treatment there was no significant difference in cell numbers. A 24h treatment time with etoposide (3µM) was chosen for study so as to allow detection of early transcriptional changes before any overt apoptosis-mediated cell loss.
Estrogen inhibits caspase-3/7 activity in osteoblasts
Caspase-3/7 activity is universally increased during apoptosis (17,18). We sought to determine whether or not estradiol affected caspase-3/7 activity in G-292 cells treated with agents which promote apoptosis. Cells were pretreated for 24h with 10nM estradiol or vehicle and then exposed to either 3µM etoposide or 10ng/mL TNFα and after 24h, caspase-3/7 activity in cellular extracts was assayed.
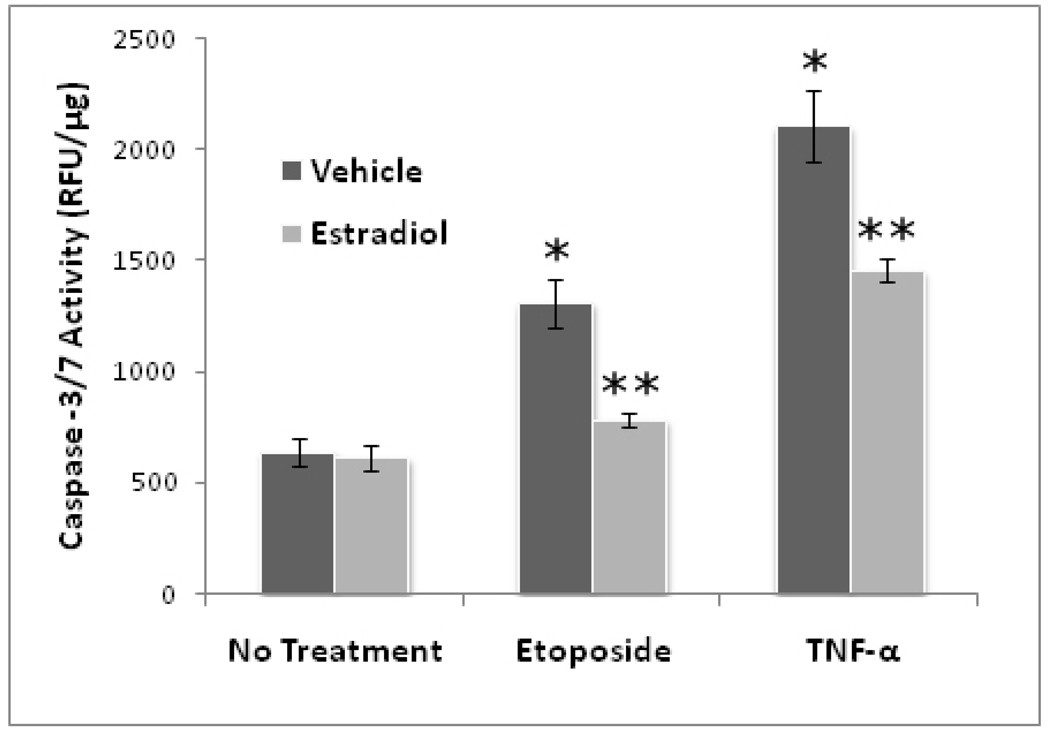
Figure 1 shows that both etoposide and TNFα stimulated caspase-3/7 activity in G-292 cells and that this activation was significantly inhibited by pretreatment of cells with estradiol. It is of interest that estradiol inhibited caspase-3/7 activation by both agents, although there was more complete inhibition etoposide-stimulated activity. Etoposide inhibits cellular topoisomerase and activates the intrinsic pathway of apoptosis, whereas TNFα binds to cell surface receptors which activate the extrinsic pathway of apoptosis.
Figure 1. Effect of Estradiol on Caspase-3/7 Activity in G-292 Cells.
G-292 cells were seeded in 12-well plates and after 2d in culture were treated with 10nM estradiol (E2) or vehicle. After 24h, cells were treated as indicated with 3µM etoposide (Etop) or 10ng/mL TNFα (TNF). After 24h, cell lysates were prepared and assayed for caspase-3/7 activity using the DEVDase fluorescent Apo-ONE system (Promega). Values are means ± SE of three separate experiments. (* p<0.01 vs no treatment, **p<0.01 vs no E2; ANOVA, Tukey’s post hoc)
Estrogen reduces apoptotic gene expression in osteoblasts
Oligoarray analysis was used to detect changes in early apoptotic gene expression in G-292 cells (Figure 2). Cells were pretreated for 24h with or without 10nM estradiol and then treated with or without 3µM etoposide for 24h. Total RNA was isolated for use in Human Apoptosis Microarrays (SABiosciences) containing 112 key apoptotic gene targets. Total RNA was isolated using Aurum mini kits (Bio-Rad) and checked for integrity of 18S and 28S rRNA by agarose gel electrophoresis. Microarrays were hybridized with purified biotinylated cRNA probes which were prepared using biotin-16-UTP, TrueLabeling-AMP linear RNA amplification (Bio-Rad), and G-292 RNA. Expression levels of the apoptosis-related genes were quantified using Fuji LAS-1000 Plus chemiluminescence imager and Image Data Extraction Applet.
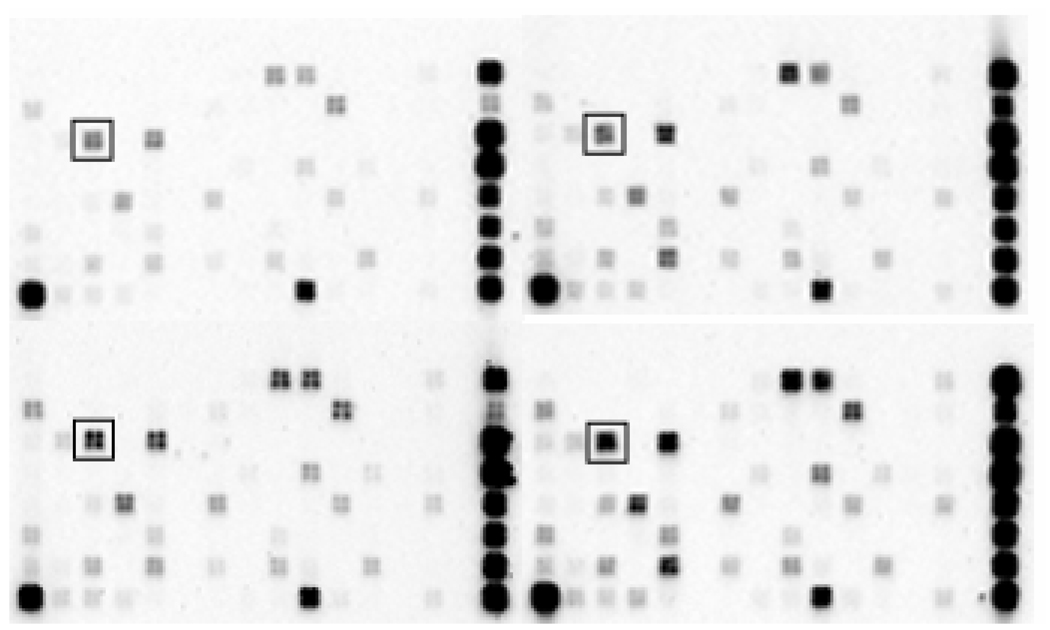
Figure 2. Apoptosis Microarray Analysis.
Oligo GEArray Human Apoptosis Microarrays were hybridized with biotinylated cRNA probes prepared from total RNA of G-292 cells treated with vehicle (lower left), 10nM estradiol (upper left), 3µM etoposide (lower right), or estradiol plus etoposide (upper right). The BID target probe is boxed, showing an example of a specific gene induced by etoposide but suppressed by estradiol. On the right of each microarray are normalization standards including those for GAPDH, beta-2-microglobulin, and beta-actin.
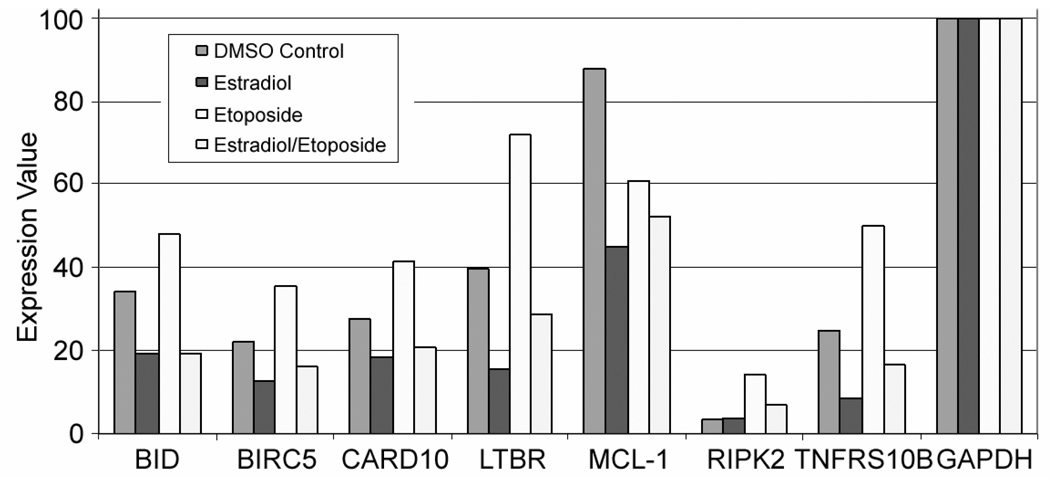
Figure 3 shows the summary analysis of duplicate determinations of apoptotic gene expression in G-292 cells using the Human Apoptosis Microarrays. Each microarray contained 12 categories of human apoptotic genes including those encoding TNF ligands and receptors, bcl-2 family proteins, caspases and caspase recruitment domain proteins, p53 and DNA damage response targets. Not every gene in the apoptotic panel was expressed in G-292 cells. However, the majority of the subset of genes which were expressed in G-292 cells showed the same regulatory pattern: gene expression was induced by etoposide and suppressed by estradiol even in the presence of etoposide. Among the genes showing significant expression in G-292 cells and exhibiting this regulatory pattern were: BID, the death agonist; CARD10, the caspase recruitment domain family member; LTBR, the lymphotoxin receptor of the TNFR superfamily; TNFRSF-12, the TNF receptor superfamily member; and RIP2, the death-inducing kinase.
Figure 3. Quantitation of Human Apoptosis Microarray Data.
Shown are average expression values from duplicate determinations for several apoptotic genes in treated G-292 cells. Image Data Extraction Applet (SABiosciences) was used to perform quantitative analyses. Gene expression values were normalized to GAPDH. Columns in each grouping are, from left to right, DMSO-control, 10nM estradiol treatment, 3µM etoposide treatment, and combined estradiol/etoposide treatment.
Quantitative real-time PCR using SYBR Green detection (SABiosciences) and MyIQ optical module (Bio-Rad) was used to substantiate the microarray data. Templates were prepared from total RNA using RT2 First Strand kits and amplified using RT2 qPCR Master Mixes and algorithm designed primers. As reported in Table 1, estradiol treatment significantly inhibited the etoposide-driven increases in levels of mRNA encoding BID (0.683 ± 0.142), LTBR (0.660 ± 0.182), and TNFRSF-12 (0.682 ± 0.168). Levels of RIP2 mRNA were also inhibited, although to an extent not achieving statistical significance.
TABLE 1.
Quantitative Real-time PCR of Apoptosis-related Genes in Etoposide-treated G-292 Cells: Effect of Estradiol
| Apoptosis Gene (Symbol - Description) | Fold-change Expression Estradiol vs No Estradiol |
|---|---|
| BID – BH3 interacting death agonist | 0.683 ± 0.142* |
| LTBR – Lymphotoxin beta receptor | 0.660 ± 0.182* |
| RIPK2 – Receptor-interacting ser-threonine kinase 2 | 0.831 ± 0.175 |
| TNFRSF-12 – TNF receptor superfamily, member 12A | 0.682 ± 0.168* |
Transcript level were determined by quantitative real-time PCR starting with total RNA derived from G-292 cells treated for 24h with or without estradiol (10 nM) followed by 24h with etoposide (3µM). The delta-delta Ct method using β-actin as housekeeping gene was used to calculate fold-change in expression for each gene of interest. Values are means ± SE (N=3).
p<0.05 estradiol vs no estradiol, Student’s t-test
Estrogen inhibits expression of InsP3R in G-292 cells
The type 1 InsP3R, encoded by the ITPR1 gene, is an intracellular calcium release channel that binds cytochrome c and sensitizes cells to apoptotic stimuli (19–23). In G-292 cells and other osteoblastic cells, exposure to apoptotic stimuli such as TNFα and IL-1β increased ITPR1 gene expression, ITPR1 mRNA, and type 1 InsP3R protein levels (24). In lymphocytes deficient in InsP3R or in CHO cells in which InsP3R expression was silenced, there was an apparent protection against apoptotic stimuli (25,26). Furthermore, these data also showed that apoptosis sensitivity in B-cells could be titrated by changes in total InsP3R expression (20). In G-292 cells, estrogen treatment inhibited type 1 InsP3R expression, suggesting that InsP3R suppression may represent a potential mechanism by which estrogens deter apoptosis in osteoblasts (27).
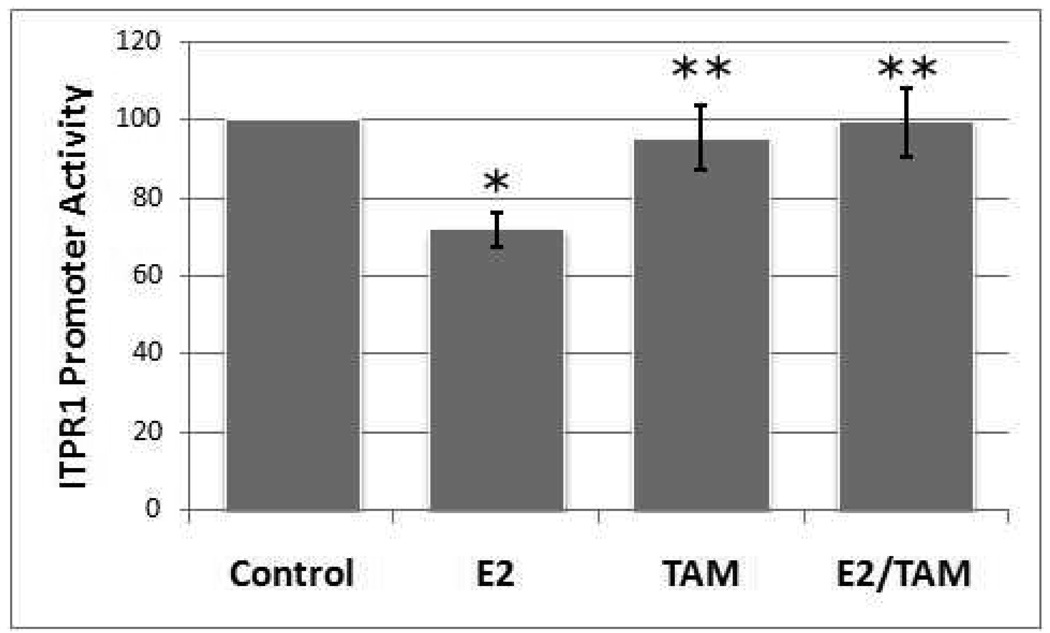
ITPR1 promoter activity was assayed in G-292 cell by lipofectamine-mediated transfection of the -174ITPR1-pCAT basic reporter vector which incorporates 174bp of the human ITPR1 promoter with endogenous TATA element upstream of the bacterial CAT gene. The data in Figure 4 show that estradiol inhibited ITPR1 promoter activity and this inhibition was reversed by tamoxifen, suggesting that the transcriptional suppression may be mediated by an ER.
Figure 4. Effects of Estradiol and Tamoxifen on ITPR1 Promoter Activity.
G-292 cells were transiently transfected with the -174ITPR1-pCAT basic reporter vector which includes 174bp of the human ITPR1 promoter and endogenous TATA element upstream of the bacterial CAT gene. Four hours post-transfection, media containing 1nM β-estradiol (E2), 100nM tamoxifen (TAM), or estradiol plus tamoxifen (E2/TAM) were added. ITPR1 promoter activities were determined after 24h. Activities were normalized for β-galactosidase activities of co-transfected pβGal vector. Values are means ± SE (N=3). (*p<0.05 vs no estradiol control, **p<0.05 vs estradiol, Student’s t-test)
To investigate the potential roles of the ER subtypes in repression of ITPR1 promoter activity, G-292 cells were transfected with expression vectors for either ERα or ERβ1 and the effect of estradiol on ITPR1 promoter activity determined. The results, summarized in Table 2, indicate that overexpression of ERβ1 potentiated the repressive action of estradiol on ITPR1 promoter activity in G-292 cells; whereas, overexpression of ERα enhanced basal promoter activity. The results suggest that ERβ may be mediating estrogen-dependent ITPR1 gene repression in these cells.
TABLE 2.
Effects of ERα or ERβ1 Overexpression on ITPR1 Promoter Activity in G-292 Cells
| Expression Vector | Treatment | ITPR1 Promoter Activity |
|---|---|---|
| pcDNA3.1 | Vehicle | 100 |
| pcDNA3.1 | 10nM Estradiol | 74.6 ± 5.4* |
| pcDNA3.1/ERα | 10nM Estradiol | 108.3 ± 5.2** |
| pcDNA3.1/ERβ1 | 10nM Estradiol | 43.9 ± 6.6** |
ITPR1 promoter activities were determined 24h after lipofectamine-mediated transfection of G-292 cells with the -174ITPR1-pCAT basic reporter vector which includes 174bp of the human ITPR1 promoter including endogenous TATA element upstream of the bacterial CAT gene. CAT activities were normalized for β-galactosidase activities from co-transfected pβGal vector. ER activities based upon ERE-luciferase reporters were increased 40±9 fold in cells transfected with ER expression vectors. Values were normalized to vehicle-treated controls and are means ± SE (N=5–6).
p<0.01 vs no estradiol,
p<0.01 vs pcDNA3.1 plus estradiol, Student’s t-test
In summary, the results demonstrate that estradiol effectively prevented caspase-3/7 activation driven by apoptotic stimuli and decreased the expression of several key apoptosis-promoting genes in G-292 osteosarcoma cells, an experimental human osteoblast model. These genes include the BH-interacting death agonist BID, the tumor necrosis receptor superfamily members LTBR and TNFRSF12, and the death-inducing kinase RIP2. It is interesting that estrogen, perhaps via ERβ1, also repressed transcription of the ITPR1 gene which encodes the intracellular calcium release channel implicated as a critical regulator of early apoptosis. Although these studies have been carried out with a human osteosarcoma cell line, the characteristics of these cells model those of primary osteoblasts and so the outcomes can provide a working framework for understanding osteoblast cell physiology.
DISCUSSION
The mature population represents the fastest growing age group and as a result bone fractures are estimated to rise substantially over the next few years. More than 5 million postmenopausal US women are currently categorized as being in the highest risk group for bone fracture. Behavioral lifestyle management currently involves appropriate diet and continuation of weight bearing exercises to build and maintain healthy bones. In addition, there are a number of therapeutic options for the management of osteoporosis, including bisphosphonates, calcitonins, raloxifene, and parathyroid hormone. Recent safety concerns about the widely-prescribed bisphosphonate drugs, especially their association with osteonecrosis of the jaw and oversuppression of bone turnover, may limit their ultimate usefulness (3–5).
Hormone therapy is a viable alternative to combat osteoporosis for some women at least during the short-term following menopause. This reconsideration of hormone therapy for the short-term management of menopausal osteoporosis stems largely from two observations. One observation is that studies of short-term (2–4 years) hormone therapy showed bone density benefits that extended to the long-term (at least 9 years) (8,9). The second observation is from the WHI data which showed clearly that hormone therapy prevented bone thinning and fractures. Moreover, follow-up analysis of the WHI data, in which women were statistically stratified by age, revealed only nonsignificant effects of hormone therapy on coronary heart disease in younger (50–59) women or women with less than 10 years since menopause, while there was a significant reduction of total mortality in these women [Hazard Ratio 0.70 (0.51–0.96)] with no increased risk of stroke (6).
We are now beginning to understand how estrogens affect bone biology. It is clear that there are direct effects of estrogens on bone-forming osteoblasts. Studies indicate that physiologic and pharmacologic estrogen exposure promotes a resistance of osteoblasts to the processes of apoptosis. Mechanistically, this involves acute inhibition of caspase-3 activity initiated through either the intrinsic or extrinsic pathways as well as the suppression of a battery of apoptosis-promoting genes, including the gene encoding the type 1 InsP3R. InsP3R appears to be a master regulator of apoptosis and its role as a proapoptotic mediator is increasingly appreciated (20–22). That estrogens suppress apoptosis-associated activities in osteoblasts at multiple cellular sites may underlie the dramatic antiosteoporotic actions of estrogen therapies observed universally in clinical trials.
ACKNOWLEDGMENT
Support from USPHS-NIH T32 DE07034
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Torgerson DJ, Bell-Syer SEM. Hormone replacement therapy and the prevention of nonvertebral fractures: A meta-analysis of randomized trials. Journal of the American Medical Association. 2001;285(22):2891–2897. doi: 10.1001/jama.285.22.2891. [DOI] [PubMed] [Google Scholar]
- 2.Writing Group for the Women’s Health Initiative investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Journal of the American Medical Association. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Ott SM. Editorial: Long-term safety of bisphosphonates. Journal of Clinical Endocrinology & Metabolism. 2005;90(3):1897–1899. doi: 10.1210/jc.2005-0057. [DOI] [PubMed] [Google Scholar]
- 4.Woo S-B, Hellstein JW, Kalmar JR. Systematic review: Bisphosphonates and osteonecrosis of the jaws. Annals of Internal Medicine. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 5.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. Journal of Oral and Maxillofacial Surgery. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Rossouw JE, Prentice RL, Manson JE, Wu LL, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Journal of the American Medical Association. 2007;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 7.Writing Group for the PEPI. Effects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. Journal of the American Medical Association. 1996;276:1389–1396. [PubMed] [Google Scholar]
- 8.Speroff L, Rowan J, Symons J, et al. The comparative effect on bone density, endometrium, and lipids of continuous hormones as replacement therapy (CHART study). A randomized control trial. Journal of the American Medical Association. 1996;276:1397–1403. [PubMed] [Google Scholar]
- 9.Bagger YZ, Tanko LB, Alexandersen P, et al. Two to three years of hormone replacement therapy in healthy women have long-term preventive effects on bone mass and osteoporotic fractures: the PERF study. Bone. 2004;34:728–735. doi: 10.1016/j.bone.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Middleton ET, Steel SA. The effects of short-term hormone replacement therapy on long-term bone mineral density. Climacteric. 2007;10:257–263. doi: 10.1080/13697130701370435. [DOI] [PubMed] [Google Scholar]
- 11.Martin RB. Targeted bone remodeling involves BMU steering as well as activation. Bone. 2007;40:1574–1580. doi: 10.1016/j.bone.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocrine Reviews. 2000;21(2):115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 13.Xing L, Boyce BF. Regulation of apoptosis in osteoclasts and osteoblastic cells. Biochemical Biophysical Research Communications. 2005;328(3):709–720. doi: 10.1016/j.bbrc.2004.11.072. [DOI] [PubMed] [Google Scholar]
- 14.Hay E, Lemonnier J, Fromique O, Marie PJ. Bone morphogenetic protein-2 promotes osteoblast apoptosis through a Smad-independent, protein kinase c-dependent signaling pathway. J Biological Chemistry. 2001;276:29028–29036. doi: 10.1074/jbc.M011265200. [DOI] [PubMed] [Google Scholar]
- 15.DeJong RS, Mulder NH, Dijksterhhuis D, DeVries E. Review of current clinical experience with prolonged (oral) etoposide in cancer treatment. Anticancer Research. 1995;15:2319–2330. [PubMed] [Google Scholar]
- 16.Boyce BF, Li P, Yao Z, et al. TNF-alpha and pathologic bone resorption. Keio Journal of Medicine. 2005;54(3):127–131. doi: 10.2302/kjm.54.127. [DOI] [PubMed] [Google Scholar]
- 17.Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–2966. doi: 10.1038/sj.onc.1207558. [DOI] [PubMed] [Google Scholar]
- 18.Walsh JG, Cullen SP, Sheridan C, et al. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proceedings of the National Academy of Sciences USA. 2008;105(35):12815–12819. doi: 10.1073/pnas.0707715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White C, Li C, Yang J, et al. The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nature Cell Biol. 7:1021–1027. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Wang X, Vais H, et al. Apoptosis regulation by Bcl-XL modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proceedings of the National Academy of Sciences USA. 2007;104(30):12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nature Reviews. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 22.Boehning D, Paterson RL, Snyder SH. Apoptosis and calcium: new roles for cytochrome c and inositol 1,4,5-trisphosphate. Cell Cycle. 2004;3:252–254. [PubMed] [Google Scholar]
- 23.Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nature Cell Biology. 2003;5:1041–1043. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- 24.Bradford PG, Maglich JM, Kirkwood KL. IL-1β increases type 1 inositol trisphosphate receptor expression and IL-6 secretory capacity in osteoblastic cell cultures. Molecular Cell Biology Research Communications. 2000;3:73–75. doi: 10.1006/mcbr.2000.0194. [DOI] [PubMed] [Google Scholar]
- 25.Jayarman T, Marks AR. T cells deficient in inositol 1,4,5-trisphosphate are resistant to apoptosis. Molecular Cellular Biology. 1997;17:3005–3012. doi: 10.1128/mcb.17.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendes CC, Gomes DA, Thompson M, et al. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biological Chemistry. 2005;280:40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- 27.Kirkwood KL, Homick K, Dragon MB, Bradford PG. Cloning and characterization of the type I inositol 1,4,5-trisphosphate receptor gene promoter. J Biological Chemistry. 1997;272:22425–22431. doi: 10.1074/jbc.272.36.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]