Abstract
In many species, females store sperm between copulation and egg fertilization, but the consequences of sperm storage and patterns of sperm use for female life history and reproductive success have not been investigated in great detail. In hymenopteran insect societies (ants, bees, wasps), reproduction is usually monopolized by one or relatively few queens, who mate only during a brief period early in life and store sperm for later use. The queens of some ants are particularly long-lived and have the potential to produce millions of offspring during their life. To do so, queens store many sperm cells, and this sperm must remain viable throughout the years of storage. Queens should also be under strong selection to use stored sperm prudently when fertilizing eggs. We used the leaf-cutter ant Atta colombica to investigate the dynamics of sperm use during egg fertilization. We show that queens are able to fertilize close to 100 per cent of the eggs and that the average sperm use per egg is very low, but increases with queen age. The robustness of stored sperm was found to decrease with years of storage, signifying that senescence affects sperm either directly or indirectly via the declining glandular secretions or deteriorating sperm-storage organs. We evaluate our findings with a heuristic model, which suggests that the average queen has sperm for almost 9 years of normal colony development. We discuss the extent to which leaf-cutter ant queens have been able to optimize their sperm expenditure and infer that our observed averages of sperm number, sperm robustness and sperm use are consistent with sperm depletion being a significant cause of mortality of mature colonies of Atta leaf-cutter ants.
Keywords: sperm survival, sperm number, fertilization, social insects, queen age, sperm senescence
1. Introduction
In many species with internal fertilization, females possess specialized storage organs where sperm is maintained between insemination and egg fertilization (e.g. insects: Pitnick et al. 1999; birds: Shugart 1988; reptiles: Olsson & Madsen 1998). Although the sperm-storage period may be as long as weeks or months, it is usually much shorter than the lifespan of a female (Birkhead & Møller 1998). The queens of eusocial Hymenoptera (ants, bees and wasps) are striking exceptions because some of them can survive for more than a decade (Pamilo 1991; Keller & Genoud 1997; Keller 1998) together with the sperm that they stored in their storage organs (spermathecae) early in adult life (Boomsma et al. 2005). Because these queens never remate (Boomsma & Ratnieks 1996; Boomsma et al. 2005), male gametes are potentially more limiting for lifetime reproductive success than female gametes, as queens produce eggs throughout their lives (Cole 1983). Long-lived ant queens, therefore, provide unusual opportunities to study extremes of adaptation, as sperm viability (the number of live sperm cells) in male ejaculates is expected to be high, and sperm senescence during storage low. The number of sperm used per fertilized egg is also expected to be minimized (Tschinkel 1987; Tschinkel & Porter 1988). In all these cases, adaptations may have reached degrees of sophistication that are never observed in organisms with normal remating promiscuity (Boomsma 2007), but direct measurements of sperm survival, sperm use and sperm senescence in eusocial insects are either not available or have not been interpreted as specific adaptations (e.g. Yu & Omholt 1999).
The present study provides direct estimates of sperm use and sperm senescence in Atta leaf-cutter ant queens. This genus is highly suitable for such studies because: (i) its colonies are headed by a single long-lived (10–20 years) queen and have more than a million workers when mature (Weber 1972), and (ii) newly established colonies can be collected in numbers and maintained under controlled laboratory conditions for many years. This allowed us to sample batches of eggs and to determine egg fertilization status, to count sperm cells present on and in the eggs and to assess sperm robustness after variable times of storage. We first tested the hypothesis that queens are expected to minimize the number of sperm used per egg while still successfully fertilizing all eggs in periods where male production (from unfertilized eggs) is not expected. We then examined whether sperm use is affected by queen age and the number and robustness of the remaining stored sperm. Finally, we assessed how the robustness and number of stored sperm changes with queen age and compared our results with a simple heuristic model generating the expected patterns of variation in relationships between sperm use, the number of stored sperm and colony longevity.
2. Material and methods
(a). Collection of Atta colombica queens and measurements of fertilization success
A total of 25 A. colombica queens (labelled A–Y) were, in different subsets, used for sperm-use estimates. Queens were approximately 1 year old when nests were excavated in Gamboa, Republic of Panama, in May 2003 (n = 5), 2004 (n = 4), 2006 (n = 3) and 2008 (n = 4). The age of these queens could be inferred because 1 year old colonies have only one or two conical entrance funnels with fungus chambers directly underneath (Weber 1972). Excavated colonies were transported to Copenhagen (Denmark) and maintained under standardized laboratory conditions (25°C and 70% relative humidity). Nine additional A. colombica queens were collected directly after their mating flight in Gamboa, Republic of Panama in May 2007, and were examined in the week following collection. To collect eggs, queens were periodically isolated in small subcolony nest-boxes with only a few tending workers and a small fragment of fungus garden, so that newly laid eggs could be harvested every few hours.
To quantify the overall fertilization success, we examined the ploidy level (haploid or diploid) of eggs from a random subset of eight queens of different age (ranging from 3.5 to 6.5 years). Freshly laid eggs (less than 2 h) were counted, collected and placed in a small subcolony with workers and fungus. All eggs were allowed to develop for 10–12 days, and then analysed using flow cytometry. This allows characterization of ploidy by measuring the degree of fluorescence reflection of stained DNA in disassociated nuclei (Aron et al. 2003; Cournault & Aron 2008). Each egg was placed in an eppendorf tube and ruptured using a fine needle, after which 200 µl of an extraction buffer (CyStain DNA 2 step, PARTEC) was added to extract nuclei and the sample was vortexed for 30 s. Subsequently, 800 µl staining solution (CyStain DNA 2 step, PARTEC) was added, containing the fluorescent stain 4′,6-diamino-2-phenylindole (DAPI) that binds strongly to DNA. The DNA content of each sample was analysed using a PA-I flow cytometer (PARTEC, Partec Gmbh, Münster, Germany) with an optical arrangement as used by Cournault & Aron (2008). Nuclei of sperm cells (haploid) and of somatic cells from workers (diploid) were used as standards for calibration. Eggs containing fewer than 2000 embryonic cells were occasionally found (but not over-represented in particular queens) and were discarded from the dataset because they would probably give ambiguous results. Final sample sizes for which the ploidy level was determined from fluorescence histograms were 20–30 eggs per queen.
(b). Sperm use during egg fertilization
We define sperm use as the number of sperm found on freshly laid eggs, assuming that all sperm released by queens adhere to the egg so that they can be visualized by DAPI staining shortly after oviposition.
Freshly laid eggs (less than 2 h old) were collected from all 25 queens and individually placed on a microscope slide and stained with 5 µl of a DAPI working solution, i.e. 2 µl of a DAPI stock solution (2 mg DAPI in 1 ml dimethylsulfoxide) in 1 ml 0.1 M NaPO4 buffer, pH 7.0. Cover slides were placed over the eggs, which made them burst and the cytoplasm flow out, allowing DAPI staining of sperm cells present both on the egg's chorion and in the cytoplasm. Eggs were examined with a fluorescence microscope (Olympus CX41, EXFO X-Cite 120, figure 1), and the number of sperm cells associated with each egg was counted for a total of 30–60 eggs per queen 1 year old or more and for eight eggs for each of the queens that had been collected directly after the mating flight. For these young queens, we could only examine the first batch of eggs owing to the very high queen mortality, which is common during the weeks following insemination (Weber 1972; Baer et al. 2006).
Figure 1.
Photographs of sperm cells present on eggs laid by A. colombica queens (only the sperm nuclei are visible). (a) More than 200 sperm cells were found on one of the eggs of queen G (figure 2g). (b) Close-up of an egg with two sperm nuclei visible (queen F, figure 2f).
For a subset of queens (n = 10) that survived long enough, we examined the effect of queen age on sperm use by repeating the same procedure after keeping these queens in laboratory nests for a series of years. Sperm use by the oldest queen was measured on three occasions, at 1.5, 4.5 and 6.5 years of age, while the remaining nine queens were measured twice, with age classes ranging from 1.5 to 5.5 years and the minimum time-span between the two measurements being 1.5 years.
(c). Robustness and number of stored sperm
A partially different subset of 10 queens of different ages (ranging from 1.5 to 6.5 years) was used to determine the number and robustness of sperm stored in the spermatheca and its potential influence on sperm use. The number of sperm used per egg was determined, as described above, for a minimum of 30 eggs per queen. We then dissected the spermatheca of each queen using watchmaker's forceps, ruptured it and diluted its contents in 1.5 ml Hayes saline (9 g NaCl, 0.2 g CaCl2, 0.2 g KCl and 0.1 g NaHCO3 in 1000 ml H2O). We used 10 µl of this stock solution and diluted it in 990 µl distilled water. Four aliquots of 1 µl each were taken from this new solution and placed on a microscope slide. The aliquots were stained with 3 µl DAPI and the total number of sperm in each aliquot was counted using a fluorescence microscope. The number of sperm in these aliquots was multiplied by the dilution factor in Hayes saline to obtain estimates of the number of stored sperm per queen (Baer et al. 2006).
Another three samples of 3 µl were taken from the same stock solution and placed on a microscope slide. The survival of sperm in these samples, after being exposed to the Hayes saline for approximately 30 min, was measured using a Live/Dead sperm viability kit (L-7011, Molecular Probes) as described by den Boer et al. (2008). To each sample, 3 µl of SYBR-14 (a membrane-permeant nucleic acid stain for live sperm) and 2 µl of propidium iodide (a dead cell stain) were added, after which the proportion of live (fluorescent green) and dead (fluorescent red) sperm among at least 300 randomly selected sperm cells was assessed under a fluorescence microscope. The number of dual-stained sperm cells was on average only 0.43 per cent of the total count, and these cells were discarded from the dataset.
It is important to note that the percentages of live and dead sperm obtained by this method are affected by the dilution of sperm in Hayes saline solution, which, on its own, does not support sperm survival (den Boer et al. 2008). The values given for the survival of sperm, therefore, do not represent absolute sperm viabilities in vivo, but should be interpreted as relative viabilities across different queen age classes. We assume that all sperm which we dissected out of spermathecae were alive because sperm cells that die naturally during undisturbed storage are likely to be few and appear to be removed by digestion or absorption through the spermathecal wall (Da Cruz-Landim et al. 2003). The relative viabilities that we measured are therefore likely to represent differences in robustness of sperm when exposed to Hayes saline, which correlate with in vivo viability differences during sperm release for egg fertilization, but not necessarily in a linear fashion. We, therefore, use the term ‘sperm robustness’ for this measure.
(d). Statistical analysis
Data were analysed using JMP 7.02 (SAS Institute). The distribution of sperm use was highly skewed and could not be fitted with standard parametric distributions (§3), so we used non-parametric statistics to compare sperm-use distributions across individuals. For subsequent parametric analyses of variation in sperm use, we used the median number of sperm per fertilized egg as an average rather than the mean. For all tests that did not explicitly include queen age effects, we only used the data for one age category per queen, normally that representing the youngest age at which her eggs were examined, because this would minimize any artefacts owing to laboratory conditions. All results were qualitatively identical when samples from the oldest age at which her eggs were sampled were used instead.
Average robustness of stored sperm per queen (±s.e.m.) was estimated by fitting a generalized linear model with binomial errors, corrected for over-dispersion, using the number of live sperm in each queen-specific 3 µl sample as the dependent variable and the total number of sperm in the sample as the binomial denominator. Correlations between queen age, sperm survival, sperm number and sperm use in the 10 queens for which all these variables had been measured were also investigated. For this analysis, the last sperm-use measure before queen sacrifice was used.
(e). Heuristic model
To compare our results with expected patterns of sperm use and sperm number, a simple heuristic model was built in RealBasic (v. 2008.1, Real Software), based on sperm-storage figures from previous studies on the same Gamboa population (Fjerdingstad & Boomsma 1998; Baer et al. 2006) and on available life-history data (Weber 1972; Fowler et al. 1986). The model assumes that the average A. colombica colony goes through a period of logistic growth during the first 5 years after establishment before achieving a size of two million workers, after which 3000 gynes are produced each year (Weber 1972). The initial number of sperm stored was drawn randomly from a normal distribution with a mean of 244 million and standard deviation of 83 million (Baer et al. 2006), and the average worker lifespan was set at four months (Fowler et al. 1986), so that the maintenance of a worker force of two million would require the annual fertilization of six million eggs. Stored sperm viability was assumed to be 100 per cent throughout. Sperm use and remaining sperm supplies were calculated for each six-month period after colony founding, based on different combinations of the initial sperm use per egg and the observed change in this number with age, so that the expected age at which queens run out of sperm could be estimated.
The model was run 10 000 times to determine whether the patterns of sperm use and storage that we observed were consistent with the model. Values of initial sperm use and change in sperm use with age were picked randomly from a lognormal and normal distribution, respectively, with means and standard deviations as obtained in our analysis (see §3 for details). All sample distributions of sperm use were truncated to avoid negative values, which are biologically impossible. The proportion of such negative values was small (less than 0.1%) and when they occurred, the simulated colony was discarded and a new one generated to replace it. The longevity of each of the 10 000 simulated colonies based purely on numerical sperm depletion was calculated, and the instantaneous sperm use and number of stored sperm for each simulated colony were sampled randomly from one of the six-month periods during the first 20 years of each model run (the maximum longevity of any of the model colonies). As mean sperm use was required as input to the model, this value was estimated from the median sperm-use data by multiplying by 1.92, the slope of the through-origin regression between observed median and mean sperm use (r = 0.976).
3. Results
(a). Fertilization success and sperm use during egg fertilization
We tested the ploidy level of 20–30 eggs per queen for eight queens of different ages. For five of these queens, no haploid eggs were found. The remaining three queens laid only one or two haploid eggs, and this frequency did not appear to be related to their age, the median number of sperm used at that age or the robustness and the number of their remaining sperm supply. In total, only four out of 207 analysed eggs were haploid, giving an overall fertilization rate of 98.1 per cent (95% CI: 95.6–99.4%). We concluded that A. colombica queens essentially fertilize all eggs in periods when male production from unfertilized eggs is undesirable because small colonies never produce males. We, therefore, added one to all sperm-use counts to compensate for the single sperm cell that merged with the haploid egg nucleus during fertilization and that could thus not be observed at the moment of counting.
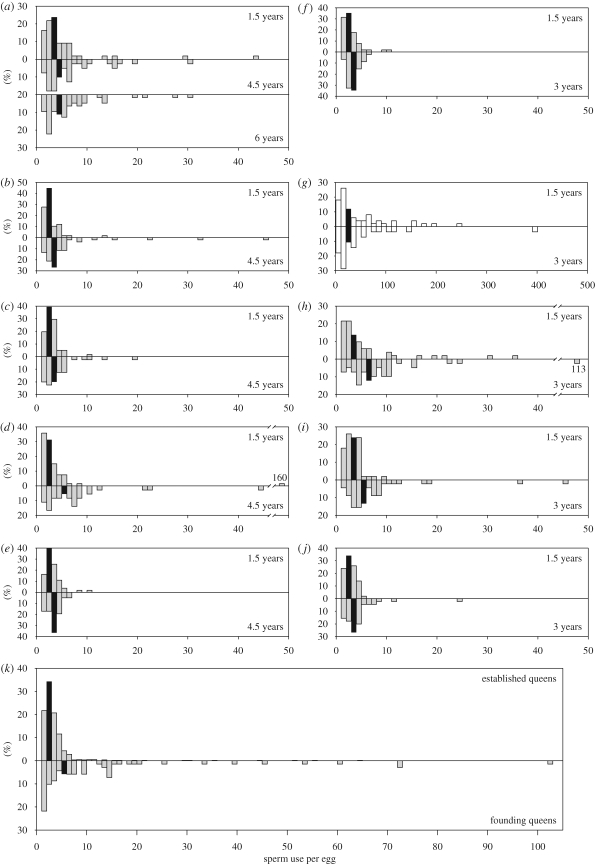
The distribution of sperm used for egg fertilization by individual queens was highly skewed to the right (figure 2) and could not be adequately fitted by standard families of distributions (lognormal, Poisson, negative binomial or exponential, including over-dispersed versions of these distributions). However, the distribution of mean sperm-use values between queens was lognormally distributed and used as such in the heuristic model. Sperm use differed significantly between queens (Kruskal–Wallis test, H = 241.33, d.f. = 15, p < 0.001). Most queens used very few sperm to fertilize their eggs, but queen G was a clear outlier, using between five and 401 sperm per egg (median 25.5), so she was excluded from subsequent analyses. Even after excluding her, there were still highly significant differences in sperm use among queens (H = 123.69, d.f. = 14, p < 0.001).
Figure 2.
(a–j) Histograms showing the frequency distributions of the number of sperm per egg in 10 queens for which multiple samples could be obtained, with earlier samples presented above samples taken at later age ((a) queen A, (b) queen B, (c) queen C, (d) queen D, (e) queen E, (f) queen F, (g) queen G, (h) queen H, (i) queen I, and (j) queen J). Note that queen A was sampled three times and that the x-axis for queen G is an order of magnitude greater than for the other queens. (k) Histograms showing the frequency distribution of the number of sperm per egg combined for 15 queens with established colonies (upper graph), based on the first sample for each queen, and for 10 founding queens collected directly after their mating flight (lower graph). The black bar in each histogram contains the median number of sperm per egg.
The overall median sperm use (with queen G excluded) was two per egg when only the youngest age class was examined (three per egg for only the oldest age class). Variation in median sperm use among founding queens was much greater than among established queens (figures 2k and 3, F-test of equality of variances; F8,14 = 12.63, p < 0.001), and median sperm use itself was also higher in founding queens (7.17 ± 1.58 s.e.) than in established queens (2.73 ± 0.34; Welch t-test with unequal variance, t = 2.74, d.f. = 8.77, p = 0.023). These patterns were also evident in the heuristic model (figures 3 and 4), which shows that colonies with high sperm use inevitably die before reaching sexual maturity at approximately 5 years of age. For established queens, median sperm use increased with queen age (figure 3; r = 0.621, n = 15, p = 0.014), with queen age explaining 39 per cent of the variance in median sperm use. This effect was even clearer for the queens from which multiple sperm samples had been collected over time, with median sperm use in the second age class measured always being higher than in the first age class (figures 2 and 3, paired t-test, t = 4.87, d.f. = 8, p1-tailed < 0.001). The average increase in mean sperm usage was 0.774 ± 0.199 sperm per egg per year (mean ± s.e.).
Figure 3.
Median number of sperm used per egg for all 25 queens, plotted as a function of queen age. Where queens have been measured at more than one age, the data points (open circles) are connected by a line. The dotted lines represent the calculated maximum sperm use per egg that queens can afford in order to produce colonies that reach sexual maturity (inferred to be at 5 years of age), with the different lines representing different initial sperm numbers after mating (see inset). The light grey background circles show sperm use of surviving queens as simulated by the heuristic model and illustrate that colony survival is expected to decline rapidly after colonies have reached sexual maturity at 5 years of age, and only queens that store a high number of sperm and use their supply prudently will be able to produce sexual offspring during multiple seasons.
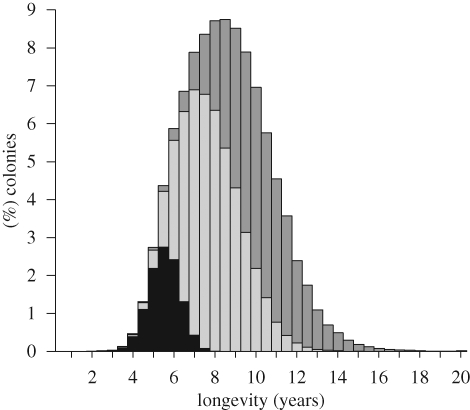
Figure 4.
Histogram of colony longevity, based purely on sperm limitation, as predicted by the heuristic model. Each bar is shaded according to the contribution of model colonies with low (dark grey) (less than two), medium (light grey) (2–10) and high (black) (more than 10) values of initial sperm use per egg.
(b). Robustness and number of stored sperm
The robustness of stored sperm when exposed to Hayes solution decreased significantly with increasing queen age (figure 5a,b; r = −0.687, n = 10, p = 0.028), with age explaining 47 per cent of the variance in observed sperm survival. However, the survival of stored sperm upon dissection was not correlated with sperm use (r = −0.301, p = 0.40) or the number of stored sperm (r = 0.059, p = 0.87).
Figure 5.
(a) Proportion of live sperm (±s.e.m.) taken from the spermathecae of 10 queens after exposure to Hayes saline. The queens are grouped in four different age classes. (b) Sperm robustness assay with live sperm cells staining green and dead sperm cells red. (c) Number of sperm (±s.e.m.) found in the spermathecae of the same 10 queens. The light grey background circles represent the equivalent data for each of the 10 000 runs of the heuristic model. (d) Mean sperm use during egg fertilization plotted against the number of sperm present in the spermatheca of the same 10 queens. The light grey background circles represent the equivalent output of the heuristic model.
The estimated number of stored sperm varied between 72 and 372 million, with a mean of 230 million, estimates that are very close to previous reports for this species (Baer et al. 2006). The four aliquots per queen were very consistent (figure 5c,d), with only 2.8 per cent of the variance in sperm number being attributable to differences between samples from the same queen. The number of stored sperm decreased with age, but the correlation was not significant (figure 5c, r = −0.267, p = 0.456). The negative correlation between sperm use and the number of remaining sperm in storage was close to significant (figure 5d, r = −0.574, p = 0.083), suggesting that this trend might have become significant if larger samples sizes had been used or if our laboratory colonies had been allowed to grow much larger over the years.
The patterns in sperm number used for fertilization were consistent with expected distributions generated by the heuristic model (figure 5c,d). When we estimated the expected colony longevity distribution as generated by our heuristic model, we found that the maximal values that we obtained were very similar to available estimates of approximately 20 years for mature colonies of Atta leaf-cutter ants (Weber 1972).
4. Discussion
The extreme adaptations of sperm survival, sperm use and sperm senescence that we expected at the onset of this study were all borne out. Many of the Atta queens investigated used only two sperm per egg on average at 1–2 years of age, and this number increased only slowly over time. The robustness of stored sperm (measured as survival in Hayes saline solution) decreased as a function of storage time, but this did not reduce the ability of older queens to essentially fertilize all eggs using an average of six sperm per egg or fewer. These statistics place the Atta leaf-cutter ants in a unique position, as we are not aware of any other animal with similar abilities to keep sperm viable during storage in the female body. These results both shed new light on long-standing but little investigated questions in social insect research and establish the leaf-cutter ants as a suitable general model system for studying mechanisms that promote viability and reduce senescence of sperm, and of the costs and trade-offs involved in the expression of these adaptations. The sections below address these issues in more detail.
(a). The economy of sperm use in Atta queens
The established queens used in this study all headed laboratory colonies that never produce haploid males, most probably because we constrained them to stay about 100-fold smaller than mature colonies in the field. We, therefore, assume that queens in our laboratory colonies ‘intended’ to produce only workers, and our analysis of egg ploidy shows that they achieve this with high efficiency. A similar degree of fertilization efficiency has been found in the honeybee Apis mellifera, where diploid (worker) and haploid (drone) larvae are raised in differently sized cells and where queens almost always lay unfertilized eggs in drone cells and fertilized eggs in worker cells (Ratnieks & Keller 1998). Given that both honeybee and leaf-cutter ant queens are surrounded and nursed by workers, the very low rate of mistakes in fertilizing eggs (and the probable need to remove or recycle such unintended unfertilized eggs) probably represents a negligible cost to overall colony efficiency, but a higher mistake rate would probably have incurred significant fitness costs.
If sperm use followed a Poisson process, as might be expected if queens open the spermathecal duct for a fixed time, then to achieve the observed fertilization success of 98.1 per cent would require a median number of four sperm released per egg, which is double the median number we observed for young A. colombica queens (two sperm per egg). This suggests that queens have some mechanism that allows them to normally dispense or assess the release of sperm more accurately than predicted from this simplistic analysis, although it is also evident that this mechanism does not always work because occasionally very large numbers of sperm are released (the long right tails of the distributions in figure 2). The average number of sperm used by A. colombica queens is similar to that estimated for the monogynous fire ant Solenopsis invicta (mean 3.2 sperm per egg; Tschinkel 2006; Tschinkel & Porter 1988), but below the lower range of all available estimates for A. mellifera (4–100; Bresslau 1905; Harbo 1979; Ruttner 1975; Yu & Omholt 1999). Our direct estimates show, to our knowledge, for the first time that sperm use varies considerably between queens and between age cohorts.
We expect that colonies of queens which use relatively many sperm per egg are unlikely to survive long in the field, as shown by the heuristic model (figure 4). Our observed overall median value of two sperm per egg, combined with an average of more than 98 per cent egg fertilization, seems likely to be typical for field colonies and may well represent a fairly optimal balance between ensuring both almost complete egg fertilization and the preservation of sperm stores for later use. While most of the queens in our study used few sperm, some were found to use many more on average, with queen G using a median of 25 sperm per egg (the maximum for this queen was approx. 400). Based on our heuristic model, this queen would be unlikely to maintain a colony for more than 4 years in the field. Our model therefore supports the notion that the reproductive success of A. colombica queens is sperm-limited (cf. Cole 1983, Tschinkel & Porter 1988). If an average queen was inseminated with 244 million sperm (Baer et al. 2006) that did not decline at all in viability, and she would use only a single sperm per egg throughout her life, then sperm use would be sufficient to allow a colony to survive 44 years. However, if sperm use per egg by such an average queen increased by the observed value of 0.774 each year, then colony longevity would be reduced to 10 years. If the observed range of sperm use by newly inseminated queens (2.4–26.9 sperm per egg) is real, this would imply a longevity range of 5–9 years, implying zero to moderate reproductive success (figure 3). The results of our heuristic model suggest that sperm limitation is likely to lead to an average queen and colony longevity of 8.54 years, meaning that sexuals will, on average, only be produced for 3–4 years and that 5 per cent of colonies will run out of sperm before producing any sexuals (figure 4).
We observed that founding queens who mated just a few days before we investigated their first eggs used more sperm per egg than 1.5 year old queens. This may indicate that none of these queens with a median sperm use of more than 2 would survive even until 1 year of age for reasons other than sperm depletion. The surplus of sperm used on these very first eggs could also represent remnants of ejaculates that were left in the queen's reproductive tract during mating and that happen to be captured by the first passing eggs, which seems possible in spite of A. colombica sperm being transferred to the spermatheca during copulation (Baer & Boomsma 2006). During the first days after mating, Atta queens make the transition from being carefully nursed winged virgins in their natal colony to being independent solitary colony founders under heavy predation pressure and with no nest-mate grooming protection against infectious diseases. There may also be disadvantages of storing large amounts of sperm at this stage, as a queen's encapsulation immune response is negatively correlated with the number of stored sperm (Baer et al. 2006). This suggests that the probability of initial solitary survival in the face of considerable disease pressure is traded-off against maximal reproductive lifespan after having survived the colony-founding stage (Baer et al. 2006).
(b). Senescence of sperm and / or sperm-storage organs
Sperm use was found to increase with queen age (figure 3), while the robustness of stored sperm decreased with queen age (which has also been found in the honeybee; Lodesani et al. 2004). As we interpret our measure of robustness as reflecting sperm quality rather than sperm viability (§2), this suggests that sperm quality deteriorates without sperm dying as long as it remains in the sperm-storage organs. This suggests an interesting parallel with recent work showing that sperm senescence may affect sexual selection in promiscuous organisms where fertilization follows relatively shortly after mating (Reinhardt 2007; Pizzari et al. 2008).
As eusocial queens store sperm for life as if it were part of their own body, it is difficult to separate the direct effects of sperm senescence from the indirect effects of ageing of the queen spermatheca and its supporting glands and muscle tissues. As in all ageing processes, both nature (the original sperm phenotype) and nurture (the storage process) are likely to be crucial for achieving the impressive longevity and viability of ant sperm. The longevity of ant sperm means that there should be opportunity for selection to act on genes that are expressed by spermatozoa when they are used for fertilization after having been stored. However, sperm is inactive during storage when, in honeybees, a complex mixture of female spermathecal proteins provides an entire metabolic network of support functions (Baer et al. 2009). We suspect that proteomic analyses of leaf-cutter ant spermathecal gland fluid will show a similar female support role (den Boer 2009; den Boer et al. 2009). In addition, spermathecal antioxidant enzymes are likely to protect sperm cells from oxidative stress (Weirich et al. 2002).
Although we found that sperm use increases with queen age and that sperm robustness decreases with queen age, we did not find a direct relationship between sperm survival in Hayes and sperm use, and the observed increase in sperm use is more than would be required to compensate for the reduction in sperm robustness. This suggests that the increased use of sperm may be more likely owing to senescence of the queen mechanism of sperm release than to the intrinsic deterioration of the sperm itself. In both honeybees and ants, the junction of the spermatheca and the spermathecal duct is associated with a set of muscle fibres (Bresslau 1905; Wheeler & Krutzsch 1994; Gobin et al. 2006), while an additional valve flap has been shown to separate the spermathecal lumen from the spermathecal duct in the ant Crematogaster opuntiae (Wheeler & Krutzsch 1994). Sperm release has, therefore, been hypothesized to be regulated by contraction of these muscles, so that histological cross-sections of queens of different age could possibly provide direct morphological evidence for ageing of these muscles being responsible for less accurate sperm allocation.
We conclude that the long-lived queens of social Hymenoptera, and ants in particular, provide excellent opportunities for explicitly studying the economy of sperm use and the resource allocation and senescence trade-offs involved in sperm storage (cf. Baer et al. 2006). Our results give significant support to the notion that sperm limitation does have the potential to constrain lifetime reproductive success in eusocial insects with long-lived colonies, no matter whether queens mate singly (Ross et al. 1988; Tschinkel 2006) or multiply (this study). Finally, our study shows that the eusocial insects may be useful models for investigating sperm senescence, which so far has only been studied in animals with variable degrees of remating promiscuity.
Acknowledgements
We thank Marlene Stürup for practical assistance. This work was supported by grants from the Danish National Research Foundation to J.J.B., an Australian Research Council (ARC) Fellowship to B.B. and grants from the Belgian National Research Foundation (FRS-FNRS) to S.A. We thank two anonymous reviewers for constructive comments on a previous version of this manuscript.
References
- Aron S., De Menten L., Van Bockstaele D.2003Brood sex ratio determination by flow cytometry in ants. Mol. Ecol. Notes 3, 471–475 (doi:10.1046/j.1471-8286.2003.00488.x) [Google Scholar]
- Baer B., Boomsma J. J.2006Mating biology of the leaf-cutting ants Atta colombica and A. cephalotes. J. Morphol. 267, 1165–1171 (doi:10.1002/jmor.10467) [DOI] [PubMed] [Google Scholar]
- Baer B., Armitage S., Boomsma J. J.2006Sperm storage induces an immunity cost in ants. Nature 41, 872–875 (doi:10.1038/nature04698) [DOI] [PubMed] [Google Scholar]
- Baer B., Eubel H., Taylor N., O'Toole N., Millar A. H.2009Insights into female sperm storage from the spermathecal fluid proteome of the honeybee Apis mellifera. Genome Biol. 10, R67 (doi:10.1186/gb-2009-10-6-r67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhead T. R., Møller A. P.1998Sperm competition and sexual selection London, UK: Academic Press [Google Scholar]
- Boomsma J. J.2007Kin selection versus sexual selection: why the ends do not meet. Curr. Biol. 17, 673–683 (doi:10.1016/j.cub.2007.06.033) [DOI] [PubMed] [Google Scholar]
- Boomsma J. J., Ratnieks F. L. W.1996Paternity in eusocial Hymenoptera. Phil. Trans. R. Soc. Lond. B 351, 947–975 (doi:10.1098/rstb.1996.0087) [Google Scholar]
- Boomsma J. J., Baer B., Heinze J.2005The evolution of male traits in social insects. Annu. Rev. Entomol. 50, 395–420 (doi:10.1146/annurev.ento.50.071803.130416) [DOI] [PubMed] [Google Scholar]
- Bresslau E.1905Der samenblasengang der Bienenkönigin. Zool. Anz. 29, 299–325 [Google Scholar]
- Cole B.1983Multiple mating and the evolution of social behavior in the Hymenoptera. Behav. Ecol. Sociobiol. 12, 191–201 (doi:10.1007/BF00290771) [Google Scholar]
- Cournault L., Aron S.2008Rapid determination of sperm number in ant queens by flow cytometry. Insectes Soc. 55, 283–287 (doi:10.1007/s00040-008-1003-8) [Google Scholar]
- Da Cruz-Landim C., Yabuki A. T., Iamonte M.2003Ultrastructure of the spermatheca of Melipona bicolor bicolor LEP (Hymenoptera, Apinae, Meliponini). Biosci. J. Uberlândia 19, 57–64 [Google Scholar]
- den Boer S. P. A.2009Dynamics of ejaculate transfer, sperm storage and sperm use in social insects. PhD thesis, University of Copenhagen, Denmark [Google Scholar]
- den Boer S. P. A., Boomsma J. J., Baer B.2008Seminal fluid enhances sperm viability in the leafcutter ant Atta colombica. Behav. Ecol. Sociobiol. 62, 1843–1849 (doi:10.1007/s00265-008-0613-5) [Google Scholar]
- den Boer S. P. A., Boomsma J. J., Baer B.2009Honey bee males and queens use glandular secretions to enhance sperm viability before and after storage. J. Insect Physiol. 55, 538–543 (doi:10.1016/j.jinsphys.2009.01.012) [DOI] [PubMed] [Google Scholar]
- Fjerdingstad E. J., Boomsma J. J.1998Multiple mating increases the sperm stores of Atta colombica leafcutter ant queens. Behav. Ecol. Sociobiol. 42, 257–261 (doi:10.1007/s002650050437) [Google Scholar]
- Fowler H. G., Pereira-da-Silva V., Forti L. C., Saes N. B.1986Population dynamics of leaf-cutting ants: a brief review. In Fire ants and leaf-cutting ants: biology and management (eds Lofgren C. S., Vander Meer R. K.), pp. 123–145 Boulder, CO: Westview Press [Google Scholar]
- Gobin B., Ito F., Peeters C., Billen J.2006Queen–worker differences in spermatheca reservoir of phylogenetically basal ants. Cell Tissue Res. 326, 169–178 (doi:10.1007/s00441-006-0232-2) [DOI] [PubMed] [Google Scholar]
- Harbo J. R.1979The rate of depletion of spermatozoa in the queen honeybee spermatheca. J. Apic. Res. 18, 204–207 [Google Scholar]
- Keller L.1998Queen lifespan and colony characteristics in ants and termites. Insectes Soc. 45, 235–246 (doi:10.1007/s000400050084) [Google Scholar]
- Keller L., Genoud M.1997Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature 389, 958–960 (doi:10.1038/40130) [Google Scholar]
- Lodesani M., Balduzzi D., Galli A.2004A study on spermatozoa viability over time in honey bee (Apis mellifera ligustica) queen spermathecae. J. Apic. Res. 43, 27–28 [Google Scholar]
- Olsson M., Madsen T.1998Sexual selection and sperm competition in reptiles. In Sperm competition and sexual selection (eds Birkhead T. R., Møller A. P.), pp. 503–577 San Diego, CA: Academic Press [Google Scholar]
- Pamilo P.1991Life-span of queens in the ant Formica exsecta. Insectes Soc. 38, 111–119 (doi:10.1007/BF01240961) [Google Scholar]
- Pitnick S., Markow T., Spicer G. S.1999Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution 53, 1804–1822 (doi:10.2307/2640442) [DOI] [PubMed] [Google Scholar]
- Pizzari T., Dean R., Pacey A., Moore H., Bonsall M. B.2008The evolutionary ecology of pre- and post-meiotic sperm senescence. Trends Ecol. Evol. 23, 131–140 (doi:10.1016/j.tree.2007.12.003) [DOI] [PubMed] [Google Scholar]
- Ratnieks F. L. W., Keller L.1998Queen control of egg fertilization in the honey bee. Behav. Ecol. Sociobiol. 44, 57–61 (doi:10.1007/s002650050514) [Google Scholar]
- Reinhardt K.2007Evolutionary consequences of sperm cell aging. Q. Rev. Biol. 82, 375–393 (doi:10.1086/522811) [DOI] [PubMed] [Google Scholar]
- Ross K. G., Vargo E. L., Fletcher D. J. C.1988Colony genetic structure and queen mating frequency in fire ants of the subgenus Solenopsis (Hymenoptera, Formicidae). Biol. J. Linn. Soc. Lond. 34, 105–117 (doi:10.1111/j.1095-8312.1988.tb01952.x) [Google Scholar]
- Ruttner F.1975Die instrumentelle Besamung der Bienenkönigin 2 Bucharest, Romania: Apimondia Publishing House [Google Scholar]
- Shugart G. W.1988Uterovaginal sperm-storage glands in sixteen species with comments on morphological differences. AUK 105, 379–384 [Google Scholar]
- Tschinkel W. R.1987Fire ant queen longevity and age: estimation by sperm depletion. Ann. Entomol. Soc. Am. 80, 263–266 [Google Scholar]
- Tschinkel W. R.2006The fire ants Cambridge, London: The Belknap Press of Harvard University Press [Google Scholar]
- Tschinkel W. R., Porter S. D.1988Efficiency of sperm use in queens of the fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 81, 777–781 [Google Scholar]
- Weber N. A.1972Gardening ants, the Attines Philadelphia, PA: The American Philosophical Society [Google Scholar]
- Weirich G., Collins A., Williams V.2002Antioxidant enzymes in the honey bee Apis mellifera. Apidologie 33, 3–14 (doi:10.1051/apido:2001001) [Google Scholar]
- Wheeler D. E., Krutzsch P. H.1994Ultrastructure of the spermatheca and its associated gland in the ant Crematogaster opuntiae (Hymenoptera, Formicidae). Zoomorphology 114, 203–212 (doi:10.1007/BF00416859) [Google Scholar]
- Yu R., Omholt S. W.1999Early developmental processes in the fertilised honeybee (Apis mellifera) oocyte. J. Insect Physiol. 45, 763–767 (doi:10.1016/S0022-1910(99)00056-6) [DOI] [PubMed] [Google Scholar]