Abstract
Vasopressin is a peptide hormone that regulates renal water excretion in part through its actions on the collecting duct. The regulation occurs in part via control of transcription of genes coding for the water channels aquaporin-2 (Aqp2) and aquaporin-3 (Aqp3). To identify transcription factors expressed in collecting duct cells, we have carried out LC-MS/MS-based proteomic profiling of nuclei isolated from native rat inner medullary collecting ducts (IMCDs). To maximize the number of proteins identified, we matched spectra to rat amino acid sequences using three different search algorithms (SEQUEST, InsPecT, and OMSSA). All searches were coupled to target-decoy methodology to limit false-discovery identifications to 2% of the total for single-peptide identifications. In addition, we developed a computational tool (ProMatch) to identify and eliminate ambiguous identifications. With this approach, we identified >3,500 proteins, including 154 proteins classified as “transcription factor” proteins (Panther Classification System). Among these, are members of CREB, ETS, RXR, NFAT, HOX, GATA, EBOX, EGR, MYT1, KLF, and CP2 families, which were found to have evolutionarily conserved putative binding sites in the 5′-flanking region or first intron of the Aqp2 gene, as well as members of EBOX, NR2, GRE, MAZ, KLF, and SP1 families corresponding to conserved sites in the 5′-flanking region of the Aqp3 gene. In addition, several novel phosphorylation sites in nuclear proteins were identified using the neutral loss-scanning LC-MS3 technique. The newly identified proteins have been incorporated into the IMCD Proteome Database (http://dir.nhlbi.nih.gov/papers/lkem/imcd/).
Keywords: vasopressin, transcription, aquaporin
the inner medullary collecting duct (IMCD) is the final portion of the renal collecting duct system. It is responsible for the controlled reabsorption of water from the tubule lumen into the interstitial space of the kidney for eventual return to the bloodstream. The main controlling factor is the peptide hormone vasopressin. Vasopressin mediates rapid regulation of IMCD water permeability by triggering redistribution of the water channel protein aquaporin-2 (Aqp2) from a largely intracellular location to the apical plasma membrane through vesicular trafficking (30). Addition of water channels to the apical plasma membrane increases its permeability to water, allowing accelerated osmotic water transport. In addition to this “classic” mode of regulation, vasopressin has long term effects on the renal collecting duct to increase the total abundance of the Aqp2 protein (10) as well as that of its basolateral counterpart aquaporin-3 (Aqp3) (13).
Vasopressin has been demonstrated to increase transcription of the Aqp2 gene (22, 28, 52), resulting in increased levels of Aqp2 mRNA (12, 16, 51) and protein (31) in kidney tissue. Water restriction increases, while water loading decreases Aqp2 mRNA levels in rat kidney (9, 29, 40). Administration of an orally acting vasopressin V2 antagonist decreased Aqp2 mRNA (16), a finding subsequently confirmed by Christensen et al. (9) and Murillo-Carretero et al. (29). Christensen et al. (9) showed in addition that treatment of rats with a vasopressin V2 receptor antagonist caused renal Aqp2 mRNA levels to fall within 30 min.
In addition to Aqp2, Aqp3 gene expression is regulated by vasopressin as well, with marked increases in levels of Aqp3 mRNA (12, 29) and Aqp3 protein (13, 45). However, the role of transcriptional mechanisms is largely unexplored for Aqp3.
The transcriptional network that governs long-term responses to vasopressin is largely unknown. Recently, we used mRNA profiling (Affymetrix) of native rat IMCD and mouse mpkCCD collecting duct cells, coupled with computational analysis (Genomatix) to identify conserved transcriptional regulator binding site motifs in the 5′-flanking region of the Aqp2 gene to identify transcriptional regulators (TRs) that potentially regulate Aqp2 gene expression (54). The findings demonstrated SF1, NFAT, FKHD, ETS, RXR, AP2, CREB, GATA, SRF, HOX, and EBOX family TR binding sites as likely components of the transcriptional network responsible for regulation of Aqp2 gene transcription. Although these conserved binding site motifs can predict what TR families may be involved in transcriptional regulation of Aqp2 and other genes, identification of the actual TR proteins expressed in the IMCD is a necessary intermediate step to the design of studies needed to fully resolve transcriptional regulatory networks involved in regulation of Aqp2 gene expression. We have previously carried out extensive transcriptomic profiling of IMCD cells using Affymetrix arrays to learn what transcripts are expressed in the IMCD (46) (see “IMCD Transcriptome Database”: http://dir.nhlbi.nih.gov/papers/lkem/imcdtr/). However, relative transcript levels are not necessarily predictive of the level of the corresponding proteins in cells and direct detection by mass spectrometry is desirable. The low abundance of many TR proteins relative to other categories of proteins has made them difficult to detect by protein mass spectrometry and the current IMCD Proteome Database (33) contains only a few TR proteins such as Stat2 and Pax8, detected in whole cell analysis. Notably, a transcription factor whose role is already established, namely Creb1 (22, 28, 52), was not detected using proteomic methods. This problem can be addressed biochemically by isolating nuclei from IMCD cells to enrich nuclear proteins prior to mass spectrometric identification, making it more likely that TRs and other nuclear proteins will be detected. In this study, we have isolated nuclei from native rat IMCDs and carried out proteomic and phosphoproteomic profiling using 1-D SDS-PAGE followed by LC-MS/MS. To increase the number of proteins identified we utilized a computational protocol that includes a combination of three search algorithms (SEQUEST, InsPecT, and OMSSA) for matching mass spectra to rat protein sequences. To limit false-positive identifications we used target-decoy analysis to set the false discovery rate (FDR) to <2% of the total for single-peptide identifications. In addition we developed a novel computational tool (ProMatch) to identify ambiguous identifications, i.e., tryptic or chymotryptic peptides that are the same in two or more proteins. Finally, since regulation of transcription is in part mediated by regulated phosphorylation of various nuclear proteins, we also carried out MS-based experiments to identify phosphoproteins in nuclei from native IMCD cells.
METHODS
IMCD Sample Preparation and Isolation of Nuclei
Twenty male Sprague-Dawley rats were euthanized (National Heart, Lung, and Blood Institute Animal Care and Use Committee-approved protocol H-0110) and their renal inner medullas (IMs) were resected and pooled. The IMs were minced and digested in a solution containing 250 mM sucrose, 2,000 units/ml hyaluronidase, and 3 mg/ml collagenase B for 75 min at 37°C with continuous stirring. IMCDs were sedimented at 70 g for 20 s, and the supernatant containing non-IMCD elements was discarded. IMCD pellet was resuspended in a homogenization solution [250 ml sucrose solution containing phosphatase inhibitor (Halt phosphatase inhibitor, Thermo Scientific, Waltham, MA) and protease inhibitor (Complete mini, Roche Diagnostics, Indianapolis, IN)]. Previous studies have shown that IMCDs isolated by this technique are at least 85% pure (46), viable, and vasopressin-responsive (35).
Isolated IMCDs were suspended in the homogenization solution and homogenized using a Potter-Elvehjem motor-driven homogenizer on ice for a total of 2 min in 15 s intervals. The homogenized solutions were centrifuged at 1,000 g for 30 min at 4°C. The supernatant from the previous step, which contained the cytoplasmic fraction of lysed IMCD cells, was kept. The cell lysis solutions Cytoplasmic Extraction Reagent I and II (CER I and CERII) from the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Thermo Scientific) were then added, according to instructions, to the pellet now containing nuclei and unbroken IMCD cells followed by centrifugation for 5 min at 16,000 g. CERI and CERII were again added to the pellet at half the original volumes and the sample was centrifuged as above. All supernatants containing the cytoplasm of lysed cells were pooled (designated “Cyto”). Nuclear extraction solution was then added to the pellet, and the sample was centrifuged according to the kit instructions. The supernatant from the final centrifugation contained soluble nuclear proteins (designated “NE”) and the pellet contained nuclear membrane/ER proteins (designated “NP”).
One-dimensional SDS-PAGE and In-gel Digestion
One-dimensional (1-D) SDS-PAGE was performed on samples from NE, NP, and Cyto fractions (400 μg protein each) using three separate 10% polyacrylamide Ready Gels (Bio-Rad, Hercules, CA). The gels were stained with Imperial Protein Stain (Thermo Scientific). Each gel was sliced into 40 fractions and cut into 1 mm3 blocks. In-gel digestion was performed as described previously (34). Briefly, 25 mM NH4HCO3/50% acetonitrile (ACN) was added to each sample three times for 10 min each to dehydrate the gels and samples were dried via a Speed Vac. 10 mM DTT in 25 mM NH4HCO3 was added to each sample at 56°C for 1 h followed by the addition of 55 mM iodoacetamide in 25 mM NH4HCO3 for 45 min in the dark. The gel blocks were then dried via a Speed Vac. The gel pieces were digested overnight at 37°C with 12.5 ng/μl of Sequencing Grade Modified Trypsin (Promega, Madison, WI). Following the in-gel digestion, peptides were extracted with 50% ACN/0.1% formic acid (FA) then desalted using a ZipTip C18 pipette tip (Millipore, Billerica, MA). In addition, a separate set of 40 NE fractions was in-gel digested overnight at 25°C with 12.5 ng/μl of Sequencing Grade Chymotrypsin (Roche Diagnostics, Indianapolis, IN). In total, 120 samples were analyzed by LC-MS/MS.
Nanospray LC-MS/MS
Each peptide sample was analyzed once via 1-D nanospray LC-MS/MS with a modified ProteomeX 2D LC/MS workstation utilizing a linear ion trap mass spectrometer, LTQ-FT (Thermo, San Jose, CA) (20). In an alternating fashion, two Zorbax 300SB-C18 peptide traps (Agilent Technologies, Wilmington, DE) chromatographically separated the peptides. A nanospray ionization source and a reverse-phase PicoFrit column [BioBasic C18, 75 μm × 10 cm, tip = 15 μm (New Objective, Woburn, MA)] were used. The peptides were eluted via an increasing 0–60% gradient of solvent B in solvent A (A = 0.1% FA, B = 100% ACN/0.1% FA) over a 30 min period at a flow rate of ∼200 nl/min.
Post-LC-MS/MS Analysis
Search algorithms.
To maximize the number of peptide identifications, we analyzed the data using three different search algorithms. In addition to utilizing two peak matching algorithms viz. SEQUEST (15) and the Open Mass Spectrometry Search Algorithm (OMSSA) (17), we also employed InsPecT (44), a so-called “hybrid” algorithm that performs partial de novo sequencing generating 3-amino acid tags that are used to narrow down the possible peptide candidates before performing the final peak matching. A fixed +57 Da modification on cysteine and variable +14 Da cysteine and +16 Da methionine modifications were included as part of the search.
The raw data files were searched, using the three search algorithms, against a concatenated forward and reverse database that included the most recent RefSeq rat protein database from the National Center for Biotechnology Information appended with a list of common contaminants (pig and bovine trypsin as well as human isoforms of keratin). Peptide identifications were sorted from best to worst using the following parameters: XCorr (SEQUEST), P value (InsPecT), and E-value (OMSSA). Target-decoy analysis was performed to limit global FDR for each peptide identified using the following formula: FDR = 2R/(F + R), where R is the number of accumulated peptide hits from reversed “decoy” sequences and F is the number of accumulated peptide hits from forward “target” sequences (14). All peptides passing the 2% FDR filter from the three different search algorithms were merged at the spectral level. For a given spectrum, a valid identification was defined if at least 67% of the passed search algorithms agree on the identification (an agreement of 3 out of 3, 2 out of 3, 2 out of 2, or 1 out of 1). If no agreement among the three search algorithms was found, the single best identification (based on FDR) across all algorithms for a given spectrum was selected. Unresolved spectra were discarded.
Peptide to protein matching.
The same peptide may map to more than one protein. However, peak matching programs like SEQUEST often report a single match even when the peptide could match to multiple protein candidates. To detect ambiguous identifications, we have used an in-house program written in Java called ProMatch (executable file available upon request). In this program, each peptide identified is matched against all proteins in the rat RefSeq database, every protein whose amino acid subsequence identically matches to that peptide is extracted.
The following rules were used in reporting the peptide-based protein identifications. A peptide that matched only to a single protein was defined as a “unique peptide” and reported as such. A peptide that matched to multiple proteins was defined as a “nonunique peptide.” If that peptide matched to multiple proteins that are splicing variants or products of alternative transcription start sites in the same gene, it was considered as a unique peptide since it maps to only a single gene. For this determination, we extracted the nucleotide sequences of all the proteins that matched to a particular peptide and then applied the ClustalW2 sequence alignment program (European Bioinformatics Institute) to compare them. Proteins with a similarity score ≥90 were considered to be derived from the same gene and a single RefSeq accession number is reported for all. A protein identification was labeled as “unambiguous” if it was derived from at least one unique peptide. A protein identification was labeled as “ambiguous” if it was derived exclusively from one or more nonunique peptides derived from different genes. Ambiguous protein identifications were not reported.
Phosphopeptide Analysis
Sample preparation.
IMCDs were isolated and pooled from 10 male Sprague-Dawley rats. Nuclear isolation was performed as described above. The NE and NP fractions (250 and 500 μg, respectively) were resuspended with 6 M guanidine solution. Protein samples were reduced with 10 mM DTT solution at 56°C for 1 h then alkylated with 40 mM iodoacetamide solution in the dark at room temperature for 1 h. DTT solution (40 mM) was added after alkylation to quench excess iodoacetamide. Samples were diluted in 25 mM NH4HCO3 solution and digested with trypsin (1:30 wt/wt) overnight at 37°C. Peptide samples were desalted with a 1 ml HLB cartridge (Oasis, Milford, MA) and then dried via a Speed Vac.
Enrichment of phosphopeptides.
Dried peptide samples were resuspended in 5% acetic acid and Ga3+-IMAC (Phosphopeptide Isolation Kit, Thermo Scientific) was performed as described previously (20). The IMAC flow-through was subjected to an additional phosphopeptide isolation protocol using TopTips with TiO2 Material (PolyLC, Columbia, MD). Phosphopeptide samples were dried with a Speed Vac, resuspended in 0.5% FA, and desalted with ZipTip C18 pipette tips (Millipore).
LC-MS3 analysis.
The neutral loss scanning LC-MS3 analysis was performed as described (20). Briefly, isolated phosphopeptide samples were analyzed on an Agilent 1100 nanoflow system (Agilent Technologies, Palo Alto, CA) connected to a Finnigan LTQ-FT mass spectrometer (Thermo Electron, San Jose, CA) equipped with a nanoelectrospray ion source. The LTQ was used for the full MS scan and subsequent spectra (MS2 and MS3). MS3 scans were triggered when the presence of a neutral loss peak (−98, −49, or −32.7 m/z from precursor ion) was detected in MS2 scans.
Post LC-MS3 analysis.
SEQUEST, InsPecT, and OMSSA searches were performed against the concatenated forward and reverse database as described above. A fixed +57 Da cysteine modification and variable +16 Da methionine and +80 Da phosphorylation on serine, threonine, and tyrosine modifications were included. In addition, a variable loss of water −18 Da modification was included for serine and threonine when searching MS3 spectra. Identified peptides (both phosphopeptides and nonphosphopeptides) were filtered for a 2% FDR using the target-decoy approach described above. The PhosphoPIC program was used to extract and compile MS2 and MS3 spectra of phosphopeptides (21). Phosphorylation site assignment was performed using Ascore (2), Phosphate Localization Score (PLscore) (1), and PhosphoScore (39).
Immunoblotting
Samples were solubilized in 1.5% SDS/Tris, pH 6.8. A BCA assay (Thermo Scientific) using BSA as the standard was used to determine total protein concentrations. Samples were then diluted in Laemmli buffer (10 mM Tris, pH 6.8, 1.5% SDS, 6% glycerol, 0.05% bromphenol blue, and 40 mM DTT). Proteins (5 μg of each sample) were separated by 1-D SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with blocking buffer (LI-COR Biotechnology, Lincoln, NE) then exposed overnight to primary antibodies diluted in the blocking buffer. After a series of washes, membranes were incubated with secondary antibodies as previously described (35) prior to image acquisition on an Odyssey Infrared Imaging System (LI-COR Biotechnology).
Antibodies
Antibodies used are listed as follows: aldose reductase (Alr2) (sc-17735, Santa Cruz Biotechnology, Santa Cruz, CA), BRM/SWI2-related gene 1 (Brg1) (sc-17796, Santa Cruz Biotechnology), and cyclic AMP response element binding protein 1 (Creb1) (9197, Cell Signaling, Danvers, MA). Species-specific secondary antibodies were obtained from Rockland Immunochemicals (Gilbertsville, PA).
Transcription Factor Binding Site Analysis
The 5′-flanking region (1,000 nucleotides) and first intron of Aqp2 and Aqp3 gene of human, rat, and mouse were extracted using UCSC Genome Browser (http://genome.ucsc.edu/). To identify conserved transcription factor binding sites (TFBS), the 5′-flanking region, and first intron sequences were analyzed using the online Genomatix software suite (http://www.genomatix.de/) as previously described (54). Sequence matches to a particular TFBS matrix were scored and filtered based on MatInd algorithm (36).
RESULTS AND DISCUSSION
Confirmation of Nuclear Isolation and Purification from Native Rat IMCD
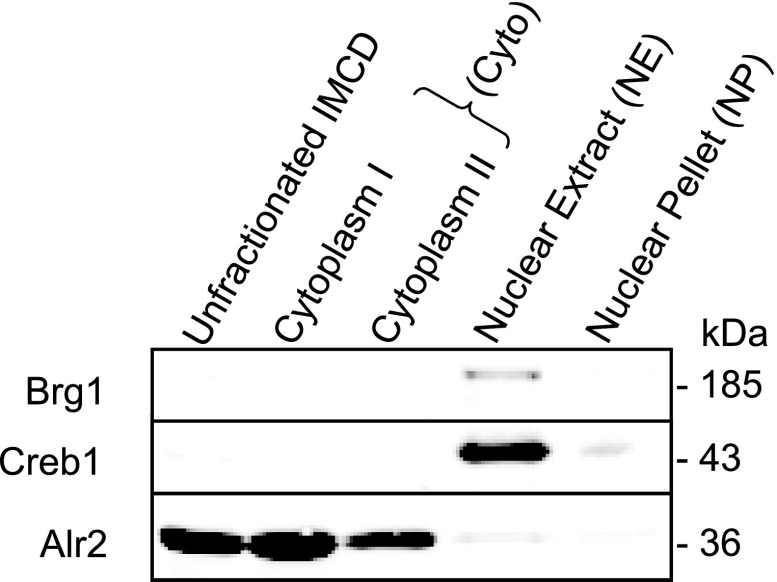
Common markers for the cytoplasm and nucleus were used to determine the efficacy of the nuclear isolation and enrichment procedure. Figure 1 demonstrates that the nuclear proteins BRM/SWI2-related gene 1 (Brg1) and cyclic-AMP response element binding protein 1 (Creb1) were almost exclusively present in the nuclear fractions. In contrast, the cytosolic marker aldose reductase (Alr2) was far more abundant in the cytoplasmic fractions.
Fig. 1.
Quality control for nuclear isolation. Nuclear marker Brg1 is found only in the nuclear extract (NE) fraction. Another nuclear marker Creb1 is chiefly present in the NE fraction but is also found in the nuclear pellet (NP) fraction. Cytoplasmic marker (Alr2) is predominantly present in the cytoplasmic fractions. (Cytoplasm I = Potter-Elvehjem supernatant, Cytoplasm II = CERI/CERII supernatant.) Note that 5 μg of each sample was loaded. In this study we carried out MS analysis of the pooled Cytoplasm I and II (Cyto) as well as NE and NP fractions.
Proteomic Profiling of Rat IMCD Fractions
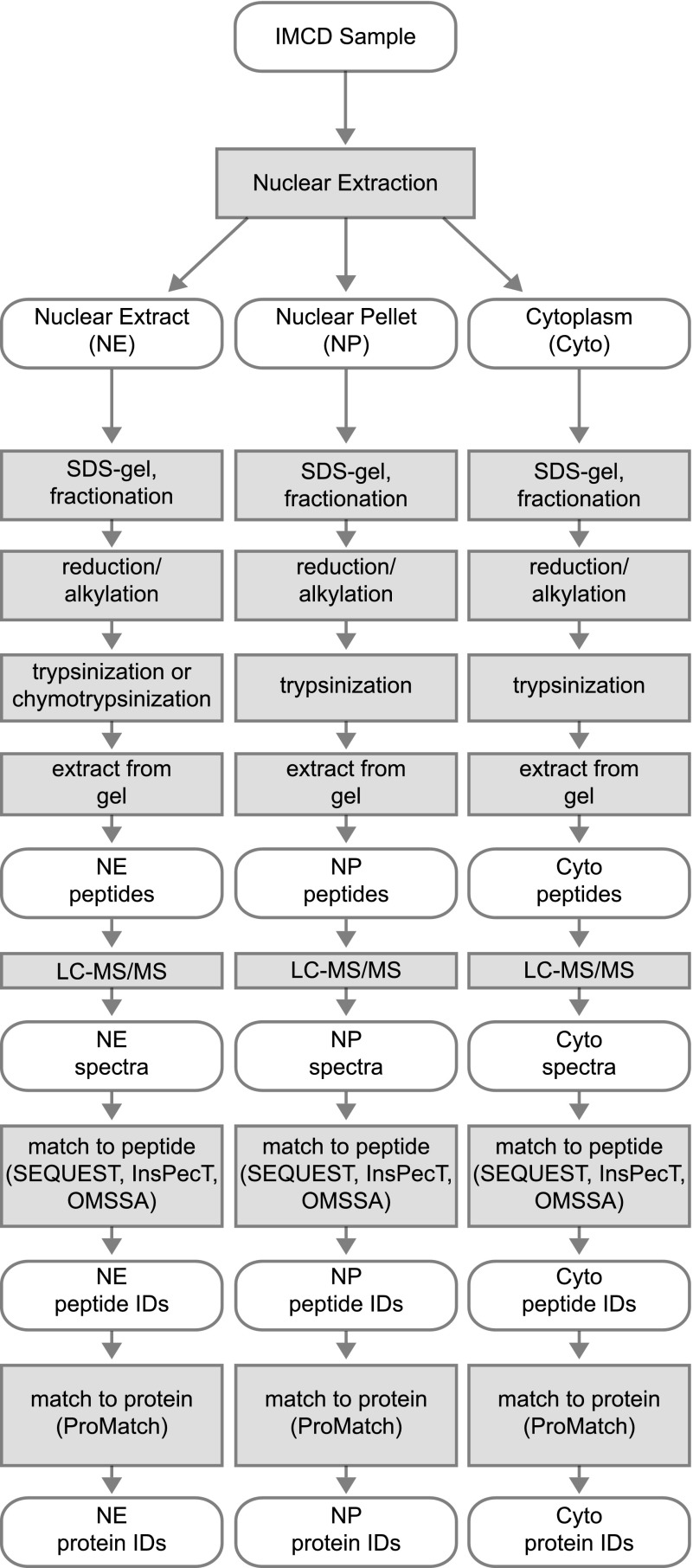
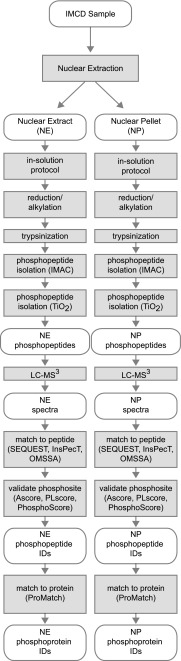
LC-MS/MS analysis was performed on three fractions derived from the nuclear isolation protocol: the soluble nuclear fraction (NE), the nuclear membrane pellet (NP), and the pooled cytoplasmic fractions (Cyto). Figure 2 summarizes the proteomic and bioinformatic workflow used for these samples. While the sample preparation protocol is relatively standard, we customized the post-LC-MS/MS data analysis for this study. Specifically, three different search algorithms (SEQUEST, InsPecT, and OMSSA) were used to increase the number of peptides identified from the spectra that were generated. The ProMatch algorithm was then used to check whether identified peptide sequences were unique to a single identified protein to allow ambiguous identifications to be eliminated.
Fig. 2.
Proteomic work flow. Graphical representation of the complete workflow used for this proteomic study. The ovals represent the experimental samples, spectra, or proteomic data. Shaded boxes represent the biochemical and computational methods that were applied to the biological samples and data.
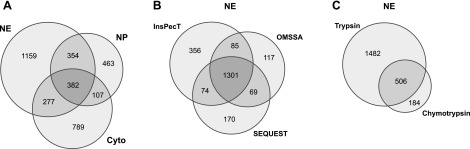
A total of 2,172 proteins were identified in the NE fraction, 1,306 proteins were identified in the NP fraction, and 1,555 proteins were identified in the cytoplasmic fraction. For all three fractions we imposed a single-peptide FDR of 2% or less using a target-decoy algorithm (Fig. 3A). A total of 3,531 proteins were identified in one or more of the three fractions (all protein identifications are listed in Supplementary Table S1).1 Overall, 45% of protein identifications in this study are based on spectra for 2 or more peptides (Supplementary Table S1). Figure 3B summarizes the number of proteins discovered by each of the three search algorithms (SEQUEST, InsPecT, and OMSSA) in the NE fraction. Also in the NE fraction, 690 proteins were identified from chymotrypsin-digested samples. This yielded 184 proteins that were not found using trypsin and confirmed the presence of 506 proteins found in the NE trypsin dataset (Fig. 3C). It is evident from Fig. 3, B and C, that the use of multiple search algorithms and different proteases are beneficial in increasing the number as well as the confidence of unique peptides and proteins identified. Among the 3,531 protein identifications made in this study, 2,163 (61%) were not identified in the IMCD in previous studies and now have been added to the IMCD Proteome Database (http://dir.nhlbi.nih.gov/papers/lkem/imcd/).
Fig. 3.
Venn diagram of proteomic results. A: the number of proteins identified in the NE, NP, and cytoplasmic fractions (Cyto) with an estimated single-peptide false discovery rate specified as 2% or less. A total of 3,531 proteins were identified in one or more of the 3 fractions. B: the number of proteins identified in the NE fraction by each searching algorithm (SEQUEST, InsPecT, and OMSSA). Intersections between each circle show the number of proteins identified by a combination of search algorithms. C: the number of proteins identified in the NE fraction using 2 different proteases (trypsin and chymotrypsin).
Transcription Factors Identified by LC-MS/MS
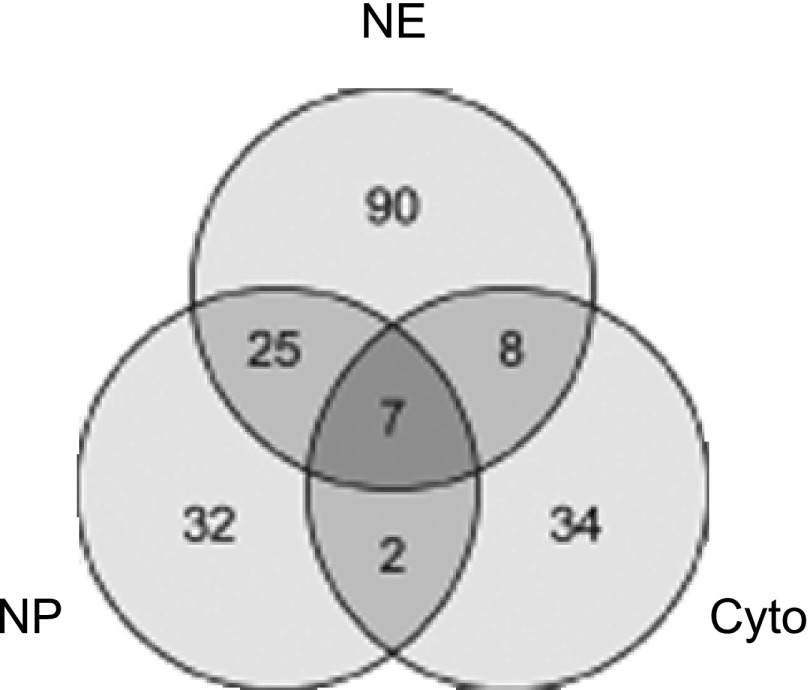
To enumerate the transcription factors found in the rat IMCD in this study, proteins identified in all the samples were combined into one dataset (Supplementary Table S1). One hundred fifty-four proteins (4.4% of total) were classified as “transcription factor” proteins (Table 1) using the Panther Classification System (http://www.pantherdb.org/). Figure 4 shows a Venn diagram of the distribution of transcription factors identified in the NE, NP, and Cyto fractions, with the majority of these proteins found in the NE fraction. One hundred twenty-eight of these proteins (83%) were new identifications for the IMCD Proteome Database.
Table 1.
Transcription factors
| Accession No. | Gene | Name | Sample |
|---|---|---|---|
| CREB transcription factors | |||
| NP_001002809 | Atf6b (Crebl1) | cAMP responsive element binding protein-like 1 | NP |
| NP_604392 | Creb1 | cAMP responsive element binding protein 1 isoform A | NE |
| NP_001099749 | RGD1565584 | similar to tyrosine kinase-associated leucine zipper protein LAZipII | Cyto |
| Basic helix-loop-helix transcription factors | |||
| NP_036913 | Arnt2 | aryl hydrocarbon receptor nuclear translocator 2 | Cyto |
| XP_001072661 | LOC684826 | similar to Basic helix-loop-helix transcription factor scleraxis | NP |
| NP_001020878 | Tfeb | transcription factor EB | NP |
| NP_113965 | Usf1 | upstream stimulatory factor 1 | NE |
| NP_112401 | Usf2 | upstream transcription factor 2 | NE |
| Homeobox transcription factors | |||
| NP_476450 | Barhl1 | BarH-like homeobox 1 | NP |
| XP_001058888 | Chx10 | similar to Homeobox protein CHX10 (Ceh-10 homeodomain-containing homolog) isoform 2 | NE |
| XP_347164 | Cutl1 | similar to Homeobox protein cut-like 1 (CCAAT displacement protein) (CDP) (Homeobox protein Cux) | NE |
| NP_036713 | Hoxa2 | homeo box A2 | NE |
| NP_001017480 | Hoxb7 | homeo box B7 | NE |
| XP_001069122 | LOC688999 | similar to Homeobox protein Hox-D8 (Hox-4.3) (Hox-5.4) | NE |
| NP_113925 | Nkx6-1 | NK6 homeobox 1 | Cyto |
| NP_001099831 | Pax2 | paired box 2 | NE |
| NP_037133 | Pax6 | paired box 6 | NE |
| NP_112403 | Pax8 | paired box 8 | NE |
| NP_001094151 | Pbx1 | preB-cell leukemia homeobox 1 isoform b | NE |
| XP_001081290 | RGD1562142 | similar to Homeobox protein Hox-B6 (Hox-2.2) (MH-22A) | NE |
| XP_001081293 | RGD1562292 | similar to Homeobox protein Hox-B5 (Hox-2.1) (MU-1) (H24.1) | Cyto |
| XP_226533 | RGD1562686 | similar to genetic suppressor element 1 | NE |
| XP_001073119 | RGD1563929 | similar to OG2 homeobox | Cyto |
| XP_234082 | RGD1564874 | similar to NK1 transcription factor related 2-like,b | Cyto |
| XP_001058915 | Zfhx4 | similar to zinc finger homeodomain 4 | NE |
| NP_001040562 | Zhx3 | zinc fingers and homeoboxes 3 | NE, NP |
| Nuclear hormone receptors | |||
| NP_599227 | Dnttip1 | deoxynucleotidyltransferase, terminal, interacting protein 1 | NE |
| NP_001008511 | Esrra | estrogen related receptor, alpha | NE |
| NP_976081 | Esrrg | estrogen-related receptor gamma | NE |
| NP_113814 | Nr1 h2 | nuclear receptor subfamily 1, group H, member 2 | NP |
| NP_542956 | Nr2f2 | nuclear receptor subfamily 2, group F, member 2 | NE |
| NP_036708 | Nr3c1 | nuclear receptor subfamily 3, group C, member 1 | NP |
| NP_478117 | Sf1 | splicing factor 1 isoform 2 | NP |
| Zinc finger transcription factors | |||
| NP_001099559 | Ash2l | ash2 (absent, small, or homeotic)-like | NE |
| NP_446008 | Bud31 | BUD31 homolog | NE |
| NP_114012 | Ctcf | CCCTC-binding factor (zinc finger protein) | NE, NP |
| NP_001101986 | Dpf2 | D4, zinc and double PHD fingers family 2 | NE, NP |
| NP_001099893 | Evi1 | ecotropic viral integration site 1 | NE |
| NP_001029098 | Fhl1 | four and a half LIM domains 1 isoform 1 | Cyto |
| NP_001101293 | Gfi1b | growth factor independent 1B transcription repressor | NE |
| XP_218939 | Hbxap | similar to remodeling and spacing factor 1 | Cyto |
| NP_001100647 | Jarid1b | jumonji, AT rich interactive domain 1B | NP |
| NP_112397 | Klf10 | Kruppel-like factor 10 | NE |
| NP_445988 | Klf15 | Kruppel-like factor 15 | Cyto |
| XP_001072513 | LOC684924 | similar to zinc finger protein 449 | NP |
| XP_001062820 | LOC685209 | similar to Zinc finger BED domain containing protein 4 | Cyto |
| XP_001067699 | LOC688634 | similar to Histone acetyltransferase MYST4 (MYST protein 4) (MOZ, YBF2/SAS3, SAS2 and TIP60 protein 4) (Querkopf protein) | NE |
| NP_001094368 | Mtf2 | metal response element binding transcription factor 2 | NE |
| NP_851595 | Myst2 | MYST histone acetyltransferase 2 | NE |
| NP_001099501 | Np | nucleoside phosphorylase | Cyto |
| NP_001103962 | Phf14 | PHD finger protein 14 | Cyto |
| NP_001100812 | Phf2 | PHD finger protein 2 | NE |
| NP_001071116 | Prdm2 | retinoblastoma protein-binding zinc finger protein | Cyto |
| NP_001005893 | Repin1 | replication initiator 1 | NE |
| XP_219346 | RGD1305903 | similar to mesenchymal stem cell protein DSC43 | NE |
| XP_001061052 | RGD1310645 | similar to Zinc finger protein 407 | NE,NP |
| XP_001057325 | RGD1359201 | similar to CG15011-PA | Cyto |
| XP_001067122 | RGD1560834 | similar to zinc finger and BTB domain containing 41 | Cyto |
| XP_233885 | RGD1561526 | similar to zinc finger protein 512 | NP |
| XP_234528 | RGD1563945 | similar to PHD finger protein 16 | NE |
| XP_001076564 | RGD1564807 | similar to zinc finger protein, subfamily 1A, 5 | NP |
| NP_001128016 | RGD1565545 | hypothetical protein LOC308337 | Cyto |
| NP_997714 | Ring1 | ring finger protein 1 | NE |
| NP_001094197 | Rufy1 | RUN and FYVE domain containing 1 | NE |
| NP_695222 | St18 | suppression of tumorigenicity 18 | NE |
| XP_223196 | Wdfy3 | similar to WD repeat and FYVE domain containing 3 | NP |
| NP_775412 | Yy1 | YY1 transcription factor | NE, NP |
| NP_001100567 | Zbtb11 | zinc finger and BTB domain containing 11 | NE |
| NP_001099350 | Zbtb20 | zinc finger and BTB domain containing 20 | NE, NP |
| NP_001124009 | Zbtb39 | zinc finger and BTB domain containing 39 | Cyto |
| NP_446454 | Zbtb7a | zinc finger and BTB domain containing 7a | NE |
| NP_001010963 | Zc3 h15 | erythropoietin 4 immediate early response | NE |
| NP_001028873 | Zeb2 | zinc finger E-box binding homeobox 2 | NE, Cyto |
| NP_001101283 | Zfp217 | zinc finger protein 217 | NE |
| XP_001059668 | Zfp236 | similar to Zinc finger protein 236 | NE, Cyto |
| NP_001100701 | Zfp278 | zinc finger protein 278 | Cyto |
| NP_001100591 | Zfp316 | zinc finger protein 316 | NE |
| NP_001128057 | Zfp426l2 | zinc finger protein 426 | NP |
| NP_877975 | Zfp472 | zinc finger protein 472 | NE |
| NP_714953 | Zfp709 | zinc finger protein 709 | NP |
| NP_001101279 | Znf512b | zinc finger protein 512B | NE |
| NP_001019429 | Znf574 | zinc finger protein 574 | Cyto |
| NP_001132956 | Znf644 | zinc finger protein 644 | NP, Cyto |
| NP_001073676 | Znf652 | zinc finger protein 652 | NP |
| XP_001080236 | Zzef1 | similar to zinc finger, ZZ type with EF hand domain 1 | Cyto |
| Other transcription factors | |||
| XP_219832 | Aff2 | similar to AF4/FMR2 family member 2 (Fragile X mental retardation protein 2 homolog) | NP |
| XP_341162 | Ahctf1 | similar to transcription factor ELYS | NE, Cyto |
| NP_446456 | Cand1 | cullin-associated and neddylation-dissociated 1 | NE, NP, Cyto |
| NP_001101391 | Casp8ap2 | caspase 8 associated protein 2 | NE |
| NP_001013209 | Cbfb | core-binding factor, beta subunit | NE |
| NP_445979 | Cdc5l | CDC5 cell division cycle 5-like | NE |
| NP_001028242 | Cip29 | cytokine induced protein 29 kDa | NE |
| NP_446118 | Dll3 | delta-like 3 | Cyto |
| NP_001011914 | Dr1 | down-regulator of transcription 1 | NE |
| NP_942046 | Eif3 h | eukaryotic translation initiation factor 3, subunit H | Cyto |
| NP_001102912 | Erh | enhancer of rudimentary homolog | NE |
| NP_001100552 | Etv5 | ets variant 5 | Cyto |
| NP_001014192 | Fam48a | transcription factor (p38 interacting protein) | NE |
| NP_001012137 | Fus | fusion, derived from t(12;16) malignant liposarcoma | NE, Cyto |
| NP_001041351 | Ilf2 (Nf45) | interleukin enhancer binding factor 2 | NE,NP |
| XP_001076183 | LOC500591 | similar to calmodulin-binding transcription activator 1 | Cyto |
| XP_001069951 | LOC503338 | similar to HBxAg transactivated protein 2 | Cyto |
| XP_001057305 | LOC681004 | similar to GC-rich sequence DNA-binding factor homolog isoform 2 | NE |
| XP_001081073 | LOC688079 | similar to TAF15 RNA polymerase II, TATA box binding protein (TBP)-associated factor | NE |
| NP_001103001 | LOC689296 | hypothetical protein LOC689296 | Cyto |
| NP_001101145 | Maml3 | mastermind like 3 | NE |
| NP_001011999 | Morf4l1 | mortality factor 4 like 1 | NE |
| NP_001007715 | Morf4l2 | mortality factor 4 like 2 | Cyto |
| NP_113754 | Nfib | nuclear factor I/B | NE |
| NP_113755 | Nfic | nuclear factor I/C | NE |
| XP_342347 | Nfkb1 | similar to Nuclear factor NF-kappa-B p105 subunit (DNA-binding factor KBF1) (EBP-1) | NE, NP |
| NP_036997 | Nfya | nuclear transcription factor Y, alpha | NE |
| NP_113741 | Nfyb | nuclear transcription factor-Y beta | NE |
| NP_001004206 | Pa2 g4 | ErbB3-binding protein 1 | NE, NP |
| NP_001099311 | Ptrf | polymerase I and transcript release factor | NE,NP, Cyto |
| NP_071786 | Pttg1 | pituitary tumor-transforming 1 | NP |
| XP_001063244 | Pura | similar to Transcriptional activator protein Pur-alpha (Purine-rich single-stranded DNA-binding protein alpha) | NE, NP, Cyto |
| NP_001017503 | Purb | purine rich element binding protein B | NE, Cyto |
| XP_001071121 | Rb1 | similar to Retinoblastoma-associated protein (PP105) (RB) | NE, NP |
| NP_001099414 | Rfx1 | regulatory factor X, 1 (influences HLA class II expression) | NE |
| XP_001056829 | RGD1308009 | similar to CCR4-NOT transcription complex, subunit 1 isoform a | NE, Cyto |
| XP_001080950 | RGD1311017 | similar to RIKEN cDNA 4732497O03 | NP |
| XP_001061499 | RGD1560020 | similar to Myb proto-oncogene protein (C-myb) | NE |
| NP_001102749 | RGD1561926 | hypothetical protein LOC500695 | NP |
| XP_220843 | RGD1562272 | similar to TAF11 RNA polymerase II, TATA box binding protein (TBP)-associated factor | Cyto |
| XP_228426 | RGD1564748 | similar to Mortality factor 4-like protein 2 (MORF-related gene X protein) | NP |
| NP_001012004 | Rnf25 | ring finger protein 25 | NE |
| NP_001008290 | Sbds | Shwachman-Bodian-Diamond syndrome homolog | NE, NP |
| NP_001007713 | Sdpr | serum deprivation response | NP |
| NP_001100834 | Sfmbt2 | Scm-like with four mbt domains 2 | NE |
| NP_001102231 | Sin3a | SIN3 homolog A, transcription regulator | NE,NP |
| NP_001108510 | Sin3b | SIN3 homolog B, transcription regulator | NE, NP, Cyto |
| NP_062064 | Smad2 | SMAD family member 2 | NP |
| NP_037227 | Smad3 | SMAD family member 3 | NE |
| NP_062148 | Smad4 | SMAD family member 4 | NE |
| NP_110485 | Smad7 | SMAD family member 7 | NE |
| NP_112383 | Ssrp1 | Structure specific recognition protein 1 | NE, NP |
| NP_036879 | Stat3 | signal transducer and activator of transcription 3 | NE, NP |
| NP_001011979 | Tardbp | TAR DNA binding protein | NE, NP, Cyto |
| NP_001099921 | Tbx15 | T-box 15 | NE |
| NP_001100504 | Tbx4 | T-box 4 | NE |
| NP_001124046 | Tcf20 | transcription factor 20 | NP |
| NP_001128186 | Tcfcp2 | transcription factor CP2 | NE, NP |
| NP_001100640 | Tcfcp2l1 | transcription factor CP2-like 1 | NP |
| NP_001032431 | Tcfcp2l2 | transcription factor CP2-like 2 | Cyto |
| XP_001069278 | Tead1 | similar to Transcriptional enhancer factor TEF-1 (TEA domain family member 1) isoform 2 | NE, NP |
| NP_001100317 | Tfdp2 | transcription factor Dp-2 (E2F dimerization partner 2) | Cyto |
| NP_001009693 | Thrap3 | thyroid hormone receptor associated protein 3 | NE, NP |
| NP_001020136 | Trappc2 | trafficking protein particle complex 2 | NE |
| NP_001128309 | Trps1 | trichorhinophalangeal syndrome I homolog | NP, Cyto |
| XP_001076644 | Ubp1 | similar to upstream binding protein 1 (LBP-1a) | NP |
| NP_001099193 | Ubtf | upstream binding transcription factor, RNA polymerase I isoform 1 | NE, NP |
| NP_620809 | Xab2 | XPA-binding protein 2 | NE |
| NP_113751 | Ybx1 | Y box binding protein 1 | NE, NP, Cyto |
| XP_001079683 | Ybx2 | similar to Y-box-binding protein 2 (Germ cell-specific Y-box-binding protein) (FRGY2 homolog) | NE |
NE, soluble nuclear fraction; NP, nuclear membrane pellet; Cyto, pooled cytoplasmic fractions.
Fig. 4.
Venn diagram of the distribution of transcription factors identified in the NE, NP, and Cyto fractions.
Computational Analysis of Conserved Transcription Factor Binding Elements in Aqp2 and Aqp3 Genes
To relate the transcription factors found (Table 1) to their possible roles in regulation of Aqp2 and Aqp3 gene transcription, we have mapped conserved binding element motifs present in the 5′-flanking region as well as the first intron of rat Aqp2 and Aqp3 genes (Fig. 5), based on analysis using the Genomatix software suite (methods). In the following, we summarize these findings in the context of existing literature.
Fig. 5.
Transcription factor binding site analysis. The 1,000 bp 5′-flanking region and 1st intron of the Aqp2 and Aqp3 genes of human, rat, and mouse were analyzed for conserved transcription factor binding sites (TFBS) using Genomatix database and software suite. This figure represents only Rat Aqp2 and Aqp3 genes. Conserved TFBS were found in the 1,000 bp 5′-flanking region of the Aqp2 and Aqp3 genes and the 1st intron of the Aqp2, however, no conserved TFBS were found in the 1st intron of the Aqp3 gene. Transcription factors identified in this study that potentially bind to these TFBS are shown above each TFBS. Abbreviations for Genomatix TFBS family name: V$SF1F = Vertebrate steroidogenic factor; V$NFAT = Nuclear factor of activated T-cells; V$FKHD = Fork head domain factors; V$ETSF = Human and murine ETS1 factors; V$RXRF = RXR heterodimer binding sites; V$AP2F = Activator protein 2; V$CREB = cAMP-responsive element binding proteins; V$GATA = GATA binding factors; V$SRFF = Serum response element binding factor; V$HOXF = Paralog hox genes 1–8 from the four hox clusters A, B, C, D; V$EBOX = E-box binding factors; V$EGRF = EGR/nerve growth factor induced protein C & related factors; V$MYT1 = MYT1 C2HC zinc finger protein; V$KLFS = Krueppel like transcription factors; V$CP2F = CP2-erythrocyte Factor related to drosophila Elf1; V$CEBP = Ccaat/Enhancer Binding Protein; V$PARF = PAR/bZIP family; V$NR2F = Nuclear receptor subfamily 2 factors; V$HAND = Twist subfamily of class B bHLH transcription factors; V$GREF = Glucocorticoid responsive and related elements; V$MAZF = Myc associated zinc fingers; V$ZBPF = Zinc binding protein factors; and V$SP1F = GC-Box factors SP1/GC.
Transcription Factors Potentially Involved in Aqp2 Gene Transcription
Figure 5A shows the transcription factors found in this study (listed in Table 1) that potentially bind conserved transcription factor binding elements in the first 1,000 bp of the 5′-flanking region or first intron of the Aqp2 gene, based on computational analysis using the Genomatix suite. As discussed in the following some of these have been examined experimentally in prior studies.
CREB.
A conserved CREB binding motif (aka CRE) is present at −222 bp from the transcription start site. Originally identified by Uchida and colleagues (48), the importance of this site has been thoroughly documented by the subsequent studies of Hozawa et al. (22), Matsumura et al. (28), Yasui et al. (52), and Cai et al. (5). There are several genes in mammalian genomes that code for proteins that could potentially bind to CRE, including the index protein Creb1, which is activated by phosphorylation at Ser-133 by Ca-calmodulin-dependent kinases, ribosomal S6 kinase, or protein kinase A (41). In this study we identify two of these, Creb1 and Crebl1 (also known as “cAMP responsive element binding protein-like 1”). These contain the so-called “bZIP domain,” comprising a basic region and a leucine zipper region. Crebl1 acts in the unfolded protein response (UPR) pathway by activating UPR target genes induced during endoplasmic reticulum (ER) stress (24). It is a single-pass integral membrane protein that undergoes regulated intramembrane proteolysis in the Golgi during the UPR to yield an ∼400 amino acid cytoplasmic product that translocates into the nucleus.
GATA.
Centered at −214 bp from the transcription start site is a conserved putative GATA sequence, a binding motif for zinc-finger transcription factors of the GATA family. Uchida et al. (47) identified Gata3 mRNA in microdissected collecting ducts and demonstrated that overexpression of Gata3 enhanced Aqp2 promoter-reporter activity. Both Gata2 and Gata3 are expressed in IMCD at many fold above the median signal (46). In the present study, we identified another GATA family member, Trps1 (Tricho-rhino-phalangeal syndrome type I protein), in nuclei from native IMCD cells. This protein is known to bind relatively specifically to GATA sequences and represses GATA-regulated genes (27). Interestingly Rai et al. (37) identified, in the 5′-flanking region of the Aqp2 gene, a region overlapping this GATA site that contained an unidentified cis-element with an apparent negative regulatory role on transcriptional activity. However, in a later study overexpression of the Gata3 transcription factor increased Aqp2 transcription pointing to an enhancer role for this binding element (47), in seeming contradiction to the findings of Rai et al. (37). A possible explanation for this conundrum is that Trps1 normally maintains repression at the GATA site, blocking the enhancer activity of Gata2 and Gata3 that would otherwise occur.
HOX.
In the present study we also identified by mass spectrometry two transcription factors that potentially bind to a conserved HOX binding element centered at −201 bp from the transcription start site, viz., Hoxa2 and Hoxb7. Homeobox or HOX transcription factors are recognized to be involved in renal tubule segmentation as well as collecting duct development (32). The 5′-flanking region of Hoxb7 has been used to target transgene expression specifically to collecting duct cells in mice (38, 42).
NFAT.
Centered at −469 bp upstream from the transcription start site is a potential NFAT binding element. Several NFAT proteins including TonEBP (Nfat5) (19) and the calcineurin-dependent Nfat proteins (Nfatc1–4) (26) have been implicated in Aqp2 transcriptional regulation. Although we did not identify these transcription factors in the present study, we found a protein (Nf45) with weak homology to them that has been previously implicated in regulation of interleukin-2 transcription in lymphocytes (23). Although mRNA levels do not necessarily correlate with protein expression, Affymetrix microarray studies of native IMCD cells demonstrated that Nfat5 (also called TonEBP) is expressed with a signal 12.5-fold above the median signal for all transcripts (46). Interestingly, the same study showed that another Nfat protein is strongly expressed in rat renal IMCD, viz. Nfatc3, which is regulated by intracellular calcium. A rise in calcium activates the Ca-dependent protein phosphatase, calcineurin, which dephosphorylates the Nfat protein allowing it to translocate into the nucleus. A member of this family has recently been implicated in the transcriptional control of the Aqp2 gene (26). The involvement of Ca2+-calcineurin-sensitive regulation of Aqp2 gene transcription identifies another mechanism by which vasopressin can regulate Aqp2 expression, since several laboratories have demonstrated that activation of the V2 vasopressin receptor is associated with an elevation of intracellular calcium (6, 8, 11, 35, 43, 53).
ETS.
Centered at −455 bp from the transcriptional start site of the Aqp2 gene is a conserved Ets binding motif that we identified in a previous study (54) as playing an enhancer role in cell-specific expression of Aqp2, and possibly playing a role in the vasopressin response. In the present study, MS analysis identified Etv5 (ets variant gene 5 or PEA3), a member of the ets family. This transcription factor is known to be selectively expressed in ureteric bud (the developmental precursor of the collecting duct system) (7).
RXR.
Centered at −342 bp from the transcription start site is a conserved putative RXR binding motif. RXR binding elements bind dimers of ligand-activated transcription factors, usually RAR/RXR heterodimers. These transcription factors are known to be involved in development of the ureteric bud (4). In addition, RXR can heterodimerize with vitamin D receptors, peroxisome proliferator activator receptors, thyroid receptors, and other ligand-activated nuclear receptors. MS analysis in the present paper found one such ligand-activated transcription factor, viz. Nr1h2 (nuclear receptor subfamily 1 group H member 2, also known as “liver X receptor”). Interestingly, this is one of two transcription factor genes (with Elf1) whose mRNA levels correlated negatively with Aqp2 mRNA levels among subcloned mpkCCD collecting duct cell lines (54). Its endogenous ligand is at present unknown.
EBOX.
A conserved EBOX motif is located at −67 bp relative to the transcription start site of Aqp2. Two transcription factors were identified that potentially bind this site, namely Usf1 and Usf2. These are classical basic helix-loop-helix transcription factors known to be involved in transcriptional regulation in a variety of tissues.
Potential binding elements in the first intron.
We identified four potential transcription factor binding elements in a conserved region of the first intron of the Aqp2 gene (Fig. 5A), viz. binding sites for EGR, MYT1, KLF, and CP2 family transcription factors. The transcription factors identified in IMCD nuclei that correspond to these sites (Zbtb7a, St18, Klf15, Tcfcp2, and Tcfcp2l1) are all of the zinc-finger or the CP2 classes of transcription factors and typically play repressor roles. Klf15 has been previously implicated in regulation of the ClC-K1 chloride channel of the thin ascending limb of Henle (49). Tcfp2l1 is associated with normal duct development in both the salivary gland and kidney (50).
Transcription Factors Potentially Involved in Aqp3 Gene Transcription
Figure 5B shows the transcription factors found in this study (Table 1) that potentially bind conserved transcription factor binding elements in the first 1,000 bp of the 5′-flanking region or first intron of the Aqp3 gene, based on computational analysis using the Genomatix suite. No conserved binding motifs were found in the first intron.
The 5′-flanking region of the Aqp3 gene contains several conserved transcription factor binding element motifs seen in the Aqp2 5′-flanking region or first intron including two EBOX motifs (potentially binding Usf1 and Usf2), an NR2 nuclear receptor site (potentially binding the orphan nuclear receptor Nrf2f), and two KLF sites (potentially binding Klf15). These sites are potentially responsible for the coordinate regulation of Aqp2 and Aqp3 seen in response to vasopressin (45). In addition, the 5′-flanking region of the Aqp3 gene contains some interesting unique sites that may provide some of the explanation for the difference in the tissue distribution of Aqp3 expression vs. Aqp2 expression, as well as differences in regulation. Foremost among these unique sites is a putative GRE (glucocorticoid response element), which can bind either the glucocorticoid receptor (Nr3c1) or the mineralocorticoid receptor (Nr3c2). Although only the former was found by MS analysis of the nuclear fraction in the current study, the latter is also known to be strongly expressed in the rat renal IMCD (46), providing a potential explanation for the large increase in Aqp3 protein abundance seen in the renal collecting duct in response to treatment of rats with the mineralocorticoid, aldosterone (25).
In addition, the 5′-flanking region of the Aqp3 gene contains two highly conserved “MAZ” or “MYC-associated zinc finger” sites at −62 and −79 bp relative to the transcriptional start site. Genomatix analysis predicts that one transcription factor from Table 1 would bind to these sites, viz. Zbtb19 (aka Zfp278 or “protein kinase A RI subunit α-associated protein”). Like other zinc-finger transcription factors, this transcription factor is associated with repressor activity. Finally, the 5′-flanking region of the Aqp3 gene contains a highly conserved SP1 binding element motif at −62 bp, which potentially binds another zinc-finger transcription factor found in this study by mass spectrometry, viz. Klf10 (also known as “TGF-β-inducible early growth response protein 1”), that likely manifests repressor function.
Transcriptional Co-regulators and Nucleic Acid Binding Proteins Identified by Proteomic Analysis of IMCD
Transcriptional co-regulators are listed in Table 2. Proteins classified as “nucleic acid binding” proteins by Panther analysis are listed in Supplementary Table S2. This category includes basal transcription factors, helicases, RNA polymerases, and other ubiquitous proteins. Both lists are made up largely of proteins that would be found in any cell type.
Table 2.
Transcriptional co-regulators
| Accession No. | Gene | Name | Sample |
|---|---|---|---|
| NP_062093 | Aes | amino-terminal enhancer of split | NP |
| NP_001101094 | Arid5b | AT rich interactive domain 5B (Mrf1 like) | NP |
| NP_001101923 | Cbfa2t3 | core-binding factor, runt domain, alpha subunit 2, translocated to, 3 | NE |
| NP_445981 | Ciita | class II transactivator | NE |
| NP_596872 | Crebbp | CREB binding protein | NE |
| NP_445787 | Ctbp2 | C-terminal binding protein 2 | NE |
| XP_341396 | Dcp1a | similar to mRNA decapping enzyme 1A (Smad4-interacting transcriptional co-activator) | NE |
| NP_001102523 | Dtx3l | deltex 3-like | NE |
| NP_001100027 | Edf1 | endothelial differentiation-related factor 1 | NE |
| NP_001100774 | Ell | elongation factor RNA polymerase II | Cyto |
| XP_576312 | Ep300 | similar to E1A binding protein p300 | NE |
| NP_598232 | Hdgfrp2 | hepatoma-derived growth factor-related protein 2 | NE |
| NP_001009625 | Ifi35 | interferon-induced protein 35 | NE |
| XP_001061895 | LOC360888 | similar to lin-9 homolog | NE |
| XP_001055295 | LOC679225 | similar to Ladybird homeobox corepressor 1 | NP |
| XP_001054608 | LOC679693 | similar to Mediator of RNA polymerase II transcription subunit 12 isoform 1 | NE |
| XP_001080941 | LOC688035 | similar to transformation related protein 53 binding protein 1 | NE |
| XP_001066953 | LOC689062 | similar to nuclear DNA-binding protein | NE |
| NP_001019932 | Lrrfip2 | leucine rich repeat (in FLII) interacting protein 2 | NE, NP |
| NP_001100271 | Med17 | mediator complex subunit 17 | NE |
| NP_001100035 | Med27 | mediator complex subunit 27 | NP |
| NP_114010 | Ncoa2 | nuclear receptor coactivator 2 | NP |
| XP_221724 | Nrip1 | similar to nuclear receptor interacting protein 1 | NE |
| NP_001101968 | Nrip3 | nuclear receptor interacting protein 3 | NE |
| NP_001094308 | Prkcbp1 | protein kinase C binding protein 1 | NE |
| NP_786941 | Psip1 | PC4 and SFRS1 interacting protein 1 | NE, NP |
| NP_001100644 | Rbbp5 | retinoblastoma binding protein 5 | NP |
| NP_001101164 | Rfx5 | regulatory factor X, 5 (influences HLA class II expression) | NE |
| XP_221241 | RGD1304870 | similar to transcription elongation factor B (SIII), polypeptide 2 | NP |
| XP_223519 | RGD1561605 | similar to Ada2b CG9638-PA, isoform A | Cyto |
| NP_671706 | Ruvbl1 | RuvB-like 1 | NE, NP |
| NP_001020576 | Ruvbl2 | RuvB-like 2 | NE, NP |
| NP_071789 | Safb | scaffold attachment factor B | NE, Cyto |
| NP_073185 | Snd1 | staphylococcal nuclease domain containing 1 | NE, Cyto |
| NP_001009618 | Sub1 | RNA polymerase II transcriptional coactivator | NE, NP |
| NP_001100731 | Supt16 h | suppressor of Ty 16 homolog | NE, NP |
| NP_001101374 | Tgs1 | trimethylguanosine synthase homolog | NE, NP |
| XP_233081 | Thoc2 | similar to THO complex subunit 2 (Tho2) | NE |
| NP_446368 | Trim28 | tripartite motif-containing 28 | NE, NP, Cyto |
| XP_345267 | Trim33 | similar to tripartite motif protein 33 | NE |
| NP_001101666 | Wtip | WT1-interacting protein | Cyto |
| NP_976244 | Zmynd11 | zinc finger, MYND domain containing 11 delta isoform | NE |
Phosphoproteomic Analysis
Figure 6 summarizes the workflow utilized for the discovery of phosphoproteins in NE and NP fractions from rat IMCD. Three phosphorylation site verification tools were used [Ascore (2), Phosphate Localization Score (PLscore) (1), and PhosphoScore (39)] to increase our confidence in the identified phosphorylation sites. Table 3 summarizes the 122 phosphorylation sites found. Of those, 63 were previously unidentified phosphorylation sites (not present in Phosphosite, http://www.phosphosite.org). Eight phosphorylation sites in six proteins were identified as a “transcription factor” or “transcription co-regulator” by Panther, namely Bclaf1 (S512), Hbxap (S608 and S1352), Lrrfip2 (S133), Ptrf (S169), Safb (S309), and Trim28 (S52 and S474). The presence of phosphorylated COOH-terminal tails of two aquaporins in the nuclear fractions raises the possibility of a role for regulated intramembrane proteolysis (3) of aquaporin proteins in transcriptional regulation in the IMCD, a possibility that needs further investigation. However, we cannot rule out the presence of these aquaporin phosphopeptides in nuclear fractions owing to contamination with small amounts of ER membranes, which are essentially extensions of the nuclear envelope.
Fig. 6.
Phosphoproteomic work flow. Graphical representation of the sample preparation, LC-MS3, and bioinformatic steps taken in the nuclear phosphoproteomic study. Ovals represent the sample or proteomic data. Shaded boxes represent the biochemical and computational methods that were applied to the sample or proteomic data. The work flow utilized 2 phosphopeptide isolation kits (IMAC and TiO2 tips) to maximize phosphopeptide recovery and 3 phosphorylation site verification programs (Ascore, PLscore, and PhosphoScore) to ensure phosphosite validity.
Table 3.
Phosphorylation sites identified in nuclear fractions
| Accession No. | Gene | Name | Sequence | PhosphoSite |
|---|---|---|---|---|
| NP_001037859 | Ablim1 | actin-binding LIM protein 1 | S*GLHRPVSTDFAQYNSYGDVSGGVR | S311^ |
| TLS*PTPSAEGFQDGR | S115^ | |||
| XP_574618 | Ahnak | similar to AHNAK nucleoprotein isoform 1 | FKAEAALPS*PK | S2257 |
| XP_216539 | Aim1l | similar to absent in melanoma 1 | AVRDYCT*PR | T1271^ |
| NP_001029156 | Ank3 | ankyrin 3, epithelial isoform 2 | RQS*FTSLALR | S1458^ |
| NP_036910 | Aqp1 | aquaporin 1 | VWTSGQVEEYDLDADDINS*R | S262^ |
| NP_037041 | Aqp2 | aquaporin 2 (collecting duct) | QS*VELHS*PQSLPR | S256, S261 |
| RQS*VELHSPQSLPR | S256 | |||
| RQSVELHS*PQSLPR | S261 | |||
| NP_001029312 | Baiap2l1 | BAI1-associated protein 2-like 1 | SIS*TVDLTEK | S423 |
| NP_001041317 | Bclaf1 | BCL2-associated transcription factor 1 | LKELFDYS*PPLHK | S512^ |
| NP_001102045 | Brd3 | bromodomain containing 3 | SES*PPPLSEPK | S263^ |
| NP_114175 | Calm1 | calmodulin 1 | HVMT*NLGEKLT*DEEVDEMIR | T111^, T118 |
| NP_446072 | Cdc42bpb | Cdc42-binding protein kinase beta | HSTPSNSSNPSGPPS*PNSPHR | S1692 |
| NP_001101634 | Cgnl1 | cingulin-like 1 | EGVGEETLS*PR | S251^ |
| NCFPKPCGS*QPNS*PTPEDLAK | S198, S202 | |||
| NCFPKPCGSQPNS*PT*PEDLAK | S202, T204^ | |||
| NP_001007146 | Ctnna1 | catenin (cadherin-associated protein), alpha 1, 102 kDa | TPEELDDS*DFETEDFDVRSRTS*VQTEDDQLIAGQSAR | S643S657 |
| NP_001101210 | Ctnnd1 | catenin (cadherin associated protein), delta 1 | GS*LASLDSLRK | S346 |
| GSLAS*LDSLRK | S349 | |||
| VGGS*SVDLHR | S268 | |||
| XP_225259 | Dsp | similar to desmoplakin isoform I isoform 2 | NLTIRS*SSLS*DPLEESSPIAAIFDTENLEK | S2613, S2617 |
| NP_114008 | Dvl1 | dishevelled, dsh homolog 1 | HHFLGIS*IVGQS*NDR | S265^, S270^ |
| NP_001013122 | Eef1d | eukaryotic translation elongation factor 1 delta | GATPAEDDEDNDIDLFGS*DEEEEDKEAAR | S531 |
| KGATPAEDDEDNDIDLFGS*DEEEEDKEAAR | S531 | |||
| NP_067713 | Epb4.1l1 | erythrocyte protein band 4.1-like 1 isoform L | HQAS*INELKR | S510 |
| XP_217039 | eplin | similar to Epithelial protein lost in neoplasm (mEPLIN) | GENEETLGRPAQPPSAGETPHS*PGVEDAPIAKRLS*ENSCSLDDLEIGAGHLSSSAFNSEK | S488S225 |
| SEAQQPIYTKPLS*PDAR | S360 | |||
| SPKPLS*PSLR | S608 | |||
| TSSVKS*PKPLS*PSLRK | S603^, S608 | |||
| TSSVKS*PKPLSPS*LRK | S603^, S610^ | |||
| NP_001124037 | Fam83 h | family with sequence similarity 83, member H | KGS*PTPAYPER | S860 |
| NP_001128071 | Flna | filamin, alpha | RAPS*VANVGSHCDLSLK | S2144 |
| XP_218939 | Hbxap | similar to remodeling and spacing factor 1 | IES*DEEEDFENVGK | S1352^ |
| TSVDKDIQRLS*PIPEEVVR | S608^ | |||
| NP_001020580 | Hdac1 | histone deacetylase 1 | MLPHAPGVQMQAIPEDAIPEES*GDEDEEDPDKR | S393 |
| NP_446159 | Hdgf | hepatoma-derived growth factor | AGDMLEDS*PKRPK | S165 |
| GNAEGS*S*DEEGKLVIDEPAKEK | S132, S133 | |||
| RAGDMLEDS*PKRPK | S165 | |||
| NP_579819 | Hist1 h1d | histone cluster 1, H1d | KAS*GPPVSELITK | S36 |
| RKAS*GPPVSELITK | S36 | |||
| NP_073177 | Hist1 h4b | histone cluster 1, H4b | RIS*GLIYEETR | S48 |
| NP_001101168 | Hist2 h3c2 | histone cluster 2, H3c2 | FQSSAVMALQEASEAY*LVGLFEDTNLCAIHAK | Y100^ |
| NP_058944 | Hnrnpa1 | heterogeneous nuclear ribonucleoprotein A1 | SES*PKEPEQLR | S6 |
| SES*PKEPEQLRK | S6 | |||
| NP_476482 | Hnrnpk | heterogeneous nuclear ribonucleoprotein K | IIPTLEEGLQLPS*PTATSQLPLESDAVECLNYQHYK | S116^ |
| NP_114176 | Hspb1 | heat shock protein 1 | QLS*S*GVSEIR | S85, S86^ |
| QLS*SGVSEIR | S85 | |||
| SPS*WEPFR | S15 | |||
| SPS*WEPFRDWYPAHSR | S15 | |||
| NP_001101412 | Lad1 | ladinin | LPS*VEEAEVSKPSPPASKDEGEEFQAILR | S62 |
| NP_001002016 | Lmna | lamin A isoform C2 | LRLS*PS*PTSQR | S389, S391 |
| LRLS*PSPTSQR | S389 | |||
| LRLSPS*PTSQR | S391 | |||
| NP_001001515 | Lmo7 | LIM domain 7 | SRS*TTELNDPLIEK | S1476^ |
| XP_001080824 | LOC360570 | similar to myosin XVIIIa | RFS*FSQR | S140 |
| SLAPDLS*DDEHDPVDSISRPR | S2020^ | |||
| XP_341387 | LOC361100 | similar to topoisomerase (DNA) II beta | KTS*FDQDSDVDIFPSDFTSEPPALPR | S367^ |
| KTSFDQDS*DVDIFPSDFTSEPPALPR | S372 | |||
| KVVEPANS*DS*DSELGNIPK | S312^, S314^ | |||
| VKAS*PITNDGEDEFVPS*DGIDKDEYAFSPGK | S189, S202^ | |||
| VKAS*PITNDGEDEFVPSDGIDKDEYAFSPGK | S189 | |||
| XP_573444 | LOC498222 | similar to specifically androgen-regulated protein | APAQPAS*PALVR | S396^ |
| XP_001056725 | LOC681423 | similar to delangin isoform A | AITSLLGGGS*PK | S2431^ |
| XP_001061576 | LOC682450 | similar to CG31613-PA | FQS*S*AVIALQEASEAYLVGLFEDTNLCAIHAK | S87^, S88^ |
| XP_001068152 | LOC684233 | similar to Putative RNA-binding protein 15 (RNA-binding motif protein 15) (One-twenty two protein) | DRT*PPLLYRLLLLERPS*PVRDR | T567S701^ |
| XP_001071565 | LOC684681 | similar to Histone H1.2 (H1 VAR.1) (H1c) | S*ET*APAAPAAAPPAEKAPAK | S108^, T110^ |
| XP_001055673 | LOC685179 | similar to SWI/SNF-related matrix-associated actin-dependent regulator of chromatin c2 isoform b isoform 2 | DMDEPS*PVPNVEEVTLPK | S347^ |
| XP_001078152 | LOC686579 | similar to AHNAK nucleoprotein isoform 2 isoform 1 | KGDRS*PEPGQTWAHEVFSSR | S94 |
| NP_001019932 | Lrrfip2 | leucine rich repeat (in FLII) interacting protein 2 | RGS*GDTSSLIDPDTSLSELR | S133^ |
| NP_037198 | Map2 | microtubule-associated protein 2 | ARVDHGAEIITQS*PSR | S1780 |
| NP_113708 | Myh10 | myosin, heavy chain 10, nonmuscle | QLHIEGAS*LELS*DDDTESKQLHIEGASLELS*DDDTESK | S1952^, S1956S1956 |
| RQLHIEGASLELS*DDDTESK | S1956 | |||
| NP_037326 | Myh9 | myosin, heavy chain 9, nonmuscle | GTGDCS*DEEVDGKADGADAK | S1944 |
| NP_631977 | Nexn | nexilin (F actin binding protein) isoform b | AEIKDMLAS*DEEEEPSKVEK | S16 |
| NP_037124 | Npm1 | nucleophosmin (nucleolar phosphoprotein B23, numatrin) | CGSGPVHISGQHLVAVEEDAES*EDEDEEDVK | S125 |
| NP_073636 | Nucks1 | nuclear casein kinase and cyclin-dependent kinase substrate 1 | TSAS*PPLEK | S223^ |
| XP_218972 | Numa1 | similar to nuclear mitotic apparatus protein 1 | AAQLQGS*PAPEKGEVLGDALQLDTLKQEAAK | S416^ |
| NP_001106799 | Pde4d | phosphodiesterase 4D isoform 1 | KSRMS*WPSSFQGLR | S137^ |
| NP_071796 | Plec1 | plectin 1 isoform 1 | KQIT*VEELVR | T4033^ |
| SSS*VGSSSSYPISSAVPR | S4389 | |||
| NP_001101576 | Plxnb2 | plexin B2 | ADPAY*RCVWCRGQNR | Y779^ |
| XP_343923 | Polr2a | similar to DNA-directed RNA polymerase II largest subunit (RPB1) | GHGGCGRY*QPRIR | Y187^ |
| NP_001099311 | Ptrf | polymerase I and transcript release factor | LPAKLS*VSK | S169^ |
| NP_001013225 | Rbm39 | RNA binding motif protein 39 | DKS*PVREPIDNLTPEER | S136 |
| XP_578547 | RGD1562028 | similar to Mitogen-activated protein kinase kinase kinase kinase 5 (MAPK/ERK kinase kinase kinase 5) (MEK kinase kinase 5) (MEKKK 5) | TAS*EINFDKLQFEPPLRK | S316^ |
| XP_001075668 | RGD1564067 | hypothetical protein | T*KVEEEPPPPPPPPPPPPPPPPPR | T104^ |
| NP_071789 | Safb | scaffold attachment factor B | APTAAPS*PEPR | S309 |
| XP_217021 | Sbf1 | similar to SET binding factor 1 isoform a | LGLGTLSS*SLS*R | S1142^, S1145^ |
| NP_476489 | Sept2 | septin 2 | IYHLPDAES*DEDEDFKEQTR | S218 |
| IYHLPDAESDEDEDFKEQT*R | T228^ | |||
| NP_789826 | Sept9 | septin 9 isoform 2 | LVDTLSQRS*PKPSLR | S67^ |
| NP_001009255 | Sfrs9 | splicing factor, arginine/serine rich 9 | GS*PHYFSPFRPY | S211 |
| NP_690921 | Slc4a9 | solute carrier family 4, sodium bicarbonate cotransporter, member 9 | WS*APHVPTLALPSLQK | S89^ |
| NP_037165 | Slc5a1 | solute carrier family 5 (sodium/glucose cotransporter), member 1 | MT*KEEEEAMKLK | T622^ |
| NP_062251 | Snip | SNAP25-interacting protein | RCRVVT*DTLAQIRR | T853^ |
| NP_001011981 | Snx17 | sorting nexin 17 | S*QEYKIVLRK | S194^ |
| NP_741984 | Spna2 | alpha-spectrin 2 | WRS*LQQLAEER | S1217 |
| NP_001101456 | Srrm1 | serine/arginine repetitive matrix 1 | EKS*PELPEPSVR | S220^ |
| RYS*PPIQR | S601 | |||
| NP_001099736 | Tjp1 | tight junction protein 1 | VQIPVSHPDPDPVS*DNEDDSYDEDVHDPR | S113^ |
| VQIPVSHPDPDPVSDNEDDS*YDEDVHDPR | S119^ | |||
| NP_446225 | Tjp2 | tight junction protein 2 | KVQVAPLQGS*PPLSHDDR | S107 |
| NP_037019 | Tmpo | thymopoietin | GPPDFSSDEEREPT*PVLGSGASVGR | T74 |
| NP_446368 | Trim28 | tripartite motif-containing 28 | RPAASSAAAASASASS*PAGGGGEAQELLEHCGVCR | S52^ |
| SRS*GEGEVSGLMR | S474^ | |||
| NP_001026829 | Trip12 | thyroid hormone receptor interactor 12 | S*ESPPAELPSLR | S310^ |
| NP_037202 | Utrn | utrophin | AAQAS*LSALNDPSAVEQALQEK | S933^ |
| NP_973718 | Xirp2 | xin actin-binding repeat containing 2 | CFET*QPLY*VIR | T507^, Y511^ |
| NP_001101423 | Zcchc11 | zinc finger, CCHC domain containing 11 | FILTS*GKPPTIVCS*ICK | S768^, S777^ |
Previously unidentified.
Kinases in Nuclear Fractions
Table 4 categorizes the kinases found in this study, which include 58 kinases present in the nuclear extract fraction. While all are candidates for regulatory roles in transcription in IMCD, some already have well documented roles in other tissues. For example, all three of the kinases known to phosphorylate Creb1 at Ser133 are present in either the NE or NP fraction, viz. protein kinase A catalytic subunit α (Prkaca), calcium/calmodulin-dependent protein kinase II δ and γ (Camk2d and Camk2g), and ribosomal protein S6 kinase (Rps6ka5 and Rps6kc1) (41). In addition several MAP kinases were detected, including Erk1 (Mapk3), Erk2 (Mapk1), Erk3 (Mapk6), and p38α (Mapk14), which are known to phosphorylate transcription factors of the ETS, AP1, and GATA families (Phosphosite: http://www.phosphosite.org/). Finally, the Hipk2 protein phosphorylates a number of transcription factors including p53, Pax6, and Zbtb4 (Phosphosite).
Table 4.
Protein kinases in nuclear fractions
| Accession No. | Gene | Name | Sample |
|---|---|---|---|
| AGC Ser/Thr protein kinase family | |||
| NP_001101987 | Ccdc88b | coiled-coil domain containing 88B | NP |
| NP_446072 | Cdc42bpb | Cdc42-binding protein kinase beta | NE, NP, Cyto |
| NP_001025082 | Cit | citron (rho-interacting, serine/threonine kinase 21) | NE |
| NP_112358 | Grk1 | G protein-coupled receptor kinase 1 | NE, NP, Cyto |
| NP_001100737 | Lats2 | large tumor suppressor 2 | NE |
| NP_851603 | Mast1 | microtubule associated serine/threonine kinase 1 | NE |
| NP_001099225 | Pkn2 | protein kinase N2 | NE |
| NP_001094392 | Prkaca | cAMP-dependent protein kinase catalytic subunit alpha | NP, Cyto |
| NP_112360 | Rock1 | Rho-associated coiled-coil containing protein kinase 1 | NE, Cyto |
| NP_037154 | Rock2 | Rho-associated coiled-coil containing protein kinase 2 | NE, Cyto |
| NP_001101518 | Rps6ka5 | ribosomal protein S6 kinase, polypeptide 5 | NP |
| NP_001099454 | Rps6kc1 | ribosomal protein S6 kinase, 52 kDa, polypeptide 1 | NP |
| NP_001076805 | Stk38l | serine/threonine kinase 38 like | NE |
| Atypical: PDK/BCKDK protein kinase family | |||
| NP_110499 | Pdk2 | pyruvate dehydrogenase kinase, isozyme 2 | NE |
| Atypical: PI3/PI4-kinase family | |||
| NP_001100291 | Atm | ataxia telangiectasia mutated homolog | NE |
| NP_063971 | Mtor | FK506 binding protein 12-rapamycin associated protein 1 | NE, Cyto |
| NP_001101797 | Prkdc | protein kinase, DNA activated, catalytic polypeptide | NE |
| CAMK Ser/Thr protein kinase family | |||
| NP_036651 | Camk2d | calcium/calmodulin-dependent protein kinase II delta | NE, NP, Cyto |
| NP_598289 | Camk2 g | calcium/calmodulin-dependent protein kinase II gamma | NE |
| NP_071991 | Dapk3 | Death-associated protein kinase 3 | NE |
| NP_067731 | Mark2 | MAP/microtubule affinity-regulating kinase 2 | NE |
| NP_570105 | Mark3 | MAP/microtubule affinity-regulating kinase 3 | NE |
| NP_001007618 | Nuak2 | NUAK family, SNF1-like kinase, 2 | NP, Cyto |
| NP_001009362 | Pask | PAS domain containing serine/threonine kinase | NP |
| NP_062015 | Prkaa1 | protein kinase, AMP-activated, alpha 1 catalytic subunit | NP |
| XP_230713 | RGD1565143 | Sperm motility kinase X | NE |
| NP_001011900 | Tssk1 | testis-specific serine kinase 1 | NE |
| CK1 Ser/Thr protein kinase family | |||
| NP_446067 | Csnk1a1 | casein kinase 1, alpha 1 | NE, NP |
| NP_001012194 | Vrk1 | vaccinia related kinase 1 | NE, NP |
| CMGC Ser/Thr protein kinase family | |||
| NP_543161 | Cdk5 | cyclin-dependent kinase 5 | NP |
| NP_059040 | Gsk3a | glycogen synthase kinase 3 alpha | NE |
| NP_114469 | Gsk3b | glycogen synthase kinase 3 beta | NE |
| NP_001102092 | Hipk2 | homeodomain interacting protein kinase 2 | NE |
| NP_446294 | Mapk1 | mitogen activated protein kinase 1 | NE, NP |
| NP_112282 | Mapk14 | mitogen-activated protein kinase 14 | NP |
| NP_059043 | Mapk3 | mitogen activated protein kinase 3 | NE |
| NP_113810 | Mapk6 | mitogen-activated protein kinase 6 | NE |
| NP_001093976 | Pctk3 | PCTAIRE protein kinase 3 | NE, NP, Cyto |
| NP_001011923 | Prpf4b | PRP4 premRNA processing factor 4 homolog B | NE, NP |
| NP_001020897 | Srpk1 | SFRS protein kinase 1 | NE |
| NEK Ser/Thr protein kinase family | |||
| XP_224971 | Nek3 | similar to Serine/threonine-protein kinase Nek3 (NimA-related protein kinase 3) | NP |
| NP_001099274 | Nek8 | NIMA-related kinase 8 | NE |
| STE Ser/Thr protein kinase family | |||
| NP_596900 | Ilk | integrin linked kinase | NE, NP |
| XP_001079680 | LOC687705 | similar to misshapen-like kinase 1 isoform 1 isoform 1 | NE |
| NP_113831 | Map2k1 | mitogen-activated protein kinase kinase 1 | NP |
| NP_579817 | Map2k2 | mitogen activated protein kinase kinase 2 | NE |
| NP_001094144 | Map2k3 | mitogen activated protein kinase kinase 3 | NP |
| NP_001100374 | Map4k4 | mitogen-activated protein kinase kinase kinase kinase 4 | NE |
| NP_001101664 | Oxsr1 | oxidative-stress responsive 1 | NP |
| NP_445758 | Pak2 | p21-activated kinase 2 | NP |
| NP_001099708 | Pak4 | p21 (CDKN1A)-activated kinase 4 | NE |
| XP_001070192 | RGD1563568 | similar to serine/threonine protein kinase MASK | NE, NP |
| NP_062222 | Slk | serine/threonine kinase 2 | NE, NP, Cyto |
| XP_577078 | Stk10 | similar to Serine/threonine-protein kinase 10 (Lymphocyte-oriented kinase) | NE |
| NP_001120966 | Stk24 | serine/threonine kinase 24 | NE |
| NP_113923 | Stk3 | serine/threonine kinase 3 | NP |
| NP_062235 | Stk39 | Ste-20 related kinase (SPAK) | NP |
| NP_775449 | Taok1 | TAO kinase 1 | NE |
| Tyr protein kinase family | |||
| XP_001079831 | Abl1 | similar to Proto-oncogene tyrosine-protein kinase ABL1 (p150) | NE |
| NP_001025210 | Csk | c-src tyrosine kinase | NE,NP |
| NP_001102447 | Epha2 | ephrin receptor EphA2 | NE, NP, Cyto |
| NP_599158 | Epha7 | ephrin receptor EphA7 | NE |
| NP_001120791 | Ephb2 | Eph receptor B2 | NE, Cyto |
| NP_077059 | Fgr | proto-oncogene tyrosine-protein kinase Fgr | NE |
| NP_077344 | Frk | fyn-related kinase | NP, Cyto |
| NP_036887 | Fyn | FYN oncogene related to SRC, FGR, YES | NE |
| NP_058767 | Insr | insulin receptor | NP |
| NP_445918 | Jak1 | Janus kinase 1 | NP |
| NP_110484 | Lyn | Yamaguchi sarcoma viral (v-yes-1) oncogene homolog isoform A | NE,NP |
| NP_001100785 | Pragmin | pragmin | NE, Cyto |
| NP_114183 | Src | v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog | NP, Cyto |
| NP_001099207 | Tek | TEK tyrosine kinase, endothelial | NE |
| NP_001100482 | Tnk1 | tyrosine kinase, nonreceptor, 1 | NP |
| NP_150640 | Yes1 | Yamaguchi sarcoma viral oncogene homolog 1 | NE, NP, Cyto |
| Other protein kinases | |||
| NP_112628 | Camkk2 | calcium/calmodulin-dependent protein kinase 2 beta | NP |
| NP_446276 | Csnk2a1 | casein kinase 2, alpha 1 polypeptide | NE, Cyto |
| NP_001100879 | Csnk2a2 | casein kinase 2, alpha prime polypeptide | NE, Cyto |
| NP_062208 | Eif2ak2 | eukaryotic translation initiation factor 2-alpha kinase 2 | NP |
| XP_226572 | RGD1306091 | similar to cDNA sequence BC021891 | NP |
| XP_577704 | RGD1560972 | similar to serine/threonine kinase | NE |
| XP_001076790 | RGD1562028 | similar to Mitogen-activated protein kinase kinase kinase kinase 5 (MEK kinase kinase 5) (MEKKK 5) | NE |
| XP_001080090 | RGD1562674 | similar to kinase suppressor of ras 2 | NE |
In general, of the 82 kinases listed in Table 4, 36 of them are classified by Gene Ontology (component) as located in “nucleus.” Among these are kinases that have housekeeping roles in the nucleus, such as DNA repair (Atm and Prkdc), mRNA splicing (Stk3), and cell cycle regulation (Cit, Cdk5, Gsk3β, and Mtor).
Phosphatases
Table 5 categorizes the 17 phosphatase proteins found in nuclear fractions in this study. These proteins include receptor and nonreceptor tyrosine phosphatases, serine/threonine phosphatases, and dual-specificity phosphatases. All could potentially play roles in transcriptional regulation in the collecting duct.
Table 5.
Protein phosphatases in nuclear fractions
| Accession No | Gene | Name | Sample |
|---|---|---|---|
| Dual-specificity phosphatases | |||
| NP_001012352 | Dusp26 | Dual-specificity phosphatase 26 | NP |
| XP_001071732 | Dusp27 | PREDICTED: similar to Dual specificity protein phosphatase 13 (Testis- and skeletal-muscle-specific DSP) | NE |
| NP_001013065 | Mtm1 | X-linked myotubular myopathy gene 1 | NP |
| NP_001101593 | Mtmr2 | myotubularin-related protein 2 | NE |
| Receptor tyrosine phosphatases | |||
| NP_062122 | Ptprf | protein tyrosine phosphatase, receptor type, F | NE, NP |
| NP_599183 | Ptprg | protein tyrosine phosphatase, receptor type, G | NE |
| NP_058965 | Ptprj | protein tyrosine phosphatase, receptor type, J | NP |
| XP_001063025 | Ptpru | PREDICTED: similar to protein tyrosine phosphatase, receptor type, L | NE |
| Nonreceptor tyrosine phosphatases | |||
| NP_446360 | Ptpn6 | protein tyrosine phosphatase, nonreceptor type 6 | NE, NP |
| NP_037220 | Ptpn11 | protein tyrosine phosphatase, nonreceptor type 11 | NE, NP |
| XP_213997 | Ptpn13 | PREDICTED: similar to Tyrosine-protein phosphatase nonreceptor type 13 | NE |
| NP_001100670 | Ptpn14 | protein tyrosine phosphatase, nonreceptor type 14 | NP |
| Serine/threonine phosphatases | |||
| NP_037197 | Ppp1cb | protein phosphatase 1, catalytic subunit, beta | NE, Cyto |
| NP_071943 | Ppp1 ml | protein phosphatase 1, catalytic subunit, gamma isoform | NE |
| NP_058735 | Ppp2ca | protein phosphatase 2a, catalytic subunit, alpha isoform | NE |
| NP_476481 | Ppp2r1a | alpha isoform of regulatory subunit A, protein phosphatase 2 | NE, NP, Cyto |
| NP_852044 | Ppp2r5b | protein phosphatase 2, regulatory subunit B', beta isoform | NE |
General Observations
In addition to investigating the regulation of the water channels Aqp2 and Aqp3, a general goal of our studies is the development of tools, including proteomics databases, that can be useful to the kidney research community. In so doing, we have developed several proteomics and transcriptomic databases profiling genes expressed in the IMCD, thick ascending limb and proximal tubule (https:http://dirweb.nhlbi.nih.gov/Centers/CBPC/LKEM_G/LKEM/Pages/--TranscriptomicandProteomicDatabases.aspx). Our nuclear proteomics data have been added to an upgraded IMCD Proteome Database website (http://dir.nhlbi.nih.gov/papers/lkem/imcd/).
An additional goal has been to develop improved computational approaches to increase the yield of peptides that can be identified from high-quality spectra without a high rate of false positive identifications. In this study, the use of multiple search algorithms [SEQUEST (15), InsPecT (44), and OMSSA (17)] gave substantial numbers of identifications unique to each search algorithm. The largest increment in identifications was found with the InsPecT algorithm, a hybrid approach that uses de novo sequencing of a portion of each peptide to narrow the search space for pattern matching. InsPecT allowed the identification of a subset of peptides often missed by strictly peak-matching software such as SEQUEST or OMSSA. Further steps, such as target-decoy analysis as well as performing ambiguity checks on identified peptides using an in-house algorithm ProMatch, limited the number of false positive identifications and eliminated ambiguous identifications. We emphasize that target-decoy analysis has been demonstrated to be superior in limiting false positive identifications compared with use of the “two-peptide rule,” requiring protein identifications to those established by two or more peptides (18).
Overall, with the new data presented in this study, we have laid the groundwork for future studies of the role of vasopressin and other hormones in transcriptional regulation in renal collecting duct cells. The data provide a guide to proteins present in the nucleus of the “cells of interest” for such studies, which become candidates for genetic manipulation in cell culture and mouse models. The ultimate goal is to identify the relevant transcriptional regulatory networks involved in the control of Aqp2 and Aqp3 gene transcription. By sharing our data in a publicly accessible database, we encourage other investigators to partake in these investigations.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Albuquerque CP, Smolka MB, Payne SH, Bafna V, Eng J, Zhou H. A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol Cell Proteomics 7: 1389–1396, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol 24: 1285–1292, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Biemesderfer D. Regulated intramembrane proteolysis of megalin: linking urinary protein and gene regulation in proximal tubule? Kidney Int 69: 1717–1721, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Burrow CR. Retinoids and renal development. Exp Nephrol 8: 219–225, 2000. [DOI] [PubMed] [Google Scholar]
- 5. Cai Q, McReynolds MR, Keck M, Greer KA, Hoying JB, Brooks HL. Vasopressin receptor subtype 2 activation increases cell proliferation in the renal medulla of AQP1 null mice. Am J Physiol Renal Physiol 293: F1858–F1864, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Champigneulle A, Siga E, Vassent G, Imbert-Teboul M. V-2-like vasopressin receptor mobilizes intracellular Ca2+ in medullary collecting tubules. Am J Physiol Renal Fluid Electrolyte Physiol 265: F35–F45, 1993. [DOI] [PubMed] [Google Scholar]
- 7. Chen Q, Xu S, Huang S, Zhang A, Feng Q, Guo X, Guo M, Chen R, Yang T. Suppression subtractive hybridization analysis of gene expression during late kidney development identifies the developmentally regulated gene rPEA3. Nephron Exp Nephrol 111: e103–e115, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Chou CL, Yip KP, Michea L, Kador K, Ferraris J, Wade JB, Knepper MA. Regulation of aquaporin-2 trafficking by vasopressin in renal collecting duct: Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275: 36839–36846, 2000. [DOI] [PubMed] [Google Scholar]
- 9. Christensen BM, Marples D, Jensen UB, Frokiaer J, Sheikh-Hamad D, Knepper M, Nielsen S. Acute effects of vasopressin V2-receptor antagonist on kidney AQP2 expression and subcellular distribution. Am J Physiol Renal Physiol 275: F285–F297, 1998. [DOI] [PubMed] [Google Scholar]
- 10. DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci USA 91: 8984–8988, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ecelbarger CA, Chou CL, Lolait SJ, Knepper MA, DiGiovanni SR. Evidence for dual signaling pathways for V2 vasopressin receptor in rat inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 270: F623–F633, 1996. [DOI] [PubMed] [Google Scholar]
- 12. Ecelbarger CA, Nielsen S, Olson BR, Murase T, Baker EA, Knepper MA, Verbalis JG. Role of renal aquaporins in escape from vasopressin-induced antidiuresis in rat. J Clin Invest 99: 1852–1863, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper MA. Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F663–F672, 1995. [DOI] [PubMed] [Google Scholar]
- 14. Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods 4: 207–214, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Eng JK, McCormack AL, Yates JR., III An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 5: 976–989, 1994. [DOI] [PubMed] [Google Scholar]
- 16. Fujita N, Ishikawa SE, Sasaki S, Fujisawa G, Fushimi K, Marumo F, Saito T. Role of water channel AQP-CD in water retention in SIADH and cirrhotic rats. Am J Physiol Renal Fluid Electrolyte Physiol 269: F926–F931, 1995. [DOI] [PubMed] [Google Scholar]
- 17. Geer LY, Markey SP, Kowalak JA, Wagner L, Xu M, Maynard DM, Yang X, Shi W, Bryant SH. Open mass spectrometry search algorithm. J Proteome Res 3: 958–964, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Gupta N, Pevzner PA. False discovery rates of protein identifications: a strike against the two-peptide rule. J Proteome Res 8: 4173–4181, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hasler U, Jeon US, Kim JA, Mordasini D, Kwon HM, Feraille E, Martin PY. Tonicity-responsive enhancer binding protein is an essential regulator of aquaporin-2 expression in renal collecting duct principal cells. J Am Soc Nephrol 17: 1521–1531, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoffert JD, Wang G, Pisitkun T, Shen RF, Knepper MA. An automated platform for analysis of phosphoproteomic datasets: application to kidney collecting duct phosphoproteins. J Proteome Res 6: 3501–3508, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hozawa S, Holtzman EJ, Ausiello DA. cAMP motifs regulating transcription in the aquaporin-2 gene. Am J Physiol Cell Physiol 270: C1695–C1702, 1996. [DOI] [PubMed] [Google Scholar]
- 23. Kao PN, Chen L, Brock G, Ng J, Kenny J, Smith AJ, Corthesy B. Cloning and expression of cyclosporin A- and FK506-sensitive nuclear factor of activated T-cells: NF45 and NF90. J Biol Chem 269: 20691–20699, 1994. [PubMed] [Google Scholar]
- 24. Kitamura M. Endoplasmic reticulum stress and unfolded protein response in renal pathophysiology: Janus faces. Am J Physiol Renal Physiol 295: F323–F334, 2008. [DOI] [PubMed] [Google Scholar]
- 25. Kwon TH, Nielsen J, Masilamani S, Hager H, Knepper MA, Frokiaer J, Nielsen S. Regulation of collecting duct AQP3 expression: response to mineralocorticoid. Am J Physiol Renal Physiol 283: F1403–F1421, 2002. [DOI] [PubMed] [Google Scholar]
- 26. Li SZ, McDill BW, Kovach PA, Ding L, Go WY, Ho SN, Chen F. Calcineurin-NFATc signaling pathway regulates AQP2 expression in response to calcium signals and osmotic stress. Am J Physiol Cell Physiol 292: C1606–C1616, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Malik TH, Shoichet SA, Latham P, Kroll TG, Peters LL, Shivdasani RA. Transcriptional repression and developmental functions of the atypical vertebrate GATA protein TRPS1. EMBO J 20: 1715–1725, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8: 861–867, 1997. [DOI] [PubMed] [Google Scholar]
- 29. Murillo-Carretero MI, Ilundain AA, Echevarria M. Regulation of aquaporin mRNA expression in rat kidney by water intake. J Am Soc Nephrol 10: 696–703, 1999. [DOI] [PubMed] [Google Scholar]
- 30. Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92: 1013–1017, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci USA 90: 11663–11667, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patterson LT, Potter SS. Atlas of Hox gene expression in the developing kidney. Dev Dyn 229: 771–779, 2004. [DOI] [PubMed] [Google Scholar]
- 33. Pisitkun T, Bieniek J, Tchapyjnikov D, Wang G, Wu WW, Shen RF, Knepper MA. High-throughput identification of IMCD proteins using LC-MS/MS. Physiol Genomics 25: 263–276, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101: 13368–13373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pisitkun T, Jacob V, Schleicher SM, Chou CL, Yu MJ, Knepper MA. Akt and ERK1/2 pathways are components of the vasopressin signaling network in rat native IMCD. Am J Physiol Renal Physiol 295: F1030–F1043, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res 23: 4878–4884, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rai T, Uchida S, Marumo F, Sasaki S. Cloning of rat and mouse aquaporin-2 gene promoters and identification of a negative cis-regulatory element. Am J Physiol Renal Physiol 273: F264–F273, 1997. [DOI] [PubMed] [Google Scholar]
- 38. Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D, Carroll T, McMahon A, Hummler E, Rossier BC. Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest 112: 554–565, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ruttenberg BE, Pisitkun T, Knepper MA, Hoffert JD. PhosphoScore: an open-source phosphorylation site assignment tool for MSn data. J Proteome Res 7: 3054–3059, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saito T, Ishikawa SE, Sasaki S, Fujita N, Fushimi K, Okada K, Takeuchi K, Sakamoto A, Ookawara S, Kaneko T, Marumo F, Saito T. Alteration in water channel AQP-2 by removal of AVP stimulation in collecting duct cells of dehydrated rats. Am J Physiol Renal Physiol 272: F183–F191, 1997. [DOI] [PubMed] [Google Scholar]
- 41. Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68: 821–861, 1999. [DOI] [PubMed] [Google Scholar]
- 42. Srinivas S, Goldberg MR, Watanabe T, D'Agati V, al-Awqati Q, Costantini F. Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev Genet 24: 241–251, 1999. [DOI] [PubMed] [Google Scholar]
- 43. Star RA, Nonoguchi H, Balaban R, Knepper MA. Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct. J Clin Invest 81: 1879–1888, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tanner S, Shu H, Frank A, Wang LC, Zandi E, Mumby M, Pevzner PA, Bafna V. InsPecT: identification of posttranslationally modified peptides from tandem mass spectra. Anal Chem 77: 4626–4639, 2005. [DOI] [PubMed] [Google Scholar]
- 45. Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rat. Am J Physiol Renal Fluid Electrolyte Physiol 271: F414–F422, 1996. [DOI] [PubMed] [Google Scholar]
- 46. Uawithya P, Pisitkun T, Ruttenberg BE, Knepper MA. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics 32: 229–253, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uchida S, Matsumura Y, Rai T, Sasaki S, Marumo F. Regulation of aquaporin-2 gene transcription by GATA-3. Biochem Biophys Res Commun 232: 65–68, 1997. [DOI] [PubMed] [Google Scholar]
- 48. Uchida S, Sasaki S, Fushimi K, Marumo F. Isolation of human Aquaporin-CD gene. J Biol Chem 269: 23451–23455, 1994. [PubMed] [Google Scholar]
- 49. Uchida S, Tanaka Y, Ito H, Saitoh-Ohara F, Inazawa J, Yokoyama KK, Sasaki S, Marumo F. Transcriptional regulation of the CLC-K1 promoter by myc-associated zinc finger protein and kidney-enriched Kruppel-like factor, a novel zinc finger repressor. Mol Cell Biol 20: 7319–7331, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamaguchi Y, Yonemura S, Takada S. Grainyhead-related transcription factor is required for duct maturation in the salivary gland and the kidney of the mouse. Development 133: 4737–4748, 2006. [DOI] [PubMed] [Google Scholar]
- 51. Yasui M, Marples D, Belusa R, Eklof AC, Celsi G, Nielsen S, Aperia A. Development of urinary concentrating capacity: role of aquaporin-2. Am J Physiol Renal Fluid Electrolyte Physiol 271: F461–F468, 1996. [DOI] [PubMed] [Google Scholar]
- 52. Yasui M, Zelenin SM, Celsi G, Aperia A. Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am J Physiol Renal Physiol 272: F443–F450, 1997. [DOI] [PubMed] [Google Scholar]
- 53. Yip KP. Coupling of vasopressin-induced intracellular Ca2+ mobilization and apical exocytosis in perfused rat kidney collecting duct. J Physiol 538: 891–899, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, Chou CL, Pisitkun T, Nelson RD, Knepper MA. Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci USA 106: 2441–2446, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.