Abstract
Remote populations are predicted to be vulnerable owing to their isolation from potential source reefs, and usually low population size and associated increased extinction risk. We investigated genetic diversity, population subdivision and connectivity in the brooding reef coral Seriatopora hystrix at the limits of its Eastern Australian (EA) distribution and three sites in the southern Great Barrier Reef (GBR). Over the approximately 1270 km survey range, high levels of population subdivision were detected (global FST = 0.224), with the greatest range in pairwise FST values observed among the three southernmost locations: Lord Howe Island, Elizabeth Reef and Middleton Reef. Flinders Reef, located between the GBR and the more southerly offshore reefs, was highly isolated and showed the signature of a recent bottleneck. High pairwise FST values and the presence of multiple genetic clusters indicate that EA subtropical coral populations have been historically isolated from each other and the GBR. One putative first-generation migrant was detected from the GBR into the EA subtropics. Occasional long-distance dispersal is supported by changes in species composition at these high-latitude reefs and the occurrence of new species records over the past three decades. While subtropical populations exhibited significantly lower allelic richness than their GBR counterparts, genetic diversity was still moderately high. Furthermore, subtropical populations were not inbred and had a considerable number of private alleles. The results suggest that these high-latitude S. hystrix populations are supplemented by infrequent long-distance migrants from the GBR and may have adequate population sizes to maintain viability and resist severe losses of genetic diversity.
Keywords: Seriatopora hystrix, subtropical, connectivity, microsatellites, Eastern Australia
1. Introduction
Coral reefs are among the most vulnerable habitats worldwide and are increasingly threatened both by chronic and acute anthropogenic disturbances (e.g. Bellwood et al. 2004; Hoegh-Guldberg et al. 2007). Dramatic deterioration of reef health has occurred over the past few decades and is predicted to continue due to rapidly increasing sea surface temperatures (SSTs) and ocean acidification, resulting in increased bleaching frequency and severity, and reduced calcification and reef accretion (e.g. Hoegh-Guldberg et al. 2007; Silverman et al. 2009). High-latitude reefs are of particular concern as they are already considered to be a marginal reefal environment (Veron 1995; Kleypas et al. 1999a; Guinotte et al. 2003). Geographical isolation (Kleypas et al. 1999a; Hughes et al. 2003), as well as their location in areas of rapidly declining aragonite saturation (e.g. Kleypas et al. 1999b; Guinotte et al. 2003) may further contribute to their vulnerability. The capacity of corals at subtropical reefs to survive disturbances, such as mass bleaching, disease, crown-of-thorns starfish (COTS) outbreaks or chronic stressors such as rapidly changing SSTs and ocean chemistry, will depend partly on their population genetic structure, as remote populations are predicted to have small effective population sizes, low genetic diversity and to suffer from inbreeding. Low genetic diversity reflects limited adaptive potential (Gates & Edmunds 1999; Frankham 2005), and inbreeding can significantly reduce fitness and increase the risk of extinction (Crnokrak & Roff 1999; Bijlsma et al. 2000).
Over an evolutionary timeframe, successful long-distance dispersal, settlement and reproduction may increase genetic diversity where the dispersed species has an existing population or, if the species is not present, constitute a founder event. Coral reefs have evolved under various intensities, modes and frequencies of disturbances, and reef coral populations have experienced severe reduction (Wakeford et al. 2008) to complete eradication throughout their evolutionary history (e.g. Veron 1995). Recovery of disturbed reefs depends on the density of survivors, their fecundity and the magnitude of recruitment of individuals from external sources (e.g. Harrison & Wallace 1990; Harrison & Booth 2007). Routine dispersal over ecological time-scales (i.e. ecological gene flow) is necessary for replenishment of severely disturbed reefs (e.g. Jones et al. 2009). If a coral species were to be completely extirpated at the isolated subtropical reefs of Lord Howe Island, Elizabeth Reef and Middleton Reef, recovery would require rafting or long-distance larval dispersal across more than 630 km of open ocean. The rapid, warm-water Eastern Australian Current (EAC) provides a vehicle for unidirectional dispersal from the Great Barrier Reef (GBR) to the subtropics, and large gyres extending several hundreds of kilometres offshore (e.g. Ridgway & Dunn 2003) could disperse larvae between subtropical inshore and offshore reefs (Wilson & Harrison 1997). Despite the potential for dispersal, previous research has demonstrated low but variable realized gene flow between coral populations from the GBR to the subtropics (Ayre & Hughes 2004) and within the subtropics (Miller & Ayre 2008). Further, no genetic variation was found in the reef coral Seriatopora hystrix population at Lord Howe Island population for five of the six allozyme loci tested, leading to the hypothesis that a founder event of one or very few colonists is responsible for the presence of this species at Lord Howe Island (Ayre & Hughes 2004). The high subdivision and low genetic diversity at subtropical sites has been interpreted as historical isolation and has led to predictions that localized extinctions will have ‘persistent impacts over very long periods’ (Ayre & Hughes 2004), and that ‘the likelihood that the GBR will act as a source of recruits to [high-latitude reefs] following catastrophic events is negligible and only likely to occur over long timescales’ (Miller & Ayre 2008).
The focus of this study is the brooding hermaphroditic reef coral S. hystrix (Pocilloporidae). Seriatopora hystrix has primarily sexually produced larvae (Ayre & Resing 1986; Sherman 2008) that are matured within the polyps and periodically released (Harrison & Wallace 1990). Most brooding corals, including S. hystrix, have larvae that are competent to settle within a few hours after release (Isomura & Nishihira 2001; Harii et al. 2002). This promotes settlement on, or close to, the natal reef (e.g. Tioho et al. 2001). Recent work on S. hystrix populations using highly variable microsatellite markers showed dispersal mostly occurred over small spatial scales (less than 100 m), although this was supplemented by less frequent longer-distance dispersal (Underwood et al. 2007). Localized dispersal generally leads to high population differentiation, and earlier research on GBR S. hystrix populations (using allozymes) confirms this (FST = 0.43, Ayre & Dufty 1994; FST = 0.15, Ayre & Hughes 2000). Despite the usually rapid settlement of larvae, some brooding corals produce a small proportion of larvae that have extended competency periods of over 100 days in the water column (e.g. Harii et al. 2002), which can translate to a dispersal potential of hundreds of kilometres (Harrison & Wallace 1990; Harrison & Booth 2007). Genetic evidence of recent longer distance dispersal events in S. hystrix (on the order of tens to hundreds of kilometres) exists from the Red Sea, Western Australia and Eastern Australia (EA) (Maier et al. 2005; Underwood et al. 2007; Van Oppen et al. 2008).
Currently, all subtropical EA reefs are Marine Parks or Nature Reserves, but, apart from Elizabeth and Middleton Reefs, are managed individually by three different states or Commonwealth departments. Whether these reefs should be managed as individual units or a network is largely dependent on the spatial scale of dispersal of focus species (Jones et al. 2009). As the integral habitat-forming component of reefs, scleractinian corals are a key target taxon for genetic studies of dispersal (Jones et al. 2009). This is, to our knowledge, the first population genetics study to include a coral species from the Middleton and Elizabeth Reefs: these subtropical atolls have the highest number of coral species (approx. 155) and the largest reef area (approx. 8000 total hectares) south of the GBR (Hutchings et al. 1992; Veron 1993; Oxley et al. 2004; A. Noreen 2007, unpublished data). This study aims to increase our understanding of the risk of the loss of genetic diversity, inbreeding and extinction of S. hystrix at the southern limits of its distribution range, as well as to estimate the temporal scale of the frequency and magnitude of long-distance dispersal from the GBR.
2. Material and methods
Ten DNA microsatellite loci were used to assess genetic diversity and connectivity in S. hystrix from four major EA subtropical reefs and three reefs in the southern GBR (figure 1). The reefs sampled in this study were subtropical Lord Howe Island, Elizabeth Reef, Middleton Reef, Flinders Reef and Broomfield, Snake and Turner Reefs from the GBR. Lord Howe Island is the southernmost true coral reef in the world (Harriott et al. 1995). The other true coral reefs of the EA subtropics, Elizabeth and Middleton Reefs, are located 180 and 235 km north of Lord Howe Island, respectively (figure 1).
Figure 1.
Map of EA showing the sampling locations of the three GBR reefs and four subtropical reefs sampled. Map details of subtropical reefs show sampling sites as points, or lines when the sampling was continuous in a given area.
Two habitats were sampled at each of these reefs: Lord Howe Island—lagoon and east coast; Elizabeth Reef—lagoon and channel; Middleton Reef—Blue Hole (=lagoon) and channel. These reefs have a unique mix of temperate, subtropical and tropical biota and represent the highest latitude species records for many tropical taxa (Hutchings et al. 1992; Veron 1993; Harriott et al. 1995; Oxley et al. 2004; P. Harrison & A. Noreen 2007, unpublished data). A large proportion of coral species sampled at these locations were sexually reproductive (Hutchings et al. 1992; Harrison 2008). These reefs lie in the path of the warm, south and east flowing EAC, which not only acts as a conduit for larval transport from further north, but provides the climate needed for these warm-water reefs to exist (Veron & Done 1979; Hutchings et al. 1992; Harriott et al. 1995; Harrison & Booth 2007).
Flinders Reef, near the south Queensland coast, supports a diverse coral community of at least 119 coral species growing on and around a small sandstone platform (Veron 1993; Harrison et al. 1998). A single site was sampled at Flinders Reef. Of all subtropical sites where S. hystrix are recorded, only one was not sampled. This was the Gneering Shoals, a low-diversity coral community growing on rocky substrate approximately 50 km north of Flinders Reef, which is known to have only sporadic occurrences of S. hystrix (Banks & Harriott 1995). An outer reef site was sampled at each of three reefs on the GBR (figure 1).
Branches of S. hystrix approximately 4 cm in length were collected by hand from individual colonies using SCUBA or snorkel and placed immediately in 95 per cent ethanol (see table 1 for sample sizes). DNA was extracted following Wilson et al. (2002). Ten loci were amplified using fluorescently labelled primers in three multiplex PCRs as described in Underwood et al. (2006). Samples with rare (frequency < 0.05) private alleles were PCR amplified and genotyped at least twice to confirm the allele's authenticity. In this study, rare alleles shared between two subtropical sites are defined as ‘semi-private’ alleles.
Table 1.
Sample size (N), proportion of unique genotypes (Ng) to total samples (N) and number and identity of fixed loci in each of the sampled sites.
| site | N | Ng/N | no. fixed loci | locus fixed |
|---|---|---|---|---|
| Lord Howe Island lagoon | 42 | 0.88 | 0 | |
| Lord Howe Island east | 22 | 0.91 | 1 | Sh2_002 |
| Elizabeth Reef lagoon | 55 | 0.98 | 1 | Sh3_003 |
| Elizabeth Reef channel | 35 | 1 | 1 | Sh4_001 |
| Middleton Reef lagoon | 17 | 1 | 1 | Sh3_003 |
| Middleton Reef channel | 37 | 0.97 | 1 | Sh3_003 |
| Flinders Reef | 33 | 0.97 | 2 | Sh3_003 Sh3_007 |
| Broomfield Reef | 27 | 1 | 0 | |
| Snake Reef | 37 | 1 | 0 | |
| Turner Reef | 29 | 1 | 0 |
(a). Data analysis
Gimlet v. 1.3.3 (Valiere 2002) was used to assess the presence of clone mates; in the case that the same multilocus genotype was found more than once, all but one of the individuals were removed from the dataset prior to analysis. Tests for Hardy–Weinberg equilibrium and linkage disequilibrium (LD) were conducted in the web-based package GenePop v. 4.0 (Raymond & Rousset 1995) under the following Markov chain parameters: 1000 dememorization, 100 batches, 10 000 iterations per batch. Fstat v. 2.9.3 (Goudet 2001) was used to generate allele frequencies, allelic richness (calculated per locus) and FST values (Weir & Cockerham 1984, Infinite Allele Model). Statistical significance of pairwise FST values was based on 45 000 permutations and p-values were corrected for multiple comparisons using strict Bonferroni. Bottleneck v. 1.2.02 (Cornuet & Luikart 1996) was used to determine whether any recent population bottlenecks were likely to have occurred. This program uses coalescent theory to provide different models for heterozygosity compared with allele richness. Allele numbers decrease faster than heterozygosity during bottlenecks, and a rapid decrease in allele numbers while maintaining heterozygosity creates a distinct signature. However, this pattern deteriorates after two to four generations, which means that Bottleneck will only detect recent events. A change in the allele frequency distribution (‘mode shift’) indicates a recent bottleneck. Bottleneck was run under the two-phased mutation model default settings with 10 000 replications. Structure v. 2.2 (Pritchard et al. 2000), a model-based clustering method, was used to find the most likely number of genetic clusters (K) and to represent these visually. Parameters used were: admixture model and independent allele frequency model with no prior information. Burn-in was 100 000 with 1 000 000 iterations for K = 1 to 12. For each K, the run was replicated five times, and the most likely value of K was determined following Evanno et al. (2005). GeneClass v. 2.0 (Piry et al. 2004) was used to detect first-generation migrants under the following conditions: likelihood ratio L_home/L_max; criterion: Rannala & Mountain (1997), simulation algorithm: Paetkau et al. (2004); 10 000 simulated individuals with a threshold p ≤ 0.01. This generated a probability of each individual being a putative migrant. These putative migrants were then removed from the original populations and reassigned to the sampled populations using the above parameters. This produced a probability for each putative migrant of originating from each of the other sampled populations.
3. Results
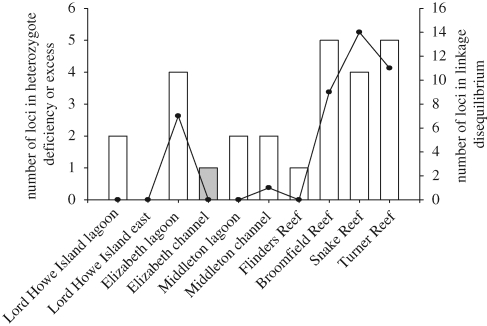
The Lord Howe Island lagoon site had the highest number of potential clone mates of any site (table 1), where three samples of one multilocus genotype and two samples of another multilocus genotype were present among the 42 colonies collected. However, the majority of samples at all sites were derived via sexual reproduction. All but one subtropical population sampled showed a significant heterozygote deficit for zero to two loci only (figure 2), indicating that null alleles, further population sub-structuring (Wahlund 1928), or inbreeding (Wright 1922) are unlikely to apply to these populations. The Elizabeth Reef lagoon site showed significant heterozygote deficits in a larger number of loci (figure 2). The single significant heterozygote excess (in the Elizabeth Reef channel population) is unlikely to have biological significance. LD and significant heterozygote deficiencies were high in the GBR populations. The pairwise FST values between the two eastern Lord Howe Island sites were not significantly different from zero (p ≥ 0.05), and these sampling sites were therefore pooled for further analysis.
Figure 2.
Number of loci with significant (p ≤ 0.01) heterozygote deficiencies (empty bars) or excess (grey bars) and number of locus pairs showing significant (p ≤ 0.01) LD (solid line).
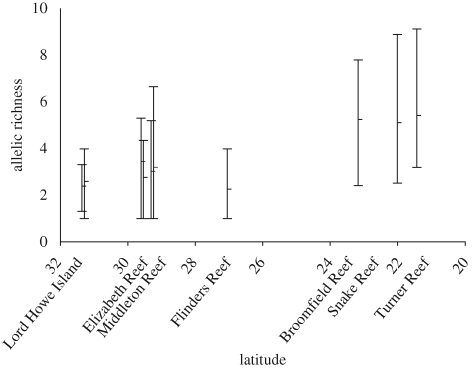
Allelic richness was significantly lower in the subtropics compared with the southern GBR (t-test, p = 0.0009) (figure 3). Despite spanning over 700 km in latitude, there was no significant difference in allelic richness between sites within the subtropics (ANOVA, p = 0.42). All subtropical sites except one (Lord Howe Island lagoon) had a locus fixed for a single allele and Flinders Reef had two (table 1). Most subtropical sites or reefs had private or semi-private alleles (table 2).
Figure 3.
Allelic richness. Bars represent the range of allelic richness for 10 loci. Subtropical reefs with two sites have been separated by 0°4′ along the x-axis for visual clarity.
Table 2.
Alleles private to one or two subtropical sites in EA.
| locus | size (bp) | location(s) | frequency |
|---|---|---|---|
| Sh2_002 | 123 | Elizabeth Reef lagoon | 0.011 |
| Elizabeth Reef channel | 0.048 | ||
| 158 | Middleton Reef lagoon | 0.031 | |
| Sh3_003 | 86 | Lord Howe Island lagoon | 0.021 |
| Lord Howe Island east | 0.024 | ||
| Sh2_005 | 129 | Middleton Reef channel | 0.027 |
| Sh3_007 | 101 | Elizabeth Reef lagoon | 0.066 |
| 113 | Elizabeth Reef lagoon | 0.009 | |
| Sh2_008 | 213 | Elizabeth Reef lagoon | 0.010 |
| Middleton Reef channel | 0.014 | ||
| Sh3_009 | 166 | Lord Howe Island lagoon | 0.128 |
| Lord Howe Island east | 0.105 | ||
| Sh4_010 | 224 | Elizabeth Reef lagoon | 0.009 |
Pairwise FST values were significantly different from zero for all comparisons except one (Broomfield Reef × Turner Reef) (table 3). Subtropical × subtropical reefs showed a larger range of pairwise FST values (FST = 0.034–0.475) than GBR × subtropical reefs (FST = 0.162–0.358), despite a similar number of pairwise comparisons (21 versus 22). The highest pairwise FST values observed were between Lord Howe Island east and lagoon × Middleton Reef lagoon (FST = 0.475 and 0.462, respectively). The global FST value was 0.224.
Table 3.
Summary of pairwise FST values (Weir & Cockerham 1984). Permutations were run 45 000 times with strict Bonferroni corrections. After this, 98% (44) of the comparisons remained significant with 89% (40) significant at p ≤ 0.001. LHI, Lord Howe Island; Eliz., Elizabeth Reef; Midd., Middleton Reef; Broom, Broomfield Reef.
| LHI lagoon | LHI east | Eliz. lagoon | Eliz. channel | Midd. lagoon | Midd. channel | Flinders Rf. | Broom. Rf. | Snake Rf. | |
|---|---|---|---|---|---|---|---|---|---|
| 0.034* | LHI east | ||||||||
| 0.233*** | 0.202*** | Eliz. lag | |||||||
| 0.130*** | 0.147*** | 0.146*** | Eliz. chan | ||||||
| 0.475*** | 0.462*** | 0.105*** | 0.391*** | Midd. lag | |||||
| 0.233*** | 0.251*** | 0.113*** | 0.190*** | 0.285*** | Midd. chan | ||||
| 0.241*** | 0.265*** | 0.229*** | 0.205*** | 0.384*** | 0.293*** | Flinders Rf. | |||
| 0.328*** | 0.294*** | 0.170*** | 0.268*** | 0.250*** | 0.232*** | 0.252*** | Broom. Rf. | ||
| 0.320*** | 0.285*** | 0.162*** | 0.252*** | 0.224*** | 0.204*** | 0.240*** | 0.040*** | Snake Rf. | |
| 0.358*** | 0.339** | 0.174*** | 0.308*** | 0.196** | 0.212*** | 0.279*** | 0.068ns | 0.095** | Turner Rf. |
*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
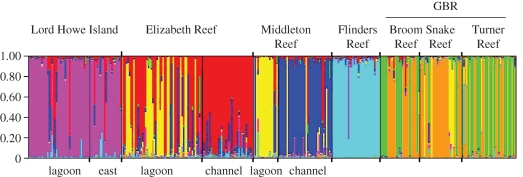
The most likely number of genetic clusters (K) as determined in Structure was seven. At all of the subtropical sites, except for the Elizabeth Reef lagoon, most individuals sampled at that site belonged to a single genetic cluster (figure 4). The Elizabeth Reef lagoon site showed a signature of admixture between two clusters: the Elizabeth Reef channel and the Middleton Reef lagoon. There were two main genetic clusters on the GBR, which were distributed among the three sampling locations, although a small number of GBR individuals also showed a genetic affinity with the Middleton and Elizabeth Reef lagoonal cluster.
Figure 4.
Structure bar plot for K = 7. Each vertical line represents the assignment probability of a single individual to one (or more) of the genetic clusters, without reference to where that individual was sampled.
The genetic assignment test detected 18 potential first-generation migrants. Eleven of those were detected in subtropical populations, and all but two of those were assigned to another subtropical population with high probability (more than 80%) (table S4 in the electronic supplementary material). One was assigned with similar probability to either another subtropical site or a GBR reef (table S4 in the electronic supplementary material). Flinders Reef did not have an immigrant or emigrant identified and is the only site sampled that showed evidence of the S. hystrix population having gone through a recent bottleneck event (table S3 in the electronic supplementary material).
4. Discussion
The long-term viability of populations depends at least partially on the genetic characteristics of the existing populations. Low recruitment into a population from outside sources may suffice to maintain genetic diversity (Tallmon et al. 2004), but is likely to be insufficient for replenishment and recovery if local populations are severely devastated (Jones et al. 2009). Stochasticity of recruitment as well as differences in life history, larval competency periods, settlement and survival requirements (Harrison & Wallace 1990; Harrison & Booth 2007) mean that different species will have different levels of connectivity to, and genetic diversity at, isolated locations (e.g. Miller & Ayre 2008; Underwood et al. 2009). Species with high population subdivision and limited dispersal are hypothesized to be more vulnerable to disturbances than populations with higher connectivity (e.g. Jones et al. 2009), despite some of these (such as S. hystrix) having a broad geographical range and large global population size. This study supports previous evidence that populations at high-latitude, isolated reefs have had historically low connectivity from each other and the GBR. Nevertheless, the data presented here show that occasional long-distance dispersal may aid in maintaining the genetic diversity of subtropical S. hystrix populations, and at least some subtropical reefs may support sufficiently large effective population sizes to enable them to resist severe losses of genetic diversity.
(a). Genetic diversity at subtropical reefs
Allelic richness was significantly lower at subtropical reefs than on the GBR (t-test, p = 0.0009), but not significantly different between subtropical sites (ANOVA, p = 0.42). There was no gradual decrease in allelic richness with latitude, which contrasts with onshore subtropical populations of the related coral Pocillopora damicornis (Miller & Ayre 2008). Allelic richness of S. hystrix at several GBR Reefs (using the same 10 loci) was similar to that recorded in this study (Van Oppen et al. 2008), demonstrating that the subtropical genetic diversity was not substantially eroded compared to some GBR populations.
The lagoonal samples of Lord Howe Island represented the sole subtropical site in this study to retain polymorphism at all loci (table 1). Lord Howe Island S. hystrix had an average allelic richness per locus of 2.50, and a maximum allelic richness of 4.47 (at locus Sh2_006). This contrasts with previous research showing that S. hystrix was fixed for five of the six allozyme loci that were polymorphic on the GBR (Ayre & Hughes 2004). Our data therefore do not support the hypothesis that the S. hystrix population at Lord Howe Island is derived from one or very few colonists (cf. Ayre & Hughes 2004).
In this study, the GBR and most subtropical sites or reefs were found to have private or semi-private alleles (table 2 and table S2 in the electronic supplementary material). The high number of private alleles on the GBR may be an artefact of the small number of GBR reefs included in this study. Collectively, the subtropical sites have nine private alleles at seven loci (table 2). Private subtropical alleles were almost always rare (less than 0.05). However, an exception was an allele at Lord Howe Island, which was present in both lagoonal and eastern populations at a frequency above 0.10. Elizabeth and Middleton Reefs had seven private alleles, which were generally site-specific, although one semi-private allele was present at both Elizabeth Reef sites, and another was shared between Elizabeth and Middleton Reef. These private alleles may have entered the population through gene flow from unsampled populations, or from mutations having occurred locally. Irrespective of the origin of the private alleles, they demonstrate that these subtropical sites have sufficiently large effective population sizes to maintain low frequency alleles, rather than losing them through genetic drift. The presence of separate private alleles at sites located less than 5 km apart indicate significant historical isolation; however, the presence of semi-private alleles (shared between two subtropical sites) support low levels of gene flow between some populations.
Despite a severe COTS outbreak at Elizabeth and Middleton Reefs in the mid-1980s (J. E. N. Veron 2008, personal communication, Hutchings et al. 1992), there was no evidence of reduced genetic diversity compared with other subtropical sites or of S. hystrix populations having undergone a recent bottleneck. Flinders Reef was the only site sampled that showed a mode shift in Bottleneck, implying that its high genetic differentiation and two fixed loci may stem from a bottleneck event ca. two to four generations ago. This is supported by field evidence: the last survey of Flinders Reef was undertaken in 1993, and S. hystrix was only recorded at a single site and in low abundance (Harrison et al. 1998), whereas during the sample collection for this study (November 2007) hundreds of colonies of S. hystrix were sighted in a relatively small area. Thus, it is possible that S. hystrix at Flinders Reef has undergone a bottleneck, followed by a recent population size expansion.
(b). Genetic differentiation of subtropical reefs
Genetic signatures of both recent and historical admixture were more prominent on the GBR than in the subtropics. First, FST values between the GBR populations were significantly lower than those among subtropical populations, indicating higher historical gene flow. These populations also displayed higher heterozygote deficiencies and LD, suggestive of more recent genetic admixture. This is probably a result of their closer proximity, and the large number of other GBR reefs that could act as stepping stones between those sampled. Evidence for recent admixture was limited at subtropical locations, with one exception. The Elizabeth Reef lagoon population exhibited higher LD and heterozygote deficiencies than other subtropical reefs (figure 2), and a mix of mainly two genetic clusters in the Structure analysis (figure 4). Despite evidence of recent admixture at Elizabeth Reef lagoon site, all sites sampled at Elizabeth and Middleton Reefs were significantly differentiated, including sites at the same reef separated by only a few kilometres (FST > 0, p < 0.001, table 3). The Structure analysis indicates a deep division between the Middleton and Elizabeth lagoonal cluster and the other subtropical sites; at K = 2, one cluster comprised all subtropical sites except the Middleton lagoonal samples and a proportion of Elizabeth lagoonal samples, while the other cluster was the GBR, the Middleton lagoon and a proportion of Elizabeth lagoon samples. Recent work on S. hystrix in isolated low-latitude North Western Australian reefs has also found highly significant differentiation between sites within a reef (Underwood et al. 2009). This is indicative of predominantly highly localized dispersal, which is supported by spatial autocorrelation analysis in Underwood et al. (2007).
Despite significant genetic subdivision between offshore subtropical sites, nine potential first-generation migrants (less than 3%) were assigned with a high probability (more than 80%) to other offshore subtropical site(s) (table S4 in the electronic supplementary material). Flinders Reef was the most genetically isolated reef in this study: it had two fixed loci (table 1), formed a distinct genetic cluster in the Structure analysis (figure 4) and did not have an immigrant or emigrant detected (table S4 in the electronic supplementary material). Hence, it is not a ‘sink’ population from the GBR nor a ‘source’ to offshore subtropical reefs further south.
The patterns of genetic subdivision and connectivity in S. hystrix presented here show both differences and similarities to patterns of genetic structure in other taxa. The pocilloporid coral P. damicornis showed high levels of subdivision among seven sites at Lord Howe Island based on allozyme data (Miller & Ayre 2004), consistent with our observation of S. hystrix in this study. Lord Howe Island populations of other marine organisms are generally also highly isolated (Benzie 1992; Ayre & Hughes 2004; Miller & Ayre 2008). For example, COTS populations from Lord Howe Island were the most genetically differentiated of all EA populations sampled (Benzie 1992). However, in contrast to our findings for S. hystrix, two other taxa sampled at Elizabeth and Middleton Reefs exhibited no significant differentiation between sites (COTS, Benzie 1992; black cod, Appleyard & Ward 2007) nor to GBR populations (Benzie 1992).
(c). Larval dispersal to subtropical reefs
Dispersal from tropical to subtropical reefs along EA over an evolutionary timeframe is supported by the occurrence of approximately 155 coral species in 42 genera shared between the GBR and the high-latitute offshore EA reefs (Veron & Done 1979; Hutchings et al. 1992; Veron 1993; Harriott et al. 1995; Oxley et al. 2004, P. Harrison & A. Noreen 2007, unpublished data). Although approximately a dozen coral genera have been found rafting (e.g. Jokiel 1990; deVantier 1992), larval dispersal is assumed to be the most likely origin of the majority of the primarily tropical coral species recorded at subtropical locations. In this study, both the GeneClass and Structure results indicate that a colony of S. hystrix sampled at Elizabeth Reef may have originated on the GBR. While this immigrant could have originated from an unsampled subtropical population, a recent analysis of these data with a much larger dataset from GBR with the same 10 loci confirms our results. This analysis, which included the four subtropical reefs and 22 GBR reefs (an additional 19 central and southern reefs), also identified an Elizabeth Reef S. hystrix individual which had low probability of originating from any subtropical site and a high probability of originating from the GBR (M. J. H. Van Oppen 2009, personal communication). Independent evidence for recent long-distance dispersal events of corals from the GBR to the subtropics comes from the observation that coral species richness at Lord Howe Island and Elizabeth and Middleton Reefs has increased since the first survey of these locations in 1977 and 1981, respectively (Veron & Done 1979; Hutchings et al. 1992). New records include distinct species unlikely to have been overlooked during the original surveys, and whose nearest population is located on the GBR (e.g. Australogyra zelli Oxley et al. 2004). Subtropical reefs are limited by low winter water temperatures (Veron 1995), whereas increasing SSTs in this region (Lough 2008) may benefit subtropical reefs by providing a more conducive reef environment and provide opportunities for species range expansions (Buddemeier et al. 2004; Figueira & Booth in press). It is not inconceivable that migration from the GBR into the subtropics has increased over the past few decades as a consequence of an increase in temperature (Lough 2008) and the intensifying of the EAC (Poloczanska et al. 2007). The summertime recruitment of tropical GBR fish species at Lord Howe Island (Figueira & Booth in press) and more southerly locations on the New South Wales coast (Booth et al. 2007; Figueira & Booth in press) support our interpretation of dispersal over these large distances.
All subtropical reefs sampled in this study are Marine Parks and are managed by three jurisdictions. For S. hystrix, the geographical boundaries of the Marine Parks are larger than the routine dispersal range, suggesting that the present management arrangement is appropriate. This study corroborates previous findings; high levels of population subdivision between high-latitude sites and reefs. Routine gene flow over ecological time-scales is extremely localized for S. hystrix and is likely to be insufficient to allow immediate recovery of subtropical reefs through reseeding from the GBR following extensive localized mortality. However, contrary to some previous studies, we suggest infrequent successful long-distance dispersal supplements coral populations on these high-latitude reefs. The levels of gene flow estimated here are likely to contribute to the maintenance of genetic diversity of these coral populations. We augment these results with the hypothesis that populations of S. hystrix at Lord Howe Island, Middleton Reef and Elizabeth Reef maintain adequately large populations to prevent inbreeding and resist acute loss of genetic diversity.
Acknowledgements
This project was funded by an International Society of Reef Studies/The Ocean Conservation Fellowship to A.M.E.N. A.M.E.N. is supported by a Southern Cross University International Postgraduate Research Scholarship and Stipend. We thank Southern Cross University and the Australian Institute for Marine Science for institutional support, Paul Anderson (DEWHA) for providing the opportunity to do fieldwork at Elizabeth and Middleton Reefs, Simon Hartley (SCU) for diving assistance, Greg Luker (SCU) for producing the map, Lesa Peplow (AIMS) for laboratory support and the AIMS PopGen discussion group for comments on an early draft. The comments of two anonymous reviewers substantially improved this manuscript.
References
- Appleyard S., Ward R.2007Genetic connectedness between black cod (E. daemelii) collections along the NSW coast and the Elizabeth & Middleton Reefs Reserve. Report to the Department of Environment and Water Resources, Australia [Google Scholar]
- Ayre D. J., Dufty S.1994Evidence for restricted gene flow in the viviparous coral Seriatopora hystrix on Australia's Great Barrier Reef. Evolution 48, 1183–1201 (doi:10.2307/2410377) [DOI] [PubMed] [Google Scholar]
- Ayre D. J., Hughes T. P.2000Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution 54, 1590–1605 [DOI] [PubMed] [Google Scholar]
- Ayre D. J., Hughes T. P.2004Climate change, genotypic diversity and gene flow in reef-building corals. Ecol. Lett. 7, 273–278 (doi:10.1111/j.1461-0248.2004.00585.x) [Google Scholar]
- Ayre D. J., Resing J. M.1986Sexual and asexual reproduction of planulae in reef corals. Mar. Biol. 90, 187–190 (doi:10.1007/BF00569126) [Google Scholar]
- Banks S. A., Harriott V. J.1995Coral communities of the Gneering Shoals and Mudjimba Island, south-eastern Queensland. Mar. Freshwater Res. 46, 1137–1144 (doi:10.1071/MF9951137) [Google Scholar]
- Bellwood D. R., Hughes T. P., Folke C., Nystrom M.2004Confronting the coral reef crisis. Nature 429, 827–833 (doi:10.1038/nature02691) [DOI] [PubMed] [Google Scholar]
- Benzie J. A. H.1992Review of the genetics, dispersal and recruitment of crown-of-thorns starfish (Acanthaster planci). Aust. J. Mar. Freshwater Res. 43, 597–610 (doi:10.1071/MF9920597) [Google Scholar]
- Bijlsma R., Bundgaard J., Boerema C. A.2000Does inbreeding affect the extinction risk of small populations?: predictions from Drosophila. J. Exp. Biol. 13, 502–514 (doi:10.1046/j.1420-9101.2000.00177.x) [Google Scholar]
- Booth D., Figueira W., Gregson M., Brown L., Beretta G.2007Occurrence of tropical fishes in temperate southeastern Australia: role of the Eastern Australian Current. Estuarine Coastal Shelf Sci. 72, 102–114 (doi:10.1016/j.ecss.2006.10.003) [Google Scholar]
- Buddemeier R. W., Kleypas J. A., Aronson R. B.2004Coral reefs and global climate change: potential contributions of climate change to stresses on coral reef ecosystems Arlington, VA: The Pew Center on Global Climate Change [Google Scholar]
- Cornuet J. M., Luikart G.1996Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144, 2001–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crnokrak P., Roff D.1999Inbreeding depression in the wild. Heredity 83, 260–270 (doi:10.1038/sj.hdy.6885530) [DOI] [PubMed] [Google Scholar]
- DeVantier L. M.1992Rafting of tropical marine organisms on buoyant coralla. Mar. Ecol. Prog. Ser. 86, 301–302 (doi:10.3354/meps086301) [Google Scholar]
- Evanno G., Regaut S., Goudet J.2005Detecting the number of clusters of individuals using the software Structure: a simulation. Mol. Ecol. 14, 2611–2620 (doi:10.1111/j.1365-294X.2005.02553.x) [DOI] [PubMed] [Google Scholar]
- Figueira W. F., Booth D. J.In press Increasing ocean temperatures allow tropical fishes to survive over winter in temperate waters. Global Change Biol. (doi:10.1111/j.1365–2486.2009.01934.x) [Google Scholar]
- Frankham R.2005Genetics and extinction. Biol. Conserv. 126, 131–140 (doi:10.1016/j.biocon.2005.05.002) [Google Scholar]
- Gates R. D., Edmunds P. J.1999The physiological mechanisms of acclimatization in tropical reef corals. Am. Zool. 39, 30–41 [Google Scholar]
- Goudet J.2001Fstat, a program to estimate and test gene diversities and fixation indices, v. 2.9.3. Available from http://www.unil.ch/izea/softwares/fstat.html [Google Scholar]
- Guinotte J. M., Buddemeier R. W., Kleypas J. A.2003Future coral reef habitat marginality: temporal and spatial effects of climate change in the Pacific basin. Coral Reefs 22, 551–558 (doi:10.1007/s00338-003-0331-4) [Google Scholar]
- Harii S., Kayanne H., Takigawa H., Hayashibara T., Yamamoto M.2002Larval survivorship, competency periods and settlement of two brooding corals, Heliopora coerulaea and Pocillopora damicornis. Mar. Biol. 141, 39–46 (doi:10.1007/s00227-002-0812-y) [Google Scholar]
- Harriott V. J., Harrison P. L., Banks S. A.1995The coral communities of Lord Howe Island. Mar. Freshwater Res. 46, 457–465 (doi:10.1071/MF9950457) [Google Scholar]
- Harrison P. L.2008Coral spawn slicks at Lord Howe Island, Tasman Sea, Australia: the world's most southerly coral reef. Coral Reefs 27, 35 (doi:10.1007/s00338-007-0302-2) [Google Scholar]
- Harrison P. L., Booth D. J.2007Coral reefs: naturally dynamic and increasingly disturbed ecosystems. In Marine ecology (eds Connell S. D., Gillanders B. M.), pp. 316–377 Melbourne, Australia: Oxford University Press [Google Scholar]
- Harrison P. L., Wallace C. C.1990Reproduction, dispersal and recruitment of Scleractinian corals. In Coral reef ecosystems, ecosystems of the world, vol. 25 (ed. Dubinsky Z.), pp. 133–207 Amsterdam, The Netherlands: Elsevier Science Publishers [Google Scholar]
- Harrison P. L., Harriott V. J., Banks S. A., Holmes N. J.1998The coral communities of Flinders Reef and Myora Reef in the Moreton Bay Marine Park, Queensland, Australia. In Moreton Bay and catchment (ed. Tibbetts I. R.), pp. 525–536 Brisbane, Australia: School of Marine Science, The University of Queensland [Google Scholar]
- Hoegh-Guldberg O., et al. 2007Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (doi:10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- Hughes T. P., et al. 2003Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933 (doi:10.1126/science.1085046) [DOI] [PubMed] [Google Scholar]
- Hutchings P., et al. 1992Reef biology: a survey of Elizabeth and Middleton Reefs, South Pacific. Kowari 3 Canberra, Australia: Australian National Parks and Wildlife Service [Google Scholar]
- Isomura N., Nishihira M.2001Size variation of planulae and its effect on the lifetime of planulae in three pocilloporid corals. Coral Reefs 20, 309–315 (doi:10.1007/s003380100180) [Google Scholar]
- Jokiel P. L.1990Transport of reef corals into the Great Barrier Reef. Nature 347, 665–667 (doi:10.1038/347665a0) [Google Scholar]
- Jones G. P., Almany G. R., Russ G. R., Sale P. F., Steneck R. S., Van Oppen M. J. H., Willis B. L.2009Larval retention and connectivity among populations of coral and reef fishes: history, advances and challenges. Coral Reefs 28, 307–325 (doi:10.1007/s00338-009-0469-9) [Google Scholar]
- Kleypas J. A., McManus J. W., Menez L. A. B.1999aEnvironmental limits to coral reef development: where do we draw the line? Am. Zool. 39, 146–159 [Google Scholar]
- Kleypas J. A., Buddemeier R. W., Archer D., Gattuso J.-P., Langdon C., Opdyke B. N.1999bGeochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284, 118–120 (doi:10.1126/science.284.5411.118) [DOI] [PubMed] [Google Scholar]
- Lough J. M.2008Shifting climate zones for Australia's tropical marine ecosystems. Geophys. Res. Lett. 35, L14708 (doi:10.1029/2008GL034634) [Google Scholar]
- Maier E., Tollrian R., Baruch R., Nurnberger B.2005Isolation by distance in the scleractinian coral Seriatopora hystrix from the Red Sea. Mar. Biol. 147, 1109–1120 (doi:10.1007/s00227-005-0013-6) [Google Scholar]
- Miller K. J., Ayre D. J.2004The role of sexual and asexual reproduction in structuring high latitude populations of the reef coral Pocillopora damicornis. Heredity 92, 557–568 (doi:10.1038/sj.hdy.6800459) [DOI] [PubMed] [Google Scholar]
- Miller K. J., Ayre D. J.2008Protection of genetic diversity and maintenance of connectivity among reef corals within marine protected areas. Conserv. Biol. 22, 1245–1254 (doi:10.1111/j.1523-1739.2008.00985.x) [DOI] [PubMed] [Google Scholar]
- Oxley W. G., Ayling A. M., Cheal A. M., Osbourne K.2004Marine surveys undertaken in the Elizabeth and Middleton Reefs Marine National Nature Reserve, December 2003. Report to the Department of Environment and Heritage, Australia [Google Scholar]
- Paetkau D., Slade R., Burden M., Estoup A.2004Genetic assignment methods for the direct, real-time estimation of migration rate using assignment methods: a simulation-based exploration of accuracy and power. Mol. Ecol. 13, 55–65 (doi:10.1046/j.1365-294X.2004.02008.x) [DOI] [PubMed] [Google Scholar]
- Piry S., Alapetite A., Cornuet J. M., Paetkau D., Baudouin L., Estoup A.2004GeneClass2: a software for genetic assignment and first-generation migrant detection. J. Hered. 95, 536–539 (doi:10.1093/jhered/esh074) [DOI] [PubMed] [Google Scholar]
- Poloczanska E. S., et al. 2007Climate change and Australian marine life. Oceanogr. Mar. Biol. 45, 407–478 [Google Scholar]
- Pritchard J. K., Stephens M., Donnolley P.2000Inference of population structure using multilocus genotype data. Genetics 155, 945–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannala B., Mountain J. L.1997Detecting immigration by using multilocus genotypes. Proc. Natl Acad. Sci. USA 94, 9197–9221 (doi:10.1073/pnas.94.17.9197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M., Rousset F.1995GenePop (version 1.2): population genetics software for exact tests and ecumenicism. Heredity 86, 248–249 [Google Scholar]
- Ridgway K. R., Dunn J. R.2003Mesoscale structure of the mean Eastern Australian Current System and its relationship with topography. Prog. Oceanogr. 56, 189–222 (doi:10.1016/S0079-6611(03)00004-1) [Google Scholar]
- Sherman C. D. H.2008Mating system variation in the hermaphroditic brooding coral, Seriatopora hystrix. Heredity 100, 296–303 (doi:10.1038/sj.hdy.6801076) [DOI] [PubMed] [Google Scholar]
- Silverman J., Lazar B., Long C., Caldeira K., Erez J.2009Coral reefs may start dissolving when atmospheric CO2 doubles. Geophys. Res. Lett. 36, (doi:10.1029/2008GL036282) [Google Scholar]
- Tallmon D. A., Luikart G., Waples R. S.2004The alluring simplicity and complex reality of genetic rescue. Trends Ecol. Evol. 19, 489–496 (doi:10.1016/j.tree.2004.07.003) [DOI] [PubMed] [Google Scholar]
- Tioho H., Tokeshi M., Nojima S.2001Experimental analysis of recruitment in a scleractinian coral at high latitude. Mar. Ecol. Prog. Ser. 213, 79–86 (doi:10.3354/meps213079) [Google Scholar]
- Underwood J. N., Souter P. B., Ballment E. R., Lutz A. H., Van Oppen M. J. H.2006Development of 10 polymorphic microsatellite markers from herbicide-bleached tissues of the brooding pocilloporid coral Seriatopora hystrix. Mol. Ecol. Notes 6, 176–178 (doi:10.1111/j.1471-8286.2005.01183.x) [Google Scholar]
- Underwood J. N., Smith L. D., Van Oppen M. J. H., Gilmour J. P.2007Multiple scales of genetic connectivity in a brooding coral on isolated reefs following catastrophic bleaching. Mol. Ecol. 16, 771–784 (doi:10.1111/j.1365-294X.2006.03187.x) [DOI] [PubMed] [Google Scholar]
- Underwood J. N., Smith L. D., Van Oppen M. J. H., Gilmour J. P.2009Ecologically relevant dispersal of corals on isolated reefs: implications for managing resilience. Ecol. Appl. 19, 18–29 (doi:10.1890/07-1461.1) [DOI] [PubMed] [Google Scholar]
- Valiere N.2002Gimlet: a computer program for analysing genetic individual identification data. Mol. Ecol. Notes 2, 377–379 [Google Scholar]
- Van Oppen M. J. H., Lutz A., De'ath G., Peplow L., Kininmonth S.2008Genetic traces of recent long-distance dispersal in a predominantly self-recruiting coral. PLoS ONE, 3e3401 (doi:10.1371/journal.pone.0003401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veron J. E. N.1993A biogeographic database of hermatypic corals: species of the Central Indo-Pacific genera of the world Australian Institute of Marine Science Monograph Series, vol. 10 Townsville, Australia: Australian Institute of Marine Science [Google Scholar]
- Veron J. E. N.1995Corals in space and time: the biogeography and evolution on the Scleractinia Sydney, Australia: University of New South Wales Press [Google Scholar]
- Veron J. E. N., Done T. J.1979Corals and coral communities on Lord Howe Island. Aust. J. Mar. Freshwater Res. 30, 203–236 (doi:10.1071/MF9790203) [Google Scholar]
- Wahlund S.1928Zusammensetzung von Population und Korrelationserscheinung vom Standpunkt der Vererbungslehre aus betrachtet. Hereditas 11, 65–106 [Google Scholar]
- Wakeford M., Done T. J., Johnson C. R.2008Decadal trends in a coral community and evidence of changed disturbance regime. Coral Reefs 27, 1–13 (doi:10.1007/s00338-007-0284-0) [Google Scholar]
- Weir B. S., Cockerham C. C.1984Estimating F-statistics for the analysis of population structure. Evolution 38, 1358–1370 (doi:10.2307/2408641) [DOI] [PubMed] [Google Scholar]
- Wilson J., Harrison P. L.1997Sexual reproduction in high latitude coral communities of the Solitary Islands, Eastern Australia. In Proc. 8th Int. Coral Reef Symp., Panama, vol. 1, pp. 533–538 [Google Scholar]
- Wilson K., et al. 2002Genetic mapping of the black tiger shrimp Penaeus monodon with amplified fragment length polymorphism. Aquaculture 204, 297–309 (doi:10.1016/S0044-8486(01)00842-0) [Google Scholar]
- Wright S.1922Coefficients of inbreeding and relationship. Am. Nat. 56, 330–338 (doi:10.1086/279872) [Google Scholar]