Abstract
In the present study, we investigated the effect of voluntary exercise on the formation and growth of human pancreas Panc-1 and prostate PC-3 tumors in immunodeficient mice. Female severe combined immunodeficient (SCID) mice were injected subcutaneously with human pancreas cancer Panc-1 cells, and male SCID mice were injected subcutaneously with human prostate cancer PC-3 cells. Voluntary running wheel exercise for 63 days starting one week before the subcutaneous injection of Panc-1 or PC-3 tumor cells into SCID mice suppressed the growth of both Panc-1 and PC-3 tumors. The exercise regimen increased food and fluid consumption in both female and male mice. Exercise also decreased the size of the parametrial fat pads in female mice and the paradidymis fat pads in male mice, but there was no effect on body weight. Mechanistic studies showed that voluntary running wheel exercise inhibited proliferation as reflected by decreased mitosis, and the exercise regimen also stimulated apoptosis as reflected by increased caspase-3 (active form) expression in both Panc-1 and PC-3 tumors. Voluntary running wheel exercise decreased the ratio of the percent mitotic cells/apoptotic cells in Panc-1 and PC-3 tumors by 38% and 32%, respectively. The present study demonstrates an inhibitory effect of voluntary exercise on the growth of pancreas and prostate tumors in a SCID mouse xenograft model.
Keywords: exercise, cancer prevention, apoptosis, mitosis, cancer growth
INTRODUCTION
Pancreas and prostate cancers are important causes of death in the United States. Pancreas cancer ranks fourth among U.S. cancer deaths (males and females), and the five year survival is less than 5% (1) whereas prostate cancer ranks second among U.S. cancer deaths in males (after lung) (1). Attempts to inhibit the formation or treat these cancers are important goals of the cancer research community. Studies in experimental animals indicate an inhibitory effect of voluntary exercise on colon, breast, skin and pancreas carcinogenesis (2-7), but to the best of our knowledge there are no studies on the effect of exercise on prostate carcinogenesis or on the growth of prostate or pancreas tumors.
Epidemiology studies on the relationship between physical activity and overall prostate cancer risk have been inconclusive (8-11), but large population-based studies suggest that physical exercise is associated with a reduced risk of advanced prostate cancer and prostate cancer death (12, 13). In additional studies, serum from exercising men inhibited the growth and increased apoptosis in prostate cancer LNCaP cells (14, 15). Although the results of epidemiology studies on a possible association between physical activity and pancreas cancer risk are inconclusive (16-18), laboratory-based studies demonstrated that voluntary exercise reduced the growth of preneoplastic pancreatic foci and inhibited azaserine-induced pancreas carcinogenesis in Lewis and F344 rats (6, 7).
The present study was undertaken to determine the effects of voluntary running wheel exercise on the formation and growth of human pancreas Panc-1 and androgen-independent prostate PC-3 xenograft tumors in SCID mice. Our study showed that voluntary running wheel exercise inhibited the growth of both pancreas Panc-1 and prostate PC-3 tumors in SCID mice. Mechanistic studies showed that voluntary running wheel exercise inhibited proliferation and stimulated apoptosis in these tumors.
MATERIALS AND METHODS
Cell culture and reagents
Panc-1 cells were kindly provided by Dr. Pamela Crowell (Indiana University-Purdue University Indianapolis, IN). PC-3 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Matrigel was obtained from BD Biosciences (Bedford, MA). RPMI-1640 and DMEM tissue culture media, penicillin-streptomycin, L-glutamine and fetal bovine serum (FBS) were from Gibco (Grand Island, NY). Panc-1 cells were maintained in DMEM, and PC-3 cells were maintained in RPMI-1640 culture medium. Both DMEM and RPMI media contained 10% FBS and were supplemented with penicillin (100 units/ml)-streptomycin (100 μg/ml) and L-glutamine (300 μg/ml). Cultured cells were grown at 37°C in a humidified atmosphere of 5% CO2 and were passaged twice a week.
Formation and growth of Panc-1 and PC-3 tumors in immunodeficient mice with or without voluntary running wheel exercise
SCID mice (6-7 weeks old) were obtained from Taconic Farms Inc. (Germantown, NY). The animals were housed in sterile filter-capped microisolator cages and provided with sterilized food (laboratory rodent diet 5010) and water. Female mice were injected subcutaneously with Panc-1 cells (2×106 cells/0.1 ml/mouse) suspended in 50% Matrigel in DMEM medium, and male mice were injected subcutaneously with PC-3 cells (2×106 cells/0.1 ml/mouse) suspended in 50% Matrigel in RPMI 1640 medium. In the experiment with Panc-1 cells, 12 female mice were placed in cages equipped with running wheels (6 mice/cage) and 10 female mice were placed in cages with no running wheel (5 mice/cage) one week before the tumor cell inoculation. In the experiment with PC-3 cells, 12 male mice were placed in cages equipped with running wheels and 12 male mice were placed in cages with no running wheel one week before the tumor cell inoculation (6 mice/cage). Mice in cages with running wheels had free access to the wheel 24 h/day during the whole experimental period (63 days). The running wheels had digital counters that measured running wheel revolutions (5)). Tumor size measurements were started on day 21 after tumor cell injection. Tumor size was measured by determining tumor length and width (expressed as cm2) once per week until the end of the experiment. Body weight was also measured once every week. All animal experiments were carried out under an Institutional Animal Care and Use Committee (IACUC)-approved protocol.
Determination of mitotic cells
Panc-1 and PC-3 tumors in mice from each experimental group were excised. The samples were fixed overnight in 10% formalin and then transferred into 70% ethanol. Paraffin-embedded tissue sections of 5-μm thickness were stained with hematoxylin and eosin (H&E). Mitotic cells were counted under a light microscope as described elsewhere (19).
Caspase-3 (active form) immunostaining
An antibody that reacts with the active form of caspase-3 was purchased from R&D Systems (Catalog number: AF835). Tumor sections used for the measurement of caspase-3 (active form) were stained by the horseradish peroxidase-conjugated avidin method with some modification (20). Briefly, sections were incubated with caspase-3 primary antibody (1:2,000 dilution) for 30 min at room temperature followed by incubation with a biotinylated anti-rabbit secondary antibody for 30 min and incubation with conjugated-avidin solution (ABC ellite kit purchased from Vector Laboratories) for 30 min. Color development was achieved by incubation with 0.02% 3,3'-diaminobenzidine tetrahydrochloride containing 0.02% hydrogen peroxide for 10 min at room temperature. The slides were then counterstained with hematoxylin, dehydrated, and coverslips were added for permanent mounting. A positive reaction was shown as a brown precipitate in the cytoplasm and/or perinuclei of the cells. The percent of caspase-3 positive cells was determined in each tumor.
Statistical analyses
The Student's t test was used to determine differences for percent mitotic cells, percent caspase-3 positive cells, and ratio of percent mitotic cells/caspase-3 positive cells between the running wheel and the no running wheel groups. The analyses of increase in tumor size were based on the mixed effect regression (repeated measurement) model (21). The treatment effects were assessed by comparing the rates of change over time between treatment groups (i.e. comparing the slopes between treatment groups).
RESULTS
Effects of voluntary running wheel exercise on the consumption of food and water in SCID mice
In female mice injected with Panc-1 cells, the running wheel group consumed 21% more food and 23% more water when compared with mice in the non-running wheel group (Table 1). The average distance the mice ran on the running wheel was 1.09±0.16 miles/mouse/day (Table 1). In the experiment with PC-3 cells in male mice, the running wheel group consumed 26% more food and 22% more water when compared with mice in the non-running wheel group (Table 1). The average distance the mice ran in the running wheel group was 1.38±0.21 miles/mouse/day (Table 1).
Table I.
Effects of voluntary exercise on food and fluid consumption in SCID mice
| Group | Gender/number of mice | Food consumption (g/mouse/day) | Fluid consumption (ml/mouse/day) | Running distance (miles/mouse/day) |
|---|---|---|---|---|
| Panc-1 | ||||
| Control | F/10 | 3.83±0.08 | 4.32±0.09 | - |
| Running wheel | F/12 | 4.62±0.09*** | 5.29±0.10*** | 1.09±0.16 |
| PC-3 | ||||
| Control | M/12 | 4.54±0.12 | 5.50±0.11 | - |
| Running wheel | M/12 | 5.71±0.10*** | 6.69±0.09*** | 1.38±0.21 |
Female (F) SCID mice were injected with human pancreas cancer Panc-1 cells (2.0 × 106 cells/mouse). Male (M) SCID mice were injected with human prostate cancer PC-3 cells (2.0 × 106 cells/mouse). Twelve female and 12 male mice were placed in cages (6 mice/cage) equipped with running wheels (1 running wheel/cage) one week before the injection of tumor cells and remained until the end of the study (56 days after the tumor cell injection). Wheel revolutions and consumption of food and fluid were recorded. Each value represents the mean ± S.E. The student's t test was used to determine the difference between the control and running wheel group.
p<0.001.
Effects of voluntary running wheel exercise on body weight and tissue fat in SCID mice
Running wheel exercise had no effect on body weight in the mice injected with Panc-1 or PC-3 cells, but there was a 30% decrease in the weight of the parametrial fat pads in the female mice injected with Panc-1 cells and a 32% decrease in the weight of paradidymis fat pads in the male mice injected with PC-3 cells (Table 2).
Table II.
Effects of voluntary exercise on body weight and parametrial/paradidymis fat pads in SCID mice
| Group | Gender/number of mice | Initial and final body weight (g/mouse) | Parametrial (F) or paradidymis (M) fat pads (g/mouse) | Percentage decrease in fat pad weight |
|---|---|---|---|---|
| Panc-1 | ||||
| Control | F/10 | 22.6±0.30 | 0.33±0.03 | - |
| 24.2±0.40 | ||||
| Running wheel | F/12 | 22.2±0.29 | 0.23±0.02* | 30.3 |
| 23.5±0.48 | ||||
| PC-3 | ||||
| Control | M/12 | 26.2±0.74 | 0.31±0.02 | - |
| 28.0±0.49 | ||||
| Running wheel | M/12 | 26.3±0.53 | 0.21±0.02** | 32.3 |
| 27.9±0.65 |
Body weights of female (F) and male (M) SCID mice in the experiments described in Table 1 were measured. Parametrial fat pads from female mice and paradidymis fat pads from male mice were weighed. Each value represents the mean ± S.E. The student's t test was used to determine the difference between the control and running wheel group.
p<0.05
p<0.01.
Effects of voluntary running wheel exercise on the growth of Panc-1 and PC-3 tumors in SCID mice
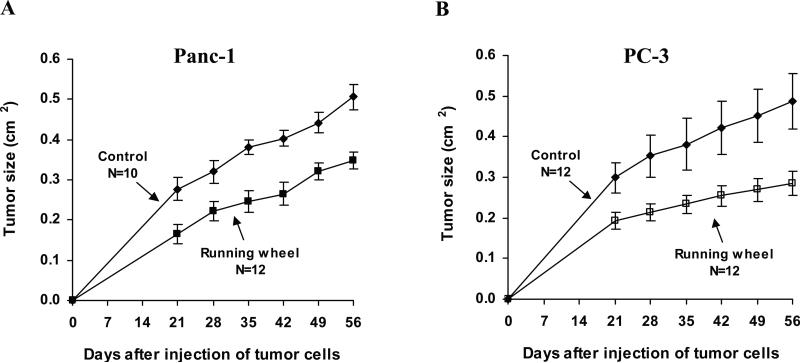
2 × 106 Panc-1 or PC-3 cells were injected subcutaneously into SCID mice as described in Fig. 1. Voluntary running wheel exercise delayed the formation of Panc-1 tumors in some mice. All mice in the control group formed a measurable tumor by 21 days after the injection of Panc-1 cells while 17% of the mice in the running wheel group were tumor free at this time. Eight percent of the animals in the running wheel group remained tumor-free at 42 days after the injection of Panc-1 cells and all mice had a tumor by 49 days.
Figure 1.
Effect of voluntary exercise on the growth of pancreas Panc-1 and prostate PC-3 tumors in SCID mice. Female SCID mice were injected subcutaneously with human pancreas cancer Panc-1 cells (2.0 × 106 cells/mouse), and male SCID mice were injected subcutaneously with human prostate cancer PC-3 cells (2.0 × 106 cells/mouse). Both male and female mice were randomly assigned into two groups. One group was placed in cages (5-6 mice/cage) equipped with running wheels (1 running wheel/cage) one week before the injection of tumor cells, and the running wheels remained until the end of the study (56 days after the tumor cell injection). Mice in another group were in cages without a running wheel (5-6 mice/cage). Starting at day 21 after tumor cell injection, tumor size (length × width; cm2) per mouse was measured once a week until the end of the experiment. In A, tumor size was measured in the control group for all 10 tumors on each day as indicated. In the running wheel group, tumor size was measured on day 21 (N=10), days 28, 35 and 42 (N=11) and on days 49 and 56 (N=12). In B, tumor size was measured in the control and running wheel groups in all 10 tumors on each day as indicated. Each value represents the mean ± S.E.
The growth of Panc-1 tumors was measured in 10 control mice and in 12 running wheel mice. In the control group, all 10 mice had a measurable tumor on day 21, and tumor size was determined on days 21, 28, 35, 42, 49, and 58 (N=10 on each day). In the running wheel group, not all animals had a tumor at 21 days after injection of Panc-1 cells, and tumor size was measured on day 21 (N=10), days 28, 35, and 42 (N=11), and on days 49 and 56 (N=12) (Fig. 1A). From the fitted regression equations, the rates of tumor growth between days 21 and 56 were 0.00654 cm2 per day for the control group, and 0.00476 cm2 for the running wheel group. This implied that, on average, the rate of tumor growth for the control group was 0.00179 cm2 per day faster than that for the running wheel group per day (the difference was significant with p=0.0331). The difference in tumor size between the control group and the running wheel group increased from 0.0930 cm2 to 0.156 cm2 from day 21 to day 56. The differences were statistically significant at the 5% level beginning on day 28 (p≤0.0069). At the end of the experiment, the average tumor size per mouse (length × width; cm2) was 0.51±0.03 for the control group and 0.35±0.02 for the running wheel group (Fig.1). Statistical analysis using the Student's t test showed that the difference for tumor size between the control and running wheel group at the end of the study was statistically significant (p<0.001). Running wheel exercise had no effect on body weight throughout the study (data not shown).
Although running wheel exercise did not delay the formation of PC-3 tumors measured at 21 days, it suppressed the growth of PC-3 tumors in the mice (Fig.1B). From fitted regression equations, the rates of tumor growth between days 21 and 56 were 0.00537 cm2 per day for the control group and 0.00263 cm2 for the running wheel group (10 mice/group). This implied that, on average, the rate of PC-3 tumor growth for the control group was 0.00274 cm2 per day faster than that for the running wheel group per day (the difference was significant with p = 0.0065). On average, the difference in tumor size between the control group and the running wheel group increased from 0.106 cm2 to 0.202 cm2 from day 21 to day 56. The differences were statistically significant at the 5% level beginning on day 28 (p < 0.0388). At the end of the experiment, the average tumor size per mouse was 0.49±0.07 cm2 for the control group and 0.28±0.03 cm2 for the running wheel group (Fig.1). Statistical analysis using the Student's t test showed that the difference in tumor size between the control and running wheel group was statistically significant (p<0.05). There was no significant difference in the body weight between the control and running wheel group throughout the study (data not shown).
Effects of voluntary running wheel exercise on mitosis and apoptosis in Panc-1 and PC-3 tumors
The effects of voluntary running wheel exercise on proliferation and apoptosis in Panc-1 and PC-3 tumors described in Figure 1 were studied by determining the percentage of mitotic cells and caspase-3 (active form) positive cells in these tumors. As shown in Table 3, the percentage of mitotic cells was decreased and the percentage of apoptotic cells was increased in Panc-1 and PC-3 tumors in mice with running wheel exercise as compared to the control group. Running wheel exercise decreased the ratio of the percent mitotic cells/percent caspase-3 (active form) positive cells in Panc-1 and PC-3 tumors by 38% and 32%, respectively (Table 3). Our results indicate a shift in the balance between cell proliferation and cell death towards decreased tumor growth. Similar results were obtained when data from the two animals that developed a measurable pancreas tumor only after 21 days post-tumor cell injection were excluded from the analysis.
Table III.
Effects of voluntary exercise on the percent of mitotic and caspase-3 positive cells in pancreas Panc-1 and prostate PC-3 tumors
| Group | Gender/number of mice | Percent mitotic cells | Percent caspase-3 positive cells | Ratio of percent mitotic cells/caspase-3 positive cells | Percent decrease in ratio of mitotic/apoptotic cells |
|---|---|---|---|---|---|
| Panc-1 | |||||
| Control | F/10 | 0.55±0.03 | 0.40±0.03 | 1.43±0.13 | - |
| Running wheel | F/12 | 0.43±0.02** | 0.49±0.02* | 0.89±0.05*** | 37.8 |
| PC-3 | |||||
| Control | M/12 | 0.47±0.02 | 0.35±0.02 | 1.37±0.07 | - |
| Running wheel | M/12 | 0.38±0.02* | 0.42±0.02* | 0.93±0.05*** | 32.1 |
Tumors from female (F) and male (M) SCID mice in experiments described in Table 1 were fixed in formalin and processed for paraffin sections. Mitotic cells were identified and counted in H&E stained sections using a light microscope. Caspase-3 positive cells were identified immunohistochemically. Each value represents the mean ± S.E. The student's t test was used to determine the difference between the control and running wheel group.
p<0.05
p<0.01
p<0.001
DISCUSSION
In the present study, we demonstrated for the first time that voluntary running wheel exercise inhibited the growth of human pancreas and prostate tumors in immunodeficient SCID mice, and these effects of exercise were paralleled by decreased proliferation and increased apoptosis. In a previous study, it was found that voluntary running wheel exercise suppressed the size and growth rate of azaserine-induced preneoplastic pancreatic foci in rats (6). Voluntary running wheel exercise was also found to inhibit the formation of N-nitrosomethylurea-induced mammary cancer (3) and the formation of azoxymethane-induced colon cancer (2) in rat models. In addition, Welsch and colleagues found that voluntary running wheel exercise reduced the growth of breast xenograft tumors in athymic mice (22). However, these earlier studies did not examine the effects of voluntary exercise on apoptosis in preneoplastic or tumor cells. Recent studies showed that voluntary exercise inhibited UVB-induced carcinogenesis, enhanced UVB-induced apoptosis in the epidermis and also enhanced apoptosis in UVB-induced tumors but not in non-irradiated normal epidermis or in the epidermis away from tumors in tumor-bearing mice (5, 23).
The mechanisms by which voluntary exercise inhibits the growth and stimulates apoptosis in tumor cells are not known. Voluntary exercise is known to modify circulating growth factors and cytokines such as insulin-like growth factor 1, interleukin 6, interleukin 10 and leptin (10, 12, 24). Recent in vitro studies showed that serum from exercising men inhibited the growth and stimulated apoptosis in cultured human prostate cancer LNCaP cells (14, 15). We have obtained similar results using serum from exercising mice (unpublished observation). Further studies are needed to determine if exercise-induced growth inhibition and enhanced apoptosis in Panc-1 and PC-3 tumors are mediated by cytokines that are modulated by running wheel exercise in SCID mice.
Although there were no differences in body weight between mice in the running wheel group and mice in the non-running wheel control group, tissue fat was decreased in mice with access to running wheels when compared to mice with no access to running wheels (Table 2). A recent study showed that voluntary running wheel exercise inhibited UVB-induced skin carcinogenesis and decreased the weight of the parametrial fat pads and the thickness of the dermal fat layer in SKH-1 mice (5). Surgical removal of the parametrial fat pads (partial lipectomy) 2 weeks before UVB irradiation enhanced UVB-induced apoptosis (23) suggesting that fat cells secrete substances that inhibit apoptosis in cells with DNA damage and possibly in tumors. Further studies are needed to determine whether the exercise-induced decrease in tissue fat plays a role in the exercise-induced inhibition of Panc-1 and PC-3 tumor growth. In epidemiology studies, exercise was reported to be associated with a decreased risk of advanced prostate cancer (12, 13, 25). Additional studies are needed to determine whether exercise decreases the risk or growth of pancreas cancer in humans.
ACKNOWLEDGEMENTS
The present study was supported in part by grants from the National Institutes of Health (CA 121391-01 and CA092268). The authors thank Ms. Florence Florek for her excellent help in the preparation of this manuscript.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Reddy BS, Sugie S, Lowenfels A. Effect of voluntary exercise on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Res. 1988;48:7079–7081. [PubMed] [Google Scholar]
- 3.Cohen LA, Choi K, Backlund JY, Harris R, Wang CX. Modulation of N-nitrosomethylurea induced mammary tumorigenesis by dietary fat and voluntary exercise. In Vivo. 1991;5:333–344. [PubMed] [Google Scholar]
- 4.Thompson HJ. Effects of physical activity and exercise on experimentally-induced mammary carcinogenesis. Breast Cancer Res Treat. 1997;46:135–141. doi: 10.1023/a:1005912527064. [DOI] [PubMed] [Google Scholar]
- 5.Michna L, Wagner GC, Lou YR, Xie JG, Peng QY, Lin Y, Carlson K, Shih WJ, Conney AH, Lu YP. Inhibitory effects of voluntary running wheel exercise on UVB-induced skin carcinogenesis in SKH-1 mice. Carcinogenesis. 2006;27:2108–2115. doi: 10.1093/carcin/bgl057. [DOI] [PubMed] [Google Scholar]
- 6.Roebuck BD, McCaffrey J, Baumgartner KJ. Protective effects of voluntary exercise during the postinitiation phase of pancreatic carcinogenesis in the rat. Cancer Res. 1990;50:6811–6816. [PubMed] [Google Scholar]
- 7.Craven-Giles T, Tagliaferro AR, Ronan AM, Baumgartner KJ, Roebuck BD. Dietary modulation of pancreatic carcinogenesis: calories and energy expenditure. Cancer Res. 1994;54:1964s–1968s. [PubMed] [Google Scholar]
- 8.Cerhan JR, Torner JC, Lynch CF, Rubenstein LM, Lemke JH, Cohen MB, Lubaroff DM, Wallace RB. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States). Cancer Causes Control. 1997;8:229–238. doi: 10.1023/a:1018428531619. [DOI] [PubMed] [Google Scholar]
- 9.Wannamethee SG, Shaper AG, Walker M. Physical activity and risk of cancer in middle-aged men. Br J Cancer. 2001;85:1311–1316. doi: 10.1054/bjoc.2001.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee IM, Sesso HD, Chen JJ, Paffenbarger RS., Jr. Does physical activity play a role in the prevention of prostate cancer? Epidemiol Rev. 2001;23:132–137. doi: 10.1093/oxfordjournals.epirev.a000778. [DOI] [PubMed] [Google Scholar]
- 11.Friedenreich CM, McGregor SE, Courneya KS, Angyalfi SJ, Elliott FG. Case-control study of lifetime total physical activity and prostate cancer risk. Am J Epidemiol. 2004;159:740–749. doi: 10.1093/aje/kwh106. [DOI] [PubMed] [Google Scholar]
- 12.Patel AV, Rodriguez C, Jacobs EJ, Solomon L, Thun MJ, Calle EE. Recreational physical activity and risk of prostate cancer in a large cohort of U.S. men. Cancer Epidemiol Biomarkers Prev. 2005;14:275–279. [PubMed] [Google Scholar]
- 13.Nilsen TI, Romundstad PR, Vatten LJ. Recreational physical activity and risk of prostate cancer: A prospective population-based study in Norway (the HUNT study). Int J Cancer. 2006;119:2943–2947. doi: 10.1002/ijc.22184. [DOI] [PubMed] [Google Scholar]
- 14.Leung PS, Aronson WJ, Ngo TH, Golding LA, Barnard RJ. Exercise alters the IGF axis in vivo and increases p53 protein in prostate tumor cells in vitro. J Appl Physiol. 2004;96:450–454. doi: 10.1152/japplphysiol.00871.2003. [DOI] [PubMed] [Google Scholar]
- 15.Ornish D, Weidner G, Fair WR, Marlin R, Pettengill EB, Raisin CJ, Dunn-Emke S, Crutchfield L, Jacobs FN, Barnard RJ, Aronson WJ, McCormac P, McKnight DJ, Fein JD, Dnistrian AM, Weinstein J, Ngo TH, Mendell NR, Carroll PR. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol. 2005;174:1065–1069. doi: 10.1097/01.ju.0000169487.49018.73. [DOI] [PubMed] [Google Scholar]
- 16.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286:921–929. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 17.Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, Albanes D. A prospective study of medical conditions, anthropometry, physical activity, and pancreatic cancer in male smokers (Finland). Cancer Causes Control. 2002;13:417–426. doi: 10.1023/a:1015729615148. [DOI] [PubMed] [Google Scholar]
- 18.Sinner PJ, Schmitz KH, Anderson KE, Folsom AR. Lack of association of physical activity and obesity with incident pancreatic cancer in elderly women.[see comment]. Cancer Epidemiol Biomarkers Prev. 2005;14:1571–1573. doi: 10.1158/1055-9965.EPI-05-0036. [DOI] [PubMed] [Google Scholar]
- 19.Tannapfel A, Geissler F, Kockerling F, Katalinic A, Hauss J, Wittekind C. Apoptosis and proliferation in relation to histopathological variables and prognosis in hepatocellular carcinoma. J Pathol. 1999;187:439–445. doi: 10.1002/(SICI)1096-9896(199903)187:4<439::AID-PATH272>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Zheng X, Chang RL, Cui XX, Avila GE, Lee S, Lu YP, Lou YR, Shih WJ, Lin Y, Reuhl K, Newmark H, Rabson A, Conney AH. Inhibitory effect of 12-O-tetradecanoylphorbol-13-acetate alone or in combination with all-trans-retinoic acid on the growth of LNCaP prostate tumors in immunodeficient mice. Cancer Res. 2004;64:1811–1820. doi: 10.1158/0008-5472.can-03-2848. [DOI] [PubMed] [Google Scholar]
- 21.Lindsey JK. Models for Repeated Measurements. Claredon Press; Oxford: 1993. [Google Scholar]
- 22.Welsch MA, Cohen LA, Welsch CW. Inhibition of growth of human breast carcinoma xenografts by energy expenditure via voluntary exercise in athymic mice fed a high-fat diet. Nutr Cancer. 1995;23:309–318. doi: 10.1080/01635589509514385. [DOI] [PubMed] [Google Scholar]
- 23.Lu YP, Lou YR, Nolan B, Peng QY, Xie JG, Wagner GC, Conney AH. Stimulatory effect of voluntary exercise or fat removal (partial lipectomy) on apoptosis in the skin of UVB light-irradiated mice. Proc Natl Acad Sci U S A. 2006;103:16301–16306. doi: 10.1073/pnas.0607789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen BK, Fischer CP. Beneficial health effects of exercise--the role of IL-6 as a myokine. Trends Pharmacol Sci. 2007;28:152–156. doi: 10.1016/j.tips.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci EL, Liu Y, Leitzmann MF, Stampfer MJ, Willett WC. A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med. 2005;165:1005–1010. doi: 10.1001/archinte.165.9.1005. [DOI] [PubMed] [Google Scholar]