Abstract
T helper-2 (TH2)-bias, the propensity of naive CD4+ T cells to differentiate into interleukin 4 (IL-4) secreting TH2 cells, is a genetic trait impacting infectious, autoimmune and allergic disease susceptibility. TH2-bias correlates with the amount of IL-4 initially secreted by newly activated TH cells that feeds back positively through the IL-4R-STAT6-GATA3 pathway to drive TH2 development. Here, we identify Mina, a JmjC family member, as a genetic determinant of TH2-bias. Mina specifically bound to and repressed the Il4 promoter. Mina overexpression in transgenic mice impaired Il4 expression, while its knockdown in primary CD4+ T cells led to Il4 derepression. Together, these findings provide mechanistic insight into an Il4 regulatory pathway controlling TH differentiation and genetic variation in TH2-bias.
Naive CD4+ T cells are multipotent sentinels of the immune system, poised to respond to instructive signals from antigen-presenting cells by differentiating into distinct effector cell lineages. These include T helper (TH) 1 and TH2 cells, differentially adapted for the control, respectively, of intra- and extracellular pathogens, in part via developmentally acquired potential for high expression of distinct cytokine genes1. Dysregulated CD4+ T cell development can promote susceptibility to infectious, autoimmune and allergic disease2–9.
Interleukin 4 (IL-4) [http://www.signaling-gateway.org/molecule/query?afcsid=A001262], the canonical TH2 effector cytokine, is also a critical developmental determinant, promoting TH2 and inhibiting TH1 differentiation10. Recently activated TH cells make low but functional amounts of IL-4 that induce positive feedback through the IL-4 receptor (IL-4R) [http://www.signaling-gateway.org/molecule/query?afcsid=A001263] and transcription factors STAT6 [http://www.signaling-gateway.org/molecule/query?afcsid=A002236] and GATA311, 12 to promote the differentiation of TH2 cells possessing the capacity to secrete copious amounts of IL-410, 13–18. Thus, regulation of autocrine IL-4 expression by activated TH cells is a key control point in T helper cell lineage commitment. Nonetheless, the molecular mechanism underlying this regulation is incompletely understood.
TH2-bias is a complex genetic trait characterizing variation in the propensity of naive TH cells to differentiate into TH2 (as opposed to TH1) cells. TH2-bias, measured experimentally as the amount of IL-4 produced by effector CD4+ T cells differentiated in vitro from naive TH cells activated under ‘neutral’ conditions (no exogenous cytokines added, except IL-2, and cultured without cytokine-specific antibodies), varies over 50-fold from the high-producer phenotype of BALB/c mice to the low-producer phenotype of B10.D2 mice and correlates with susceptibility to TH2-dependent diseases such as bronchial asthma and leishmaniasis14–16, 19–22. Various cellular mechanisms have been suggested as the basis for TH2-bias, including variation in the sensitivity to prostaglandin 2 (PGE2)-dependent inhibition of interferon-γ (IFN-γ) production23, the timing of IL-12Rβ2 downregulation24, 25 and the capacity of activated TH cells to produce autocrine IL-4 (refs. 14–16, 20). Genetic approaches to dissect TH2-bias have yielded numerous quantitative trait loci (QTL) spread across mouse chromosomes 5, 12, 14 15, 16, 17, 18 and 19 (refs. 14, 26–29) and a single discrete genetic locus on chromosome 11 (refs. 24, 25). Several QTLs have been confirmed and isolated as discrete genetic loci by interval specific congenic mapping14, 26, 28. However, to date none of the underlying genetic determinants have been identified.
Here, we combine classical genetic and transcriptional profiling analyses to identify Mina, a member of the Jumonji (Jmj) family, as a determinant of the TH2-bias regulatory locus Dice1.2 that was identified through its activity in the autocrine IL-4 pathway of activated TH cells28. We found that TH2-bias and autocrine Il4 expression correlated inversely with Mina transcriptional rate that, in turn, correlated with Mina locus haplotype. Consistent with these findings, we show that Mina bound to the Il4 promoter where it repressed Il4 expression. We propose that a regulatory polymorphism controlling Mina expression in activated TH cells determines the strength of Mina-dependent Il4 repression and hence the degree of autocrine IL-4 production and ultimately the extent of TH2 differentiation, thereby accounting, at least in part, for strain-specific differences in TH2-bias.
Results
Mina is a Dice1.2 candidate gene
Using QTL and interval specific congenic mapping, we previously mapped an Il4 regulatory locus controlling TH2-bias, Dice1.2, to a 26.8 Mb chromosome 16 genomic interval (Fig. 1a and 14, 28). To resolve this interval further, we generated sub-congenic strain C16D2/8D that bisects the Dice1.2-spanning B10.D2 (low TH2-bias)-derived genomic segment contained in the BALB/c (high TH2-bias) background congenic strain C16D2/8 (ref. 28) to 14.1 Mb (Fig. 1a, D16MIT138-MB04). Splenic CD4+ T cells from BALB/c, B10.D2 and C16D2/8D were primed for 16 h before analysis of Il4 expression by quantitative reverse transcriptase PCR (RT-PCR). As expected, control BALB/c and B10.D2 cells exhibited, respectively, high and low TH2-bias phenotypes (Fig. 1b). Production of Il4 transcripts by C16D2/8D cells was similar to B10.D2 and significantly less than BALB/c (P = 0.0317). This result indicates that Dice1.2 occurs within the 13.7 Mb C16D2/8D congenic interval that spans D16MIT138 to MB04 and contains 121 predicted or known genes30. Twenty nine of these encode olfactory receptors, leaving 92 Dice1.2 candidates.
Figure 1.
Mina is a Dice1.2 candidate gene. (a) Location of chromosome 16 congenic intervals in C16D2/8 and C16D2/8D. Shown for each strain are the chromosomal regions inherited from B10.D2 (grey), BALB/c (white) or undetermined (striped). Genotyped locations are indicated with cyan dots. Genotyping markers are indicated on the right axis, with Mina in red. Long and short vertical bars indicate the original and new Dice1.2 genetic intervals, respectively. (b) Relative Il4 expression (Hprt1-normalized, arbitrary units) in TCR-activated CD4+ T cells from BALB/c (BALB), B10.D2 (D2) and C16D2/8D mice (n = 2–5; each symbol represents an individual mouse and the mean is indicated by a horizontal line). * Statistical significance was determined using a t-test; P = 0.0317. Data are representative of 2 experiments with similar results.
The polymorphism(s) in Dice1.2 underlying genetic variability in TH2-bias may reside in coding or regulatory sequence. To test for the latter, we interrogated genes in the Dice1.2 interval by transcriptional profiling using mRNA isolated from BALB/c and C16D2/8D CD4+ T cells stimulated by T cell receptor (TCR) cross-linking for 16 h, a time point when differential Il4 expression was nearly maximal. The Affymetrix gene chip used for initial screening contained 30 Dice1.2 candidate genes. Of these, half were undetectable and of the remainder, all but one displayed similar expression in BALB/c and C16D2/8D cells. The one exception was Mina which exhibited two-to-three-fold higher expression in C16D2/8D than in BALB/c cells (Supplementary Fig. 1 online). Although expression profile analysis of the remaining genes in the Dice1.2 interval could reveal additional Dice1.2 candidates, we explored here the possibility that Mina acts as a negative regulator of Il4 gene expression and TH2-bias.
Inverse correlation in IL-4 and Mina levels
First, to investigate whether the inverse correlation in Mina and Il4 expression observed in BALB/c and B10.D2 was generalizable, we used quantitative RT-PCR to explore their expression in recently activated CD4+ T cells isolated from a panel of independent inbred mouse strains representing high and low TH2-bias phenotypes. Whereas the kinetics of Mina and Il4 transcriptional induction was similar across the tested strains, peak magnitudes were markedly dissimilar, segregating the tested strains into two discrete groups (Fig. 2a). In low TH2-bias C57BL/6, B10.D2 and C3H/HeN strains, high Mina expression correlated with low Il4 expression; while in high TH2-bias BALB/c, DBA/1J, DBA/2J and DBA/2N strains, the converse was true (Fig. 2a). Thus, the inverse correlation in Il4 and Mina expression was generalizable across strains with varying TH2-bias phenotypes.
Figure 2.
Inverse correlation in Mina and Il4 expression in naïve TH cells. (a) Mina (left) and Il4 (right) mRNA expression (Actb-normalized, arbitrary units) time course from TCR cross-linking-activated CD4+ T cells isolated from low (B10.D2, C57BL/6, and C3H/HeN; filled symbols) and high (BALB/c, DBA/1J, DBA/2J, and DBA/2N; open symbols) TH2-biased strains. Data are the mean of three independent experiments and error bars indicate SEM. (b) Mina, Il4 and Ifng mRNA expression (Hprt1-normalized, arbitrary units) time course from (top) TCR cross-linking-activated BALB/c (open symbol) and C57BL/6 (closed symbol) naive TH cells or from (bottom) TH1- (closed symbol) and TH2- (open symbol) primed C57BL/6 naive TH cells. Data are representative of two independent experiments and error bars indicate SEM for triplicate PCR measurements. (c) Mina expression (Actb-normalized, arbitrary units) in CD4+ T cells stimulated with TCR cross-linking for 24 h in the presence of graded doses of IL-4 (B10.D2) or anti-IL-4 (BALB/c). Data are the mean of three independent experiments and error bars indicate SEM. (d) Mina expression (Actb-normalized, arbitrary units) in CD4+ T cells from B10.D2 mice stimulated for 24 h in the presence of anti-IL12. Data are the mean of three independent experiments and error bars indicate SEM.
From the previous analysis of splenic CD4-enriched T cells, it is not clear whether the inverse correlation in Il4 and Mina expression occurs in naïve progenitor TH or effector TH cells. To investigate this question, we compared cytokine gene expression in highly purified naive TH cells from BALB/c and C57BL/6. Quantitative RT-PCR analysis of naive TH cells (99% CD4+CD62LhiCD44lo; less than 0.2 % natural killer T cell contamination) stimulated for 24 h with plate-bound antibodies to TCR and CD28 revealed an inverse correlation in Mina and Il4 expression, similar to that observed in CD4-enriched cells (Fig. 2b, top). Next, we compared restimulated memory effector cells generated from C57BL/6 TH cells primed under TH1 and TH2 conditions. As expected, Il4 expression was low and high in TH1- and TH2-primed cells, respectively (Fig. 2b, bottom). However, in contrast to naive TH cells from C57BL/6 and BALB/c, TH1 and TH2 cells displayed similar kinetics and magnitude for Mina expression. Thus, the disparate Il4 expression potential of TH1 and TH2 cells is not inversely correlated with Mina expression. By contrast, natural variation in the capacity of naive TH cells to express Il4 is inversely correlated with Mina expression.
Mina acts upstream of IL-4 and IL-12
Two possibilities could account for the inverse correlation between Il4 and Mina expression, either negative regulation of Il4 by Mina or negative regulation of Mina by IL-4 signaling. To distinguish these, we compared Mina mRNA abundance from activated B10.D2 CD4+ T cells in the presence and absence of exogenously added IL-4. The addition of IL-4 to B10.D2 CD4+ T cells did not diminish its high Mina phenotype. Conversely, IL-4 neutralization with an IL-4-specific antibody did not enhance the low Mina phenotype of BALB/c CD4+ T cells (Fig. 2c). Similar results were obtained with highly purified naive TH cells (Supplementary Fig. 2a online). Given the ability of IL-12 to promote and inhibit TH1 and TH2 development, respectively, it was also possible that Mina expression was regulated by IL-12. However, neutralization of IL-12 with an IL-12-specific antibody did not diminish the high Mina expression of B10.D2 cells (Fig. 2d). Furthermore, addition of IL-12 did not enhance the low Mina expression of BALB/c naive TH cells (Supplementary Fig. 2b). Together, these results indicate that neither the IL-4 nor the IL-12 signaling pathways regulate Mina expression, providing support for the idea that Mina acts as a negative regulator of Il4 expression in naive TH cells.
Mina is transcriptionally regulated
We next asked whether differential Mina transcript abundance led to differential protein abundance. Purified BALB/c and C57BL/6 naive TH cells were activated over a 72-h time course with plate-bound antibodies to the TCR and CD28 and the resulting nuclear and cytoplasmic extracts were analyzed by immunblot using a Mina-specific antibody (Fig. 3a). Whereas cytosolic Mina increased with time, nuclear Mina appeared transiently, peaking around 24 h in both BALB/c and C57BL/6. In both subcellular compartments Mina protein amounts were consistently higher (~2–5 fold) in C57BL/6 than in BALB/c, demonstrating tight linkage between Mina protein and mRNA abundance. To determine whether Mina was regulated transcriptionally, we exploited the fact that pre-mRNA abundance correlates with transcriptional rate31. Measurement of Mina pre-mRNA abundance revealed higher expression in C57BL/6 than in BALB/c newly activated TH cells, correlating with differential Mina mRNA abundance, (Fig. 3b). Taken together, these results indicate that Mina protein abundance is transcriptionally regulated.
Figure 3.
Mina is transcriptionally regulated. (a) Mina and Actin immunoblots from cytosolic and nuclear extracts of BALB/c and C57BL/6 naive TH cells activated by PMA-ionomycin over the indicated time course. The upper band in the nuclear Mina immunoblot is non-specific. Actin-normalized data are plotted to the right of each set of blots. Data are representative of two independent experiments. (b) Mina pre-mRNA expression (Hprt1-normalized, arbitrary units) time course from TCR-crosslinking activated naïve TH cells isolated from pools of BALB/c (open squares, n = 5) and C57BL/6 (closed squares, n = 20) mice. Expression was measured by quantitative PCR using primers targeting sequences from Mina intron 1. Data are representative of 2 independent experiments and error bars indicate SEM for triplicate PCR measurements.
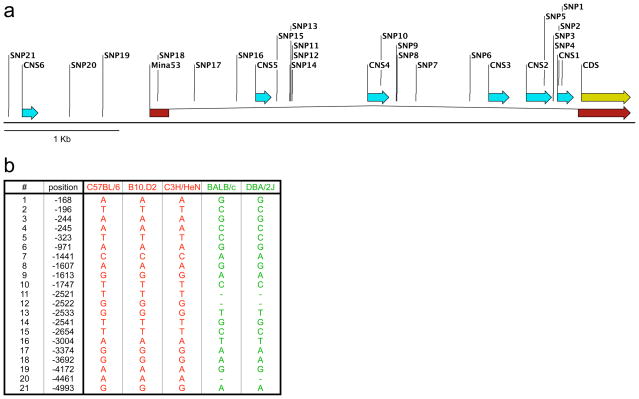
To explore whether regulatory polymorphisms could account for differential Mina transcription between high and low TH2-biased strains, we sequenced the 5′ end of the Mina genomic locus (including intron 1 and 1304 bp of the promoter) from high (BALB/c and DBA/2J) and low (C57BL/6, B10.D2/OsnJ, C3H/HeN) TH2-bias phenotype strains. We identified 21 SNPs that defined precisely two haplotypes (Fig. 4), correlating with TH2-bias and Mina expression phenotypes (Fig. 2). Thus, Mina haplotypes are a useful genetic marker for TH2-bias and may contain regulatory polymorphism(s) responsible for differential Mina expression across strains with distinct TH2-bias phenotypes.
Figure 4.
Mina haplotype predicts TH2-bias phenotype. (a) Map of the promoter- proximal end of the Mina genomic locus. Indicated are the 21 SNPs identified across this interval, along with exons (red), coding region (yellow) and conserved non-coding sequences (CNS; cyan). (b) Position of each SNP (relative to the translational start site in exon2) and its allelic identity for 3 low (red) and 2 high (green) TH2-bias strains.
Mina specifically binds and represses the Il4 promoter
To investigate whether Mina can repress transcription from the Il4 promoter, we performed transient reporter assays, cotransfecting 68-41 murine T cell hybridoma cells with a Mina or empty expression vector and a luciferase reporter driven by either an Il4 or an Il2 promoter32. Mina inhibited luciferase activity driven by the Il4 but not the Il2 promoter (Fig. 5a). To define the Mina responsive region in the 766 bp Il4 promoter, we used the transient reporter assay to interrogate a series of Il4 promoter deletion mutants. Neither truncation of the Il4 promoter to −300 bp nor to −140 bp relative to the translational start site was sufficient to eliminate sensitivity to Mina-dependent repression (Fig. 5a). Thus, a target site for the repressive activity of Mina occurs within the first 140 bp of the Il4 promoter.
Figure 5.
Mina can bind to and repress transcription from the Il4 promoter. (a) Identification of a Mina-responsive region in the Il4 promoter. The transcriptional response to Mina of the Il4 promoter (left; varying in length from 766 to 140 bp, indicated below the graph) and the Il2 promoter (right) is shown. Reporter activity was measured from transductants stimulated (open bars) or not (filled bars) with anti-TCR for 12 h. Data are the average of three independent experiments and error bars indicate SEM (* P < 0.05; t test). (b) Nucleotide sequence of the proximal Il4 promoter with regions (indicated as horizontal lines) used as oligonucleotide probes (A, B, C, and D) in electrophoretic mobility shift assays (EMSAs). (c) Mina interacts with the proximal Il4 promoter in EMSAs. Nuclear extracts from 24 h TCR-stimulated B10.D2 CD4+ T cells were incubated with end-labeled probes A-D in the absence (left) or presence (right, probes A and B only) of 2 μg anti-Mina. Arrows indicate the location of the Mina-containing nucleoprotein complex. Data are representative of 3 independent experiments. (d) Differential binding of Mina53 to the Il4 promoter. ChIP plot depicting fold-enrichment for chromatin-bound Mina53 at five sites (numbered relative to the transcriptional start site) across the Il4 promoter and at the Cd3e locus (as a negative control) in 24 h TCR-stimulated CD4+ T cells from strains BALB/c (open bars) and B10.D2 (filled bars). Data are the mean of five independent experiments and error bars indicate SEM (* P < 0.05, t test).
To further refine the Mina responsive region, we performed electrophoretic mobility shift assays (EMSA). Nuclear extracts isolated from TCR-stimulated B10.D2 CD4+ T cells were tested against a series of end-labeled DNA probes spanning the 140 bp Il4 promoter (Fig. 5b). Nucleoprotein complexes were formed with probes A and B but not C or D (Fig. 5c); however, only the probe B complex was sensitive to disruption by a Mina specific antibody. Thus, Mina can associate with the Il4 promoter over the 30 bp probe B region that extends from −157 to −128 bp.
To determine whether Mina binds to the Il4 promoter in vivo, we performed anti-Mina chromatin immunoprecipitation (ChIP) analysis of BALB/c and B10.D2 CD4+ T cells that had been activated for 24 h. Consistent with the binding activity detected by EMSA in the −157 to −128 region, we observed Mina enrichment in the Il4 promoter but not in the CD3 epsilon promoter (Fig. 5d). Furthermore, the magnitude of binding activity at the Il4 promoter was consistently higher from B10.D2 as compared to BALB/c cells, correlating with the relative expression of Mina in these two strains (Fig. 2e). A broad ChIP survey of the entire TH2 locus in 24 h activated B10.D2 CD4+ T cells also detected a strong peak of Mina binding at the Il4 promoter (Supplementary Fig. 3). Taken together, these data support a model in which Mina is recruited to the proximal Il4 promoter where it exerts a concentration-dependent transcriptional repressive effect.
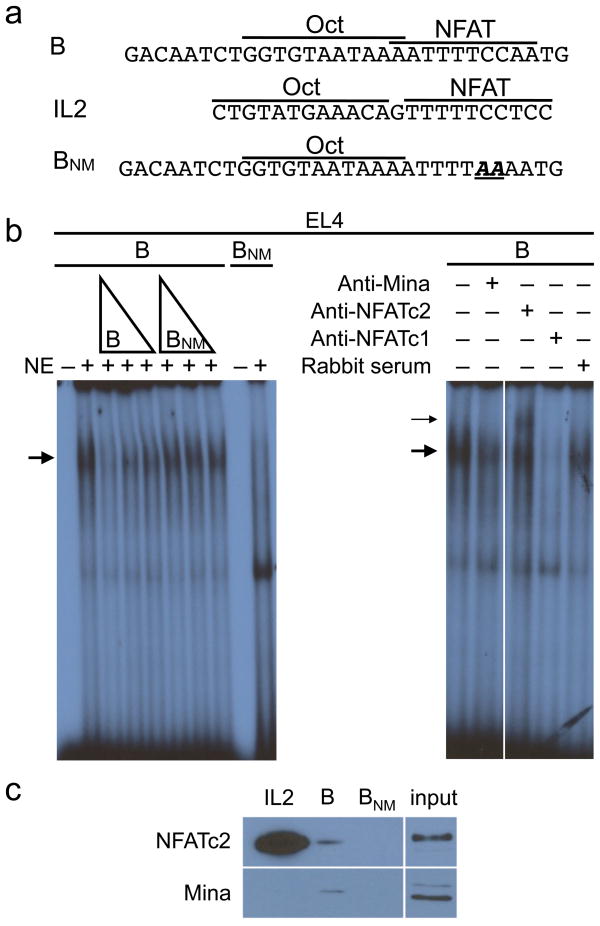
Mina recruitment to the Il4 promoter requires NFAT
As Mina lacks a predicted DNA binding domain, it is likely that its recruitment to the Il4 promoter is indirect. Probe B contains previously described binding sites for the transcription factors NFAT [http://www.signaling-gateway.org/molecule/query?afcsid=A001640; http://www.signaling-gateway.org/molecule/query?afcsid=A000024] and Oct33, 34, raising the possibility of their involvement in recruiting Mina to the Il4 promoter (Fig. 6a). To test this, we used wild-type and mutant probe B to perform EMSA analysis with nuclear extracts from the Mina-expressing T cell thymoma line EL4 (ref. 35). Nuclear extracts from activated but not resting EL4 cells (and C57BL/6 CD4+ T cells) formed a nucleoprotein complex with wild-type probe B (Supplementary Fig. 4 online). This complex was sensitive to disruption by a Mina-specific antibody, indicating that the probe B complex formed with EL4 extract (like that formed with B10.D2 CD4+ T cell extract) contained Mina (Fig. 6b). Whereas a wild-type NFAT consensus sequence could compete with probe B for complex formation, a wild-type Oct consensus sequence could not (data not shown), suggesting that the Mina complex contains NFAT but not Oct. Furthermore, a probe B mutant incapable of binding Oct was still able to form the Mina complex (data not shown). Thus, Oct is neither a constituent of, or required for Mina recruitment to the Il4 promoter.
Figure 6.
Mina recruitment to the Il4 promoter requires NFAT. (a) Nucleotide sequence of oligonucleotide probes from the Il4 (B and BNM) and Il2 (IL2) promoters used in EMSA and DNA pull down experiments in b and c. NFAT and Oct binding sites are labeled. Probe BNM is identical to probe B except for a dinucleotide mutation in the NFAT-binding site, indicated in bold italicized type. (b) The Mina nucleoprotein complex with Probe B requires NFATc1 and NFATc2 binding. Left, nuclear extracts from 4 h PMA-ionomycin induced EL4 cells were incubated with end-labeled probes B and BNM in the presence or absence of 100, 33, or 11 ng of ‘cold’ Probe B or BNM. Right, nuclear extracts from 4 h PMA- ionomycin induced EL4 cells were incubated with end-labeled Probe B and rabbit serum, 3 μg anti-Mina, 2 μg anti-NFATc1 or 2 μg of anti-NFATc2. Thick and thin arrows indicate the locations of the Mina complex and the anti-NFAT ‘supershifted’ complex, respectively. Data are representative of 3 independent experiments. (c) NFATc2 and Mina protein blots of ‘pull-down’ products formed by combining nuclear extracts (input) from 4 h PMA-ionomycin-induced EL4 cells with biotin-labeled probes (IL2, B and BNM). DNA-protein complexes were isolated using streptavidin-agarose beads. Data are representative of 2 independent experiments.
Formation of higher-order complexes using antibodies specific for NFATc1 and NFATc2 confirmed the presence of both NFAT proteins in the probe B-Mina complex (Fig. 6b and data not shown). A mutant probe B (BNM), unable to bind NFAT34, could not form the Mina complex and failed to compete probe B for complex formation (Fig. 6b), suggesting a requirement of NFAT for Mina recruitment. To confirm this notion, we performed DNA ‘pull-down’ assays with biotinylated probes B and BNM. Whereas both Mina and NFAT (c1 and c2) could be coprecipitated with the biotinylated wild-type probe B, BNM failed to coprecipitate with either NFAT protein or Mina (Fig. 6c and data not shown). Interestingly, a biotinylated NFAT-binding sequence from the Il2 promoter failed to coprecipitate Mina despite binding both NFATc1 and NFATc2 (Fig. 6c and data not shown), indicating that NFATc1 and NFATc2 were insufficient for Mina recruitment. Finally, nuclear extracts from cells treated with the calcineurin inhibitor cyclosporin A to prevent NFAT dephosphorylation and nuclear translocation, failed to form the Mina nucleoprotein complex (Supplementary Fig. 4). Taken together, these data suggest that NFAT is necessary, but not sufficient, to recruit Mina into the Il4 promoter-specific nucleoprotein complex.
Mina is necessary and sufficient to constrain IL-4 expression
To test directly whether Mina can repress Il4 transcription in CD4+ T cells, we generated 3 independent BDF1XB6 mouse lines expressing a Mina transgene driven by a lymphocyte-specific proximal Lck promoter-Eμ enhancer36. We analyzed mice after 3 backcrosses to BALB/c, at which point they displayed normal T cell development and activation marker expression (data not shown). Using quantitative RT-PCR to compare Mina expression in CD4+ T cells, we found that all three transgenic lines displayed high basal Mina expression that diminished over a 48 h activation time course to lower expression comparable to or higher than that exhibited at the ‘peak of expression’ by their respective wild-type littermate controls (Fig. 7). To determine the effect of transient enforced elevation of Mina abundance on cytokine gene expression, we examined the transcriptional induction of Il2, Il4 and Ifng. In comparison to wild-type littermate controls expression of Il4, but not Il2 and Ifng, was markedly impaired in all three Mina transgenic lines (Fig. 7). Thus, transient enforced elevation of Mina in CD4+ T cells specifically impairs expression of Il4 but not Il2 or Ifng.
Figure 7.
Transient enforced Mina elevation impairs Il4 expression in CD4+ T cells. CD4+T cells isolated from three independent Mina transgenic lines (Lines #6, #38 and #21; closed symbols, 2 mice each) and their corresponding littermate controls (open symbols, 2 mice each) were stimulated with antibodies to the TCR and CD28. RNA harvested at the indicated times post-stimulation was analyzed for Mina, Il4, Il2 and Ifng by quantitative real-time RT-PCR. Data, expressed as relative expression (Actb normalized, arbitrary units), are from two independent experiments. Each trace represents an individual mouse.
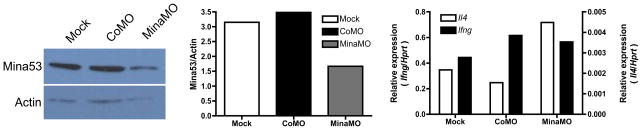
To test whether Mina-dependent repression is required to maintain normal expression of Il4, we used anti-sense morpholino technology to knockdown Mina protein in primary CD4+ T cells. C57BL/6 CD4+ T cells pre-treated for 16 h with Mina-morpholino (MinaMO) expressed half the amount of Mina protein as mock or control-morpholino (CoMO) pre-treated cells 24 h after activation (Fig. 8). By contrast, MinaMO pre-treated cells expressed ~2-fold higher amounts of Il4 (but not Ifng) mRNA than mock or CoMO pre-treated cells (Fig. 8). Thus, the quantitative potential of CD4+ T cells to express Il4 is normally constrained by tight Mina-dependent negative regulation.
Figure 8.
Il4 expression in CD4+ T cells is constrained by Mina-dependent repression. (left) Protein immunoblots of Mina and Actin expression in total cellular extracts from 4–6 × 106 CD4+ T cells activated for 24 h following 16 h mock, CoMO or MinaMO pre-treatment. (middle) Quantification of Mina expression from the protein blot. (right) Relative Il4 and Ifng expression (Hprt1-normalized, arbitrary units) in the same cells shown in the previous two panels. Shown is the mean of duplicate PCR reactions. Data are representative of 2 independent experiments.
Discussion
We have identified Mina as a genetic determinant of the Il4–TH2-bias regulatory locus Dice1.2. Our data suggest that Mina is a necessary and sufficient dose-dependent Il4-specific repressor in naive TH cells, affecting the magnitude of the early autocrine IL-4 burst required to program TH2 development. Furthermore, our results show how natural variation in Mina transcription (likely due to regulatory SNPs at the Mina locus) contributes to natural variation in TH2-bias.
Mina belongs to the Jmj protein family whose hallmark JmjC domain is homologous to the family of Fe(II) and 2-oxoglutarate (2OG)-dependent dioxygenase enzymes. The catalytic dioxgyenase core comprises three iron- and two 2OG-binding residues, of which 4 are conserved in Mina37. Dioxygenases can catalyze a variety of post-translational modifications on substrate proteins, including demethylation and hydroxylation. For example, JMJD6, a member of the JmjC-only subfamily (to which Mina belongs), was shown recently to possess histone arginine demethylase activity38 and FIH (also a member of the JmjC-only subfamily) is an asparagine hydroxylase, responsible for inactivating the transcriptional activation domain of hypoxia inducing factor HIF39, 40. Despite imperfect conservation of its core catalytic residues, Mina is a functional Fe(II)–2OG-dependent dioxygenase enzyme (personal communication, Christopher Schofield). Thus, an attractive hypothesis is that Mina acts as an Il4 repressor by demethylating histones at key positive regulatory elements such as the 3′ HSV site41, reported to be required for Notch-mediated activation of autocrine IL-4 expression in naive TH cells42, 43. Preliminary results suggest that H3K4me3 abundance is similar at HSV, HSVa, HSIV and HSS3 between naive CD4+ T cells from BALB/c and C57BL/6, inconsistent with a histone demethylating role for Mina. Another attractive hypothesis is that Mina catalyzes post-translational changes that modulate key Il4 transcription factors. In this regard, it is tantalizing that arginine methylation augments the ability of the transcription factor NIP45 to promote Il4 transcription44. Finally, it is possible that the Il4 repressive effect of Mina may be mediated by a mechanism independent of its dioxygenase activity.
Mina, which lacks an obvious DNA binding domain, associates with a region of the Il4 promoter that contains binding sites for NFAT and Oct. Our results indicate that while Oct is excluded from the Mina complex, an NFAT-dependent interaction is required for Mina recruitment to the Il4 promoter. However, though necessary, NFAT is not sufficient for Mina recruitment to the Il4 complex as it does not associate with an Il2 promoter fragment capable of recruiting both NFATc1 and NFATc2. This result implies the existence of specificity-determining sequence elements in addition to those required for NFAT recruitment. Identification of these elements may assist identification of factors required with NFAT to recruit Mina to the Il4 promoter.
Mina was first identified as a Myc-induced nuclear antigen of 53 kDa, found to be overexpressed and associated with poor prognosis in a variety of human cancer cells45–48. Studies using ChIP and inducible Myc-ERTam showed that Mina is a direct Myc target in human promyelocytic leukaemia and human glioblastoma cells48. Comparison of the Mina promoter sequence between high and low TH2-biased strains failed to reveal a polymorphism in a presumptive Myc binding site (data not shown), suggesting that differential Myc activity may not be responsible for differential Mina expression in high and low TH2-biased strains. Furthermore, Myc expression in activated naive TH cells is similar in high and low TH2-biased strains and its acute deletion does not impair Mina expression in naive TH cells (M. Koyanagi, unpublished data). Thus, in naive TH cells Mina may not be a bona fide Myc target.
21 Mina promoter and intron-1 SNPs define two distinct haplotypes that correlate with Mina expression phenotype. The polymorphism(s) underlying differential Mina expression is likely to reside in a key regulatory element whose function is modulated by one or several of the 21 or, perhaps additional, haplotype-defining SNPs. Phylogenetic sequence conservation used to identify gene regulatory elements as regions of so-called conserved non-coding sequence (CNS49). We have identified 6 CNSs in the 5 kb region of the Mina locus containing the 21 SNPs. Four of the 21 haplotype-defining SNPs (SNP-1, 2, 5 and 10), occur in 3 CNSs (CNS-4, 2 and 1) and are thus candidates for the functional regulatory polymorphism underlying differential Mina expression that distinguishes high and low TH2-biased strains. Identification of Mina regulatory polymorphism(s) will provide candidates for the causative Dice1.2 genetic lesion and a foothold to dissect critical upstream pathways.
Although Mina can explain the Il4–TH2-bias regulatory activity of Dice1.2, it remains possible that other genes in the minimal Dice1.2 interval contribute to its activity. Furthermore, Dice1.2 is one of two (the other being Dice1.1) Il4–TH2-bias regulatory loci originally identified on chromosome 16 (ref. 28). Thus, the identification of the molecular basis of Dice1.1 is likely to add further mechanistic insight into the complex regulation of Il4 and TH2-bias. Interestingly, Dice1.2 (but not Dice1.1) is tightly linked genetically to a host response modifying locus (LmrA) for the protozoan parasite Leishmania major, responsible for leishmaniasis disease28. As TH2-bias plays a critical role in determining leishmania disease susceptibility, it is possible that Mina is LmrA. In this regard, it is perhaps relevant that among hematopoietic cell types Mina is expressed in CD4+ T cells and dendritic cells, the latter cell type recently implicated, too, as a contributor to the differential TH2-bias phenotypes of BALB/c and B10.D2. A dual TH2-bias regulatory role of Mina (via separate activities in naive TH and dendritic cells) could explain the linkage of Dice1.2, but not Dice1.1, to LmrA. Investigation of the role of Mina in dendritic cells may thus provide further insight into the mechanism of TH2-bias.
Together, our data reveal a novel IL-4 repressive pathway in which Mina plays a key role regulating the amount of IL-4 produced by naive TH cells, thereby controlling the extent of TH2 differentiation and TH2-bias. Natural genetic variation in this pathway contributes to the variation in TH2-bias characteristic of distinct inbred mouse strains. It will be important to determine the upstream regulators, the downstream targets and the repressive mechanism of Mina and the extent to which the Mina pathway may be conserved and manipulated in humans.
Methods
Mice
Animals were bred and maintained in specific pathogen-free conditions in accordance with the guidelines of the Institutional Animal Care and Use Committees of St. Jude Children’s Research Hospital and the RIKEN Institute. BALB/c, C57BL/6, C3H/HeN, B10.D2, DBA/2J, DBA/1J and DBA/2N mice were purchased from Jackson Lab, Taconic Farms, CLEA and Charles River Laboratory.
Reagents and antibodies
Anti-TCRβ was purified from hybridoma H57.597. Anti-CD28 was purified, respectively, from hybridomas PV-1 and 37N51.1, kind gifts, respectively from R. Abe (Science University of Tokyo) and J. Allison (Memorial Sloan Kettering Cancer). Anti-IL-4 was used as purified protein or 10% culture supernatant from hybridoma 11B11. Anti-IL-12 was used as purified protein or 10% culture supernatant from hybridoma C17.8 (gift from G. Trinchieri, NIH). Anti-Mina antibody was purchased from (Zymed, clone M532, ref. 46, 47). Anti- NFATc2 (NFAT1/NFATp; Tamar Laboratory Supplies; clone 25A10.D6.D2), anti-NFATc1 (NFAT2; Santa Cruz, sc-13033), anti-Oct1 (Santa Cruz, sc-232), anti-Actin (Santa Cruz sc-1616), anti-rabbit secondary antibody (Santa Cruz), anti-goat HRP secondary (Santa Cruz), anti-H3K4me3 (Upstate 07-030) and isotype control IgG (Abcam AB46540-1) were purchased. Human recombinant IL-2 was used at 20 U/ml. Mouse recombinant IL-4 was purchased from PeproTech.
Expression profiling
CD4+ T cells isolated from spleen and lymph nodes were stimulated with plate-bound anti-TCRβ mAb (1 μg/ml) and anti-CD28 mAb (10 μg/ml). After 24 h, total RNA was extracted (Tri reagent, Sigma-Aldrich) and purified on RNeasy mini-columns (Qiagen). cRNA were synthesized from the isolated RNA according to Affymetrix protocols. Biotinylated cRNAs were hybridized to Affymetrix GeneChips (mouse Genome 430 version 2, Affymetrix). Microarray data were globally normalized using GeneSpring software (Agilent Technologies).
Quantitative RT-PCR
cDNA was synthesized from total cellular RNA using SuperScript III Reverse Transcriptase (Invitrogen). Quantitative RT-PCR analysis was performed on MX4000 (Stratagene) and 7500 (Applied Biosystems) real-time PCR systems. See Supplementary Table 1 online for primer sequences. mRNA expression was normalized to either Actb or Hprt1, as indicated in Figure legends.
Congenic mouse strains and phenotypic screening of Th2-bias
Generation of congenic strain C16D2/8 was described previously28. C16D2/8D was selected from the backcross progeny of [C16D2/8 × BALB/c]. The congenic interval in C16D2/8D was bred to homozygosity by intercrossing and characterized by genotyping with the markers shown in Fig. 1a and Supplementary Table 1. Splenic CD4+ T cells, isolated by MACS (CD4+ T cell Isolation kit, Miltenyi), were stimulated with plate-bound anti-TCRβ (1 μg/ml) and anti-CD28 (10 μg/ml) antibodies for 16 h prior to harvesting RNA (Tri reagent, Sigma-Aldrich) and determining Il4 and Hprt1 expression by reverse transcriptase real time PCR, as described41, 51. In some experiments, splenic CD4+ T cells were isolated by iMag (CD4 T cell Isolation kit, Sigma) and stimulated with plate-bound anti-TCR (1 μg/ml) and soluble anti-CD28 (PV-1, 10 μg/ml) antibodies for 24 h prior to harvesting RNA.
Immunoblots
Naive TH cells were stimulated with phorbol myristate acetate (PMA) and ionomycin. Nuclear and cytoplasmic protein were isolated from 15 × 106 cells using NE-PER Nuclear and Cytoplasmic Extraction Reagent (Pierce, 78833). 15 μg of nuclear and cytoplasmic protein were separated by 12% SDS-PAGE, transferred to nitrocellulose membranes and reacted with anti-Mina and anti-Actin antibody. After reaction with HRP conjugated anti rabbit secondary antibody, blots were visualized by chemiluminescence substrate (Roche). Mina expression was normalized with Actin expression.
Transient reporter assay
Strain B10.D2 Mina cDNA was inserted into pCMV10 (Sigma-Aldrich) to generate pCMV FLAG-Mina expression construct. 68-41 T cell hybridoma cells were cotransfected with plasmids encoding Firefly luciferase driven by the Il4 or Il2 promoters (pIL-4 Luc and pGL-2), pCMV10 or pCMV FLAG-Mina and a pancreatic alkaline phosphotase (PAP) expression plasmid (pSVPAP, included to control for transfection efficiency). 40 h post-transfection, cells were stimulated for 12 h with plate-bound anti-TCR mAb and total cell lysates were prepared. Luciferase activity measured with a Microplate luminometer (Promega) was normalized to PAP activity.
EMSA
EL4 cells or CD4+ T cells (enriched from B10.D2 or C57BL/6 spleen and lymph nodes) were stimulated with either PMA (Sigma) and Ionomycin (Sigma) or plate-bound anti-TCRβ (10 μg/ml) and anti-CD28 (20 μg/ml), in the presence or absence of cyclosporin A (1 μg/ml, Calbiochem52). Nuclear extracts were prepared with the NER-PER extraction kit according to manufacturers directions (Pierce). Protein concentrations were determined using the Bradford Assay (Pierce) with bovine serum albumin as a standard. Oligonucleotides were annealed in annealing buffer (100 mM Tris pH 7.5, 10 mM EDTA, 2 M NaCl, 50 mM MgCl2) by heating to 95 °C followed by slow cooling to 22°C. For gel shifts, annealed oligonucleotides were end-labeled with ATP-γ32P and T4 polynucleotide kinase (Promega). Nuclear extracts (10 μg) or bovine serum albumin (BSA), 32P-labeled oligonucleotide (1 × 104 c.p.m.), and poly(dI:dC) (125 ng, Sigma) were incubated together at 22°C for 30 min in 20 μl binding buffer (10 mM Tris pH 7.5, 60 mM KCl, 2 mM MgCl2, 0.15 mM dithiothreitol). For supershifts, antibody (2–3 μg) was included in the reaction. Binding reactions were resolved in 1 X TAE 4% polyacrylamide gels.
DNA pull-down
Pull-down assays were performed as described previously53. Briefly, streptavidin-agarose beads (300 μl, Pierce) were pre-absorbed with BSA (500 μl, 1 mg/ml), polydI:C (50 μg, Sigma), and sheared salmon sperm DNA (50 μg), then washed three times and resuspended in EMSA binding buffer (300 μl). Annealed 5′ biotin-labeled oligonucleotides (1 μg) were incubated for 30 min at 22°C with nuclear extract (300 μg) from 4 h PMA/IONO-stimulated EL4 cells in EMSA binding buffer with polydI:C (10 μg). 30 μl of pre-absorbed beads were added to the oligonucleotide–protein mixture and incubated at 4 °C for 4 h. DNA-protein-streptavidin-agarose complexes were washed three times with EMSA binding buffer, eluted in Laemmeli buffer, resolved on 12% SDS-PAGE gels, transferred to nitrocellulose membranes and blotted with anti-Mina (1 μg/ml) and anti-NFATc2 (1:5000 dilution).
ChIP
ChIP was performed using both the equivalent mass and equivalent volume methods. Briefly for the equivalent mass method (described previously54), DNA recovered from an aliquot of sheared chromatin was used as the “input” sample. The remaining chromatin was pre-cleared with protein A- and protein G-agarose (Upstate, cat. 16–156 and 16–266) and then incubated with anti-Mina antibody (2 μg/ml) overnight at 4 °C. DNA recovered after immunoprecipitation (IP) was isolated using a PCR purification kit (QIAGEN) and quantified using picogreen fluorescence (Molecular Probes). Equivalent mass of IP and input DNA was analyzed by real time PCR as described above for RT-PCR with the following modifications. Taq polymerase was from Qiagen (Hot Start) and cycling conditions were: 94 °C for 15 min followed by 45 cycles of 94 °C for 20 sec, 61 °C for 1 min and 72 °C for 40 sec. Data are presented as the ratio of IP to input CT values.
For the equivalent volume method (described previously55), chromatin was prepared as described above and then incubated with anti-Mina or control IgG (10 ug/ml) overnight at 4 °C. Input DNA and DNA recovered after immunoprecipitation (IP) were isolated using a PCR purification kit (QIAGEN). 10 μl of IP or 10 μl of a 1:20 diluted input DNA was analyzed by real time PCR as described above for RT-PCR with the following modifications. Taq polymerase was from Sigma and cycling conditions were: 94 °C for 15 min followed by 45 cycles of 94 °C for 20 sec, 61 °C for 1 min and 72 °C for 40 sec. Data are presented as the ratio of IP to input CT values.
Mina transgenic mice
To establish Mina transgenic mice, a B10.D2 Mina cDNA was cloned downstream of the Lck proximal promoter and Eμ enhancer in the p1026X expression vector56. PCR of genomic tail DNA identified ten independent BDF1XB6 transgenic founders. Transgene copy number was determined by Southern blot analysis. Three founders harboring over 10 copies of the transgene were selected and backcrossed for three generations to BALB/c prior to further analysis. Protein expression in splenic CD4+ T cells was confirmed by immunoblot analysis with anti-Mina.
Anti-sense morpholino mediated knock-down
CD4 T cells (enriched from C57BL/6 spleen and lymph nodes using complement mediated lysis with anti-HAS (J11d), anti-classII (BP107), anti-CD8 (53.67.2) were incubated overnight with endoporter reagent57 (6 μl/ml) and FITC-labeled standard control or Mina morpholino (Supplementary Table 1) (10 μM, Gene-Tools) in the presence of IL-7 (3 ng/ml). Transfection efficiency was greater than 80% as determined by FACs. CD4+ T cells were then activated with plate-bound H57/37N in the presence of IL-2 (25 U/ml) for 24 h prior to immunoblot analysis for knock-down of Mina and Actin and quantitative RT-PCR expression analysis of Il4 and Ifng.
Gene annotation
Gene annotation in the D16MIT138-MB04 interval was retrieved from the Mouse Genome Database, Mouse Genome Informatics, The Jackson Laboratory, Bar Harbor, Maine. World Wide Web (URL: http://www.informatics.jax.org); May, 2009)30.
Statistical analysis
Prism software was used for non-parametric t-tests.
Supplementary Material
Acknowledgments
We thank A. Matsuno, M. Nakamura, M. Natsume, J. Epler, Y. Zhang, N. Li, S. Brown and R. Cross for technical help; J. Partridge for discussions; D. Green, H. Beere, and J. Kang (U. Mass. Medical School) for comments on the manuscript. Supported by the Cancer Research Institute (M.B.), the Burroughs Wellcome Fund (M.B.), American Lebanese Syrian Associated Charities (M.B.), MEXT of Japan (M.Ku.), the RIKEN RCAI International Collaboration Award Program (M.B. and M.Ku.), a Grant-in-Aid for Scientific Research (B) (M.K), a Grant-in-Aid for Scientific Research on Priority Areas of the Ministry of Education, Culture, Sports, Science, and Technology (Japan) (M.Ku.), the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (M.Ku.), and the National Institutes of Health (M.B., AI048636).
Footnotes
Accession codes
The microarray data are deposited in RCAI RefDIC (URL: http://refdic.rcai.riken.jp/welcome.cgi)50 under the following accession codes: RMSPTB007001 and RMSPTB008001.
Author contributions
M.O. and M.V. did the experiments; L.C. did the Mina transcriptional analysis and supplemental figure 2; M.K. did the Mina immunoblots; X.S. analyzed the C16D2/8D mice; Y.S. did the transgenic experiments; Y.S. and L.C. maintained the mouse colonies; O.O., H.K. and A.H. did the expression profiling; M.O., M.V., M.Kubo and M.B. designed and conceptualized the research and analyzed the data; and M.B. prepared the manuscript.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual review of immunology. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Rook GA. Th2 cytokines in susceptibility to tuberculosis. Current molecular medicine. 2007;7:327–337. doi: 10.2174/156652407780598557. [DOI] [PubMed] [Google Scholar]
- 3.Wilson KT, Fantry GT. Pathogenesis of Helicobacter pylori infection. Current opinion in gastroenterology. 1999;15:66–71. doi: 10.1097/00001574-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Umetsu DT, Dekruyff RH. Immune dysregulation in asthma. Current opinion in immunology. 2006;18:727–732. doi: 10.1016/j.coi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Crane IJ, Forrester JV. Th1 and Th2 lymphocytes in autoimmune disease. Critical reviews in immunology. 2005;25:75–102. doi: 10.1615/critrevimmunol.v25.i2.10. [DOI] [PubMed] [Google Scholar]
- 6.Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy, and asthma. Current opinion in allergy and clinical immunology. 2005;5:161–166. doi: 10.1097/01.all.0000162309.97480.45. [DOI] [PubMed] [Google Scholar]
- 7.Pearce EJ, et al. Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunological reviews. 2004;201:117–126. doi: 10.1111/j.0105-2896.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 8.Maizels RM, et al. Helminth parasites--masters of regulation. Immunological reviews. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 9.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 11.Mowen KA, Glimcher LH. Signaling pathways in Th2 development. Immunological reviews. 2004;202:203–222. doi: 10.1111/j.0105-2896.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell research. 2006;16:3–10. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
- 13.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4- producing cells. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bix M, Wang ZE, Thiel B, Schork NJ, Locksley RM. Genetic regulation of commitment to interleukin 4 production by a CD4(+) T cell-intrinsic mechanism. J Exp Med. 1998;188:2289–2299. doi: 10.1084/jem.188.12.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noben-Trauth N, Hu-Li J, Paul WE. IL-4 secreted from individual naive CD4+ T cells acts in an autocrine manner to induce Th2 differentiation. European journal of immunology. 2002;32:1428–1433. doi: 10.1002/1521-4141(200205)32:5<1428::AID-IMMU1428>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Yagi R, et al. The IL-4 production capability of different strains of naive CD4(+) T cells controls the direction of the T(h) cell response. International immunology. 2002;14:1–11. doi: 10.1093/intimm/14.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, et al. Requirements for the development of IL-4-producing T cells during intestinal nematode infections: what it takes to make a Th2 cell in vivo. Immunological reviews. 2004;201:57–74. doi: 10.1111/j.0105-2896.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh CS, Macatonia SE, O’Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yagi J, et al. Genetic background influences Th cell differentiation by controlling the capacity for IL-2-induced IL-4 production by naive CD4+ T cells. International immunology. 2006;18:1681–1690. doi: 10.1093/intimm/dxl102. [DOI] [PubMed] [Google Scholar]
- 21.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annual review of immunology. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita M, Onodera A, Nakayama T. Immune mechanisms of allergic airway disease: regulation by transcription factors. Critical reviews in immunology. 2007;27:539–546. doi: 10.1615/critrevimmunol.v27.i6.40. [DOI] [PubMed] [Google Scholar]
- 23.Kuroda E, Sugiura T, Zeki K, Yoshida Y, Yamashita U. Sensitivity difference to the suppressive effect of prostaglandin E2 among mouse strains: a possible mechanism to polarize Th2 type response in BALB/c mice. J Immunol. 2000;164:2386–2395. doi: 10.4049/jimmunol.164.5.2386. [DOI] [PubMed] [Google Scholar]
- 24.Gorham JD, et al. Genetic mapping of a murine locus controlling development of T helper 1/T helper 2 type responses. Proc Natl Acad Sci U S A. 1996;93:12467–12472. doi: 10.1073/pnas.93.22.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guler ML, et al. Tpm1, a locus controlling IL-12 responsiveness, acts by a cell-autonomous mechanism. J Immunol. 1999;162:1339–1347. [PubMed] [Google Scholar]
- 26.Kosarova M, Havelkova H, Krulova M, Demant P, Lipoldova M. The production of two Th2 cytokines, interleukin-4 and interleukin-10, is controlled independently by locus Cypr1 and by loci Cypr2 and Cypr3, respectively. Immunogenetics. 1999;49:134–141. doi: 10.1007/s002510050472. [DOI] [PubMed] [Google Scholar]
- 27.Zhang F, et al. A murine locus on chromosome 18 controls NKT cell homeostasis and Th cell differentiation. J Immunol. 2003;171:4613–4620. doi: 10.4049/jimmunol.171.9.4613. [DOI] [PubMed] [Google Scholar]
- 28.Baguet A, Epler J, Wen KW, Bix M. A Leishmania major Response Locus Identified by Interval-specific Congenic Mapping of a T Helper Type 2 Cell Bias-controlling Quantitative Trait Locus. J Exp Med. 2004;200:1605–1612. doi: 10.1084/jem.20040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi P, et al. Linkage analysis of the genetic determinants of T-cell IL-4 secretion, and identification of Flj20274 as a putative candidate gene. Genes and immunity. 2005;6:290–297. doi: 10.1038/sj.gene.6364192. [DOI] [PubMed] [Google Scholar]
- 30.Eppig JT, Blake JA, Bult CJ, Kadin JA, Richardson JE. The mouse genome database (MGD): new features facilitating a model system. Nucleic acids research. 2007;35:D630–637. doi: 10.1093/nar/gkl940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipson KE, Baserga R. Transcriptional activity of the human thymidine kinase gene determined by a method using the polymerase chain reaction and an intron-specific probe. Proc Natl Acad Sci USA. 1989;86:9774–9777. doi: 10.1073/pnas.86.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokoyoda K, Takemoto Y, Nakayama T, Arai T, Kubo M. Synergism between the calmodulin-binding and autoinhibitory domains on calcineurin is essential for the induction of their phosphatase activity. J Biol Chem. 2000;275:11728–11734. doi: 10.1074/jbc.275.16.11728. [DOI] [PubMed] [Google Scholar]
- 33.Bruhn KW, Nelms K, Boulay JL, Paul WE, Lenardo MJ. Molecular dissection of the mouse interleukin-4 promoter. Proc Natl Acad Sci US A. 1993 doi: 10.1073/pnas.90.20.9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chuvpilo S, et al. Multiple closely-linked NFAT/octamer and HMG I(Y) binding sites are part of the interleukin-4 promoter. Nucleic acids research. 1993;21:5694–5704. doi: 10.1093/nar/21.24.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farrar JJ, et al. Thymoma production of T cell growth factor (Interleukin 2) J Immunol. 1980;125:2555–2558. [PubMed] [Google Scholar]
- 36.Iritani BM, Forbush KA, Farrar MA, Perlmutter RM. Control of B cell development by Ras-mediated activation of Raf. The EMBO journal. 1997;16:7019–7031. doi: 10.1093/emboj/16.23.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7:715–727. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- 38.Chang B, Chen Y, Zhao Y, Bruick RK. JMJD6 is a histone arginine demethylase. Science. 2007;318:444–447. doi: 10.1126/science.1145801. [DOI] [PubMed] [Google Scholar]
- 39.Dann CE, Bruick RK, Deisenhofer J. Structure of factor-inhibiting hypoxia-inducible factor 1: An asparaginyl hydroxylase involved in the hypoxic response pathway. Proc Natl Acad Sci USA. 2002;99:15351–15356. doi: 10.1073/pnas.202614999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elkins JM, et al. Structure of factor-inhibiting hypoxia-inducible factor (HIF) reveals mechanism of oxidative modification of HIF-1 alpha. J Biol Chem. 2003;278:1802–1806. doi: 10.1074/jbc.C200644200. [DOI] [PubMed] [Google Scholar]
- 41.Baguet A, Bix M. Chromatin landscape dynamics of the Il4-Il13 locus during T helper 1 and 2 development. Proc Natl Acad Sci U S A. 2004;101:11410–11415. doi: 10.1073/pnas.0403334101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka S, et al. The interleukin-4 enhancer CNS-2 is regulated by Notch signals and controls initial expression in NKT cells and memory-type CD4 T cells. Immunity. 2006;24:689–701. doi: 10.1016/j.immuni.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Amsen D, et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 44.Mowen KA, Schurter BT, Fathman JW, David M, Glimcher LH. Arginine methylation of NIP45 modulates cytokine gene expression in effector T lymphocytes. Mol Cell. 2004;15:559–571. doi: 10.1016/j.molcel.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 45.Nishimune Y, Ohta K, Tanaka H, Iida H, Inokuchi T. Expression of Mina53, a product of a Myc target gene in mouse testis. Int J Androl. 2006;29:323–330. doi: 10.1111/j.1365-2605.2005.00572.x. [DOI] [PubMed] [Google Scholar]
- 46.Tsuneoka M, et al. Mina53 as a potential prognostic factor for esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:7347–7356. doi: 10.1158/1078-0432.CCR-03-0543. [DOI] [PubMed] [Google Scholar]
- 47.Teye K, et al. Increased expression of a Myc target gene Mina53 in human colon cancer. Am J Pathol. 2004;164:205–216. doi: 10.1016/S0002-9440(10)63111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuneoka M, Koda Y, Soejima M, Teye K, Kimura H. A novel myc target gene, mina53, that is involved in cell proliferation. J Biol Chem. 2002;277:35450–35459. doi: 10.1074/jbc.M204458200. [DOI] [PubMed] [Google Scholar]
- 49.Dubchak I, et al. Active conservation of noncoding sequences revealed by three-way species comparisons. Genome research. 2000;10:1304–1306. doi: 10.1101/gr.142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hijikata A, et al. Construction of an open-access database that integrates cross-reference information from the transcriptome and proteome of immune cells. Bioinformatics (Oxford, England) 2007;23:2934–2941. doi: 10.1093/bioinformatics/btm430. [DOI] [PubMed] [Google Scholar]
- 51.Seki N, et al. IL-4-induced GATA-3 expression is a time-restricted instruction switch for Th2 cell differentiation. J Immunol. 2004;172:6158–6166. doi: 10.4049/jimmunol.172.10.6158. [DOI] [PubMed] [Google Scholar]
- 52.Rooney JW, Sun YL, Glimcher LH, Hoey T. Novel NFAT sites that mediate activation of the interleukin-2 promoter in response to T-cell receptor stimulation. Molecular and Cellular Biology. 1995;15:6299–6310. doi: 10.1128/mcb.15.11.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ragione FD, et al. p21Cip1 gene expression is modulated by Egr1: a novel regulatory mechanism involved in the resveratrol antiproliferative effect. J Biol Chem. 2003;278:23360–23368. doi: 10.1074/jbc.M300771200. [DOI] [PubMed] [Google Scholar]
- 54.Koyanagi M, et al. EZH2 and Histone 3 Trimethyl Lysine 27 Associated with Il4 and Il13 Gene Silencing in TH1 Cells. J Biol Chem. 2005;280:31470–31477. doi: 10.1074/jbc.M504766200. [DOI] [PubMed] [Google Scholar]
- 55.Weinmann AS, Bartley SM, Zhang T, Zhang MQ, Farnham PJ. Use of chromatin immunoprecipitation to clone novel E2F target promoters. Molecular and Cellular Biology. 2001;21:6820–6832. doi: 10.1128/MCB.21.20.6820-6832.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Attar RM, Macdonald-Bravo H, Raventos-Suarez C, Durham SK, Bravo R. Expression of constitutively active IkappaB beta in T cells of transgenic mice: persistent NF-kappaB activity is required for T-cell immune responses. Mol Cell Biol. 1998;18:477–487. doi: 10.1128/mcb.18.1.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Summerton JE. Endo-Porter: a novel reagent for safe, effective delivery of substances into cells. Ann N Y Acad Sci. 2005;1058:62–75. doi: 10.1196/annals.1359.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.