Abstract
The implication of the various lipoprotein classes in the development of atherosclerotic cardiovascular disease has served to focus a great deal of attention on these particles over the past half-century. Using knowledge gained by the sequencing of the human genome, recent research efforts have been directed toward the elucidation of the proteomes of several lipoprotein subclasses. One of the challenges of such proteomic experimentation is the ability to initially isolate plasma lipoproteins subsequent to their analysis by mass spectrometry. Although several methods for the isolation of plasma lipoproteins are available, the most commonly utilized techniques require large sample volumes and may cause destruction and dissociation of lipoprotein particle-associated proteins. Fast protein liquid chromatography (FPLC) is a nondenaturing technique that has been validated for the isolation of plasma lipoproteins from relatively small sample volumes. In this study, we present the use of FPLC in conjunction with nano-HPLC-ESI-tandem mass spectrometry as a new integrated methodology suitable for the proteomic analysis of human lipoprotein fractions. Results from our analysis show that only 200 μl of human plasma suffices for the isolation of whole high density lipoprotein (HDL) and the identification of the majority of all known HDL-associated proteins using mass spectrometry of the resulting fractions.
Keywords: proteomics, high-density lipoprotein, liquid chromatography, fast protein liquid chromatography
lipoproteins, which circulate primarily in the plasma, encompass a group of highly heterogeneous particles which differ in size, charge, lipid, apolipoprotein, and protein composition, and physiological function. The discovery of a pathological association between various lipoprotein classes and cardiovascular disease risk has sustained the impetus for research in this field. In particular, high-density lipoproteins (HDLs) have been shown to possess antiatherogenic properties (4), and it is a well-established fact that plasma HDL-cholesterol (HDL-C) levels are inversely correlated with the risk of developing atherosclerotic cardiovascular disease (3). However, the molecular mechanisms underlying the atheroprotective properties of HDL are unclear.
To further elucidate the role of HDL in the etiology of cardiovascular disease, several analyses of the human HDL proteome using mass spectrometry-based approaches have emerged within the past few years (13, 17, 26, 29). Collectively, these studies have identified >80 different HDL-associated proteins, many of which were not previously known to associate with these particles. Several of these HDL-associated proteins and enzymes, which likely mediate the atheroprotective functions of HDL, are altered in cardiovascular and metabolic diseases. An analysis of the HDL proteome in coronary artery disease patients revealed enrichment in inflammatory and complement pathway proteins such as paraoxonase-1 (PON1) and complement component 3 (29). PON1 activity levels were also found to be significantly decreased in patients with the metabolic syndrome (2). Under inflammatory conditions, serum amyloid A, an important mediator of the inflammatory response that is preferentially associated with HDL, is capable of replacing, and thereby reducing, HDL-associated apolipoprotein A-I (7).
Several lipoprotein separation techniques have been developed that allow for the isolation of HDLs and their subclasses from whole plasma as preparative work-ups prior to analysis by mass spectrometry. Although preparative ultracentrifugation (12) remains the method of choice for isolating HDL subclasses, it has been suggested that the high centrifugal forces and salt concentrations required for this technique may cause structural damage and the dissociation of HDL-associated proteins (21). This could impair the detection and analysis of relevant proteins in subsequent mass spectrometric experiments. Furthermore, the majority of ultracentrifugal methods require relatively large starting volumes of plasma (2–5 ml). However, a recent study (28), in which a D2O/sucrose method was applied in lieu of KBr and which requires relatively small sample volumes (up to 500 μl), has shown to be a promising alternative to the traditional ultracentrifugal options as it appears to have reduced the loss of protein content from low-density lipoprotein (LDL) and HDL fractions. Such studies, including the one we describe here, serve as examples of unbiased, potential improvements toward the goal of elucidating the lipoprotein proteomes that have been shown to include a vast array of nonstructural, noncovalently associated, exchangeable proteins and apolipoproteins critical to their function (13, 15, 17, 26–29).
The subsequent analysis of HDL-associated proteins has mostly relied on two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) followed by the identification of protein(s) in a single spot by peptide mass fingerprinting with a matrix-assisted laser desorption/ionization-time of flight mass spectrometer (13, 17, 26, 28). However, there are several disadvantages associated with the use of 2D-PAGE. Besides the fact that this technique can be both technically challenging and time-consuming, it requires relatively high amounts of protein and the gel spot resolution can be poor with multiple proteins migrating to the same spot (13, 17).
Fast protein liquid chromatography (FPLC), which is based on the principle of gel filtration, has proven to be a rapid, nondenaturing method for the isolation of lipoproteins, including HDL subclasses, from human plasma samples (5, 16, 24, 25). However, FPLC separation has never been used directly in conjunction with mass spectrometry for the identification of HDL-associated proteins. It was the purpose of our study to demonstrate the suitability of FPLC fractions for mass spectral analysis and the feasibility of a comprehensive characterization of the HDL-associated proteome using small plasma sample volumes. Using FPLC, in conjunction with a size-exclusion chromatography (SEC) column, we have isolated the HDL fraction from whole human plasma. In this study, we demonstrate the suitability of using the FPLC-SEC-derived lipoprotein fractions for subsequent analysis by nano-Liquid chromatography (LC)-tandem mass spectrometry (MS/MS). Using only 200 μl of human plasma for the FPLC-SEC fractionation, we characterize each FPLC-SEC-derived lipoprotein fraction by both Western blot analysis and by scan number, which has been shown to be a reliable measure of relative protein abundance in a complex mixture (22). Our data show that the combination of FPLC and MS/MS allows for a comprehensive analysis of lipoproteins.
MATERIALS AND METHODS
Plasma samples.
The protocol for the acquisition of plasma samples from volunteers was approved by an Institutional Review Board at the Medical College of Wisconsin. Written consents were obtained from each volunteer prior to inclusion in this study. Plasma samples were stored at −20°C.
Materials.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Isolation of plasma lipoproteins by FPLC-SEC.
FPLC analysis was carried out on a BioLogic DuoFlow QuadTec 10 System equipped with a BioFrac fraction collector (Bio-Rad Laboratories, Hercules, CA). All FPLC runs were performed at 4°C. All of the elutions were monitored at an absorbance of 280 nm. The size-exclusion chromatography was carried out on a single Superdex 200 10/300 GL column (GE Healthcare, Uppsala, Sweden). Other groups have used a combination of different SEC columns in conjunction with FPLC carried out on different systems (5, 16, 24, 25). The selection of a single Superdex 200 column was based on the observation that any increase in resolution achieved by combining several different SEC columns was not significant enough to warrant the extended elution times and increased potential for high back-pressure.
Elutions were performed in a 1 mM EDTA, 150 mM NaCl, 0.02% NaN3 phosphate-buffered saline solution, pH 7.4, as prepared by Ordovas and Osgood (25). This eluent buffer was degassed in a strong-side Erlenmeyer flask for 20 min under vacuum on a stir plate. The eluent buffer was also sterile filtered through a 0.22 μm vacuum-driven GP Express PLUS membrane (Millipore, Billerica, MA). Prior to each sample injection into the FPLC-SEC system, 12 ml of eluent buffer, run at 0.3 ml/min, was used to ensure equilibration of the column. The column was washed with degassed and filtered 20% ethanol between runs. Immediately prior to injection, the thawed human plasma samples were centrifuged at 10,000 g for 5 min. Plasma sample aliquots of 200 μl were used for each injection. To completely fill the injection loop, 200 μl of eluent buffer was also added to each injection. The elutions were carried out at a flow rate of 0.30 ml/min with a maximum pressure of 218 psi. The BioFrac fraction collector was used to collect fractions of 0.5 ml throughout the analysis. Fractions were collected in polypropylene collection tubes. To improve sample recovery and to prevent sample adhesiveness to the fraction collection tubes, the polypropylene tubes were pretreated with a 1 ml/l Tween 20 solution as described by Nanjee and Brinton (24). Fractions corresponding to discrete elution peaks and/or troughs were pooled and saved for subsequent analysis. The fraction sets were then concentrated using Vivaspin 500 spin columns (Vivascience, Hannover, Germany). To improve the protein recovery from these spin columns, they were preblocked with 5% SDS and rinsed following the manufacturer's recommendations.
Isolation of lipoproteins by density gradient ultracentrifugation.
Plasma lipoproteins were isolated by density gradient ultracentrifugation (DGUC) on a Sorvall Ultracentrifuge (Thermo Scientific) with a Beckman 50.4 Ti rotor (Beckman Coulter, Fullerton, CA). Two milliliters of plasma were added to a salt solution with a density of 1.1818 g/ml. This solution was centrifuged at 40,000 rpm at 10°C for 18 h. The top 2 ml were removed by aspiration. This top layer contained the very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), and LDL classes. To the remaining solution, 2 ml of a salt solution with a density of 1.4744 g/ml was added. This solution was centrifuged at 40,000 rpm at 10°C for 24 h. The top 1 ml, which contains whole HDL, was removed by aspiration.
Comparison of whole plasma elution profile with lipoprotein standards isolated by DGUC and with protein molecular weight standards.
Aliquots of 200 μl from the VLDL, IDL, and LDL DGUC standard and from the HDL DGUC standard were injected onto the Superdex 200 column. The elution profiles from both of these standards were overlaid with that for whole plasma. Protein standards, ranging in size from 29 to 669 kDa, from a protein molecular weight standard kit (Sigma Aldrich) were prepared according to the manufacturer's recommendations and individually injected onto the Superdex 200 column in aliquots of 200 μl. The elution profile for each standard was overlaid with that of whole plasma, and the peak elution position for each standard was noted.
Nondenaturing Western blot analysis.
A single, 200 μl aliquot of plasma was fractionated by FPLC on a Superdex 200 column. The whole plasma elution profile was divided into a total of eight fraction sets based on their correspondence with either a discrete elution peak or trough. The fraction sets were collected and concentrated. The protein concentration of each fraction set was assessed by a microBCA assay (Pierce, Rockford, IL). A total of 10 μg from each fraction set was separated on a 3–20% nondenaturing polyacrylamide gel (PAGE gel, San Diego, CA) at 100 V overnight at 4°C. The gels were transferred to a 0.45 μm pore size nitrocellulose membrane (Invitrogen, Carlsbad, CA) at 25 V for 4 h at 4°C, which was blocked for 12 h at 4°C in 0.001% bovine serum albumin (BSA) diluted in Tris-buffered saline (TBS; 4 mM Tris base, 6 mM Tris · HCl, and 150 mM NaCl, pH 8) with 0.08% Tween 20. Membranes were incubated with primary antibody [mouse anti-human apoA-I and apoB-100 (Santa Cruz Biotechnology, Santa Cruz, CA)] at a dilution of 1:500 in TBS-T with 0.001% BSA for 90 min at room temperature. The blots were then washed and incubated with goat-anti-mouse horseradish peroxidase-conjugated secondary antibody (Pierce, Rockford, IL) at a dilution of 1:5,000 in TBS-T with 0.001% BSA for 2 h at room temperature. They were then subjected to SuperSignal West Femto chemiluminescence Substrate (Pierce) detection system.
Chloroform extractions and trypsin digestion.
Following the concentration of the fraction sets obtained by FPLC-SEC, a series of chloroform extractions was performed on each of the eight fraction sets to isolate the lipid-embedded and associated proteins from the lipoprotein particles [protocol adapted from Mirza et al. (23)]. In brief, an equal volume of chloroform (1:1 vol/vol) was added to the sample, and it was placed on a shaker at room temperature for 1 h. An equal volume of methanol was then added to the mixture, and it was then vigorously vortexed at room temperature for 30 min. An equal volume of double-distilled water was added to the mixture, forming a visible bilayer. The sample was then centrifuged at 10,000 g for 2 min, after which the chloroform layer was discarded. One more volume of chloroform was added to the solution, and the samples were sonicated in a bath sonicator containing ice-cold water for 30 min. Following sonication, the samples were centrifuged at 10,000 g for 5 min and the chloroform layer was discarded. The proteins were then precipitated with 4× (vol/vol) acetone at −20°C for 12 h. Following the precipitation, the protein was pelleted by centrifugation. The pellet was washed with acetone, allowed to dry, and then dissolved in 250 mM ammonium bicarbonate. The proteins from each fraction set were reduced with 10 mM dithiothreitol at 37°C for 30 min. The samples were then incubated in 55 mM iodoacetamide at 37°C for 45 min in the dark. The proteins from each fraction set were then digested with trypsin (Promega, Madison, WI) at a ratio of 1:50 trypsin to protein for 12 h at 37°C. Digestions were quenched with the addition of 1 μl of 10% formic acid. The samples were then cleaned with C18 Zip-Tips (Millipore) prior to mass spectral analysis.
Nano-HPLC-MS/MS.
Protein digests were analyzed on a ThermoFinnigan LTQ ion trap mass spectrometer interfaced with a nano-LC system (Thermo Electron) equipped with an autosampler through which samples were loaded onto a C18 capillary column (100 × 0.1 mm). The capillary column was packed in-house with 5 μm C18 RP particles (Phenomenex, Cheshire, UK). The compositions of solvents A and B, used for the chromatographic separation of peptides, were 5% acetonitrile in 0.1% formic acid and 95% acetonitrile in 0.1% formic acid, respectively. The protein digest injected onto the microcapillary column was resolved at a rate of 150 μl/min, by the following gradient conditions: 0–120 min 0–25% B, 120–180 min 25–75% B, 180–190 min 75–100% B, 190–200 min 100% B, 215–220 min 100% A, 220–300 min 100% A.
Peak lists were generated from raw LC-MS/MS spectra using ExtractMS v. 3. The raw data acquired by the mass spectrometry experiment were searched against the human UniProt database (Uniprot v. 49.1, which contains 13,488 proteins and 7,360,189 amino acids) using the SEQUEST (9) (TurboSEQUEST v. 27 rev. 12) search engine. Additional search parameters included a precursor-ion mass tolerance of ±2.5, and a fragment-ion mass tolerance of 0. The output files from the database search were filtered and summarized by the program Epitomize (11). The filter allows for a positive peptide, and hence protein, identification based on Protein Probability scores. The algorithm that generates the Protein Probability scores utilizes decoy database searching and is a modified version of the Peptide Prophet (19) algorithm. The Protein Probability scores are calculated with a modified version of the algorithm Peptide Prophet. The criteria for the positive identification of a protein were identification of the protein in at least two of the six FPLC runs and a Protein Probability score of ≥0.95.
RESULTS
As outlined in the introduction, it was the purpose of the current study to examine whether FPLC-based isolation of lipoprotein fractions, and especially the HDL subfraction, from small sample volumes is amenable to subsequent characterization of the HDL-associated proteome using nano-HPLC-MS/MS. We evaluated reproducibility of FPLC separation, characterized the obtained subfractions, analyzed the HDL-associated proteome using mass spectrometry, and compared the results to previous results describing these proteins.
Reproducibility of the FPLC elution profile.
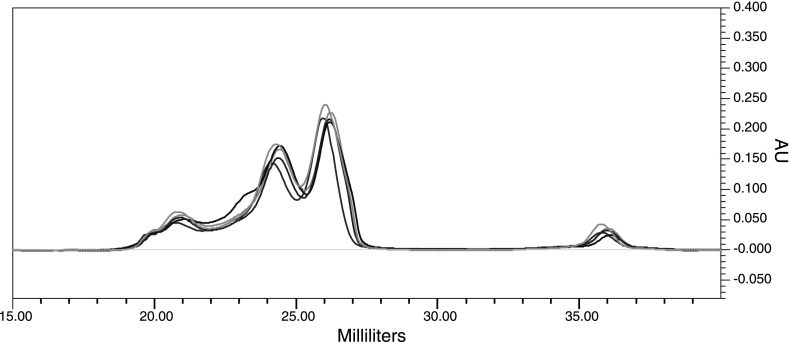
To evaluate the reproducibility of the FPLC separation, we examined 200 μl aliquots of whole human plasma from five different individuals. All samples were chromatographed over the same Superdex 200 column. As seen in Fig. 1, the elution profiles are highly reproducible, and all peak locations match perfectly, suggesting that different lipoprotein subclasses are eluting reproducibly at the same time.
Fig. 1.
The fast protein liquid chromatography-size-exclusion chromatography (FPLC-SEC) elution profile is reproducible. Five overlaid, randomly chosen, elution profiles are shown here to illustrate the reproducibility of the elution profile achieved when 200 μl of human plasma from 5 different individuals is chromatographed through a Superdex 200 column. The elution volume, in milliliters, is represented on the x-axis and the UV absorbance units (AU), measured at 280 nm, are represented by the y-axis.
Identification and characterization of HDL subfractions.
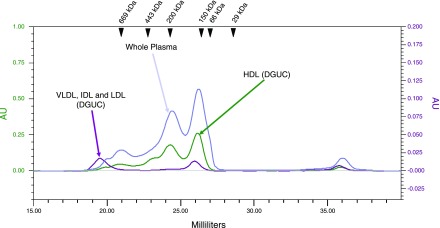
Although it has been suggested that isolation of lipoprotein particle subfractions by DGUC may result in potential sample destruction and protein dissociation, the method is still considered to be the gold standard by which one should compare other lipoprotein isolation techniques. To this end, whole HDL, obtained by DGUC, was separated by FPLC to determine the elution volumes for the HDL subclasses. The combined VLDL, IDL, and LDL fraction obtained by DGUC was also chromatographed to determine the elution volumes of these lipoprotein classes.
The chromatograms for both of these standards are overlaid with a representative chromatogram from a whole plasma sample in Fig. 2. As the elution profiles show, the DGUC-derived HDL fraction is resolved into three main peaks. Based on the principle of size-exclusion chromatography, one would expect that the particles with larger diameters would elute from the column before particles with relatively smaller diameters. Therefore, it is reasonable to infer that the first two peaks, at ∼24.5 and 26.0 ml, most likely represent the HDL subclasses. The final peak, which begins to elute at ∼35.5 ml, may represent dissociated HDL proteins and/or other plasma proteins. These findings confirm our prediction that the large bifurcated peak, which begins to elute at ∼23.5 ml, contains the HDL subclasses. Interestingly, the VLDL, IDL, and LDL fraction appears to elute in three main peaks. The first peak, which begins to elute at ∼18.5 ml, most likely represents a combination of VLDL, IDL, and LDL. The other two peaks, which elute at ∼25.5 and 35.5 ml, may represent dissociated VLDL, IDL, or LDL proteins or contaminants from the HDL layer.
Fig. 2.
Comparison of whole plasma elution profile (blue) with the elution profiles for high-density lipoprotein (HDL) isolated by density gradient ultracentrifugation (DGUC, green) and very-low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), and low-density lipoprotein (LDL) isolated by DGUC (purple). The arrowheads indicate the peak elution positions of protein standards of the given molecular weights. The left-hand y-axis represents the UV absorbance units for the HDL DGUC sample, while the right-hand y-axis represents the UV absorbance units for the VLDL, IDL, and LDL DGUC sample.
Protein standards, individually loaded onto and chromatographed over the Superdex 200 column, provide molecular weight estimates of the HDL subclasses and lend greater insight into the locations of these subclasses under the whole plasma elution profile. The arrowheads shown in Fig. 2 indicate the peak elution volumes of the protein standards of the provided molecular weights. The full elution profiles for each of the protein standards can be seen in Supplemental Fig. S1.1 The peak elution volume for apoferritin (443 kDa), which has a molecular weight greater than that of the largest HDL subclass (HDL2b, 410 kDa), is located at ∼22.5 ml. The peak elution volume for carbonic anhydrase (29 kDa), which has a molecular weight less than that of the smallest HDL subclass (HDL3c, 160 kDa), is located at ∼28.5 ml. The fact that these elution volumes flank those of the large, bifurcated peak, which is located between 24.5 and 26.0 ml, lends confidence to our inference that this peak corresponds with the HDL subclasses.
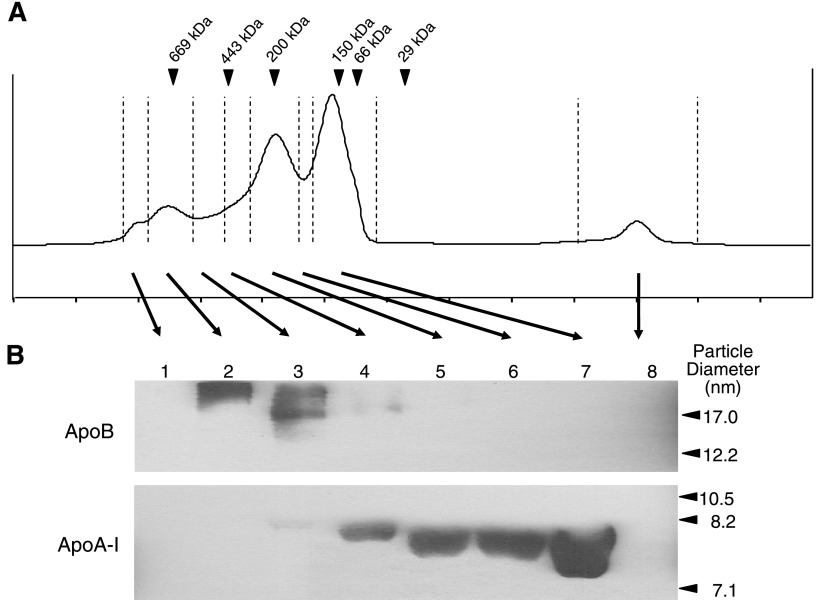
To further validate the location of the HDL subclasses under the whole plasma FPLC elution profile, a nondenaturing Western blot analysis of all eight fraction sets, collected across the elution profile, was performed. Since the major protein constituent of LDL is apolipoprotein B-100 (apoB-100) and the major protein component of HDL is apolipoprotein A-I (apoA-I), the presence of either HDL or LDL in the discrete elution peaks can be detected by immunoblotting with anti-apoB and anti-apoA-I antibodies. The results shown in Fig. 3 indicate that the apoB-containing particles are present in fraction sets 2–4, which elute between ∼20.2 and 23.5 ml. This implies that the LDL subclasses are eluting in those fraction sets. Figure 3 also illustrates that the apoA-I-containing particles are present in fraction sets 3–7, which elute between ∼21.5 and 28.0 ml. This indicates that the HDL subclasses are eluting in those fraction sets. Although there is an overlap between the elutions of the apoA-I- and apoB-containing particles (fraction sets 3–4, which elute between ∼21.5 and 23.5 ml), these data, nonetheless, clearly demonstrate the elution volume of the lipoprotein subclasses. Furthermore, the migration patterns of the apoA-I-containing particles indicate that the larger diameter particles are eluting from the column before the smaller diameter particles. This provides more evidence to support our notion that the HDL subclasses are contained in the large, bifurcated peak.
Fig. 3.
Nondenaturing Western blot analysis of the FPLC-SEC fraction sets. A: representative FPLC elution profile of 200 μl of human plasma chromatographed over a Superdex 200 column. The FPLC chromatogram is divided into 8 discrete fraction sets, each separated by a vertical dashed line. The arrowheads indicate the peak elution positions of protein standards of the given molecular weights. B: ApoB and ApoA-I Western blots of the 8 FPLC-SEC fraction sets. Particle diameters from protein standards are shown on the right.
Comprehensive characterization of proteome of lipoprotein particles from human plasma using FPLC and nano-HPLC-MS/MS.
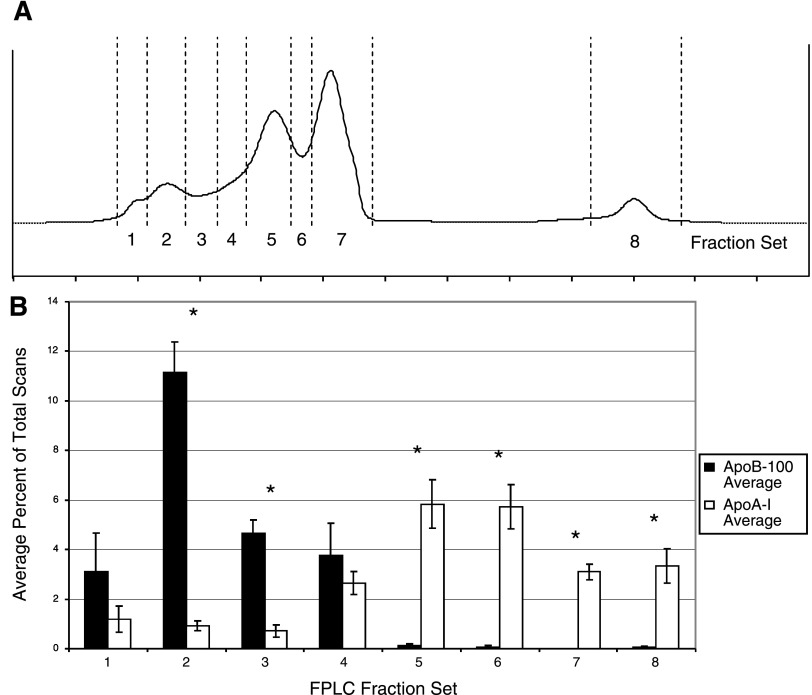
Next, we examined the suitability of the obtained FPLC fractions for direct mass spectrometric analysis. Isolated lipoprotein fractions were delipidated, as described by Mirza et al. (23), and the resulting protein samples were digested by trypsin prior to nano-HPLC-MS/MS analysis. Each fraction illustrated in Fig. 4 was analyzed separately. The results from our mass spectrometric analysis are shown in Supplemental Tables S1–S8. For all proteins included in these tables, the criteria for positive identification required detection of the protein in at least two FPLC-SEC isolations and an average Protein Probability score ≥0.95. For fraction sets 1–8, the total number of proteins meeting these criteria was 37, 71, 63, 93, 115, 113, 76, and 50, respectively.
Fig. 4.
Average percentage of total scans leading to a positive peptide identification according to our criteria for ApoB-100 and ApoA-I across all 8 FPLC fraction sets. A: representative FPLC elution profile. Vertical dashed lines divide the profile into 8 discrete fraction sets. B: average percentage of total scan counts by each FPLC fraction set. *P < 0.001 as assessed by Student's t-test.
The MS data corroborate the results from the Western blots depicted in Fig. 3. To summarize the apoB-100 and apoA-I content of each fraction, Fig. 4B shows the average percentage of total MS scans for both apoB-100 and apoA-I in each fraction set. These results clearly indicate that the initial FPLC peaks contain the majority of apoB-100-containing particles, while the latter peaks contain the majority of the apoA-I-containing particles, as evidenced by the relative abundances of the apoB-100 and apoA-I scan counts from each fraction set. In fraction sets 4, 5, 6, and 7 we detected 30, 32, 31, and 29, respectively, known HDL-associated proteins identified by other MS-based studies (13, 17, 26, 29). These include 14 of the known HDL-associated apolipoproteins (apos A-I, A-II, A-IV, B-100, C-I, C-II, C-III, D, E, F, M, L-1, H, and J) and all of the complement proteins (C3, C4-A, C4-B, and C9) identified by Vaisar et al. (29). Collectively, the studies described by Vaisar, Karlsson, Rezaee, Heller, and colleagues (13, 17, 26, 29) have identified a total of 86 different HDL-associated proteins, 26 of which have been confirmed by at least two of these studies. In our FPLC-SEC fraction sets 4–7, we identified 81, 81, 69, and 65%, respectively, of these 26 proteins. Given the fact that, collectively, these studies, which employed either shotgun proteomics or peptide mass fingerprinting, agreed on only 26 proteins strongly suggests that the HDL proteome is far from being extensively characterized, and it also highlights the fact that variations in sample handling, fractionation and protein identification workflows can lead to disparate, potentially biased, results. The fact that we were able to identify the majority of the known HDL-associated proteins, and several potentially novel HDL-associated proteins, suggests that the FPLC separation of lipoprotein particles successfully isolated HDL from the remaining lipoprotein particles, even from small volumes of plasma (200 μl). Furthermore, the resulting isolated HDL particle fractions are suitable for direct MS analysis without prior two-dimensional gel electrophoresis.
DISCUSSION
The aim of the present study was to assess the suitability of using FPLC-SEC-derived lipoprotein fractions for analysis by nano-HPLC-MS/MS and describe the experimental procedure for this integrated proteomics approach. Several methods have been established for the isolation of plasma lipoproteins. Recent interest in exploring the HDL proteome has led to the use of lipoprotein isolation techniques, such as DGUC and 2D-PAGE, as preparative methods for the subsequent analysis of purified HDL by mass spectrometry. Although DGUC has been the most popular method for the isolation of plasma lipoproteins, its association with the potential problems of sample destruction (21), the potential loss of surface apolipoproteins from specific HDL subclasses due to their differential stabilities during centrifugation (6), and the requirement for relatively large sample volumes have prompted the search for alternative methods of lipoprotein isolation (5, 16). The use of FPLC-SEC offers a validated, nondenaturing, reproducible and rapid method for the isolation of HDL from small volumes of frozen plasma (5, 16, 24, 25). These advantageous characteristics make FPLC-SEC an attractive alternative method for lipoprotein isolation prior to mass spectrometric analysis, especially when sample volume and integrity are concerns. To our knowledge, this is the first study to use FPLC-SEC in conjunction with nano-HPLC-MS/MS to characterize plasma lipoprotein fractions.
As highlighted in Fig. 1, the separation is highly reproducible. We were able to achieve nearly identical profiles from plasma samples of different individuals, suggesting that the isolation of specific lipoprotein fractions from multiple samples is easily achievable.
Although FPLC-SEC is a well-established method for the isolation of lipoproteins, a potential limitation of this technique for isolating lipoprotein fractions from plasma, as described here, is related to the concern that SEC will inherently lead to the coelution of free plasma proteins of matching size with the lipoprotein particles. Indeed, it has been shown that there is a potential for contamination of lipoprotein fractions by nonstructural, noncovalently associated proteins when purified by SEC (18). However, it is well established that, besides the established structural proteins, HDL particles also contain a wide variety of weakly associated proteins that carry out many of the various functions attributed to HDL particles (13, 17, 26, 29). Although it may be difficult to evaluate whether plasma proteins identified by our analysis are coeluting contaminants in the SEC fractions or are actually associated with the lipoprotein particles, we can at least infer the association of certain proteins with actual lipoprotein particles rather than coelution based on their identification in a wide range of fraction sets covering a wide range of molecular weights. For example, the proteins α2-macroglobulin, complement C3, and haptoglobin, with molecular weights of 163, 187, and 45 kDa, respectively, are found in all eight FPLC-SEC fraction sets (Supplemental Tables S1–S8). Similarly, the proteins serotransferrin (77 kDa), vitronectin (54 kDa), transthyretin (16 kDa), and serum albumin (69 kDa) are found in at least six of the eight fraction sets. Given the fact that the elution peaks for each of the protein standards (Supplemental Fig. S1) are relatively narrow (spanning one or few fraction sets), we believe that finding a particular protein across a wide range of fractions (i.e., 6–8 fraction sets) would strongly suggest that such a protein is actually associated with a lipoprotein particle, and not just freely circulating in the plasma and coeluting in the chromatography.
To improve the detection of lipoprotein-associated proteins, numerous arguments can be made for the depletion of several of the highly abundant plasma proteins and for the selective enrichment of lipoprotein-specific proteins such as apoB-100 and apoA-I via immunoaffinity chromatography. However, for each of these arguments, there are noteworthy counterarguments against such practices. First of all, as the results of others have indicated (13, 17, 26, 29), several of the most abundant plasma proteins, including serum albumin, haptoglobin, serotransferrin, and α2-macroglobulin, have also been shown to be HDL-associated proteins. Therefore, their removal from plasma may also remove the very particles at which the analysis is aimed. As Echan et al. (8) have indicated, the plasma proteome is so complex, that even after the depletion of the six most abundant proteins, one is still left with an extremely broad range of protein abundances. Furthermore, the depletion of serum albumin poses significant concerns (1, 8, 10, 15, 30), as it functions as an important transport protein in plasma, binding a vast array of other proteins and hormones, and it has been shown to be bound to several apolipoproteins, including apoA-I (10). Therefore, the removal of albumin may remove lipoprotein particles as well. A recent study (14) has also shown that haptoglobin, one of the most abundant plasma proteins, binds to apoA-I and may play a role in reverse cholesterol transport.
Although other studies (5, 16, 24) have analyzed FPLC fractions using the same or similar SEC columns for their lipoprotein content, we performed validation experiments to reliably locate HDL under the whole plasma FPLC elution profiles obtained under our experimental conditions. Using protein standards to estimate the elution position of lipoproteins with similar molecular weights and whole HDL isolated by density gradient ultracentrifugation, we were able to identify the elution positions for the HDL fraction (Fig. 2). These positions correspond to the large, bifurcated peak that begins to elute at ∼23.5 ml and is completed at ∼28 ml. It is important to note that unlike plasma lipoproteins, the protein standards used here are not spherical in shape. However, they provide for an effective estimation of the apparent molecular weights of the plasma lipoproteins isolated by SEC, and these standards have been used by others for the same purpose (24).
Upon dividing the whole plasma FPLC elution profile into eight discrete fraction sets as seen in Fig. 3A, we analyzed each fraction set via nondenaturing Western blot analysis. The results reveal that the majority of the apoA-I-containing particles is located in fraction sets 4–7 (Fig. 3B), which correspond to the bifurcated peak described above. Therefore, HDL is well separated from other lipoprotein subfractions and thus is suitable for further analysis.
The results of our comprehensive profiling of the FPLC-SEC-derived lipoprotein fractions by MS/MS serve not only to confirm the elution positions of the HDL subclasses and the apoB-containing particles but also to illustrate that such protein profiles can be obtained with as little as 200 μl of plasma. A large percentage of previously reported HDL-associated proteins was identified in fraction sets 4–7. Although we were unable to identify all of the known HDL-associated proteins as reported in the literature (13, 17, 26, 29), evidence from others has shown that repeated analyses of the same sample could improve our results with respect to the total number of proteins identified. Liu and colleagues (22) have shown that in performing a total of nine LC/LC/MS/MS experiments, under the same conditions on the same sample, new proteins can be identified with each experiment. Vaisar et al. (29) have shown that in performing three replicate analyses of the same HDL protein sample, the total number of proteins identified increased by ∼20%. Therefore, it is likely that further detailed analysis of FPLC-derived HDL fractions will increase the number of identified HDL-associated proteins.
The results from our study show that HDL particles can be successfully isolated from whole human plasma using FPLC-SEC. We have also demonstrated that the FPLC-SEC-isolated lipoprotein fractions, derived from only 200 μl of plasma, are suitable for analysis by nano-HPLC-MS/MS. Given the fact that we were able to identify the majority of all known HDL-associated proteins, and several potentially novel HDL-associated proteins, with high confidence suggests that our experimental approach is ideally suited for in-depth analysis of lipoprotein particles where sample volume is a limiting factor. In future studies, the method reported here could be applied to the analysis of lipoprotein proteomes in disease versus normal controls, such as dyslipidemias and obesity or cardiovascular disease, and may help provide insights into the underlying functional mechanisms by which changes in lipoprotein composition mediate functional changes and disease risk.
GRANTS
The work was supported by National Institutes of Health (NIH) Grants R01 HL-74168 and N01 HV-28182 (to M. Olivier). L. A. Collins is supported through NIH Grant T32 HL-07852.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Molly Pellitteri-Hahn and Regina Cole for technical assistance. We also thank Scarlet Shi and Hao Xu for assistance in collecting plasma samples and in their initial assistance with the Western blot analysis. We thank Dr. Ronald Krauss and Katie Wojnoonski for assistance in isolating HDL by density gradient ultracentrifugation. Finally, we are thankful to Dr. Russell Wilke for helpful discussions of the project.
The complete elution profiles for each protein standard can be seen in Supplemental Fig. S1. The results from our mass spectrometric analysis of all eight FPLC fraction sets can be found in Supplemental Tables S1–S8.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, Smith RD, Springer DL, Pounds JG. Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry. Mol Cell Proteomics 1: 947–955, 2002. [DOI] [PubMed] [Google Scholar]
- 2. Blatter Garin MC, Moren X, James RW. Paraoxonase-1 and serum concentrations of HDL-cholesterol and apoA-I. J Lipid Res 47: 515–520, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Boden WE. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs High–Density Lipoprotein Intervention Trial. Am J Cardiol 86: 19L–22L, 2000. [DOI] [PubMed] [Google Scholar]
- 4. Castelli WP. Epidemiology of coronary heart disease: the Framingham study. Am J Med 76: 4–12, 1984. [DOI] [PubMed] [Google Scholar]
- 5. Chetiveaux M, Nazih H, Ferchaud-Roucher V, Lambert G, Zair Y, Masson M, Ouguerram K, Bouhours D, Krempf M. The differential apoA-I enrichment of prebeta1 and alphaHDL is detectable by gel filtration separation. J Lipid Res 43: 1986–1993, 2002. [DOI] [PubMed] [Google Scholar]
- 6. Cheung MC, Wolf AC. Differential effect of ultracentrifugation on apolipoprotein A-I-containing lipoprotein subpopulations. J Lipid Res 29: 15–25, 1988. [PubMed] [Google Scholar]
- 7. Coetzee GA, Strachan AF, van der Westhuyzen DR, Hoppe HC, Jeenah MS, de Beer FC. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J Biol Chem 261: 9644–9651, 1986. [PubMed] [Google Scholar]
- 8. Echan L, Hsin-Yao T, Ali-Khan N, KiBeom L, Speicher DW. Depletion of multiple high-abundance proteins improves protein profiling capacities of human serum and plasma. Proteomics 5: 3292–3303, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Eng JK, McCormack AL, Yates JR., 3rd An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 5: 976–989, 1994. [DOI] [PubMed] [Google Scholar]
- 10. Gundry R, Qin F, Jelinek CA, Van Eyk JE, Cotter RJ. Investigation of an albumin-enriched fraction of human serum and its albuminome. Proteomics Clin Appl 1: 73–88, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halligan BD, Slyper RY, Twigger SN, Hicks W, Olivier M, Greene AS. ZoomQuant: an application for the quantitation of stable isotope labeled peptides. J Am Soc Mass Spectrom 16: 302–306, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest 34: 1345–1353, 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heller M, Stalder D, Schlappritzi E, Hayn G, Matter U, Haeberli A. Mass spectrometry-based analytical tools for the molecular protein characterization of human plasma lipoproteins. Proteomics 5: 2619–2630, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Henderson R, Wasan K, Leon C. Haptoglobin inhibits phospholipid transfer protein activity in hyperlipidemic human plasma. Lipids Health Dis 8: 27, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoofnagle AN, Heinecke JW. Lipoproteomics: using mass spectrometry-based proteomics to explore the assembly, structure, and function of lipoproteins. J Lipid Res 50: 1967–1975, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Innis-Whitehouse W, Li X, Brown WV, Le NA. An efficient chromatographic system for lipoprotein fractionation using whole plasma. J Lipid Res 39: 679–690, 1998. [PubMed] [Google Scholar]
- 17. Karlsson H, Leanderson P, Tagesson C, Lindahl M. Lipoproteomics II: mapping of proteins in high-density lipoprotein using two-dimensional gel electrophoresis and mass spectrometry. Proteomics 5: 1431–1445, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Karlsson H, Leanderson P, Tagesson C, Lindahl M. Lipoproteomics I: Mapping of proteins in low-density lipoprotein using two-dimensional gel electrophoresis and mass spectrometry. Proteomics 5: 551–565, 2005. [DOI] [PubMed] [Google Scholar]
- 19. Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74: 5383–5392, 2002. [DOI] [PubMed] [Google Scholar]
- 20. Kunitake ST, Carilli CT, Lau K, Protter AA, Naya-Vigne J, Kane JP. Identification of proteins associated with apolipoprotein A-I-containing lipoproteins purified by selected-affinity immunosorption. Biochemistry 33: 1988–1993, 1994. [DOI] [PubMed] [Google Scholar]
- 21. Kunitake ST, Kane JP. Factors affecting the integrity of high density lipoproteins in the ultracentrifuge. J Lipid Res 23: 936–940, 1982. [PubMed] [Google Scholar]
- 22. Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem 76: 4193–4201, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Mirza SP, Halligan BD, Greene AS, Olivier M. Improved method for the analysis of membrane proteins by mass spectrometry. Physiol Genomics 30: 89–94, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nanjee MN, Brinton EA. Very small apolipoprotein A-I-containing particles from human plasma: isolation and quantification by high-performance size-exclusion chromatography. Clin Chem 46: 207–223, 2000. [PubMed] [Google Scholar]
- 25. Ordovas JM, Osgood D. Preparative isolation of plasma lipoproteins using fast protein liquid chromatography (FPLC). Methods Mol Biol 110: 105–111, 1998. [DOI] [PubMed] [Google Scholar]
- 26. Rezaee F, Casetta B, Levels JH, Speijer D, Meijers JC. Proteomic analysis of high-density lipoprotein. Proteomics 6: 721–730, 2006. [DOI] [PubMed] [Google Scholar]
- 27. Segrest JP, Jones MK, De Loof H, Brouillette CG, Venkatachalapathi YV, Anantharamaiah GM. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J Lipid Res 33: 141–166, 1992. [PubMed] [Google Scholar]
- 28. Stahlman M, Davidsson P, Kanmert I, Rosengren B, Boren J, Fagerberg B, Camejo G. Proteomics and lipids of lipoproteins isolated at low salt concentrations in D2O/sucrose or in KBr. J Lipid Res 49: 481–490, 2008. [DOI] [PubMed] [Google Scholar]
- 29. Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 117: 746–756, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Veenstra TD, Conrads TP, Hood BL, Avellino AM, Ellenbogen RG, Morrison RS. Biomarkers: mining the biofluid proteome. Mol Cell Proteomics 4: 409–418, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.