Abstract
Background
Emerging evidence in obesity and diabetes demonstrates that excessive myocardial fatty acid (FA) uptake and oxidation contribute to cardiac dysfunction. Transgenic mice with cardiac-specific overexpression of the FA-activated nuclear receptor PPARα (MHC-PPARα mice) exhibit phenotypic features of the diabetic heart, which are rescued by deletion of CD36, a FA transporter, despite persistent activation of PPARα gene targets involved in FA oxidation.
Methods and Results
To further define the source of FA that leads to cardiomyopathy associated with lipid excess, we crossed MHC-PPARα mice with mice deficient for cardiac lipoprotein lipase (hsLpLko). MHC-PPARα/hsLpLko mice exhibit improved cardiac function and reduced myocardial triglyceride content when compared with MHC-PPARα mice. Surprisingly, in contrast to MHC-PPARα/CD36ko mice, the activity of the cardiac PPARα gene regulatory pathway is normalized in MHC-PPARα/hsLpLko mice suggesting that PPARα ligand activity exists in the lipoprotein particle. Indeed, LpL-mediated hydrolysis of very low-density lipoprotein activated PPARα in cardiac myocytes in culture. The rescue of cardiac function in both models was associated with improved mitochondrial ultrastructure and re-activation of transcriptional regulators of mitochondrial function.
Conclusions
1) MHC-PPARα mouse hearts acquire excess lipoprotein-derived lipids, 2) LpL-deficiency rescues mycocyte triglyceride accumulation, mitochondrial gene regulatory derangements, and contractile function in MHC-PPARα mice, 3) LpL serves as a source of activating-ligand for PPARα in the cardiomyocyte.
Keywords: cardiomyopathy, lipotoxicity, diabetes mellitus
INTRODUCTION
Type 2 diabetes and its associated cardiovascular complications comprise a worldwide health threat.1,2 Although patients with obesity-related diabetes have an increased incidence of heart failure following myocardial infarction,3 they are also prone to develop heart failure in the absence of significant coronary artery disease.4 These observations suggest that myocardial dysfunction in diabetes, metabolic syndrome and obesity has distinct pathogenic features.
Multiple mechanisms have been proposed to drive diabetes-associated heart dysfunction including: 1) glucose toxicity (advanced glycation end-products);5 2) microvascular disease;6 3) mitochondrial dysfunction7,8 and; 4) lipid toxicity.9,10 Evidence supports a role for lipid metabolic derangements in the development of cardiomyocyte dysfunction in the insulin-resistant and diabetic heart;9,10 both lipid accumulation and excessive fatty acid (FA) oxidation (FAO) are postulated to cause cardiomyocyte toxicity. Human studies and animal models demonstrate that diabetes and obesity are associated with accumulation of myocyte fat.11,12 Additionally, the insulin-resistant heart is unable to fully utilize glucose, forcing the organ to rely on FAs, leading to a vicious cycle of increased myocyte FA import, oxidation and triglyceride (TG) accumulation;10 signatures of a metabolic cardiomyopathy termed “lipotoxic cardiomyopathy.”
Reprogramming of the insulin-resistant heart towards FA utilization involves gene regulatory mechanisms. Peroxisome proliferator-activated receptor alpha (PPARα), a fasting-induced nuclear receptor, is chronically activated in the insulin-resistant and diabetic heart;8,13 coordinately up-regulating genes involved in cellular FA uptake and oxidation.14 PPARα is a ligand-activated transcription factor, but the endogenous ligand(s) has not been fully determined. It is well-established that FA moieties and fibrates activate PPARs.15 Transgenic mice with cardiac-specific overexpression of PPARα (myosin heavy chain [MHC]-PPARα mice) exhibit a phenotype similar to that of the diabetic heart: ventricular dysfunction associated with increased FA uptake and FAO, myocyte TG deposition, and reduced glucose utilization.13 This cardiomyopathy of MHC-PPARα mice is exacerbated by high fat (HF) diet, consistent with the importance of FA overload in driving lipotoxic effects.
FAs are delivered to cardiomyocytes from two major sources: 1) albumin-bound free FA (FFA) released from adipose tissue; 2) FA incorporated into TG within very low-density lipoproteins (VLDL) or from intestines within chylomicrons. Both FFA and lipoprotein-derived FA are substrates for human hearts via receptor-mediated uptake. While there are several FA transporters, CD36 is well established to mediate FFA uptake into rodent16 and human hearts.17 Previously, we crossed the MHC-PPARα mice with CD36-deficient mice (MHC-PPARα/CD36ko).18 CD36-deficiency prevented development of cardiomyopathy and myocardial TG overload, and normalized glucose oxidation rates in MHC-PPARα hearts.18 However, FAO rates and expression of PPARα gene targets involved in FAO remained elevated in the MHC-PPARα/CD36ko mice suggesting that PPARα ligand was still being delivered to the MHC-PPARα hearts.
Lipoprotein lipase (LpL) associated with the capillary endothelium is primarily responsible for liberation of TG-derived FA.19 Cardiac-specific deletion of this enzyme reduced heart lipoprotein-derived FA uptake without altering FFA uptake.20 To further define the FA source that drives lipotoxic cardiomyopathy, MHC-PPARα mice were crossed with heart-specific LpL knockout (hsLpLko) mice.20 We found that LpL-deficiency rescues the lipotoxic cardiomyopathy and reverses the chronic activation of PPARα, identifying a source of PPARα ligand, which must be delivered via a CD36-independent pathway. Additionally, we found that the rescue of cardiomyopathy by either CD36- or LpL-deficiency correlates with reactivation of genes involved in regulating mitochondrial function.
METHODS
Animal Generation and Experiments
Generation of MHC-PPARα/hsLpLko mice is described in Supplemental Methods. MHC-PPARα/CD36ko mice have been described.18 For HF diet studies, one month-old male and female mice were fed a diet with 43% calories from fat containing TG composed of long-chain FA (TD01381, Harlan Teklad, Madison, WI) for 4 weeks. All animal experiments were conducted in accordance with NIH guidelines for humane treatment of animals and reviewed by the Animal Studies Committee of Washington University School of Medicine (WUSM).
Histologic Analyses
Oil Red O staining was performed as described by the morphology core of the Digestive Diseases Research Core Center (DDRCC) at WUSM.18
Myocardial Triglyceride Quantification and Plasma Chemistries
Tissue TG and serum FA, TG, and cholesterol levels were determined by enzymatic, colorimetric assays (Thermo Scientific, Waltham, MA and Wako Pure Chemical Industries, Ltd. Osaka, Japan) by the Clinical Research Nutrition Unit (CNRU) Core at WUSM.
Echocardiographic Studies
Transthoracic M-mode and two-dimensional echocardiography was performed on conscious mice (n=3–4 male and 3–4 female/group) using an Acuson Sequoia 256 Echocardiography system (Acuson Corp., Mountain View, CA) as described.21
Electron Microscopy
Papillary muscle was dissected from the left ventricle (LV) of the heart, fixed as described,22 and sectioned for electron microscopy.
RNA Isolation and Quantitative Real-time RT-PCR Analyses
Total RNA was isolated from male and female hearts (n=7–10/group) and subjected to quantitative real-time RT-PCR (Q-rtPCR) using mouse-specific primer-probe sets as previously described.8,23,24
Mouse Isolated Working Heart Preparation
Low-expressing MHC-PPARα/hsLpLko animals were used for isolated working heart (IWH) studies. Eight-week male and female mice (n=6–9/group) on standard diet had IWH preparations performed as described.25 The Krebs-Henseleit perfusate solution contained 5mmol/L glucose, 100μU/mL insulin, and 0.4mmol/L palmitate.
VLDL Uptake Studies
VLDL was isolated from normolipidemic human donors by ultracentrifugation at d=1.006 g/ml for 22 hrs at 39,000 rpm and subjected to labeling as previously described.26 Details regarding VLDL preparation and injection are in Supplemental Materials.
Neonatal Rat Ventricular Myocyte Preparation
Neonatal rat ventricular myocytes (NRVM) were isolated from 1 day-old Sprague-Dawley rat pups using the Worthington Biochemical Corporation kit (Lakewood, NJ). Cells were resuspended in medium (10% horse serum, 5% fetal calf serum, 100μmol/L BrdU, 2mmol/L L-glutamine in DMEM containing 4.5g/L glucose).
Transient Transfection Assays
NRVM were plated at a concentration of approximately 3,000,000 cells/ml in 12-well gelatin-coated (Millipore, Billerica, MA) plates. At the time of plating, cells were transfected with plasmid DNA using the calcium-phosphate method as described.27 Detailed description of plasmid constructs and concentrations, reagents and cell harvest can be found in Supplemental Materials.
Statistics
For quantitative data, statistical comparisons were made using analysis of variance coupled to Tukey’s test or Student’s t test assuming unequal variances. All data are presented as means ± standard error (SE), with a statistically significant difference defined as p<0.05.
RESULTS
LpL deficiency rescues cardiomyopathy in MHC-PPARα mice
Four experimental groups were evaluated: LpLf/f (WT), LpLf/f MHC-Cre(hsLpLko), LpLf/f MHC-PPARα (MHC-PPARα), LpLf/f MHC-PPARα–MHC-Cre(MHC-PPARα/hsLpLko). Similar levels of PPARα gene expression were confirmed among the MHC-PPARα genotypes and absence of LpL was confirmed in the LpLf/f-Cre genotypes by Q-rtPCR and assessment of myocardial LpL enzymatic activity (Supplemental Figure 2). Plasma levels of FFA, TG and cholesterol were not significantly different between any genotype after HF diet (Supplemental Table 1). Although plasma TG trended towards being higher in hsLPLko animals, this was not further altered in the setting of the MHC-PPARα transgene.
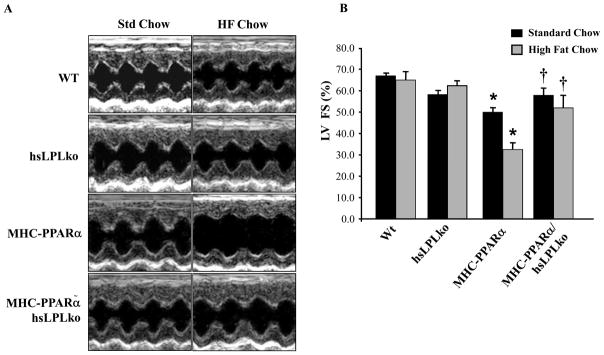
As expected, MHC-PPARα mice exhibited increased LV mass index, reduced LV fractional shortening, and increased LV systolic and diastolic diameters (Figure 1, Supplemental Table 2), a phenotype that worsened following HF diet. In contrast, MHC-PPARα/hsLpLko mice had normal ventricular function, LV mass index, and chamber size on both diets. Thus, LpL is necessary for the development of cardiomyopathy in MHC-PPARα mice.
Figure 1. LpL-deficiency rescues ventricular dysfunction in MHC-PPARα mice.
A) Representative M-mode echocardiographic images of the LV from each genotype at baseline (one month) and after 4 weeks of HF diet. B) Bars represent mean (±SE) percent LV fractional shortening (FS), as determined by echocardiographic analyses. *p<0.05 vs WT and hsLpLko, †p<0.05 vs. MHC-PPARα mice on matched diet (n=6–8/group).
LpL deficiency rescues the myocardial metabolic derangements of MHC-PPARα mice
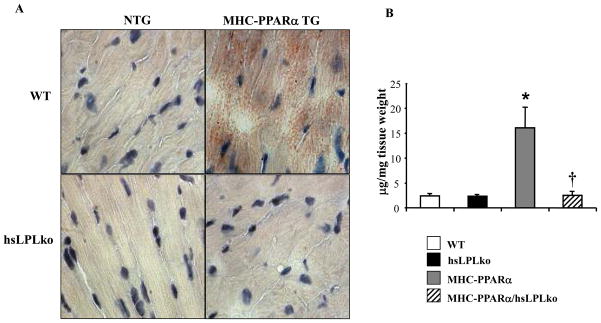
After 4 weeks of HF diet, oil red O staining, a marker of neutral lipid overload, demonstrated abnormal lipid accumulation in myocardial sections from MHC-PPARα mice compared to WT controls (Figure 2A). In striking contrast, lipid staining was similar in hearts from MHC-PPARα/hsLpLko and WT mice. Consistent with these results, TG levels in the hearts of MHC-PPARα mice were 15-fold greater than those of WT controls, but were normal in MHC-PPARα/hsLpLko hearts (Figure 2B).
Figure 2. Reversal of myocardial lipid accumulation in MHC-PPARα/hsLpLko mice on HF diet.
A) Representative photomicrograph depicting oil red O–stained ventricular tissue prepared from all genotypes following 4 weeks of HF diet. Red droplets indicate neutral lipid staining. B) Mean (±SE) myocardial TG levels for each genotype after HF diet (n=3–5/group). *p<0.05 vs WT and hsLpLko, †p<0.05 vs. MHC-PPARα.
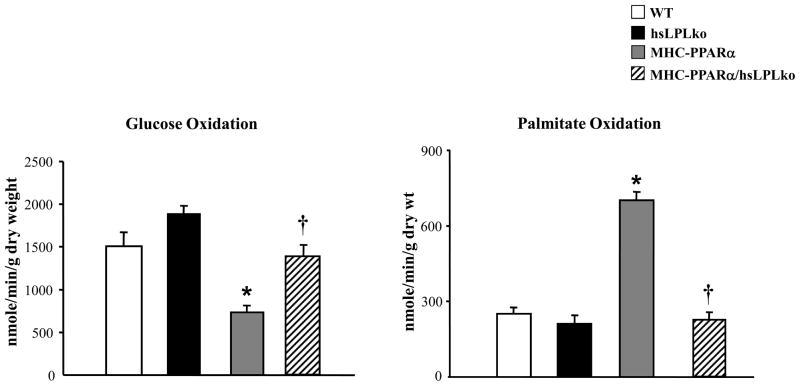
To evaluate the effects of LpL-deficiency on myocardial fuel utilization we used an IWH preparation. Low-expressing MHC-PPARα mice13 were chosen for these experiments because in the absence of HF diet, this line does not develop ventricular dysfunction, which can independently influence myocardial substrate utilization rates. As previously shown, MHC-PPARα mice exhibited high rates of palmitate oxidation and reduced glucose oxidation rates (Figure 3).13,28 In striking contrast, myocardial glucose and palmitate oxidation rates of MHC-PPARα/hsLpLko mice were not different from those of WT animals, indicating a complete reversal of the cardiac fuel utilization abnormalities characteristic of MHC-PPARα mice. Taken together with the results of the myocardial TG measurements, these results indicate that cardiac-specific LpL-deficiency corrects elevated myocardial TG levels and fuel utilization derangements in MHC-PPARα mice.
Figure 3. LpL-deficiency normalizes fuel utilization of MHC-PPARα hearts.
The oxidation rates of [U-14C]glucose (left) and [9,10-3H]palmitate (right) were assessed in IWHs (WT, n=8; hsLpLko, n=6; MHC-PPARα, n=9; MHC-PPARα/hsLpLko, n=6). Bars represent mean (±SE) oxidation rates expressed as nanomoles of substrate oxidized per minute per gram dry weight. *p<0.05 vs WT and hsLpLko, †p<0.05 vs. MHC-PPARα.
Rescue of the cardiomyopathy phenotype of MHC-PPARα mice correlates with normalization of mitochondrial ultrastructural abnormalities
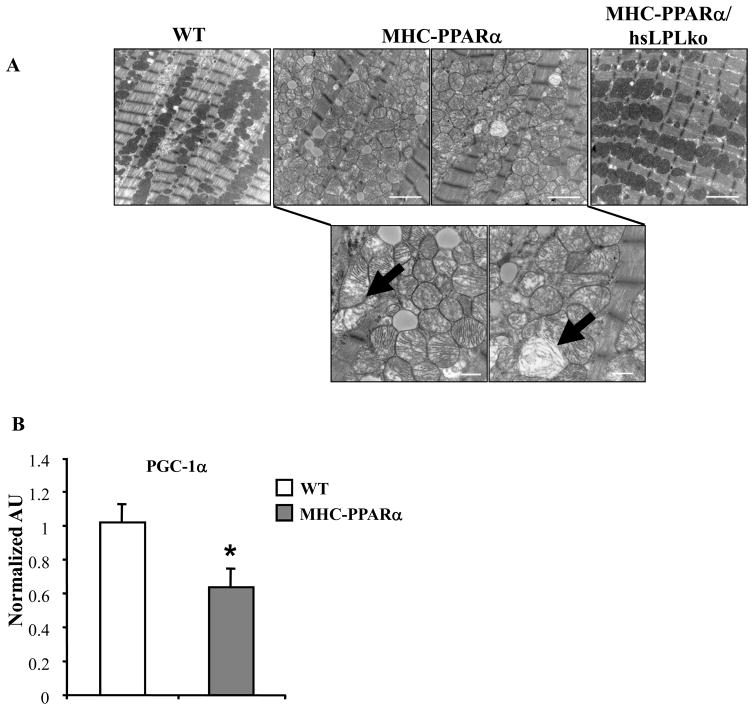
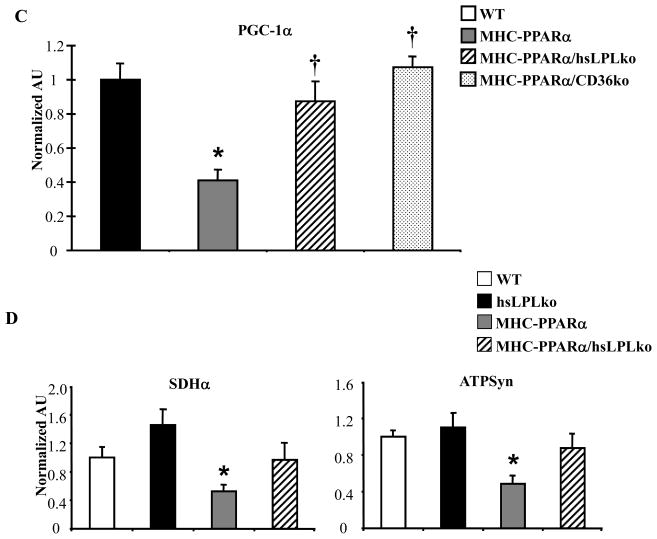
The results above, together with our previously published work,18 demonstrate that the cardiomyopathy associated with MHC-PPARα mice is rescued by either CD36-deficiency or LpL-deficiency. The metabolic phenotypes of the two rescue models display both overlapping and distinct features, potentially providing a clue about the basis for lipotoxic forms of cardiomyopathy. In both cases, TG accumulation is reversed, but only LpL-deficiency reverses the high FAO rates driven by PPARα. These results suggest that TG accumulation rather than increased FAO rates is linked to the cardiac dysfunction. Recent data demonstrates an association between cellular lipid accumulation and mitochondrial dysfunction.7,8,29 In addition, balanced fuel utilization requires appropriately matched mitochondrial capacity to oxidize fuels via the tricarboxylic acid (TCA) cycle and oxidative phosphorylation pathways.30 Evaluation of electron micrographs (EMs) prepared from LVs of MHCPPARα hearts demonstrated disorganized mitochondria with altered cristae density and architecture (black arrows, Figure 4A). Increased mitochondrial volume density was also noted. These changes were strikingly absent in EMs of MHC-PPARα/hsLpLko heart muscle (Figure 4A). The transcriptional coactivator PPARγ coactivator-1α (PGC-1α) serves a critical role in controlling mitochondrial biogenesis and function in the postnatal heart.22,31,32 The expression of PGC-1α is dysregulated in several mouse models of heart failure.33,34 We found that levels of PGC-1α mRNA were significantly reduced in the MHC-PPARα hearts (Figure 4B). Both LpL-deficiency and CD36-deficiency reversed the downregulation of PGC-1α expression (Figure 4C). Furthermore, expression of additional genes involved in mitochondrial metabolism, including the TCA cycle (succinate dehydrogenase, SDHα) and oxidative phosphorylation (ATP synthase β) was reduced in hearts from MHC-PPARα mice but was normalized in MHCPPARα/hsLpLko hearts (Figure 4D). Taken together, these data suggest that myocardial lipid accumulation leads to mitochondrial dysfunction related, at least in part, to suppression of PGC-1α gene expression.
Figure 4. Mitochondrial gene expression and ultrastructure are improved in MHC-PPARα/hsLpLko hearts.
A) Representative EMs from papillary muscle of WT, MHC-PPARα, and MHC-PPARα/hsLpLko hearts. White bars=2 microns. B) Q–rtPCR analysis of cardiac transcripts encoding PGC-1α in WT and MHC-PPARα hearts (n=9/genotype), C) PGC-1α in MHC-PPARα/hsLpLko and MHC-PPARα/CD36ko compared to WT and MHC-PPARα (n=6–7/group) and D) additional gene targets (abbreviations in text) in hearts from WT, MHC-PPARα, hsLpLko, MHC-PPARα and MHC-PPARα/hsLpLko (n=7–9/group). All bars represent mean (± SE) arbitrary unit (AU) normalized to the WT value (=1.0) in each case. *p<0.05 vs WT and hsLPLko, †p<0.05 vs. MHC-PPARα.
LpL is required for the delivery of PPARα ligand to the cardiac myocyte
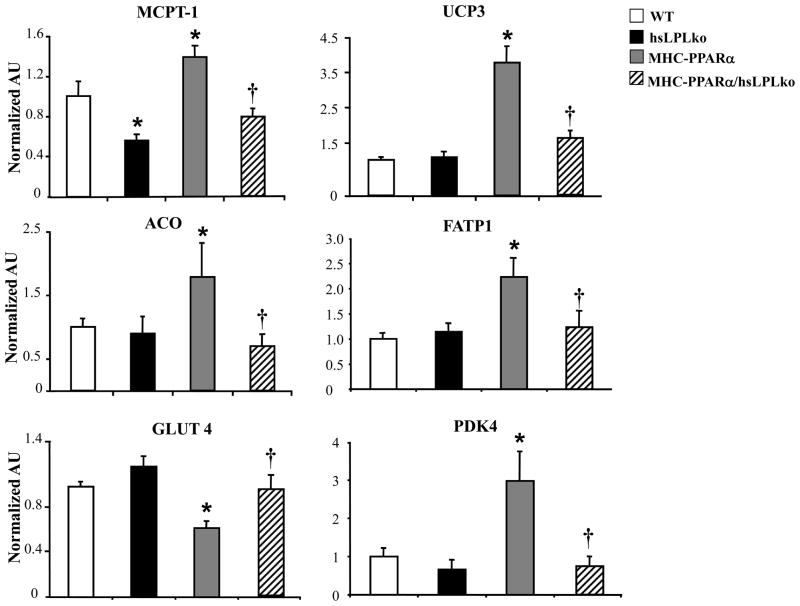
LpL-deficiency rescued both glucose and FA utilization abnormalities in MHC-PPARα mice, unveiling a striking phenotypic difference compared to CD36-deficiency. Specifically, CD36-deficiency did not reverse the high FAO rates and the increased expression PPARα FAO target genes in MHC-PPARα hearts, indicating that PPARα is still chronically stimulated in this model.18 In contrast, cardiac expression of PPARα target genes in the MHC-PPARα/hsLpLko mice was not different than WT. Specifically, the expression of PPARα target genes involved in FAO (muscle-type carnitine palmitoyl-transferase-1, MCPT-1; uncoupling protein 3, UCP3; acyl-coA oxidase, ACO), FA uptake (FA transport protein 1, FATP1) and glucose metabolism (pyruvate dehydrogenase kinase 4, PDK4; glucose transporter 4, GLUT4) in hearts from HF-fed MHC-PPARα/hsLpLko mice was normalized to the level of WT animals (Figure 5). CD36 expression was not significantly different between genotypes (Supplemental Figure 3). PPARβ also plays a role in regulating cardiac metabolism and we have found that the ratio of PPARα/PPARβ drives lipid accumulation and cardiomyopathy.35 Gene expression for PPARβ was mildly increased in MHC-PPARα hearts (2.2 fold) but unchanged in MHC-PPARα/hsLpLko hearts compared to WT (data not shown).
Figure 5. Cardiac metabolic PPARα target gene expression is normalized in LpL-deficient MHC-PPARα hearts.
A) mRNA levels of FA metabolism gene targets and B) glucose metabolism gene targets (abbreviations in text) as determined by Q-rtPCR using RNA from hearts of 2 month-old mice after HF diet (n=7–9/genotype). Bars represent mean (± SE) AU normalized to the WT value (=1.0). *p<0.05 vs WT and hsLpLko, †p<0.05 vs. MHC-PPARα.
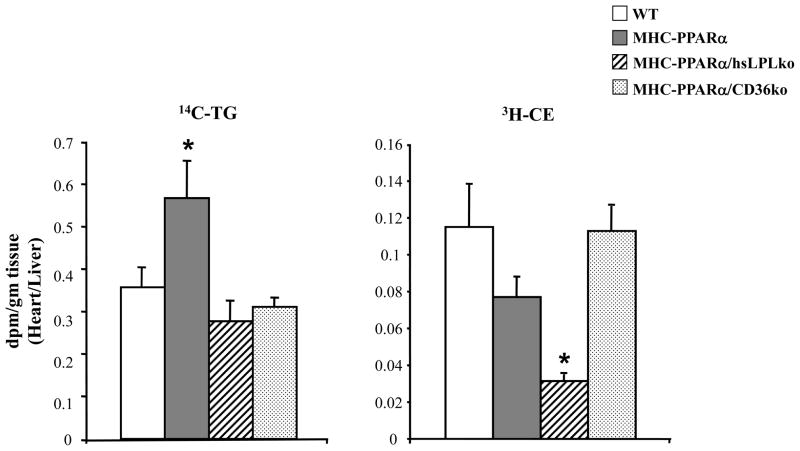
The gene expression data demonstrating LpL-deficiency normalizes expression of PPARα metabolic gene targets, suggests that LpL plays a more important role than CD36 in delivering FA or other PPARα ligands to cardiomyocytes. To assess the impact on lipoprotein-derived FA uptake in the two models, radiolabeled lipid uptake studies were performed in WT, MHC-PPARα, MHC-PPARα/hsLpLko, and MHC-PPARα/CD36ko mice using double-labeled VLDL; TG was labeled with 14C and cholesteryl ester (CE) was labeled with 3H. 8–10 week old animals were injected with the dual-labeled VLDL and tissue was harvested 30 minutes following injection. Cardiac uptake of 14C-labeled TG by MHC-PPARα mouse hearts was increased compared to WT animals. TG-derived lipid uptake was normalized in both LpL- and CD36-deficient MHC-PPARα mice (Figure 6). In contrast, 3H-CE uptake was dramatically reduced in LpL-deficient MHC-PPARα mice but not in CD36-deficient hearts (Figure 6). A similar reduction in CE uptake was seen in hsLpLko animals compared to WT animals (data not shown).
Figure 6. Heart uptake of VLDL-TG and -CE is altered in MHC-PPARα/hsLpLko hearts.
Bars represent mean (±SE) cardiac lipid uptake as represented by radioactive count (dpm/gram of tissue), normalized to liver radioactive counts, for TG (14C-TG, left) and CE (3H-CE, right) in WT (n=6), MHC-PPARα (n=7), MHC-PPARα/hsLpLko (n=8), and MHC-PPARα/CD36ko (n=8) animals. *p<0.05 compared to all other groups.
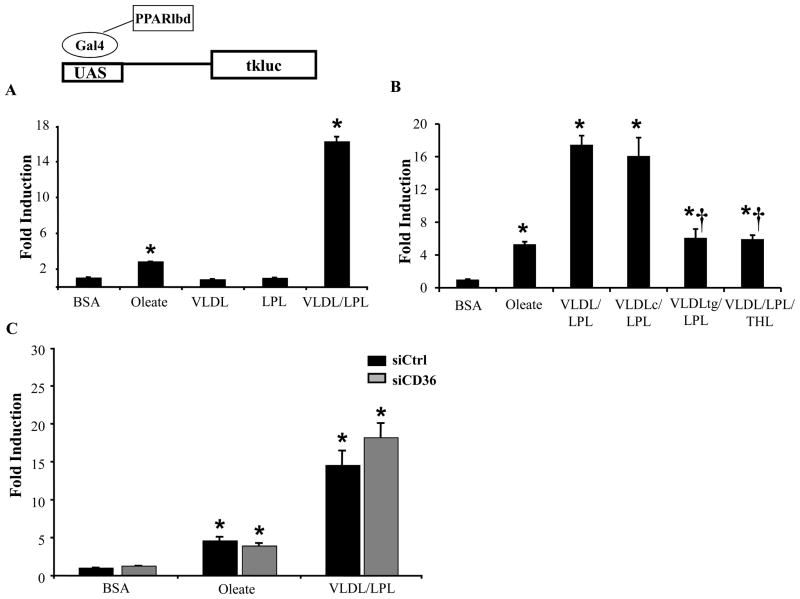
LpL- and CD36-deficient hearts differed in uptake of “core lipids” that are not hydrolyzed and remain associated with the VLDL remnant. Thus, we postulated that lipid contained in lipoprotein remnants activates PPARα in heart and that the ligand enters the cardiomyocyte independent of CD36, in an LpL-dependent manner. To test this hypothesis, a cell culture assay was employed in which the Gal4-PPARα-LBD fusion reporter was used in primary NRVMs. As expected, the FA oleate activated Gal4-PPARα-LBD to a modest (3-fold) but significant extent (Figure 7A). Docohexanoic acid (DHA) had an effect similar to oleate, but palmitate was unable to activate the Gal4-PPARα-LBD (Supplemental Figure 5). VLDL alone had no effect on Gal4-PPARα-LBD activitation. However, in the presence of LpL, VLDL resulted in a striking 15-fold activation of Gal4-PPARα-LBD, consistent with the hypothesis that LpL is necessary for VLDL-mediated PPARα activation. Studies done using higher concentrations of oleate resulted in a slight additional effect at 400umoles/L, but the VLDL/LpL effect was still much greater (data not shown). To assess the necessity of LpL enzymatic activity in VLDL activation of PPARα, the experiments were repeated using VLDL that had been depleted of TG by pretreatment with LpL or in the presence of the LpL inhibitor tetrahydrolipostatin (THL). Both the TG-depleted VLDL remnant (which contains core lipids), and VLDL+THL+LpL were only able to activate the reporter to a level similar to oleate (Figure 7B). These results indicate that the mechanism whereby LpL increases PPARα activation requires TG lipolysis and intact LpL enzymatic activity.
Figure 7. Lipolysis of VLDL by LpL results in activation of PPARα.
Transient co-transfections using the UAS3tk.luc reporter and the Gal4-PPARα-LBD performed in NRVM. Cells were stimulated for the last 12–14 hours with A) BSA, 100mmol/L oleate, VLDL alone, LpL alone, or VLDL+LpL. B) BSA, or LpL+VLDL (original), re-isolated VLDL control (VLDLc) or TG-depleted VLDL (VLDLtg), or VLDL+LpL+THL. C) Same co-transfection conditions as in A, but with either siCtrl or siCD36. The bars represent mean (±SE) relative light units (RLU) for three or more experiments each done in triplicate, corrected for Renilla luciferase activity, and normalized to the activity of cells stimulated with BSA (=1.0). *p<0.05 compared to BSA treatment for each siRNA, †p≤0.05 compared to VLDL+LpL.
Our results indicated that the LpL-mediated delivery of PPARα ligand was CD36-independent. Thus, the NRVM experiments were repeated following “knockdown” of CD36. CD36 siRNA (siCD36) reduced CD36 mRNA expression by greater than 90% compared to cells transfected with control siRNA (siCtrl) (Supplemental Figure 4). NRVM were transfected with either siCtrl or siCD36 in the presence of oleate or VLDL/LpL. Cells treated with oleate or VLDL/LpL and siCD36 had a visible decrease in intra-cellular lipid droplets compared to cells transfected with siCtrl, providing evidence for a reduction of CD36 function (data not shown). However, even in the presence of siCD36, oleate or VLDL/LpL activated the Gal4-PPARα-LBD (Figure 7C). These data are consistent with the in vivo data and strongly suggest that ligand(s) generated by LpL-mediated hydrolysis of VLDL is transported into the myocyte independent of CD36.
DISCUSSION
To understand the contributions of increased FA utilization and lipid accumulation to diabetic cardiac dysfunction, several transgenic mouse models have been generated including: cardiac-specific LpL,36 acyl-CoA-synthetase and,37 FATP1 overexpression,38 and the MHC-PPARα model used in this study.28 MHC-PPARα mice reproduce the diabetic metabolic signatures of increased FAO and cardiac TG stores combined with reduced glucose utilization. Characterization of these models has led to new questions relevant to the role of lipotoxicity in the development of cardiomyopathy including: 1) What is the relative contribution of FAO and TG accumulation to the cardiomyopathy? 2) Why, despite greater FAO rates, does TG accumulate in the myocyte? 3) What is the source of excess lipid (FFA or lipoproteins) and does it also activate PPARα?
We have previously shown that cardiac function can be restored in MHC-PPARα mice bred into a CD36-deficient background.18 CD36-deficiency reduces uptake of both FFA and lipoprotein-derived FAs. The current study addressed the specific contribution of LpL to lipotoxic cardiomyopathy, when FA transporters remained intact. The study revealed three key findings: 1) cardiac LpL-deficiency rescues contractile dysfunction and myocardial TG accumulation in MHC-PPARα mice; 2) lipoprotein-derived FA is a major source of lipid for this form of cardiomyopathy; 3) delivery of PPARα ligand is dependent on LpL-mediated hydrolysis of VLDL and independent of CD36.
Many mechanisms have been proposed for cardiac dysfunction associated with excessive lipid uptake, including: 1) direct toxicity due to FAs, TG, or other lipids;39,40 2) excessive FAO resulting in reactive oxygen species and/or increased oxygen consumption;39 3) decreased glucose oxidation; 4) altered mitochondrial function.7,8,29 The current rescue with LpL-deficiency is similar to our previous MHC-PPARα/CD36ko animals in the rescue of TG accumulation, confirming that lipid excess contributes to contractile dysfunction. Correction of the lipotoxicity by LpL deletion is further proof that lipoprotein-TG, and not FFA, is the primary culprit.
Our data also point to derangements in mitochondrial function, mediated by downregulation of PGC-1α, as a potential determinant of cardiomyopathy in this lipotoxic model. Consistent with this notion, we found that LpL-deficiency corrected the reduction in mitochondrial metabolic target gene expression and mitochondrial ultrastructural derangements in hearts of MHC-PPARα mice. Based on these results, we speculate that myocyte lipid overload leads to reduced PGC-1α signaling, which in turn contributes to ventricular dysfunction in lipotoxic cardiomyopathy. The mechanism whereby PGC-1α gene expression is downregulated by cellular lipid overload is unknown but is likely a temporal phenomenon, because in insulin-resistant hearts we have found that both PPARα and PGC-1α expression are upregulated,8 perhaps an adaptive response that fails once lipotoxic effects ensue. We have also found that PPARα is capable of activating the PGC-1α promoter in certain cell types (Duncan and Kelly, unpublished data), suggesting an autoregulatory loop exists between PPARα and its coactivator PGC-1α. We suspect that lipid overload combined with PPARα activation results in downregulation of PGC-1α via mechanisms that have not been delineated. Our current data cannot exclude a contribution of reduced myocardial glucose oxidation to contractile dysfunction, as both CD36-deficiency and LpL-deficiency normalized glucose oxidation rates in MHC-PPARα mice. However, in another model of cardiac lipotoxicity (MHC-PPARγ), glucose uptake is not altered.41
A novel finding in this study was that LpL-deficiency reversed the excess FAO and chronic activation of PPARα gene targets involved in FAO. This observation contrasts with CD36-deficiency which did not affect activation of PPARα targets or FAO in the MHC-PPARα mice.18 These results identify a fundamental difference in the pathways involved in delivery and generation of ligand for PPARα in the heart. Because CD36 reduces uptake of both FFA and lipoprotein-TG, the reduction of total FA uptake should be greater in MCH-PPARα/CD36ko; our data implicate a ligand other than FFA in PPARα activation. Previous in vitro studies have shown that VLDL/LpL can activate PPARs in endothelial cells and macrophages.42–44 Our data extend these findings to an in vivo model, and suggest that myocardial LpL participates in PPARα ligand generation. NRVM studies confirmed that VLDL, in the presence of LpL, leads to robust activation of a PPARα-reporter. Furthermore, activation of PPARα by VLDL/LpL or oleate occurs in the absence of CD36, confirming that the ligand-delivery pathway is independent of CD36. Possibly, mechanisms such as diffusion45 or alternative transporters, (e.g. FATP family members) are important for transporting and targeting PPARα ligand in the CD36-deficient animals. Notably, LpL-deficiency does not eliminate PPARα activation, reflected by target gene expression and FAO rates similar to those of WT, suggesting that some ligand is available for basal PPARα activation. In addition, the lipid uptake studies demonstrate that MHC-PPARα/hsLPLko animals have TG uptake similar to WT, suggesting that basal VLDL-TG uptake is preserved. This may occur via several mechanisms including: 1) non-cardiomyocyte LpL activity (e.g. macrophages);46 2) peripheral lipolysis leading to FA uptake by non-LpL mediated pathways; or 3) uptake of VLDL “remnant” lipoproteins generated via peripheral lipolysis.
The specific cardiac PPARα ligand was not defined in this study, but several clues were provided. The VLDL uptake studies performed in vivo revealed a dramatic difference in CE uptake between CD36-deficient and LpL-deficient MHC-PPARα animals. CE is a marker of whole particle uptake, suggesting that the ligand activity could reside in lipid moieties other than TG-derived FA. However, the NRVM experiments using TG-depleted VLDL demonstrated a decreased ability to activate the PPARα-LBD, suggesting that TG lipolysis is necessary for the full PPARα-activating effect; providing remnant-like lipoproteins to the cells alone is not sufficient. These latter results are consistent with previous findings suggesting that intact LpL enzymatic activity was necessary for PPARα activation.43 Possibly, ligand generation from VLDL particle components is a multi-step process requiring TG hydrolysis and components of the core lipid. LpL hydrolysis results in the elaboration of a number of other lipid species, including oxidized lipids (e.g. HODEs47) that could activate PPARα. It should also be noted that we have not excluded the possibility that activating ligand(s) for related PPARs, (PPARδ, PPARγ) may be carried by the VLDL particle. Further studies are warranted to evaluate the specific components of VLDL lipolysate that are capable of activating PPARα and their downstream effect on the cardiomyocyte. Identification of the specific PPARα ligand could lead to tissue-specific strategies to block chronic activation of the cardiac PPARα pathway and thus develop preventive or therapeutic strategies for lipotoxic cardiomyopathy in humans.
Supplementary Material
Acknowledgments
The authors thank Zhouji Chen for assistance with VLDL experiments, Bill Kraft for expert technical assistance with electron microcopy, and Teresa Leone for critical review of the manuscript.
SOURCES OF FUNDING
JGD is supported by NHLBI K08 award (HL084093) and is a Scholar of the Child Health Research Center of Excellence in Developmental Biology at WUSM (K12-HD001487). This work was also supported by the CNRU (P30 DK56341), DDRCC (P30 DK052574) and NIH grants HL45095 and HL73029 (IJG) and 5P50HL07711304 (DPK).
Footnotes
Clinical Perspective
Metabolic syndrome and type 2 diabetes are dramatically increasing in prevalence world-wide. Cardiovascular disease is the leading cause of death amongst patients with diabetes, including a unique form of heart failure that is independent of coronary risk factors. Accumulation of myocardial lipid and alterations in cardiac fuel metabolism likely play an important role in cardiomyopathy in diabetics, a problem referred to as “lipotoxic cardiomyopathy.” This manuscript explores several key questions related to the cause of diabetic heart disease including: 1) What is the contribution of excess fatty acid burning to contractile dysfunction? 2) What is the source of excess fat and does it activate PPARa, an important transcriptional mediator of increased FA uptake and utilization in the diabetic heart? We demonstrate in an animal model of lipotoxic cardiomyopathy, that lipoprotein-triglyceride rather than free fatty acid is the primary contributor to cardiomyopathy. Furthermore, we link lipoprotein lipase (LpL) hydrolysis of very low density lipoproteins to activation of PPARa, suggesting that LpL plays a critical role in both driving lipid excess and generating ligand for activation of PPARa; thus promoting excess FA utilization pathways. Finally, our data suggest that lipid excess is associated with mitochondrial dysfunction, a likely mechanism for cardiac failure in patients with lipotoxic cardiomyopathy. Future studies to identify the specific PPARa ligand generated by LpL may help guide therapeutic strategies for diabetic heart failure.
DISCLOSURES
DPK serves on the Scientific Advisory Boards for Lilly and Johnson and Johnson.
References
- 1.Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, Imperatore G, Narayan KM. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 2.Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Endocrinol Metab Clin North Am. 2008;37:663–684. ix. doi: 10.1016/j.ecl.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Abbott RD, Donahue RP, Kannel WB, Wilson PW. The impact of diabetes on survival following myocardial infarction in men vs. women. JAMA. 1988;260:3456–3460. [PubMed] [Google Scholar]
- 4.Cohen-Solal A, Beauvais F, Logeart D. Heart failure and diabetes mellitus: epidemiology and management of an alarming association. J Card Fail. 2008;14:615–625. doi: 10.1016/j.cardfail.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Cooper ME. Importance of advanced glycation end products in diabetes-associated cardiovascular and renal disease. Am J Hypertens. 2004;17:31S–38S. doi: 10.1016/j.amjhyper.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Orasanu G, Plutzky J. The pathologic continuum of diabetic vascular disease. J Am Coll Cardiol. 2009;53:S35–42. doi: 10.1016/j.jacc.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 8.Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-α/PGC-1α gene regulatory pathway. Circulation. 2007;115:909–917. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leichman JG, Lavis VR, Aguilar D, Wilson CR, Taegtmeyer H. The metabolic syndrome and the heart-a considered opinion. Clin Res Cardiol. 2006;95:i134–141. doi: 10.1007/s00392-006-1119-7. [DOI] [PubMed] [Google Scholar]
- 10.Lopaschuk GD. Metabolic abnormalities in the diabetic heart. Heart Fail Rev. 2002;7:149–159. doi: 10.1023/a:1015328625394. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 12.Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res. 2007;101:759–767. doi: 10.1161/CIRCRESAHA.107.160457. [DOI] [PubMed] [Google Scholar]
- 13.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 15.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 17.Nozaki S, Tanaka T, Yamashita S, Sohmiya K, Yoshizumi T, Okamoto F, Kitaura Y, Kotake C, Nishida H, Nakata A, Nakagawa T, Matsumoto K, Kameda-Takemura K, Tadokoro S, Kurata Y, Tomiyama Y, Kawamura K, Matsuzawa Y. CD36 mediates long-chain fatty acid transport in human myocardium: complete myocardial accumulation defect of radiolabeled long-chain fatty acid analog in subjects with CD36 deficiency. Mol Cell Biochem. 1999;192:129–135. [PubMed] [Google Scholar]
- 18.Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN, Kelly DP. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res. 2007;100:1208–1217. doi: 10.1161/01.RES.0000264104.25265.b6. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res. 1996;37:693–707. [PubMed] [Google Scholar]
- 20.Augustus A, Yagyu H, Haemmerle G, Bensadoun A, Vikramadithyan RK, Park SY, Kim JK, Zechner R, Goldberg IJ. Cardiac-specific knock-out of lipoprotein lipase alters plasma lipoprotein triglyceride metabolism and cardiac gene expression. J Biol Chem. 2004;279:25050–25057. doi: 10.1074/jbc.M401028200. [DOI] [PubMed] [Google Scholar]
- 21.Rogers JH, Tamirisa P, Kovacs A, Weinheimer C, Courtois M, Blumer KJ, Kelly DP, Muslin AJ. RGS4 causes increased mortality and reduced cardiac hypertrophy in response to pressure overload. J Clin Invest. 1999;104:567–576. doi: 10.1172/JCI6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1α deficient mice exhibit multi-system energy metabolic derangements: Muscle dysfunction, abnormal weight control, and hepatic steatosis. PLoS Biol. 2005;3:672–687. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly DP, Gordon JI, Alpers R, Strauss AW. The tissue-specific expression and developmental regulation of the two nuclear genes encoding rat mitochondrial proteins: medium-chain acyl-CoA dehydrogenase and mitochondrial malate dehydrogenase. J Biol Chem. 1989;264:18921–18925. [PubMed] [Google Scholar]
- 24.Huss JM, Imahashi KI, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguäre V, Murphy E, Kelly DP. The nuclear receptor ERRα is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Belke DD, Larsen TS, Lopaschuk GD, Severson DL. Glucose and fatty acid metabolism in the isolated working mouse heart. Am J Physiol. 1999;277:R1210–R1217. doi: 10.1152/ajpregu.1999.277.4.R1210. [DOI] [PubMed] [Google Scholar]
- 26.Seo T, Al-Haideri M, Treskova E, Worgall TS, Kako Y, Goldberg IJ, Deckelbaum RJ. Lipoprotein lipase-mediated selective uptake from low density lipoprotein requires cell surface proteoglycans and is independent of scavenger receptor class B type 1. J Biol Chem. 2000;275:30355–30362. doi: 10.1074/jbc.M910327199. [DOI] [PubMed] [Google Scholar]
- 27.Carter ME, Gulick T, Raisher BD, Caira T, Ladias JA, Moore DD, Kelly DP. Hepatocyte nuclear factor-4 activates medium chain acyl-CoA dehydrogenase gene transcription by interacting with a complex regulatory element. J Biol Chem. 1993;268:13805–13810. [PubMed] [Google Scholar]
- 28.Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP. A critical role for PPARα-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: Modulation of phenotype by dietary fat content. Proc Natl Acad Sci USA. 2003;100:1226–1231. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–2695. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- 30.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros D, Kelly DP. PPARγ coactivator-1 (PGC-1) promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, Medeiros DM, Kovacs A, Kelly DP. Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes Dev. 2008;22:1948–1961. doi: 10.1101/gad.1661708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garnier A, Fortin D, DelomÇnie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sano M, Wang SM, Shirai M, Scaglia F, Xie M, Sakai S, Tanaka T, Kulkarni PA, Barger PM, Youker KA, Taffet GE, Hamamori Y, Michael LH, Craigen WJ, Schneider MD. Activation of cardiac Cdk9 represses PGC-1 and confers a predisposition to heart failure. EMBO J. 2004;23:3559–3569. doi: 10.1038/sj.emboj.7600351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP. Nuclear receptors PPARβ/δ and PPARα direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest. 2007;117:3930–3939. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, Seo T, Hu Y, Lutz EP, Merkel M, Bensadoun A, Homma S, Goldberg IJ. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–426. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS, Schaffer JE. Transgenic expression of FATP1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–233. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- 39.Dyntar D, Eppenberger-Eberhardt M, Maedler K, Pruschy M, Eppenberger HM, Spinas GA, Donath MY. Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes. 2001;50:2105–2113. doi: 10.2337/diabetes.50.9.2105. [DOI] [PubMed] [Google Scholar]
- 40.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human disease. Proc Natl Acad Sci USA. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed W, Orasanu G, Nehra V, Asatryan L, Rader DJ, Ziouzenkova O, Plutzky J. High-density lipoprotein hydrolysis by endothelial lipase activates PPARalpha: a candidate mechanism for high-density lipoprotein-mediated repression of leukocyte adhesion. Circ Res. 2006;98:490–498. doi: 10.1161/01.RES.0000205846.46812.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziouzenkova O, Perrey S, Asatryan L, Hwang J, MacNaul KL, Moller DE, Rader DJ, Sevanian A, Zechner R, Hoefler G, Plutzky J. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase. Proc Natl Acad Sci USA. 2003;100:2730–2735. doi: 10.1073/pnas.0538015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chawla A, Lee CH, Barak Y, He W, Rosenfeld J, Liao D, Han J, Kang H, Evans RM. PPARI is a very low-density lipoprotein sensor in macrophages. Proc Natl Acad Sci USA. 2003;100:1268–1273. doi: 10.1073/pnas.0337331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton JA, Guo W, Kamp F. Mechanism of cellular uptake of long-chain fatty acids: Do we need cellular proteins? Mol Cell Biochem. 2002;239:17–23. [PubMed] [Google Scholar]
- 46.Babaev VR, Fazio S, Gleaves LA, Carter KJ, Semenkovich CF, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J Clin Invest. 1999;103:1697–1705. doi: 10.1172/JCI6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res. 2009;50:204–213. doi: 10.1194/jlr.M700505-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.