Abstract
Chromogranin A (CHGA) has a crucial role in formation of regulated secretory granules in neuroendocrine tissues and is also a prohormone that is proteolytically processed into peptides with diverse and complex actions. CHGA and several of its peptide products, including catestatin and pancreastatin, are implicated in pathogenesis of essential hypertension, insulin resistance, and the metabolic syndrome. The Chga knockout mouse (Chga KO) displays severe hypertension coupled with reduction in size, number, and density of regulated secretory granules. We performed genome-wide transcriptome profiling in Chga KO adrenal gland and liver for insight into biochemical and physiological systems altered in this monogenic mouse model of hypertension. Adrenal gene expression pathway prediction of enhanced insulin sensitivity (P = 0.03) in Chga KO was confirmed with glucose, insulin, and homeostasis model assessment of insulin resistance (HOMA-IR) measurements: blood glucose was normal in Chga KO, blood insulin was reduced 4.5-fold (P < 0.0001), and HOMA-IR was decreased 3.8-fold (P < 0.002). Remarkably, such observations conclusively dissociate fundamental features of the metabolic syndrome in this monogenic hypertension model. Exogenous pancreastatin treatment restored insulin sensitivity in the Chga KO to near-normal levels. Gene expression predictions of decreased adrenal cholesterol biosynthesis (P < 0.001) and increased hepatic cholesterol biosynthesis (P < 0.001) were verified with tissue total cholesterol assays: Chga KO adrenal cholesterol decreased 1.8-fold (P = 0.039) and hepatic cholesterol increased 1.8-fold (P = 0.018). Transcriptional regulatory network prediction identified sets of transcription factors that may provide insight into the unclear mechanistic links among CHGA, cholesterol, insulin sensitivity, and the metabolic syndrome. These experiments demonstrate, for the first time, that genetic variation at the CHGA locus impacts insulin sensitivity and tissue cholesterol levels in an intact, living organism. The Chga KO may constitute a unique model for studying the relationship between the CHGA locus and disease phenotypes of the metabolic syndrome.
Keywords: microarray, transcriptome, gene expression, essential hypertension, insulin resistance, cholesterol, metabolic syndrome
chromogranin a (CHGA = human; Chga = mouse) is a 48-kDa acidic protein crucial for the biogenesis of regulated secretory granules in chromaffin cells and neuroendocrine tissues (27). In addition, CHGA is a prohormone that can be proteolytically processed to yield a set of peptide hormones (e.g., catestatin, pancreastatin, vasostatin, chromacin) with diverse and complex actions (27). The expression of CHGA is heritable in humans, and its plasma levels are elevated in human essential (genetic) hypertension (26). Interestingly, the plasma concentration of catestatin, a potent nicotinic, cholinergic antagonist that inhibits catecholamine release, is decreased in patients with essential hypertension and in normotensive patients with a positive family history of the disease (17). Pancreastatin, another bioactive peptide derived from CHGA, has numerous potentially dysglycemic actions, including inhibition of glucose-stimulated insulin release from pancreatic islet beta cells (24) and inhibition of glucose uptake by adipocytes and hepatocytes (7); the peptide is elevated in the plasma of patients with type 2 diabetes (16, 30) and essential hypertension (25). Common genetic variation in the CHGA locus is associated with hypertension (2, 3, 18) and hypertensive end-organ damage (19), and naturally occurring amino acid variation within catestatin (14, 18) and pancreastatin (16, 30) has been shown to change potency of these peptide hormones. In humans, there is a strong link between CHGA, hypertension, insulin resistance (type 2 diabetes), and the metabolic syndrome—defined as a cluster of metabolic risk factors for cardiovascular disease, including essential hypertension (elevated blood pressure), insulin resistance, and dyslipidemia (e.g., high plasma triglycerides and high plasma total cholesterol) (8).
Recently, Mahapatra et al. (13) presented a report on the Chga knockout mouse that established, for the first time, a direct link between the Chga locus, secretory granule size, number, density, and cargo storage, and blood pressure homeostasis in an intact, living organism. The Chga knockout mouse displayed substantial hypertension with normalization of blood pressure through administration of exogenous catestatin peptide (13). To deepen our understanding of the role of Chga and its proteolytically derived bioactive peptides in the regulation of metabolic syndrome phenotypes, we performed microarray analysis on two tissues from the Chga knockout mouse: the adrenal gland and the liver. We chose the adrenal gland because it endogenously expresses Chga and can influence metabolic syndrome traits through secretion of its cortical steroids and its medullary catecholamines and hormone peptides. We selected the liver for two reasons: 1) the liver does not endogenously express Chga and is therefore a target of Chga and its bioactive peptides released from other tissues; and 2) the liver is central in regulation of intermediary metabolic and glucose homeostatic aspects of the metabolic syndrome. Genome-wide, transcriptome profiling in the adrenal gland and liver, with subsequent transcriptional regulatory network prediction, was used to predict changes in biochemical and physiological systems, with the overall objective being deeper understanding of the mechanistic link between the Chga locus and metabolic syndrome traits in an intact, living organism.
METHODS
Mouse strains.
Age-matched adult (∼12 wk old) male chromogranin A global knockout mice (Chga−/−) and wild-type control mice (Chga+/+) were obtained from colonies at the University of California, San Diego, in La Jolla, CA. Development of the Chga knockout mouse has previously been described by Mahapatra et al. (13). Mice were studied according to a protocol approved by the Animal Subjects Committee of the University of California at San Diego, and research was conducted in accordance with institutional guidelines.
Preparation of RNA.
Total RNA was extracted from isolated adrenal glands and livers from wild-type (n = 3) and Chga knockout (n = 3) mice with the RNAzol (guanidinium thiocyanate) kit (TelTest, Friendswood, TX), followed by RNase-free DNase I (Qiagen, Valencia, CA) treatment to eliminate residual genomic DNA. Integrity of the RNA was confirmed through 28S and 18S rRNA profiles on Agilent (Palo Alto, CA) columns and ethidium bromide-stained gels (data not shown).
Microarray experiments.
Gene expression in the adrenal gland and liver of each mouse was measured with Affymetrix (Santa Clara, CA) protocols and Affymetrix GeneChips (MG-U74Av2 GeneChips for the adrenal glands; Mouse 430A 2.0 GeneChips for the livers), as previously described (5, 6). The MG-U74Av2 chip contains 12,422 probe sets (excluding quality controls) corresponding to all functionally characterized sequences (∼6,000) in the mouse UniGene database (Build 74) and thousands of expressed sequence tag (EST) clusters. The Mouse 430A 2.0 chip contains 22,626 probe sets (excluding quality controls) corresponding to well-characterized sequences (∼14,000) in the mouse UniGene database (Build 107) and thousands of EST clusters. Tab-delimited text files of all chip spot features and probe design information are publicly available on the Affymetrix website: //www.affymetrix.com. In accordance with MIAME guidelines (//www.mged.org), the microarray data and a detailed description of experimental conditions and parameters are available at the NCBI Gene Expression Omnibus database (//www.ncbi.nlm.nih.gov/geo) under the following accession number: GSE18332. We have previously used quantitative real-time polymerase chain reaction (qRT-PCR) to confirm the fidelity of gene expression as determined by Affymetrix GeneChips (5, 6).
Microarray data analysis.
Statistical analysis of the microarray data was performed with a Bayesian statistical method known as variance-modeled posterior inference with regional exponentials (VAMPIRE) (9). Probe sets were considered significantly differentially expressed at P < 0.05 to minimize false negatives and gain a broad perspective on transcriptional and biochemical systems perturbed in the Chga knockout mouse. Two functional clustering methods, GenMAPP (20) and DAVID (4, 10), were used to perform a global analysis of adrenal and hepatic gene expression and sort differentially expressed genes into pathways and clusters of functionally related genes.
The GenMAPP program maps gene expression data onto biochemical pathways and uses nonparametric permutation tests and Westfall-Young multiple testing adjustments to identify significantly perturbed pathways. The DAVID algorithm addresses the redundant nature of genomic annotation that tends to dilute biological meaning during interpretation of gene expression data and is based on the hypothesis that similar functional annotations should have similar gene members. DAVID functional annotation clustering integrates kappa statistics and fuzzy heuristic clustering to group similar annotations into functional clusters. The P value associated with each annotation term is a modified Fisher exact score. The functional cluster enrichment score is the −log10 transformation of the geometric mean of all annotation terms within the functional cluster.
Blood glucose, insulin, and homeostasis model assessment.
Blood glucose was measured in fasting Chga knockout (n = 12) and wild-type (n = 15) mice with microcuvettes (HemoCue, Lake Forest, CA) and the Glucose 201 analyzer (HemoCue Sweden). Plasma insulin was measured in fasting Chga knockout (n = 13) and wild-type (n = 15) mice with a mouse insulin ELISA kit (Linco Research, St. Charles, MI). The homeostasis model assessment (HOMA) index of insulin resistance (HOMA-IR) (29) was computed with the standard equation: HOMA-IR = [fasting glucose (mg/dl) × fasting insulin (μU/ml)]/405. Wild-type and Chga knockout mice were administered mock (saline) or synthetic pancreastatin peptide (human CHGA273–301 fragment; GenScript, Piscataway, NJ) at 40 μg/g body wt via intraperitoneal (IP) injection 30 min before determination of the HOMA-IR index. Data were analyzed with a Student's t-test or ANOVA followed by pairwise comparisons corrected for multiple comparisons (Bonferroni).
Cholesterol assays.
Both adrenal glands and a small portion of the liver (∼200 mg) were harvested from Chga knockout (n = 6) and wild-type control (n = 6) mice, homogenized in 400 μl (for adrenal gland) or 1 ml (for liver) of 10 mM HEPES buffer, pH 7, and stored on ice. Homogenized tissues were subjected to three rounds of centrifugation at 14,000 rpm for 10 min at 4°C, in a tabletop centrifuge, with the pellet discarded after each round. Total cholesterol in plasma, adrenal gland, and liver was measured with an enzymatic, colorimetric kit (Roche US no. 450061) and the COBAS MIRA Plus Chemistry Analyzer (Roche, Basel, Switzerland). Liver cholesterol measurements were normalized by wet tissue mass (g). Adrenal gland cholesterol measurements were normalized by total protein (mg), as determined by a colorimetric assay based on the Bradford method (Bio-Rad, Hercules, CA; no. 500-0006). Data were analyzed with a Student's t-test.
Triglyceride assays.
Plasma, both adrenal glands, and a small portion of the liver were harvested from Chga knockout (n = 8 plasma, n = 6 adrenal gland, n = 7 liver) and wild-type (n = 8 plasma, n = 6 adrenal gland, n = 6 liver) control mice. Triglycerides were measured in plasma or tissue lysate with an enzymatic, colorimetric kit (Wako Diagnostics, Richmond, VA) and the COBAS MIRA Plus Chemistry Analyzer (Roche). Liver triglyceride measurements were normalized by wet tissue mass (g). Adrenal gland triglyceride measurements were normalized by total protein (mg), as determined by a colorimetric assay based on the Bradford method (Bio-Rad, no. 500-0006). Data were analyzed with a Student's t-test.
Computational transcription factor interaction network prediction.
We used the PAINT (Promoter Analysis and Interaction Network Toolset, v3.5) algorithm (//www.dbi.tju.edu/dbi/tools/paint) (28) to identify the set of transcription factors predicted to regulate expression of the genes contained in the DAVID and GenMAPP functional clusters. In brief, PAINT analyzes a given set of promoter sequences for the presence of transcription factor binding sites. The algorithm utilizes the transcription factor binding matrixes in the TRANSFAC database to produce a gene/transcription factor interaction matrix that represents a candidate transcriptional regulatory network. For the GenMAPP cholesterol biosynthesis pathway and the DAVID lipid functional clusters, all of the genes were used as input for PAINT. For the GenMAPP insulin signaling pathway, only the differentially expressed genes (P < 0.05) were used as input for PAINT.
RESULTS
Microarray statistical results.
Comparison of the adrenal gland and liver microarray experiments revealed a similar percentage of differentially expressed genes in both tissues: 15.5% for adrenal gland vs. 14.6% for liver (Table 1). In both the adrenal gland and liver, ∼50% of the differentially expressed genes were overexpressed while the other 50% were underexpressed. We have previously used qRT-PCR to confirm the fidelity of gene expression as determined by Affymetrix GeneChips (5, 6).
Table 1.
Summary of microarray statistical analysis
| Chga−/− vs. Chga+/+ | Chga−/− vs. Chga+/+ | |
|---|---|---|
| Tissue | Adrenal gland | Liver |
| Microarray | Affymetrix MG-U74Av2 | Affymetrix Mouse 430A 2.0 |
| Total no. of probe sets on microarray | 12,422 | 22,626 |
| Differentially expressed genes | 1,930 (15.5% of total) | 3,309 (14.6% of total) |
| Overexpressed genes | 909 (47%) | 1,401 (42.3%) |
| Underexpressed genes | 1,021 (53%) | 1,908 (57.7%) |
A summary of parameters and statistical results for the adrenal gland and liver microarray experiments is presented. Chga−/−, chromogranin A knockout mouse; Chga+/+, wild-type mouse.
Adrenal and hepatic gene expression patterns.
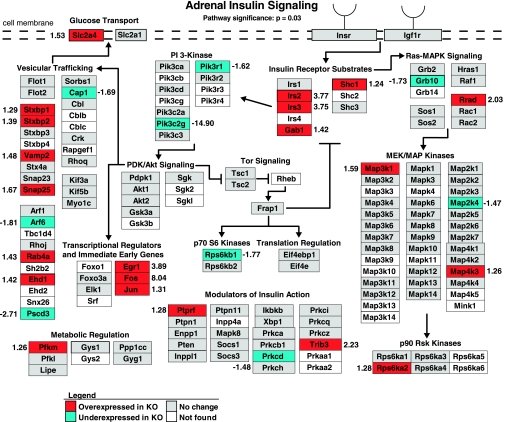
Pathway analysis (GenMAPP) of adrenal gene expression identified global changes in the insulin signaling pathway (pathway significance: P = 0.03; Fig. 1). The distribution of differentially expressed genes between overexpressed and underexpressed was significantly skewed toward overexpression (overexpressed genes: 21/30, 70%; underexpressed genes: 9/30, 30%; P = 0.0157, Fisher's exact test). Specific insulin signaling changes included overexpression of the insulin receptor substrates [insulin receptor substrate 2 (Irs2), +3.77-fold; insulin receptor substrate 3 (Irs3), +3.75-fold; Grb2-associated binding protein 1 (Gab1), +1.42; Src homology 2 domain-containing transforming protein C1 (Shc1), +1.24-fold] and overexpression of the primary glucose transporter [solute carrier family 2, facilitated glucose transporter, member 4 (Slc2a4, synonym = Glut4), +1.53-fold] in the Chga knockout mouse.
Fig. 1.
Adrenal insulin signaling and glucose transport gene expression is overexpressed in the chromogranin A (Chga) knockout mouse. Adrenal expression patterns of genes involved in insulin signaling and trans-plasma membrane glucose transport were mapped onto a biochemical pathway diagram with GenMAPP. The overall pattern of expression, specifically, the overexpression of the insulin receptor substrates (Irs2, Irs3, Gab1, Shc1) and primary glucose transporter [Glut4 (Slc2a4)], suggests enhanced insulin signaling and glucose transport in the Chga knockout mouse adrenal gland. Genes overexpressed in the Chga knockout are colored red, genes underexpressed in the Chga knockout are colored blue, genes lacking a statistically significant change are colored gray, and genes without expression data (i.e., the gene lacked a probe on the microarray) are shown in white. Numbers adjacent to genes indicate the fold change of differential expression. A positive number indicates a gene that is overexpressed in the knockout (Chga KO mean signal/wild-type mean signal), and a negative number refers to a gene that is underexpressed in the knockout (−wild-type mean signal/Chga KO mean signal).
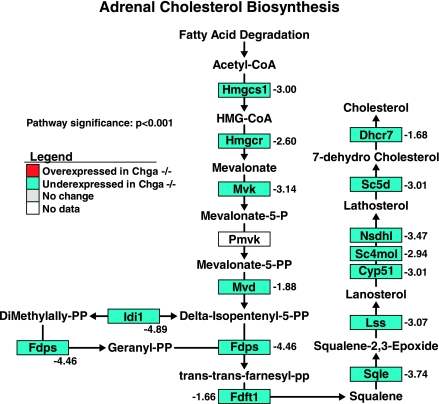
Functional cluster analysis (DAVID) in the adrenal gland revealed one statistically significant functional cluster of genes involved in adrenal sterol/steroid/lipid metabolism (P = 0.00134; Table 2). Pathway analysis (GenMAPP) of adrenal gene expression uncovered that the cholesterol biosynthesis pathway was globally underexpressed in the Chga knockout mouse (93.3% of all genes are underexpressed; 14 underexpressed genes/15 total genes) (pathway significance: P < 0.001; Fig. 2). HMG-CoA reductase (Hmgcr), the rate-limiting enzyme in cholesterol biosynthesis, was underexpressed 2.60-fold in the Chga knockout mouse.
Table 2.
Adrenal gene expression functional clusters suggest sterol/steroid/lipid abnormalities in adrenal gland of Chga knockout mouse
| Annotation Database | Database Category | Differentially Expressed Genes in Category | Category P Value | Category Benjamini P value |
|---|---|---|---|---|
| Functional Cluster 1: Sterol/steroid/lipid metabolism (104 unique genes) | ||||
| Enrichment score: 2.87; P value = 1.34E-3 | ||||
| KEGG Pathway | Biosynthesis of steroids (mmu00100) | 16 | 2.1E-6 | 4.1E-4 |
| GO: Biological Process | Sterol biosynthetic process (GO:0016126) | 17 | 6.2E-6 | 2.9E-2 |
| GO: Biological Process | Cholesterol biosynthetic process (GO:0006695) | 14 | 2.5E-5 | 5.6E-2 |
| PIR Keyword | Cholesterol biosynthesis | 10 | 4.8E-5 | 1.0E-2 |
| PIR Keyword | Sterol biosynthesis | 12 | 5.6E-5 | 9.3E-3 |
| PIR Keyword | Steroid biosynthesis | 15 | 1.6E-4 | 1.7E-2 |
| GO: Biological Process | Sterol metabolic process (GO:0016125) | 23 | 1.9E-3 | 7.7E-1 |
| PIR Keyword | Lipid synthesis | 21 | 1.9E-3 | 1.1E-1 |
| GO: Biological Process | Steroid biosynthetic process (GO:0006694) | 21 | 3.4E-3 | 8.6E-1 |
| GO: Biological Process | Lipid metabolic process (GO:0006629) | 103 | 6.7E-3 | 8.6E-1 |
| GO: Biological Process | Steroid metabolic process (GO:0008202) | 34 | 7.4E-3 | 8.4E-1 |
| GO: Biological Process | Cholesterol metabolic process (GO:0008203) | 20 | 7.9E-3 | 8.3E-1 |
| GO: Biological Process | Cellular lipid metabolic process (GO:0044255) | 90 | 8.7E-3 | 8.3E-1 |
| GO: Biological Process | Isoprenoid biosynthetic process (GO:0008299) | 7 | 1.0E-2 | 8.3E-1 |
| GO: Biological Process | Lipid biosynthetic process (GO:0008610) | 47 | 1.3E-2 | 8.3E-1 |
| GO: Biological Process | Isoprenoid metabolic process (GO:0006720) | 9 | 2.8E-2 | 9.3E-1 |
| KEGG Pathway | Terpenoid biosynthesis (mmu00900) | 4 | 5.5E-2 | 5.4E-1 |
| PIR Keyword | Isoprene biosynthesis | 3 | 1.7E-1 | 8.8E-1 |
One statistically significant gene expression cluster of functional annotation terms related to adrenal sterol/steroid/lipid metabolism was identified with DAVID. Statistical significance of the functional cluster is presented as an enrichment score (geometric mean in −log10 scale of database category P values) that is used to rank the overall biological significance of the functional cluster. The name of the annotation database for each term, the corresponding database category, and the number of differentially expressed genes in each database category are listed (note that a given gene can appear in more than 1 database category within the functional cluster, i.e., the database categories are not independent). P value, modified Fisher exact P value for each database category; Benjamini P value, P value corrected for multiple comparisons; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; PIR, Protein Information Resource.
Fig. 2.
Adrenal cholesterol biosynthesis pathway gene expression is globally underexpressed in the Chga knockout mouse. Adrenal expression patterns of genes involved in cholesterol biosynthesis were mapped onto a biochemical pathway diagram with GenMAPP. The statistically significant and global underexpression of the pathway, including 2.60-fold underexpression of HMG-CoA reductase (Hmgcr), the rate-limiting enzyme in cholesterol biosynthesis, suggests a reduction in de novo cholesterol biosynthesis in the adrenal gland of the Chga knockout mouse. Genes overexpressed in the Chga knockout are colored red, genes underexpressed in the Chga knockout are colored blue, genes lacking a statistically significant change are colored gray, and genes without expression data (i.e., the gene lacked a probe on the microarray) are shown in white. Numbers adjacent to genes indicate the fold change of differential expression. A positive number indicates a gene that is overexpressed in the knockout (Chga KO mean signal/wild-type mean signal), and a negative number refers to a gene that is underexpressed in the knockout (−wild-type mean signal/Chga KO mean signal).
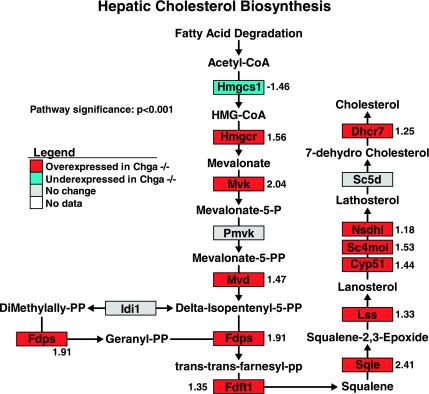
Functional cluster analysis (DAVID) of hepatic gene expression identified two statistically significant clusters of genes involved in sterol/steroid/lipid metabolism (cluster 1 P value = 2.96E-10; cluster 2 P value = 4.3E-5; Table 3). Pathway analysis (GenMAPP) revealed a pattern of global overexpression of the hepatic cholesterol biosynthesis pathway in the Chga knockout mouse (73.3% of all genes are overexpressed; 11 overexpressed genes/15 total genes) (pathway significance: P < 0.001; Fig. 3). The rate-limiting enzyme in the cholesterol biosynthesis pathway, HMG-CoA reductase (Hmgcr), was overexpressed 1.56-fold in the Chga knockout mouse.
Table 3.
Hepatic gene expression functional clusters suggest sterol/steroid/lipid abnormalities in liver of Chga knockout mouse
| Annotation Database | Database Category | Differentially Expressed Genes in Category | Category P Value | Category Benjamini P Value |
|---|---|---|---|---|
| Functional Cluster 1: Lipid metabolism (178 unique genes) | ||||
| Enrichment score = 9.53; P value = 2.96E-10 | ||||
| GO: Biological Process | Lipid metabolic process (GO:0006629) | 178 | 2.3E-11 | 5.8E-8 |
| GO: Biological Process | Cellular lipid metabolic process (GO:0044255) | 158 | 6.2E-11 | 1.0E-7 |
| GO: Biological Process | Lipid biosynthetic process (GO:0008610) | 81 | 1.8E-08 | 1.5E-5 |
| Functional Cluster 2: Sterol/steroid metabolism (77 unique genes) | ||||
| Enrichment score = 4.36; P value = 4.3E-5 | ||||
| GO: Biological Process | Steroid metabolic process (GO:0008202) | 58 | 6.5E-08 | 4.7E-5 |
| GO: Biological Process | Cholesterol metabolic process (GO:0008203) | 34 | 7.0E-08 | 4.4E-5 |
| GO: Biological Process | Sterol metabolic process (GO:0016125) | 35 | 2.7E-07 | 1.5E-4 |
| GO: Biological Process | Steroid biosynthetic process (GO:0006694) | 33 | 2.2E-06 | 1.1E-3 |
| GO: Biological Process | Cholesterol biosynthetic process (GO:0006695) | 16 | 4.7E-06 | 2.2E-3 |
| GO: Biological Process | Sterol biosynthetic process (GO:0016126) | 18 | 8.9E-06 | 3.2E-3 |
| PIR Keyword | Steroid biosynthesis | 17 | 6.3E-05 | 8.9E-3 |
| PIR Keyword | Lipid synthesis | 29 | 2.9E-04 | 2.2E-2 |
| KEGG Pathway | Biosynthesis of steroids (mmu00100) | 14 | 4.8E-04 | 9.0E-2 |
| PIR Keyword | Sterol biosynthesis | 11 | 1.0E-3 | 5.7E-2 |
| PIR Keyword | Cholesterol biosynthesis | 9 | 1.4E-3 | 6.0E-2 |
| GO: Biological Process | Isoprenoid biosynthetic process (GO:0008299) | 7 | 3.5E-2 | 9.1E-1 |
| GO: Biological Process | Isoprenoid metabolic process (GO:0006720) | 11 | 3.8E-2 | 9.1E-1 |
Two statistically significant gene expression clusters of functional annotation terms related to hepatic sterol/steroid/lipid lipid metabolism were identified with DAVID. Statistical significance of the functional cluster is presented as an enrichment score (geometric mean in −log10 scale of database category P values) that is used to rank the overall biological significance of the functional cluster. The name of the annotation database for each term, the corresponding database category, and the number of differentially expressed genes in each database category are listed (note that a given gene can appear in more than 1 database category within the functional cluster, i.e., the database categories are not independent). P value, modified Fisher exact P value for each database category; Benjamini P value, P value corrected for multiple comparisons.
Fig. 3.
Hepatic cholesterol biosynthesis pathway gene expression is globally overexpressed in the Chga knockout mouse. Hepatic expression patterns of genes involved in cholesterol biosynthesis were mapped onto a biochemical pathway diagram with GenMAPP. The statistically significant and global overexpression of the pathway, including 1.56-fold overexpression of HMG-CoA reductase (Hmgcr), the rate-limiting enzyme in cholesterol biosynthesis, suggests an increase in de novo cholesterol biosynthesis in the liver of the Chga knockout mouse. Genes overexpressed in the Chga knockout are colored red, genes underexpressed in the Chga knockout are colored blue, genes lacking a statistically significant change are colored gray, and genes without expression data (i.e., the gene lacked a probe on the microarray) are shown in white. Numbers adjacent to genes indicate the fold change of differential expression. A positive number indicates a gene that is overexpressed in the knockout (Chga KO mean signal/wild-type mean signal), and a negative number refers to a gene that is underexpressed in the knockout (−wild-type mean signal/Chga KO mean signal).
Glucose, cholesterol, and triglyceride assays.
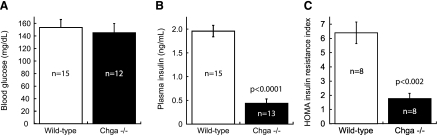
Plasma glucose concentration did not differ between wild-type (153.5 ± 12.8 mg/dl) and Chga knockout (145.3 ± 14.6 mg/dl) mice (Fig. 4A). Plasma insulin concentration was reduced 4.5-fold in Chga knockout mice (0.44 ± 0.09 ng/ml) compared with wild-type mice (1.96 ± 0.12 ng/ml) (P < 0.0001; Fig. 4B). The HOMA index of insulin resistance in Chga knockout mice (1.7 ± 0.3) was 3.8-fold lower than the HOMA index in wild-type mice (6.4 ± 0.7) (P < 0.002; Fig. 4C).
Fig. 4.
Glucose, insulin, and homeostasis model assessment (HOMA)-insulin resistance (IR) measurements confirm that the Chga knockout mouse is insulin sensitive. A: no difference was detected in blood glucose concentration between wild-type (153.5 ± 12.8 mg/dl) and Chga knockout (Chga−/−) mice (145.3 ± 14.6 mg/dl). B: plasma insulin concentration was reduced 4.5-fold in Chga knockout mice (0.44 ± 0.09 ng/ml) compared with wild-type mice (1.96 ± 0.12 ng/ml). C: the HOMA index of IR in Chga knockout mice (1.7 ± 0.3) was 3.8-fold lower than the HOMA index in wild-type mice (6.4 ± 0.7). Data are presented as means ± SE.
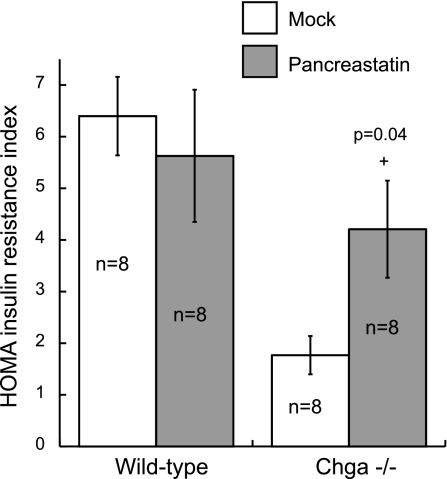
Acute treatment (30 min) with exogenous pancreastatin peptide (PST) had no effect on the HOMA-IR index in wild-type mice (6.4 ± 0.7 without PST vs. 5.6 ± 1.3 after PST) but resulted in a 2.5-fold increase in HOMA in Chga knockout mice (1.7 ± 0.3 without PST vs. 4.2 ± 0.9 after PST) (P = 0.04; Fig. 5). There was no statistical difference between wild-type mice without pancreastatin treatment (6.4 ± 0.7) and Chga knockout mice after pancreastatin treatment (4.2 ± 0.9) (Fig. 5).
Fig. 5.
Acute pancreastatin treatment increases insulin resistance in Chga knockout mice. Wild-type and Chga knockout mice were administered mock (saline) or synthetic pancreastatin peptide (PST) at 40 μg/g body wt via intraperitoneal injection 30 min before determination of the HOMA-IR index. Pancreastatin had no effect on the HOMA index in wild-type mice (6.4 ± 0.7 without PST vs. 5.6 ± 1.3 after PST) but resulted in a 2.5-fold increase in HOMA in Chga knockout mice (1.7 ± 0.3 without PST vs. 4.2 ± 0.9 after PST; +P = 0.04). There was no statistical difference between wild-type mice without PST and Chga knockout mice after PST. Data are presented as means ± SE.
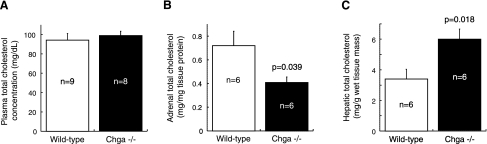
There was no difference in plasma total cholesterol between Chga knockout (99.0 ± 4.4 mg/dl) and wild-type (94.3 ± 6.8 mg/dl) mice (Fig. 6A). Measurement of adrenal total cholesterol levels revealed a 1.8-fold reduction of cholesterol levels in Chga knockout adrenal gland (0.41 ± 0.05 mg/mg tissue protein) compared with wild-type adrenal gland (0.72 ± 0.12 mg/mg tissue protein) (P = 0.039; Fig. 6B). Measurement of hepatic total cholesterol levels revealed a 1.8-fold elevation in Chga knockout liver (6.0 ± 0.7 mg/g wet tissue mass) compared with wild-type liver (3.4 ± 0.6 mg/g wet tissue mass) (P = 0.018; Fig. 6C).
Fig. 6.
Confirmation of gene expression predictions: total cholesterol is reduced in the adrenal gland and increased in the liver of the Chga knockout mouse. A: no difference was detected in plasma cholesterol between Chga knockout (99.0 ± 4.4 mg/dl) and wild-type (94.3 ± 6.8 mg/dl) mice. B: adrenal total cholesterol was reduced 1.8-fold in Chga knockout mice (0.41 ± 0.05 mg/mg tissue protein) compared with wild-type mice (0.72 ± 0.12 mg/mg tissue protein). C: hepatic total cholesterol was elevated 1.8-fold in Chga knockout (6.0 ± 0.7 mg/g wet tissue mass) compared with wild-type (3.4 ± 0.6 mg/g wet tissue mass) mice. Data are presented as means ± SE.
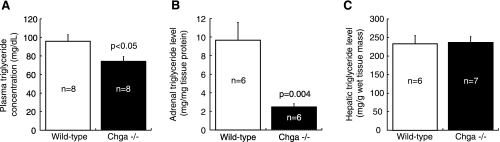
The plasma concentration of triglycerides was reduced 1.3-fold in Chga knockout mice (74.3 ± 5.1 mg/dl) compared with wild-type mice (95.9 ± 7.2 mg/dl) (P < 0.05; Fig. 7A). Adrenal triglyceride level was reduced 3.9-fold in Chga knockout mice (2.5 ± 0.3 mg/mg tissue protein) compared with wild-type mice (9.7 ± 1.9 mg/mg tissue protein) (P = 0.004; Fig. 7B). No difference in hepatic triglyceride level was detected between Chga knockout (236.5 ± 15.9 mg/g wet tissue mass) and wild-type (233.1 ± 22.1 mg/g wet tissue mass) mice (Fig. 7C).
Fig. 7.
Triglyceride concentration is decreased in the plasma and adrenal gland of the Chga knockout mouse. A: plasma concentration of triglycerides was reduced 1.3-fold in Chga knockout mice (74.3 ± 5.1 mg/dl) compared with wild-type mice (95.9 ± 7.2 mg/dl). B: adrenal triglyceride level was decreased 3.9-fold in Chga knockout mice (2.5 ± 0.3 mg/mg tissue protein) compared with wild-type mice (9.7 ± 1.9 mg/mg tissue protein). C: no difference in hepatic triglyceride level was detected between Chga knockout (236.5 ± 15.9 mg/g wet tissue mass) and wild-type (233.1 ± 22.1 mg/g wet tissue mass) mice. Data are presented as means ± SE.
Transcriptional regulatory networks.
PAINT analysis of the DAVID and GenMAPP functional clusters produced one candidate transcriptional regulatory network for each gene cluster. The insulin signaling pathway in the adrenal gland (Fig. 1) was predicted to be regulated by a network of 15 transcription factors, 5 of which were differentially expressed in Chga knockout adrenal glands: Egr1 (overexpressed +3.89-fold), Myc (underexpressed −1.39-fold), Nfia (underexpressed −1.54-fold), Nfic (overexpressed +1.24-fold), and Tcf3 (overexpressed +1.76-fold) (Table 4).
Table 4.
Transcriptional regulatory network for adrenal insulin signaling pathway
| Transcription Factors Predicted to Regulate Adrenal Insulin Signaling Pathway | Adrenal Microarray Fold Change |
|---|---|
| Egr1 (V$EGR1_01) | +3.89-fold |
| Egr3 (V$EGR3_01) | No data |
| Egr4 (V$NGFIC_01) | No data |
| Elk1 (V$ELK1_02) | No change |
| Ets1 (V$CETS1P54_01) | No change |
| Hand1/Tcf3 (V$HAND1E47_01) | No change/+1.76-fold |
| Myc/Max (V$MYCMAX_02) | −1.39-fold/No change |
| Myog/Nfia (V$MYOGNF1_01) | No change/−1.54-fold |
| Myog/Nfib (V$MYOGNF1_01) | No change/No change |
| Myog/Nfic (V$MYOGNF1_01) | No change/+1.24-fold |
| Myog/Nfix (V$MYOGNF1_01) | No change/No change |
| Nfkb1 (V$NFKAPPAB50_01) | No change |
| Srf (V$SRF_01) | No data |
| Tcfcp2 (V$CP2_01) | No change |
| Zbtb6 (V$ZID_01) | No data |
PAINT analysis of the GenMAPP-derived adrenal insulin signaling pathway identified a set of 15 transcription factors predicted to regulate expression of genes in the pathway. Five of the transcription factors were differentially expressed in the adrenal microarray experiments: Egr1 (+3.89-fold in Chga KO), Myc (−1.39-fold in Chga KO), Nfia (−1.54-fold in Chga KO), Nfic (+1.24-fold in Chga KO), and Tcf3 (+1.76-fold in Chga KO). The standard transcription factor gene symbol and the TRANSFAC binding matrix to which the transcription factor binds are shown; a “/” symbol indicates a transcription factor heterodimer. Fold change for the transcription factors in the adrenal microarray experiments is also listed: positive fold change (+) indicates statistically significant overexpression in Chga knockout (Chga KO mean signal/wild-type mean signal); negative fold change (−) indicates significantly significant underexpression in Chga knockout (−wild-type mean signal/Chga KO mean signal); “no data” indicates lack of a probe for the transcription factor on the microarray; “no change” indicates lack of a statistically significant fold change on the microarray.
PAINT identified two partially overlapping transcriptional networks regulating the adrenal (Table 2) and hepatic (Table 3) DAVID lipid/sterol/steroid functional clusters (Table 5). The adrenal network contained 17 transcription factors, and the hepatic network contained 29 transcription factors. The adrenal and hepatic networks have 10 transcription factors in common, while 7 transcription factors are unique to the adrenal network and 19 transcription factors are unique to the hepatic network.
Table 5.
Transcriptional regulatory network for lipid functional clusters
| Transcription Factors Predicted to Regulate DAVID Lipid Functional Clusters | Adrenal Microarray Fold Change | Hepatic Microarray Fold Change |
|---|---|---|
| Arnt (V$ARNT_01) | No change | – |
| Atf2 (V$CREBP1_01) | No change | −1.20-fold |
| Atf2/Jun (V$CREBP1CJUN_01) | – | −1.20-fold/no change |
| Cebpa (V$CAAT_01) | No change | No change |
| Cebpb (V$CAAT_01) | No change | No change |
| Cebpd (V$CAAT_01) | +1.23-fold | No change |
| Cebpg (V$CAAT_01) | No change | No change |
| Cebpz (V$CAAT_01) | No change | No change |
| Comp1 (V$COMP1_01) | No data | – |
| Creb1 (V$CREB_01) | – | No change |
| Dbp (V$VBP_01) | +1.43-fold | −1.47-fold |
| Esr1 (V$ER_Q6) | – | No change |
| Foxd3 (V$FOXD3_01) | – | No change |
| Gata1 (V$GATA1_02) | – | No change |
| Hlf (V$HLF_01) | No data | – |
| Hnf4a (V$COUP_01) | – | No change |
| Myc/Max (V$MYCMAX_02) | – | No change/no change |
| Myog/Nfia (V$MYOGNF1_01) | – | No change/no change |
| Myog/Nfib (V$MYOGNF1_01) | – | No change/no change |
| Myog/Nfic (V$MYOGNF1_01) | – | No change/+1.42-fold |
| Myog/Nfix (V$MYOGNF1_01) | – | No change/no change |
| Nfe2 (V$NFE2_01) | – | No change |
| Nfe2l1/Mafg (V$TCF11MAFG_01) | – | −1.31-fold/ no change |
| Nfe2l2 (V$NRF2_01) | – | No change |
| Nfkb1 (V$NFKAPPAB_01) | No change | – |
| Nfya (V$NFY_Q6) | No change | – |
| Nfyb (V$NFY_Q6) | No change | – |
| Nfyc (V$NFY_Q6) | No change | – |
| Nr2f1 (V$COUP_01) | – | No change |
| Nr2f2 (V$ARP1_01) | – | No change |
| Pbx1 (V$PBX1_02) | – | No change |
| Pou3f2 (V$BRN2_01) | – | No change |
| Rel (V$CREL_01) | No change | No change |
| Srebf1 (V$SREBP1_01) | – | +1.27-fold |
| Usf1 (V$USF_Q6) | −1.28-fold | −1.57-fold |
| Usf2 (V$USF_Q6) | No change | No change |
PAINT analysis of the DAVID-derived adrenal and hepatic lipid/sterol/steroid functional clusters identified sets of 17 and 29 transcription factors in the adrenal and hepatic transcriptional regulatory networks, respectively. The standard transcription factor gene symbol and the TRANSFAC binding matrix to which the transcription factor binds are listed; a “/” symbol indicates a transcription factor heterodimer. Fold change for the transcription factors in adrenal microarray experiments and in hepatic microarray experiments is also listed: positive fold change (+) indicates statistically significant overexpression in Chga knockout (Chga KO mean signal/wild-type mean signal); negative fold change (−) indicates statistically significant underexpression in Chga knockout (−wild-type mean signal/Chga KO mean signal); “no data” indicates lack of a probe for the transcription factor on the microarray; “no change” indicates lack of a statistically significant fold change on the microarray; “–” indicates that the specific transcription factor was not found in either the adrenal or the hepatic transcriptional regulatory network.
The transcriptional regulatory network for the GenMAPP-derived cholesterol biosynthesis pathway (Figs. 2 and 3) contains 26 transcription factors (Table 6). Of the 26 transcription factors predicted to regulate the pathway, 10 showed differences in expression between the adrenal gland and liver: Atf2 (adrenal gland: no change; liver: underexpressed −1.20-fold), Jun (adrenal gland: overexpressed +1.31-fold; liver: no change), Cebpd (adrenal gland: overexpressed +1.23-fold; liver; no change), Dbp (adrenal gland; overexpressed +1.43-fold; liver: underexpressed −1.47-fold), E2f1 (adrenal gland: underexpressed −2.12-fold; liver: overexpressed +2.64-fold), E2f5 (adrenal gland: no change; liver: underexpressed −1.28-fold), Hivep2 (adrenal gland: underexpressed −1.43-fold; liver: no change), Nfya (adrenal gland: no change; liver: underexpressed −1.61-fold), Nfyb (adrenal gland: no change; liver: overexpressed +1.30-fold), and Pax6 (adrenal gland, no change; liver: overexpressed +2.60-fold).
Table 6.
Transcriptional regulatory network for cholesterol biosynthesis pathway
| Transcription Factors Predicted to Regulate Cholesterol Biosynthesis Pathway | Adrenal Microarray Fold Change | Hepatic Microarray Fold Change |
|---|---|---|
| Atf2 (V$CREBP1_01) | No change | −1.20-fold |
| Atf2/Jun (V$CREBP1CJUN_01) | No change/+1.31-fold | −1.20-fold/no change |
| Cebpa (V$CAAT_01) | No change | No change |
| Cebpb (V$CAAT_01) | No change | No change |
| Cebpd (V$CAAT_01) | +1.23-fold | No change |
| Cebpg (V$CAAT_01) | No change | No change |
| Cebpz (V$CAAT_01) | No change | No change |
| Comp1 (V$COMP1_01) | No data | No data |
| Creb1 (V$CREB_01) | No change | No change |
| Dbp (V$VBP_01) | +1.43-fold | −1.47-fold |
| E2f1 (V$E2F_02) | −2.12-fold | +2.64-fold |
| E2f2 (V$E2F_02) | No data | No data |
| E2f3 (V$E2F_02) | No change | No change |
| E2f4 (V$E2F_02) | No data | No change |
| E2f5 (V$E2F_02) | No change | −1.28-fold |
| Elk1 (V$ELK1_02) | No change | No change |
| Evi1 (V$EVI1_06) | No change | No change |
| Gata1 (V$GATA1_02) | No change | No change |
| Hnf1a (V$HNF1_01) | No change | No change |
| Hnf4a (V$HNF4_01) | No change | No change |
| Hivep2/Rfx1 (V$MIF1_01) | −1.43-fold/No change | No change/No change |
| Nfya (V$NFY_Q6) | No change | −1.61-fold |
| Nfyb (V$NFY_Q6) | No change | +1.30-fold |
| Nfyc (V$NFY_Q6) | No change | No change |
| Pax6 (V$PAX6_01) | No change | +2.60-fold |
| Rfx1 (V$RFX1_01) | No change | No change |
PAINT analysis of the GenMAPP-derived cholesterol biosynthesis pathway identified a set of 26 transcription factors in the transcriptional regulatory network. The standard transcription factor gene symbol and the TRANSFAC binding matrix to which the transcription factor binds are listed; a “/” symbol indicates a transcription factor heterodimer. Fold change for the transcription factors in adrenal microarray experiments and in hepatic microarray experiments is also listed: positive fold change (+) indicates statistically significant overexpression in Chga knockout (Chga KO mean signal/wild-type mean signal); negative fold-change (−) indicates statistically significant underexpression in Chga knockout (−wild-type mean signal/Chga KO mean signal); “no data” indicates lack of a probe for the transcription factor on the microarray; “no change” indicates lack of a statistically significant fold change on the microarray.
DISCUSSION
The initial investigation of the Chga knockout mouse by Mahapatra et al. (13) showed that Chga gene ablation resulted in a striking increase in blood pressure and catecholamine secretion, coupled with a decrease in neuroendocrine (e.g., adrenal) regulated secretory granule size, number, density, and cargo storage in vivo. In addition, through administration of exogenous catestatin, a catecholamine release inhibitory peptide proteolytically derived from full-length CHGA protein, Mahapatra et al. were able to normalize the elevated blood pressure of the Chga knockout mouse and demonstrate, for the first time, the crucial role of CHGA in blood pressure homeostasis in an intact, living organism. The work of Mahapatra et al. demonstrated that CHGA and its proteolytically derived bioactive peptides (e.g., catestatin) can affect biochemical systems at the local (tissue) level as well as induce changes in whole body physiological systems. To gain insight into CHGA-mediated regulation of functional processes at the local level of neuroendocrine cells/tissues and at the level of whole body physiological systems (global level), we performed microarray analysis on the adrenal gland and liver of the Chga knockout mouse and then sought biochemical verification of the predicted consequences of gene expression alterations. We found that this monogenic model of severe hypertension actually exhibits enhanced insulin sensitivity and reduced triglyceride concentrations, thereby effectively dissociating features of the human metabolic syndrome.
Microarray statistical results.
Ablation of the Chga gene in the mouse resulted in differential expression of a large number of genes in the adrenal gland (1,930 genes, or 15.5% of all genes assayed) and liver (3,309 genes, or 14.6% of all genes assayed), with the distribution of genes split approximately evenly between overexpressed (∼50%) and underexpressed (∼50%) in each tissue (Table 1). Such numerous gene expression changes in the adrenal gland, a neuroendocrine tissue known to express CHGA at a very high level (27), are not surprising. However, large-scale perturbations in the liver, a tissue lacking endogenous CHGA expression, suggest that CHGA and its proteolytically derived bioactive peptides can modulate nonneuroendocrine (i.e., non-Chga expressing) tissues and physiological systems at a distance throughout the body, perhaps as a consequence of alterations in endocrine hormone secretion.
Microarray prediction of increased insulin sensitivity.
Pathway analysis (GenMAPP) of adrenal gene expression identified global changes in the insulin signaling pathway (P = 0.03, Fig. 1). The fact that the vast majority of differentially expressed insulin signaling genes were overexpressed rather than underexpressed (P = 0.0157) and that four insulin receptor substrates (crucial signaling molecules phosphorylated by the insulin receptor) and the primary trans-plasma membrane, insulin-responsive glucose transporter were overexpressed [Irs2, +3.77-fold; Irs3, +3.75-fold; Gab1, +1.42-fold; Shc1, +1.24-fold; Slc2a4 (Glut4), +1.53-fold] suggested that reduction (knockout) of Chga in the mouse results in increased signaling (sensitivity) in response to insulin.
Experimental confirmation of increased insulin sensitivity.
To confirm that ablation of the mouse Chga gene results in increased sensitivity to insulin, we measured the concentration of blood glucose and plasma insulin and computed the HOMA index of insulin resistance (Fig. 4). The Chga knockout mouse displayed a normal blood glucose concentration (Fig. 4A), yet the concentration of plasma insulin was 4.5-fold less than the concentration in wild-type mice (P < 0.0001; Fig. 4B). The Chga knockout mouse thus requires only ∼20% of the insulin that wild-type mice need in order to maintain normal blood glucose levels, an indication that CHGA can regulate sensitivity to insulin and that the Chga knockout mouse is hypersensitive to insulin. The 3.8-fold reduction of the HOMA index [a standard metric of insulin resistance (29)] (P < 0.002; Fig. 4C) provides additional evidence that Chga knockout mice are more sensitive to insulin than wild-type mice.
Since plasma triglyceride concentration is positively and strongly correlated with insulin sensitivity/resistance as part of the metabolic syndrome (8, 15), the 1.3-fold reduction of plasma triglyceride concentration (P < 0.05; Fig. 7A) is consistent with an insulin sensitivity phenotype in the Chga knockout mouse. Interestingly, the liver, which is the main source of triglycerides in the body, showed no difference in triglyceride levels between Chga knockout and wild-type mice (Fig. 7C), suggesting that other tissues (e.g., adipose) are responsible for the reduction in plasma triglyceride concentration. The extent to which the 3.9-fold reduction of triglycerides in the Chga knockout adrenal gland (P = 0.004; Fig. 7B) contributed to the decreased plasma triglyceride concentration is uncertain.
Additional experiments, such as insulin tolerance tests (ITT) and glucose tolerance tests (GTT), can probe the kinetic and temporal characteristics of increased insulin sensitivity in the Chga knockout mouse and perhaps identify cell- and tissue-specific mechanisms whereby CHGA and/or its proteolytically derived peptides regulate glucose homeostasis and influence insulin sensitivity.
The notion that CHGA can regulate insulin sensitivity is not new; however, these results are the first to show a direct effect of genetic variation at the Chga locus (i.e., gene knockout) on insulin sensitivity in an intact, living organism. The most likely mechanism whereby CHGA can regulate insulin sensitivity is through its proteolytically derived peptide fragment pancreastatin (human CHGA250-301-amide), the so-called “dysglycemic” peptide. A diverse set of potentially dysglycemic actions of pancreastatin has been reported in isolated cells and tissues (23, 30), including inhibition of glucose-stimulated insulin release from pancreatic islet beta cells (24), inhibition of glucose uptake by adipocytes and hepatocytes (7), and inhibition of insulin signaling in adipocytes (23). Dysglycemic effects have also been reported in vivo during intramesenteric vein injection of pancreastatin in the rat (21, 22) and during infusion of the peptide into the brachial artery while studying skeletal muscle glucose uptake in the human forearm model (1). Thus lack of pancreastatin is likely to be the major factor responsible for enhanced insulin sensitivity in the Chga knockout mouse, although additional experiments are required to identify involvement of specific cell and tissue types and to elucidate cellular mechanisms of pancreastatin action; for example, identification of a pancreastatin receptor has remained elusive.
Mahapatra et al. (13) previously demonstrated that administration of exogenous catestatin peptide (an inhibitor of catecholamine secretion) normalized hypertension (elevated blood pressure) in the Chga knockout mouse. With a similar rationale, we administered exogenous pancreastatin peptide (a hormone with “dysglycemic” effects) to Chga knockout mice and were able to restore the state of insulin sensitivity to near-normal levels (Fig. 5). Pancreastatin treatment did not completely normalize insulin sensitivity to wild-type levels (although there was no statistical difference in HOMA between wild-type mice without pancreastatin treatment and Chga knockout mice after pancreastatin treatment). It is possible that the IP dose of pancreastatin resulted in a circulating concentration below the normal physiological level, and/or that a longer treatment period (>30 min) is required to achieve complete normalization. More intriguingly, it is possible that other functional peptides derived from CHGA (e.g., catestatin, vasostatin) without a currently known role in glucose homeostasis can also regulate insulin sensitivity. It is also important to note that exogenous pancreastatin had no effect in wild-type mice, an indication that the normal, physiological concentration of endogenous pancreastatin is near or at the level required to saturate the system. Although the genomic and nongenomic mechanisms of action by pancreastatin are incompletely understood, our gene expression pathway mapping (Figs. 1–3) and transcriptional interaction networks (Tables 4–6) provide avenues for additional investigation.
Transcriptomic prediction of altered cholesterol biosynthesis.
Functional cluster analysis (DAVID) suggested alterations in adrenal sterol/steroid/lipid metabolism (Table 2). Pathway analysis (GenMAPP) revealed that the adrenal cholesterol biosynthesis pathway is globally underexpressed in the Chga knockout mouse (P < 0.001, Fig. 2), including 2.60-fold underexpression of HMG-CoA reductase, the rate-limiting enzyme in the pathway. In contrast, functional cluster (DAVID) and pathway (GenMAPP) analysis of hepatic gene expression identified functional clusters of differentially expressed genes involved in sterol/steroid/lipid metabolism (Table 3) and identified specific, global overexpression of the hepatic cholesterol biosynthesis pathway (P < 0.001, Fig. 3), including 1.56-fold overexpression of HMG-CoA reductase. This global, yet directionally opposite differential expression in the adrenal and hepatic cholesterol biosynthesis pathways indicates highly tissue-specific changes in multiple transcripts to regulate cholesterol biosynthesis in the Chga knockout mouse.
Experimental confirmation of decreased adrenal and increased hepatic cholesterol.
We confirmed that cholesterol levels were indeed altered in the adrenal gland and liver in the direction predicted by differential gene expression patterns: adrenal cholesterol was decreased 1.8-fold in Chga knockout mice (P = 0.039; Fig. 6B); hepatic cholesterol was increased 1.8-fold in Chga knockout mice (P = 0.018; Fig. 6C). Plasma cholesterol concentration was unchanged (Fig. 6A). The concentration of cholesterol in the plasma reflects the net contribution of cholesterol synthesis, secretion, and absorption from various tissues, including the adrenal glands and liver. Relative to Chga knockout mice, wild-type mice have decreased hepatic cholesterol and increased adrenal cholesterol; relative to wild-type mice, Chga knockout mice have increased hepatic cholesterol and decreased adrenal cholesterol. It seems that the intrastrain relationship between adrenal and hepatic cholesterol, although directionally opposite in wild-type and knockout mice, results in the same plasma concentration in each strain. The contribution of additional tissues (such as the intestines) to the maintenance of normal plasma cholesterol in this environment of adrenal and hepatic changes is also unexplored.
In the adrenal gland, the most direct relationship between CHGA and cholesterol is the regulated secretory granule. CHGA plays a major role in the biogenesis of neuroendocrine regulated secretory granules (12, 27); cholesterol plays a crucial role in regulated secretory granule membrane formation (11). The biosynthesis and concentration of cholesterol in the adrenal gland might have been reduced in response to the diminished adrenal regulated secretory granule number, size, and density caused by ablation (i.e., knockout) of the Chga gene (13). The rather large 1.8-fold decrease in adrenal cholesterol level might also impact the synthesis and secretion of cholesterol-derived steroid hormones in the adrenal cortex.
The liver does not express CHGA, so the elevation of hepatic cholesterol biosynthesis and concentration is most likely a downstream effect cascading from Chga gene ablation in neuroendocrine (i.e., Chga expressing) cells and tissues. Since the liver is the primary site of de novo cholesterol biosynthesis and an important regulator of whole body intermediary metabolism and glucose homeostasis, changes in hepatic cholesterol have potential for widespread effects, yet plasma cholesterol concentration is unchanged. Nonetheless, the hepatic cholesterol changes may result from or contribute to enhanced insulin sensitivity of the Chga knockout mouse.
Transcriptional regulatory networks.
PAINT analysis of the adrenal insulin signaling pathway, adrenal and hepatic functional clusters, and adrenal and hepatic cholesterol biosynthesis pathways identified transcriptional regulatory networks containing sets of transcription factors computationally predicted to control the expression of the differentially expressed genes within each pathway/functional cluster. Changes in mRNA expression and/or posttranslational modification of some or all of the transcription factors predicted within the networks could contribute to the differential expression of genes within each pathway/functional cluster. The microarray technology we utilized is capable of detecting differences in abundance of transcription factor mRNA; however, simple quantification of prevailing transcript levels cannot detect changes in posttranslational modification.
The adrenal insulin signaling pathway transcriptional regulatory network contained 15 transcription factors predicted to control the expression of the 29 differentially expressed genes in the pathway (Table 4). We detected a difference in mRNA abundance for 5 of the 15 transcription factors in the network (Egr1, +3.89-fold; Myc −1.39-fold; Nfia −1.54-fold; Nfic, +1.24-fold; Tcf3 +1.76-fold), and the differential expression of these 5 transcription factors could provide a mechanistic link between Chga gene knockout and altered insulin sensitivity. Since the target receptor and mechanism of action of the dysglycemic pancreastatin fragment derived from CHGA remain largely unknown, further investigation of Egr1, Myc, Nfia, Nfic, and Tcf3 could provide novel insights into pancreastatin signaling.
The adrenal lipid/sterol/steroid functional cluster (Table 2) transcriptional regulatory network contained 17 transcription factors, while the hepatic lipid/sterol/steroid functional cluster (Table 3) transcriptional regulatory network contained 29 transcription factors (Table 5). The disparity in the number of transcription factors between the adrenal gland and the liver highlights the quite different responses of lipid metabolism in each tissue to CHGA depletion. In fact, just seven transcription factors showed the same direction of response (i.e., no change, overexpression, underexpression) in both the adrenal gland and the liver.
The cholesterol biosynthesis pathway (Figs. 2 and 3) transcriptional regulatory network contained 26 transcription factors (Table 6). We observed a difference in the direction of mRNA differential expression (i.e., no change, overexpression, or underexpression) between the adrenal gland and the liver for 10 of the 26 transcription factors: Atf2, Jun, Cebpd, Dbp, E2f1, E2f5, Hivep2, Nfya, Nfyb, and Pax6. The tissue-specific changes in direction of differential expression for these 10 transcription factors could contribute to the dramatic differences in gene expression patterns in the cholesterol biosynthesis pathway between the adrenal gland (global underexpression) and liver (global overexpression) and the differences in cholesterol level in the adrenal gland (decreased 1.8-fold) and liver (increased 1.8-fold). These 10 transcription factors provide avenues for additional investigation into the poorly understood link between CHGA, adrenal and hepatic cholesterol, and insulin sensitivity.
Conclusions and perspectives.
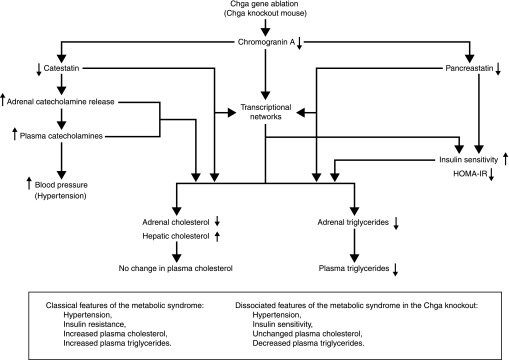
Genome-wide transcriptome profiling in the Chga knockout mouse uncovered patterns of adrenal and hepatic gene expression suggesting changes in tissue-specific and whole body biochemical and physiological systems. Prediction of increased organismal insulin sensitivity was confirmed with glucose, insulin, and HOMA-IR index measurements, while tissue-specific changes in cholesterol biosynthesis were verified with tissue cholesterol assays. These microarray studies demonstrate the utility of transcriptome profiling in predicting changes in biochemical and physiological systems, which can then be verified experimentally. The effect of CHGA and its pancreastatin peptide fragment on insulin sensitivity has previously been documented; however, our experiments demonstrate, for the first time, that genetic variation in the Chga locus directly impacts insulin sensitivity in an intact, living organism. Striking and directionally opposite differences in gene expression patterns in adrenal and hepatic cholesterol biosynthesis were identified and highlight a novel and poorly understood relationship between CHGA, cholesterol, and insulin sensitivity. Transcriptional regulatory network prediction identified sets of transcription factors that may provide insight into the yet unexplored mechanistic links between CHGA, cholesterol, and insulin sensitivity and yield additional avenues for investigation. Finally, the Chga knockout mouse may provide a valuable model for studying the relationships among disease phenotypes of the metabolic syndrome (Fig. 8); indeed, the dissociation between severe hypertension and enhanced insulin sensitivity in this model may yield unique insights into factors governing the coupling of such traits in humans.
Fig. 8.
Model of global metabolic changes in the Chga knockout mouse. An integrated model to explain how ablation of the Chga gene in the mouse leads to global metabolic changes and dissociation of metabolic syndrome phenotypes is presented. The loss of Chga protein also causes a loss of its bioactive peptides—catestatin and pancreastatin are shown here. Catestatin and pancreastatin are likely to have both genomic (through the PAINT-identified transcriptional networks) and nongenomic effects on metabolism. The effects of lack of catestatin on adrenal catecholamine release, plasma catecholamine levels, and blood pressure were previously demonstrated by Mahapatra et al. (13).
GRANTS
These studies were carried out in part in the General Clinical Research Center, University of California, San Diego, with funding provided by the National Center for Research Resources (M01-RR-000827), the Comprehensive Research Center of Excellence in Minority Health and Health Disparities (MD00020), the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases DK-007671 Nephrology Training Grant, the Department of Veterans Affairs (S. K. Mahata, D. T. O'Connor), and the NIH (R01-DA-011311 to S. K. Mahata; DK-60702 to D. T. O'Connor; P01-HL-58120 to S. K. Mahata and D. T. O'Connor).
DISCLOSURES
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
We thank Joseph Juliano for his help with the cholesterol and triglyceride assays.
REFERENCES
- 1.Cadman PE, Rao F, Mahata SK, O'Connor DT. Studies of the dysglycemic peptide, pancreastatin, using a human forearm model. Ann NY Acad Sci 971: 528–529, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Rao F, Rodriguez-Flores JL, Mahapatra NR, Mahata M, Wen G, Salem RM, Shih PA, Das M, Schork NJ, Ziegler MG, Hamilton BA, Mahata SK, O'Connor DT. Common genetic variants in the chromogranin A promoter alter autonomic activity and blood pressure. Kidney Int 74: 115–125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Rao F, Rodriguez-Flores JL, Mahata M, Fung MM, Stridsberg M, Vaingankar SM, Wen G, Salem RM, Das M, Cockburn MG, Schork NJ, Ziegler MG, Hamilton BA, Mahata SK, Taupenot L, O'Connor DT. Naturally occurring human genetic variation in the 3'-untranslated region of the secretory protein chromogranin A is associated with autonomic blood pressure regulation and hypertension in a sex-dependent fashion. J Am Coll Cardiol 52: 1468–1481, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4: P3, 2003 [PubMed] [Google Scholar]
- 5.Friese RS, Mahboubi P, Mahapatra NR, Mahata SK, Schork NJ, Schmid-Schoenbein GW, O'Connor DT. Neuroendocrine transcriptome in genetic hypertension: multiple changes in diverse adrenal physiological systems. Hypertension 43: 1301–1311, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Friese RS, Mahboubi P, Mahapatra NR, Mahata SK, Schork NJ, Schmid-Schoenbein GW, O'Connor DT. Common genetic mechanisms of blood pressure elevation in two independent rodent models of human essential hypertension. Am J Hypertens 18: 633–652, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Yanes C, Sanchez-Margalet V. Pancreastatin modulates insulin signaling in rat adipocytes: mechanisms of cross-talk. Diabetes 49: 1288–1294, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Groop L, Orho-Melander M. The dysmetabolic syndrome. J Intern Med 250: 105–120, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Hsiao A, Ideker T, Olefsky JM, Subramaniam S. VAMPIRE microarray suite: a web-based platform for the interpretation of gene expression data. Nucleic Acids Res 33: W627–W632, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Kim T, Gondre-Lewis MC, Arnaoutova I, Loh YP. Dense-core secretory granule biogenesis. Physiology 21: 124–133, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell 106: 499–509, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Mahapatra NR, O'Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest 115: 1942–1952, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahata SK, Mahata M, Wen G, Wong WB, Mahapatra NR, Hamilton BA, O'Connor DT. The catecholamine release-inhibitory “catestatin” fragment of chromogranin A: naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses. Mol Pharmacol 66: 1180–1191, 2004 [DOI] [PubMed] [Google Scholar]
- 15.McBride P. Triglycerides and risk for coronary artery disease. Curr Atheroscler Rep 10: 386–390, 2008 [DOI] [PubMed] [Google Scholar]
- 16.O'Connor DT, Cadman PE, Smiley C, Salem RM, Rao F, Smith J, Funk SD, Mahata SK, Mahata M, Wen G, Taupenot L, Gonzalez-Yanes C, Harper KL, Henry RR, Sanchez-Margalet V. Pancreastatin: multiple actions on human intermediary metabolism in vivo, variation in disease, and naturally occurring functional genetic polymorphism. J Clin Endocrinol Metab 90: 5414–5425, 2005 [DOI] [PubMed] [Google Scholar]
- 17.O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens 20: 1335–1345, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Rao F, Wen G, Gayen JR, Das M, Vaingankar SM, Rana BK, Mahata M, Kennedy BP, Salem RM, Stridsberg M, Abel K, Smith DW, Eskin E, Schork NJ, Hamilton BA, Ziegler MG, Mahata SK, O'Connor DT. Catecholamine release-inhibitory peptide catestatin (chromogranin A352-372): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation 115: 2271–2281, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Salem RM, Cadman PE, Chen Y, Rao F, Wen G, Hamilton BA, Rana BK, Smith DW, Stridsberg M, Ward HJ, Mahata M, Mahata SK, Bowden DW, Hicks PJ, Freedman BI, Schork NJ, O'Connor DT. Chromogranin A polymorphisms are associated with hypertensive renal disease. J Am Soc Nephrol 19: 600–614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salomonis N, Hanspers K, Zambon AC, Vranizan K, Lawlor SC, Dahlquist KD, Doniger SW, Stuart J, Conklin BR, Pico AR. GenMAPP 2: new features and resources for pathway analysis. BMC Bioinformatics 8: 217, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Margalet V, Calvo JR, Goberna R. Glucogenolytic and hyperglycemic effect of 33–49 C-terminal fragment of pancreastatin in the rat in vivo. Horm Metab Res 24: 455–457, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Margalet V, Calvo JR, Lucas M, Goberna R. Pancreastatin and its 33–49 C-terminal fragment inhibit glucagon-stimulated insulin in vivo. Gen Pharmacol 23: 637–638, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Margalet V, Gonzalez-Yanes C, Santos-Alvarez J, Najib S. Pancreastatin. Biological effects and mechanisms of action. Adv Exp Med Biol 482: 247–262, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Margalet V, Lucas M, Goberna R. Pancreastatin: further evidence for its consideration as a regulatory peptide. J Mol Endocrinol 16: 1–8, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Margalet V, Valle M, Lobon JA, Maldonado A, Escobar-Jimenez F, Olivan J, Perez-Cano R, Goberna R. Increased plasma pancreastatin-like immunoreactivity levels in non-obese patients with essential hypertension. J Hypertens 13: 251–258, 1995 [PubMed] [Google Scholar]
- 26.Takiyyuddin MA, Parmer RJ, Kailasam MT, Cervenka JH, Kennedy B, Ziegler MG, Lin MC, Li J, Grim CE, Wright FA, O'Connor DT. Chromogranin A in human hypertension. Influence of heredity. Hypertension 26: 213–220, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med 348: 1134–1149, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Vadigepalli R, Chakravarthula P, Zak DE, Schwaber JS, Gonye GE. PAINT: a promoter analysis and interaction network generation tool for gene regulatory network identification. OMICS 7: 235–252, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama H, Emoto M, Fujiwara S, Motoyama K, Morioka T, Komatsu M, Tahara H, Shoji T, Okuno Y, Nishizawa Y. Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment in normal range weight and moderately obese type 2 diabetic patients. Diabetes Care 26: 2426–2432, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Rao F, Wen G, Salem RM, Vaingankar S, Mahata M, Mahapatra NR, Lillie EO, Cadman PE, Friese RS, Hamilton BA, Hook VY, Mahata SK, Taupenot L, O'Connor DT. Catecholamine storage vesicles and the metabolic syndrome: the role of the chromogranin A fragment pancreastatin. Diabetes Obes Metab 8: 621–633, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]