Abstract
The Frank–Starling mechanism is a fundamental property of the vertebrate heart, which allows the myocardium to respond to increased filling pressure with a more vigorous contraction of its lengthened fibres. In mammals, myocardial stretch increases cardiac nitric oxide (NO) release from both vascular endothelium and cardiomyocytes. This facilitates myocardial relaxation and ventricular diastolic distensibility, thus influencing the Frank–Starling mechanism.
In the in vitro working heart of the eel Anguilla anguilla, we previously showed that an endogenous NO release affects the Frank–Starling response making the heart more sensitive to preload. Using the same bioassay, we now demonstrate that this effect is confirmed in the presence of the exogenous NO donor S-nitroso-N-acetyl penicillamine, is independent from endocardial endothelium and guanylate cyclase/cGMP/protein kinase G and cAMP/protein kinase A pathways, involves a PI(3)kinase-mediated activation of endothelial NO synthase and a modulation of the SR-CA2+ATPase (SERCA2a) pumps. Furthermore, we show that NO influences cardiac response to preload through S-nitrosylation of phospholamban and consequent activation of SERCA2a. This suggests that in the fish heart NO modulates the Frank–Starling response through a beat-to-beat regulation of calcium reuptake and thus of myocardial relaxation.
We propose that this mechanism represents an important evolutionary step for the stretch-induced intrinsic regulation of the vertebrate heart, providing, at the same time, a stimulus for mammalian-oriented studies.
Keywords: heterometric regulation, myocardial relaxation, endothelial nitric oxide synthase, SERCA2a, stroke volume
1. Introduction
A fundamental property of the vertebrate heart is the Frank–Starling mechanism (heterometric regulation), which allows the myocardium to respond to increased venous return (preload) with a more vigorous contraction of its lengthened fibres, performing more work through augmented stroke volume (SV) and consequent cardiac output (CO). This rapidly occurring stretch-related increase in developed force has been attributed to a length-dependent increase in cross-bridge formation and myofilament calcium responsiveness (Katz 2002).
In mammals, myocardial stretch increases cardiac nitric oxide (NO) release from both vascular endothelium and cardiomyocytes (Pinsky et al. 1997; Petroff et al. 2001; Balligand et al. 2009) facilitating myocardial relaxation, ventricular diastolic distensibility and hence the Starling response (Casadei & Sears 2003). In the past, the effect of NO on preload-induced increases in CO has been attributed to a paracrine effect of endothelial-derived NO on myofilament Ca2+ sensitivity secondary to troponin I phosphorylation by the cGMP-dependent protein kinase G (PKG) (Prendergast et al. 1997). Accordingly, Layland et al. (2002) showed that phosphorylation of troponin I increased in the presence of NO donors, this being associated with increased diastolic cell length and accelerated myocyte relaxation. More recently, an autocrine pathway of NO, related to the specific subcellular localizations and regulation of the different isoforms involved in the production of NO and in its target proteins, has also been described (for references, see Seddon et al. 2007). Even though the relative autocrine role of nitric oxide synthase (NOS) isoforms in the cardiomyocyte remains to be fully clarified, there is evidence in mammals that both myocardial endothelial NOS (eNOS) and neuronal NOS (nNOS), through cGMP-independent mechanisms, modulate the preload-induced increase of the contractile force. In details, it has been suggested that the autocrine nNOS, first observed in the sarcoplasmic reticulum (SR) (Xu et al. 1999) and subsequently also found bound to sarcolemmal membrane proteins (Williams et al. 2006), mainly promotes left ventricular relaxation by regulating the protein kinase A (PKA)-mediated phosphorylation of phospholamban (PLN) and the rate of SR Ca2+ reuptake by SR Ca2+ ATPase (SERCA2a) (Zhang et al. 2008). On the other hand, myocardial eNOS, mostly expressed into caveolae, a site where several signal transduction pathways have been shown to be modulated by NO (reviewed in Shaul 2002), primarily mediates the inotropic response to sustained stretch through a mechanism which involves S-nitrosylation of thiol residues in the Ryanodine receptors Ca2+ release channels (Massion et al. 2005). In addition, Froehlich et al. (2008) have recently shown that nitroxyl may stimulate SERCA2a pumps activity by modifying critical thiol residues in its regulatory protein PLN.
As in mammals, also in fishes the end-diastolic volume and the consequent stretch-related increase in developed force is a key regulator of cardiac performance; however, unlike mammals, fishes respond to different hemodynamic loads increasing CO mainly through an increased SV rather than heart rate (HR) (Tota & Gattuso 1996; Olson 1998). This elevated sensitivity of the fish hearts to the Starling response, well documented in both temperate eurytherm and cold-adapted teleosts (gilthead seabream, Icardo et al. 2005; eel, Imbrogno et al. 2001; icefish, Tota et al. 1991), has been in part attributed to a greater myocardial extensibility of the highly trabeculate fish heart, coupled to a maintained increase in myofilament Ca2+ sensitivity over a large range of sarcomere lengths (Di Maio & Block 2008; Shiels & White 2008).
It is interesting to note we have previously documented that in the eel A. anguilla heart, used as a paradigm of a highly trabeculate and endoluminally supplied cardiac design (Tota et al. 1983), a basal release of endogenous NO increases the sensitivity of the Frank–Starling response (Imbrogno et al. 2001). These studies using avascular working heart preparations, including the amphibian heart (Sys et al. 1997), showed that this effect cannot be attributed to a rise in coronary flow or to factors released from vascular tissues. However, neither the NOS isoforms involved nor the mechanism of NO action was directly addressed.
Therefore, the aim of this work was to investigate the mechanism through which NO modulates cardiac performance in the isolated and perfused working heart of the European eel A. anguilla under loading conditions. As in previous studies (Imbrogno et al. 2001, 2003, 2004, 2006), we took the advantage of using eel hearts in which the heart is mostly perfused endoluminally (lacunary intracardiac supply) so that the nitrergic influences on myocardial performance can be directly assessed, independently from both the coronary reactivity and the vascular endothelium-produced NO, typical of mammalian paradigms. We demonstrate, to our knowledge, for the first time in a lower vertebrate heart, that the nitrergic modulation of the Frank–Starling response neither involves the endocardial endothelium (EE)/cGMP/PKG and cAMP/PKA pathways nor the Ryanodine receptors and L-type calcium channels, but acts through an Akt-mediated activation of eNOS-dependent NO production, which through PLN-S-nitrosylation-dependent mechanism can modulate the rate of SR Ca2+ reuptake and thus myocardial relaxation. Conceivably, this mechanism represents an important evolutionary step for the stretch-induced intrinsic regulation of the vertebrate heart.
2. Material and methods
(a). Animals
We used specimens of freshwater European eel (A. anguilla L.), weighing 78.5 ± 2.3 g (mean ± s.e.m., n = 98). Fish were provided by a local hatchery and kept at room temperature (18–20°C) for 5–7 days. Each eel was anaesthetized with tricaine methane sulphonate (Sigma Chemical Co., St Louis, MO, USA). In accordance with the accepted standards of animal care, the experiments were organized to minimize stress and number of animal used.
(b). Isolated and perfused working heart preparations
The hearts, removed and cannulated, were transferred to a perfusion chamber filled with Ringer's solution and connected to a perfusion apparatus as described by Imbrogno et al. (2001). Experiments were carried out at room temperature (18–20°C). For paced experiments, hearts were stimulated with an LE 12006 stimulator (frequency identical to that of control, non-paced hearts; pulse width fixed at 0.1 ms; voltage: 1.2 ± 0.1 V, means ± s.e.m.).
(c). Calculations
HR was calculated from pressure recording curves. CO was collected over 1 min and weighed; values were corrected for fluid density and expressed as volume measurements. The afterload (mean aortic pressure) was calculated as two-thirds diastolic pressure plus one-third maximum pressure. SV (ml kg−1; CO/HR) was used as a measure of ventricular performance; changes in SV were considered to be inotropic effects.
(d). Experimental protocols
(i). Basal conditions
Isolated perfused hearts were allowed to maintain a spontaneous rhythm for up to 15–20 min. In all experiments, the control conditions were a mean output pressure of about 3.00 kPa, with a CO set to 10 ml min−1 kg body mass by appropriately adjusting the filling pressure. These values are within the physiological range (for references, see Imbrogno et al. 2001).
(ii). Drug application
After the 15–20 min of control period, hearts were perfused for 20 min with Ringer's solution enriched with S-nitroso-N-acetyl penicillamine (SNAP) or Ryanodine (RYR) or Thapsigargin at increasing concentrations to construct cumulative concentration–response curves.
(iii). Frank–Starling response
To assess the interaction between NO and the Frank–Starling response, a Starling curve was generated (baseline condition). After baseline assessment, the input pressure was returned to the control condition and a second Starling curve was generated in the presence of the nNOS inhibitors vinyl-l-N-5-(1-imino-3-butenyl)-l-ornithine (l-VNIO) and 7-nitroindazole (7-NI), the NO donor SNAP and its non-nitrosylated parent molecule N-acetylpenicillamine (NAP), the phosphatidilinositol-3-kinase (PI3K) antagonist Wortmannin, the cGMP analogue 8-bromoguanosine 3′,5′-cyclic monophosphate (8-Br-cGMP), the soluble guanylyl cyclase (sGC) specific inhibitor 1H-(1,2,4)oxadiazole-(4,3-a)quinoxalin-1-one (ODQ), the PKG and the PKA antagonists KT5823 and KT5720, respectively, the cGMP-inhibited PDE3 blocker Milrinone, and after inhibition of L-type calcium channels by Diltiazem, or Ryanodine receptors by RYR, or SERCA2a pumps by Thapsigargin. In addition, the Frank–Starling response was also studied after inducing functional damage of the ventricular EE with the detergent Triton X-100. After baseline curve, 0.1 ml of Triton X-100 at a concentration of 0.05 per cent was injected through a needle inserted into the posterior ventral region of the ventricular wall (for further details, see Imbrogno et al. 2001).
The time factor (i.e. the ‘memory’ of the heart) of loading stimulation was excluded according to our previous studies (Imbrogno et al. 2001, 2003).
(iv). Western blotting
To compare the differences in the protein expression pattern, eel ventricle samples (n = 3 for each condition) were rapidly immersed in liquid nitrogen and stored at −80°C. The ventricles were prepared according to Amelio et al. (2006). The proteins were separated on 6 per cent SDS–PAGE gels (for eNOS and phospho-eNOS detection) or 15 per cent SDS–PAGE gels (for phospho-Ser16PLN), transferred to membrane, blocked with non-fat-dried milk and incubated overnight at 4°C with polyclonal rabbit anti-eNOS antibody (Sigma) or polyclonal rabbit anti phospho-eNOS antibody (Santa Cruz Biotechnology) or polyclonal rabbit anti phospho-Ser16PLN antibody (Santa Cruz Biotechnology) diluted 1 : 500 in TBS-T containing 5 per cent non-fat dry milk. The anti-rabbit secondary antibody peroxidase linked (Amersham) was diluted 1 : 5000 in TBS-T containing 5 per cent non-fat dry milk.
(v). Biotin switch assay
Ventricles were homogenized on ice in 20 mM Tris pH 7.5, 150 mM NaCl, 1 per cent Igepal CA 630, 0.5 per cent sodium deoxycholate, 1 mM EDTA, 0.1 per cent SDS, 200 mM sodium orthovanadate and Protease Inhibitor Cocktail, using a polytron tissue grinder. The homogenate was centrifuged at 4°C for 40 min at 13 000g. The supernatant containing cytosolic proteins was collected and proteins were quantified with Bradford reagent; the pellet containing membrane proteins was resuspended in homogenization buffer and proteins were quantified with Bradford reagent.
The biotin switch assay was performed as previously described (Jaffrey & Snyder 2001).
To detect biotinylated proteins, samples from the biotin switch assay were separated on 15 per cent SDS–PAGE gels, transferred to membrane, blocked with non fat dried milk and incubated with streptavidin-peroxidase diluted 1 : 5000 for 1 h. In additional experiments, the membrane for S-nitrosylation detection was stripped and reprobed using an anti-PLN antibody (Santa Cruz Biotechnology).
(vi). Immunodetection and densitometric analysis
The immunodetection was performed using an enhanced chemiluminescence kit (ECL PLUS, Amersham). Autoradiographs were obtained by exposure to X-ray films (Hyperfilm ECL, Amersham). Immunoblots were digitalized and the densitometric analysis of the bands was carried out using NIH Image 1.6 for a Macintosh computer based on 256 grey values (0 = white; 256 = black).
(e). Statistics
Percentage changes were evaluated as means ± s.e.m. of percentage changes obtained from individual experiments. Because each heart acted as its own control, the statistical significance of differences within group was assessed using the paired Student's t-test (p < 0.05). Comparisons between groups were made using two-way analysis of variance (ANOVA). Significant differences were detected using Duncan's multiple-range test (p < 0.05).
The results of absorbance measurements and the grey values obtained from the densitometric analysis were expressed as means ± s.e.m. of determinations for each sample. To test the difference between the groups, Student's t-test was performed. Statistical significance was established at p < 0.001.
(f). Drugs and chemicals
8-Br-cGMP, ODQ, KT5720, NAP and Triton X-100 were purchased from Sigma Chemical Company. RYR, Wortmannin, Diltiazem, KT5823 and Thapsigargin were purchased from Calbiochem (Milan). l-VNIO, 7-NI and SNAP were purchased from Vinci-Biochem (Florence).
3. Results
(a). Effects of S-nitroso-N-acetyl penicillamine and nitric oxide synthase inhibition
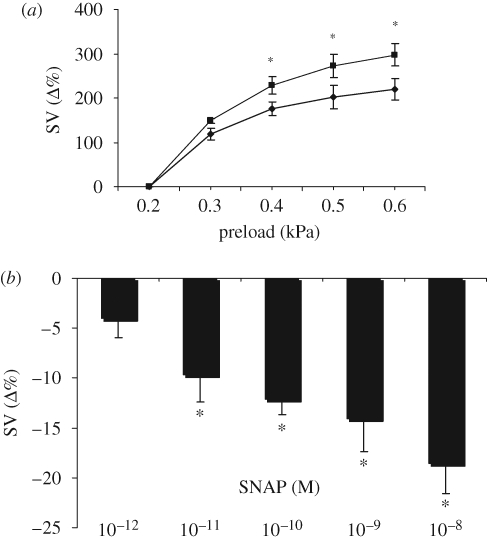
We have previously reported that intracardiac NO increases the sensitivity of the in vitro eel heart to filling pressure changes. This was demonstrated by the reduction of the preload-induced increases of SV obtained in the presence of the NOS inhibitor l-N5(1-iminoethyl)ornithine (l-NIO) (Imbrogno et al. 2001). To establish whether exogenous NO also affects the preload response, this was evaluated before and after treatment with the NO donor SNAP (10−9 M). Pre-treatment with SNAP induced a significant increase of Starling response (figure 1a). Of note, the non-nitrosylated parent molecule of SNAP, NAP (10−9 M) did not modify Starling response (at the max preload of 0.6 kPa, the percentage increment of SV was 214 ± 32% in NAP-treated hearts versus 222 ± 28% for control). Under basal conditions, SNAP decreased SV, the effect being significant from 10−11 M (figure 1b).
Figure 1.
(a) Effect of preload elevation on SV under control conditions (filled diamonds) and after treatment with the NO donor SNAP (10−9 M, filled squares). Percentage changes were evaluated as mean ± s.e.m. (n = 5). Comparison between groups was made using two-way ANOVA analysis, *p < 0.05. (b) Dose–response curve for SNAP (10−12–10−8 M). Percentage changes were evaluated as means ± s.e.m. (n = 4). Significance of differences from control values (t-test); *p < 0.05.
To discriminate the NOS isoform involved in the NO-dependent modulation of Frank–Starling response, heart preparations were exposed to two specific nNOS antagonists, l-VNIO (10−5 M) and 7-NI (10−5 M). nNOS inhibition did not influenced the response to preload changes, ruling out the involvement of nNOS and supporting a role for eNOS. At the max preload of 0.6 kPa, the percentage increments of SV were: 154 ± 22% in l-VNIO-treated hearts versus 167 ± 19% for control; 148 ± 22% in 7-NI-treated hearts versus 172 ± 28% for control.
(b). Role of endocardial endothelium-dependent nitric oxide production
The heart of A. anguilla possesses a highly trabeculated ventricle with an extensive EE surface that, being an important source of NO, modulates basal and chemically stimulated cardiac performance (Imbrogno et al. 2001, 2003). To assess the role of EE-derived NO on the eel heart response to preload, double Starling curves were generated in the presence of Triton X-100. The EE impairment caused by Triton X-100 (0.05%) did not modify the response to preload increases, thus excluding an EE source of NO in the short-term nitrergic modulation of Starling response (at the max preload of 0.6 kPa, the percentage increment of SV was 206 ± 38% in Triton-treated hearts versus 207 ± 21% for control).
(c). Preload-induced endothelial nitric oxide synthase activation
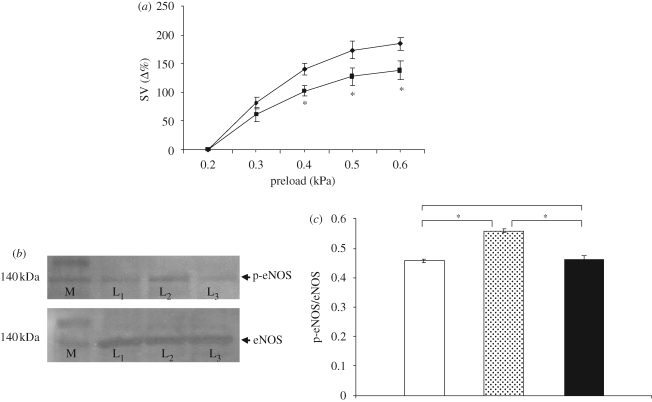
It has been reported in mammals that stretching of cardiac muscle induces a PI3K-dependent phosphorylation of the eNOS (Petroff et al. 2001). This mechanism, which involves membrane caveolae (regarding their occurrence in fish heart, see Di Maio & Block 2008) and cytoskeleton (Casadei & Sears 2003), causes a sustained increase in enzyme activity, probably by reducing the dissociation of calmodulin from activated eNOS (McCabe et al. 2000), thus enhancing NO production. To verify our hypothesis, i.e. the PI3K involvement in the preload-induced NO production, we studied the Frank–Starling response before and after treatment with the PI3K antagonist Wortmannin (10−9 M). The results obtained showed a significant decrease of Starling response, suggesting that also in the eel, mechanical stimuli could activate, via a PI3K-dependent pathway, the release of NO, which in turn modulates the cardiac heterometric mechanism (figure 2a).
Figure 2.
(a) Effect of preload on SV under control conditions (filled diamond) and after treatment with Wortmannin (10−9 M, filled square) . Percentage changes were evaluated as mean ± s.e.m. (n = 5). Comparison between groups was made using two-way ANOVA analysis, *p < 0.05. (b) Western blotting of p-eNOS and total eNOS in heart extracts (M, marker; L1, basal conditions; L2, Starling response; L3, Starling response in the presence of Wortmannin). (c) Densitometric quantification of p-eNOS over total eNOS ratio (unfilled bar, basal conditions; grey bar, Starling response; black bar, Starling + Wortmannin). Data are means ± s.e.m. of five determinations for each animal (n = 3). Statistical differences were evaluated by Student's t-test; *p < 0.001.
(d). Phospho-endothelial nitric oxide synthase expression
Western blotting analysis showed the presence of phopsho-eNOS in the hearts of A. anguilla either under basal conditions (physically unstimulated hearts) or after Starling response or after Starling response performed in the presence of Wortmannin (10−9 M). In fact, an immunoreactive band of approximatively 140 kDa, corresponding to the known p-eNOS molecular weight, was detected. The p-eNOS expression has been normalized by Western blotting analysis performed on the same extracts incubated with polyclonal anti-eNOS antibody (figure 2b). Densitometric quantification of the blots revealed an increase of 22 per cent (p < 0.001) of phospho-eNOS expression after the Starling response with respect to basal conditions. This value significantly decreased by 17 per cent (p < 0.001) when Starling curves were performed after Wortmannin treatment, showing a non-significant decrement of 1.5 per cent with respect to basal conditions (figure 2c).
(e). Nitric oxide intracellular signalling
(i). Role of cyclic guanosine monophosphate-mediated pathways
In the heart, NO is known to activate sGC to produce cGMP, which in turn may signal through the cGMP-dependent PKG or by modulating PKA via the cGMP-modulated phosphodiesterases of cAMP (PDE). We have previously shown that also in the working eel heart, intracardiac NO exerts an important modulation of mechanical performance via sGC signalling (see Imbrogno et al. 2001, 2003, 2004, 2006). Of note, treatments with either a specific inhibitor of sGC ODQ (10−5 M), or with the cGMP-activated PKG inhibitor KT5823 (10−6 M), or with cGMP analogue 8-Br-cGMP (10−6 M), or with KT5720 (10−6 M) had no effects on the Frank–Starling response. At the max preload of 0.6 kPa, the percentage increments of SV were: 174 ± 21% in ODQ-treated hearts versus 198 ± 23% for control; 176 ± 20% in 8-Br-cGMP-treated hearts versus 164 ± 11% for control; 180 ± 16% in KT5823-treated hearts versus 200 ± 8% for control; 204 ± 45% in KT5720-treated hearts versus 211 ± 20% for control (see the corresponding figure in the electronic supplementary material). Similarly, block of the cGMP-inhibited PDE3 with Milrinone (10−6 M) (data not shown) had no influence on the eel heart response to preload increases. Taken together, these findings exclude the involvement of cGMP signalling downstream of myocardial NO production in the nitrergic modulation of the Frank–Starling response.
(ii). Role of calcium
Recent literature in mammals has designed NO as a key modulator of Ca2+ cycling in the stretched myocytes. Under stretch conditions, NO through S-nitrosylation of reactive thiols has been shown to stimulate L-type Ca2+ channel function (Sun et al. 2006) and to enhance the Ryanodine receptor open probability (Petroff et al. 2001). Moreover, by activating SERCA2a pumps, it can increase calcium reuptake into the SR (Massion et al. 2005).
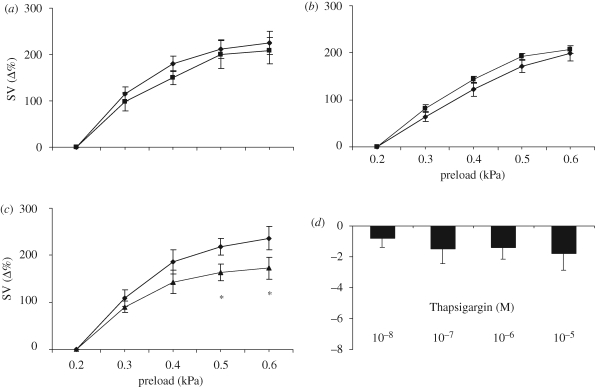
In the eel heart, the role of Ca2+ in response to preload increases was pharmacologically tested before and after treatment with inhibitors of either L-type calcium channels (Diltiazem, 10−7 M) or Ryanodine receptors (RYR, 10−7 M) or SERCA2a pumps (Thaspigargin, 10−7 M). While RYR and Diltiazem pre-treatments did not influence the preload-induced increases in SV, Thapsigargin significantly reduced them, suggesting that the nitrergic modulation of the Frank–Starling response in the eel heart occurs via an NO modulation of the rate of SR Ca2+ reuptake (figure 3a–c). Under basal conditions, RYR significantly decreased SV at the concentration of 10−7 M (data not shown); on the contrary, Thapsigargin per se did not modify basal mechanical performance (figure 3d). Concentration–response curves for Diltiazem significantly decreased SV from the concentration of 25 × 10−9 M (Imbrogno et al. 2004).
Figure 3.
Effect of preload on SV under control conditions (filled diamonds) and after treatment with (a) Diltiazem (10−7 M) (filled squares), (b) RYR (10−7 M) (filled squares) and (c) Thapsigargin (10−7 M) (filled triangles). Percentage changes were evaluated as mean ± s.e.m. (n = 5 for each group). Comparison between groups was made using two-way ANOVA analysis, *p < 0.05. Dose–response curve for (d) Thapsigargin (10−8–10−5 M). Percentage changes were evaluated as mean ± s.e.m. (n = 4).
(f). Analysis of S-nitrosylated proteins
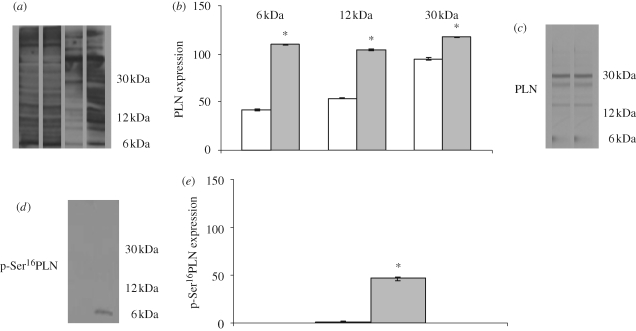
To assess whether the Starling response induces protein S-nitrosylation in the perfused hearts, using the biotin switch method, we analysed the pattern of proteins containing S-nitrosylated cysteines in homogenates of control hearts and Starling-treated hearts. The analysis of S-nitrosylated proteins in the eel cardiac tissues clearly revealed that stretch increases the degree of S-nitrosylation of a broad range of proteins. This range includes the protein band that migrates at the same location where PLN was determined by Western blot, as a monomer (6 kDa), dimer (12 kDa) and pentamer (30 kDa) (figure 4a). Densitometric quantification of the blots revealed statistically significant differences between control hearts and preload-treated hearts (figure 4b). The increment in the S-nitrosylation signal at this precise location was corroborated using an anti-PLN antibody (figure 4c).
Figure 4.
(a) Western blot of S-nitrosylated proteins in heart homogenates: cytosolic (lanes 1 and 2) and membrane (lanes 3 and 4) protein fractions; control heart (lanes 1 and 3) and Starling-treated heart (lanes 2 and 4). (b) Amount of S-nitrosylation at the migration position corresponding to the PLN as a monomer (6 kDa), dimer (12 kDa) and pentamer (30 kDa). (c) Membrane stripped and incubated with anti PLN antibody. Western blot of (d) phospholamban phosphorylated at Ser16 and (e) densitometric analysis. Data are means ± s.e.m. of five determinations for each animal (n = 3). Statistical differences were evaluated by Student's t-test; *p < 0.001. (b) and (e) Unfilled bar, control; grey bar, Starling.
(g). Phospho-Ser16-phospholamban expression
Western blotting analysis showed the phosphorylation of PLN in the Starling-treated hearts. A band of approximatively 6 kDa, corresponding to the PLN monomer molecular weight, was detected (figure 4d). The densitometric analysis of the intensity signal is shown in figure 4e.
4. Discussion
Using the in vitro working heart of the eel A. anguilla, in which the influences of coronary reactivity and vascular-generated autacoids (e.g. NO) are excluded, we demonstrate that the endogenous NO-induced modulation of the Frank–Starling response involves an Akt-mediated activation of eNOS, is independent from the cGMP/PKG and cAMP/PKA pathways, as well as from Ryanodine receptors and L-type calcium channels, but functions through an S-nitrosylation of PLN with consequent activation of SERCA2a pumps.
(a). Role of exogenous nitric oxide
Under basal conditions (physically unstimulated hearts), treatment with SNAP (from 10−12 to 10−8 M) elicited a concentration-dependent decrease of SV, significant from the concentration of 10−11 M. This result confirms previous findings (Imbrogno et al. 2001), showing that another NO donor, 3-morpholinosydnonimine (SIN-1), at the same concentrations induced effects of similar magnitude. The confirmation in the eel heart that NO tonically modulates mechanical performance in absence of loading and agonists stimulations is an important point in view of the apparently contrasting findings on the NO-induced contractile myocardial effects, probably owing to species-related differences and also to problems related to the kind of experimental design employed (Shah 1996; Tota et al. 2005). The lower vertebrate avascular heart preparations, working ‘physiologically’ (reproducing the haemodynamic responses of the in vivo heart), free of extrinsic nervous and humoral influences, appear well suited to analyse directly the cardiac autocrine/paracrine NO role (Imbrogno et al. 2001, 2003; Acierno et al. 2008).
The significant differences between the Starling curves obtained with and without the exogenous NO donor SNAP (10−9 M), evaluated by two-way ANOVA analysis, but not with the non-nitrosylated analogue of SNAP, NAP (10−9 M), highlight the relevance of the nitrergic modulation of the cardiac heterometric mechanism in the eel. Noteworthy, however, some controversy about the interpretation of the NO-elicited effects has been recently related to the use of NO donors as experimental tools, since these agents may not necessarily reproduce the physiological effects of the constitutively expressed NOSs, which generate NO in a temporally and spatially restricted manner and also depend upon the specific stimulus for NO release (Zhang et al. 2008 and references therein). Therefore, to study the NO-activated downstream transduction mechanism in response to stretch, we focused our attention on the endogenously produced NO, deliberately avoiding the use of NO donors.
(b). Stretch-dependent activation of endothelial nitric oxide synthase
In mammals, the stretch-induced NO modulation of cardiac performance has been ascribed to NO generation by both the constitutive NOS isoforms (eNOS and nNOS) (Massion et al. 2005). In the eel, we observed that while pre-treatment with an unspecific NOS inhibitor, l-NIO, significantly reduced the preload-induced increase in SV (see Imbrogno et al. 2001), this was not influenced by the two nNOS antagonists l-VNIO and 7-NI, ruling out the nNOS involvement while supporting a role for eNOS. In line with these results, Tota et al. (2005) reported the presence of eNOS, but not of nNOS, in the ventricular myocardium of the eel. Although these results do not allow us to exclude the presence of nNOS in the eel myocardiocytes, they, however, suggest an exclusive involvement of eNOS in the Frank–Starling response. Interestingly, while myocardial nNOS is considered to exert a primary role in the regulation of basal and adrenergically stimulated contractions, eNOS seems preferentially activated by mechanical stimuli, acting as a major myocardial regulator in the stretch-induced response (Sears et al. 2004 and references therein).
The exact mechanism responsible for the stretch-induced augmentation of NO production in mammals has not yet been completely elucidated, however there is evidence suggesting an implication of a tyrosine kinase inhibitor-sensitive pathway, involving membrane caveolae and cytoskeleton (Fleming & Busse 1999). In the teleost heart, the presence of caveolae, junctional SR profiles and the network of free SR has been described in detail by Di Maio & Block (2008). It is known that the serine–threonine kinase Akt phosphorylates eNOS in vitro as well as in vivo (Dimmeler et al. 1999), thereby causing a sustained increase in the enzyme activity (McCabe et al. 2000). Also nNOS possesses an Akt phosphorylation motif, but the phosphorylation effects on enzyme activity are inconsistent or mostly inhibitory (Dinerman et al. 1994). On the basis of this mammalian framework, we found that in the eel heart, the nitrergic modulation of the Starling response involves a PI3K-Akt-mediated phosphorylation of eNOS. This was demonstrated by the significant reduction of Starling response observed after inhibition of PI3K by Wortmannin pre-treatment and further confirmed by Western blotting analysis on heart homogenates incubated with anti phospho-eNOS antibody, showing a significant increase in the phosphorylated isoform in Starling-treated hearts with respect to the basal conditions. In addition, the loss of phospho-eNOS expression increment in the Starling-treated hearts in presence of Wortmannin strongly supports the hypothesis of a stretch-induced PI3K-Akt-eNOS pathway.
(c). Intracellular signalling and nitric oxide downstream effectors
Several in vitro (Paolocci et al. 2000) and intact animal (Preckel et al. 1997) studies suggested that both cGMP-dependent and -independent mechanisms contribute to the NO-mediated influence on myocardial function. NO activates sGC by binding to its haeme moiety, leading to cGMP production, consequent PKG activation and a cascade of biological signalling events (Layland et al. 2002). Moreover, NO can react with thiol residues of numerous compounds or proteins. cGMP/PKG activation is considered a major transduction pathway for NO cardiac regulation, whereby, among other effects, it induces myofilament desensitization to Ca2+, thought to be responsible for accelerated myocardial relaxation through troponin I phosphorylation at Ser23/24 (Layland et al. 2002). In the working eel heart, the NO-induced cGMP-PKG pathway remarkably modulates mechanical performance (see, for references, Tota et al. 2005). For example, it tonically decreases SV either under basal condition (Imbrogno et al. 2001) or following endoluminal chemical stimulations of AT1 angiotensin II receptors (Imbrogno et al. 2003), or β3 adrenergic receptors (Imbrogno et al. 2006), or exposure to the anti-adrenergic cardio-inhibitory peptide vasostatin I (Imbrogno et al. 2004). Importantly, however, the present data strongly suggest that the cGMP-PKG mediated mechanisms are not involved in the eel heart under loading conditions, since the Frank–Starling response is not influenced by pre-treatment with either the sGC inhibitor ODQ or the cGMP analogue 8-Br-cGMP or the PKG antagonist KT5823. This, together with the data that exclude the involvement of the EE in the stretch-induced NO production, implies the activation of different cardiac NO-signal processes, such as those related to NOS isoforms compartmentation and differences in their mode of stimulation, as well as the diffusion distance of NO within intra-myocyte molecular targets and final effectors, all of which providing now the rationale for resolving previous contradictions existing in NO cardiac biology (Seddon et al. 2007).
Recent evidence in mammals has designated NO as a key modulator of Ca2+ cycling in the stretched myocytes. In rat LV myocytes, stretch-induced increase in intracellular Ca2+ transient appears correlated to an increase in Ryanodine receptors open probability and SR Ca2+ release, an effect attributed to a direct S-nitrosylation of reactive thiols associated with a stretch-induced NO production (see Casadei & Sears 2003 for references). NO-induced regulation of calcium entry on a beat-to-beat basis is also suggested by S-nitrosylation of thiols on L-type Ca2+ channels associated with modulation of their calcium current and of the amplitude of contractile shortening (Sears et al. 2003). That myocardial NO production by nNOS and/or eNOS may tonically stimulate SERCA2a activity is also supported by an NO-dependent myocardial relaxation associated with SERCA2a-induced increase in calcium reuptake into the SR (Massion et al. 2005), as well as by a decrease in both SR Ca2+-ATPase activity and Ca2+ uptake in SR microvescicles from nNOS and/or eNOS knockout mice (Zhou et al. 2002).
Interestingly, the idea that NO may influence SERCA2a activity is also now supported in the eel heart by our results, which therefore suggest that this NO-signalling mechanism has been maintained over evolutionary time, being properly integrated into an established developmental and physiological cardiac system. In fact, we observed that the preload-induced increases in SV were not significantly affected by pre-treatment with Diltiazem or RYR, excluding a role for both L-type and RYR calcium channels, respectively, while they were significantly reduced by Thapsigargin-dependent inhibition of SERCA2a pumps. Although calcium transients were not directly measured in this study, we hypothesize that the effects of NO on the stretch-dependent increase in SV is primarily attributable to a quickening of calcium removal and thus of muscle relaxation.
The cardiac Ca-ATPase, SERCA2a, is regulated by PLN, a small membrane protein which, in its dephosphorylated state, inhibits SR Ca2+ sequestration by SERCA2a (Kimura et al. 1997). As supported by mutagenesis studies, the PLN monomer is the active isoform, dissociates from the pentamer, which acts as a reservoir of monomers, binds to SERCA2a and inhibits the pump by direct protein interaction (Reddy et al. 1999). PLN phosphorylation at Ser16 by PKA relieves its inhibitory action on SERCA2a, causing an increased rate of myocardial relaxation (Schmidt et al. 2001). However, recent evidence suggests an alternative, phosphorylation-independent mechanism of SERCA2a-regulated Ca2+ reuptake. In fact, Froehlich et al. (2008) showed that activation of SERCA2a could be achieved by modifying critical thiol residues in the PLN, which through protein conformation change relieves the inhibition of the pump. In particular, the covalent adduction of a nitroso group to a cysteine thiol side chain has recently emerged as a major mechanism by which NO mediates a large number of intracellular processes (Hess et al. 2005) and an increase in proteins S-nitrosylation by NO was recently observed, also in eel cardiac tissues (Cerra et al. 2009).
In the fish heart, both the presence of PLN in myocyte SR and the role of PLN phosphorylation in SERCA2a activation have been reported (Will et al. 1985; Castilho et al. 2007), but the role of PLN S-nitrosylation in the pump activation is heretofore completely unknown. As in mammals, also in fish, the amino acid sequence of PLN (see GenBank accession numbers for zebrafish: XM_701636.1 and puffer fish: CAG06667, DeWitt et al. 2006) includes cysteine residues, which are a potential target for S-nitrosylation. Using the biotin switch method (Jaffrey & Snyder 2001), we demonstrated, to our knowledge, for the first time in a non-mammalian heart, that preload increases can induce a S-nitrosylation of PLN. Moreover, Western blotting analysis showed a Starling-induced phosphorylation of PLN monomeric form (figure 4d). These results suggest that in fish heart, in conjunction with the classical PLN phosphorylation-dependent pathway, the SERCA2a pump catalytic efficiency can be directly modulated by NO through a mechanism which involves PLN S-nitrosylation. The possibility that, in addition to a nitrosylation of PLN, NO may directly inactivate specific phosphatases (Zhang et al. 2008) could also account for the Starling-induced phosphorylation of PLN. This issue challenges further study.
In conclusion, the present study demonstrates the existence of an important nitrergic modulation of the Frank–Starling response in the fish heart, which occurs via a cGMP-independent pathway, involves an Akt-mediated activation of eNOS-dependent NO production that, in turn, modulates the rate of SR Ca2+ reuptake through PLN S-nitrosylation. This supports the hypothesis that in the fish heart the ‘beat-to-beat’ regulation, i.e. the principal mechanism of intrinsic regulation of CO, is directly modulated by myocardial autocrine NO through a non-classical signalling that by-passes the activation of transduction cascades and the involvement of second messengers. Therefore, the challenging question arises whether, and to what extent, this mechanism represents an old evolutionary fish-specific or, instead, a well conserved (for its importance) universal trait of the vertebrate heart. Since, despite the rapidly growing number of data published on NO biology during the last 20 years, about 99 per cent of the studies are referred to (few) mammalian species (Moroz & Kohn 2007), here we propose that the investigation of evolutionary models, commonly recognized as ‘non-model species’, can provide highly useful natural tools to enhance our multilevel phylogenetic and molecular understanding regarding the involvement of myocardial S-nitrosylation processes in the NO-modulated regulation of the vertebrate heart.
Acknowledgements
In accordance with the accepted standards of animal care, the experiments were organized to minimize stress and number of animals used.
This work was supported by ‘Ministero dell'Istruzione, dell'Università e della Ricerca’ (e.g. MURST 60%) and by the Italian National Research Program in Antarctica (PNRA).
References
- Acierno R., Gattuso A., Guerrieri A., Mannarino C., Amelio D., Tota B.2008Nitric oxide modulates the frog heart ventricle morphodynamics. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 151, 51–60 (doi:10.1016/j.cbpa.2008.05.012) [DOI] [PubMed] [Google Scholar]
- Amelio D., Garofalo F., Pellegrino D., Giordano F., Tota B., Cerra M. C.2006Cardiac expression and distribution of nitric oxide synthases in the ventricle of the cold-adapted Antarctic teleosts, the hemoglobinless Chionodraco hamatus and the red-blooded Trematomus bernacchii. Nitric Oxide 15, 190–198 (doi:10.1016/j.niox.2005.12.007) [DOI] [PubMed] [Google Scholar]
- Balligand J. L., Feron O., Dessy C.2009eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol. Rev. 89, 481–534 (doi:10.1152/physrev.00042.2007) [DOI] [PubMed] [Google Scholar]
- Casadei B., Sears C. E.2003Nitric-oxide-mediated regulation of cardiac contractility and stretch responses. Prog. Biophys. Mol. Biol. 82, 67–80 (doi:10.1016/S0079-6107(03)00006-3) [DOI] [PubMed] [Google Scholar]
- Castilho P. C., Landeira-Fernandez A. M., Morrissette J., Block B. A.2007Elevated Ca2+ ATPase (SERCA2) activity in tuna hearts: comparative aspects of temperature dependence. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 148, 124–132 (doi:10.1016/j.cbpa.2007.03.033) [DOI] [PubMed] [Google Scholar]
- Cerra M. C., Angelone T., Parisella M. L., Pellegrino D., Tota B.2009Nitrite modulates contractility of teleost (Anguilla anguilla and Chionodraco hamatus, i.e. the Antarctic hemoglobinless icefish) and frog (Rana esculenta) hearts. Biochim. Biophys. Acta 1787, 849–855 (doi:10.1016/j.bbabio.2009.03.008) [DOI] [PubMed] [Google Scholar]
- DeWitt M. M., MacLeod H. M., Soliven B., McNally E. M.2006Phospholamban R14 deletion results in late-onset, mild, hereditary dilated cardiomyopathy. J. Am. Coll. Cardiol. 48, 1396–1398 (doi:10.1016/j.jacc.2006.07.016) [DOI] [PubMed] [Google Scholar]
- Di Maio A., Block B. A.2008Ultrastructure of the sarcoplasmic reticulum in cardiac myocytes from Pacific bluefin tuna. Cell Tissue Res. 334, 121–134 (doi:10.1007/s00441-008-0669-6) [DOI] [PubMed] [Google Scholar]
- Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A. M.1999Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399, 601–605 (doi:10.1038/21224) [DOI] [PubMed] [Google Scholar]
- Dinerman J. L., Steiner J. P., Dawson T. M., Dawson V., Snyder S. H.1994Cyclic nucleotide dependent phosphorylation of neuronal nitric oxide synthase inhibits catalytic activity. Neuropharmacology 33, 1245–1251 (doi:10.1016/0028-3908(94)90023-X) [DOI] [PubMed] [Google Scholar]
- Fleming I., Busse R.1999Signal transduction of eNOS activation. Cardiovasc. Res. 43, 532–541 (doi:10.1016/S0008-6363(99)00094-2) [DOI] [PubMed] [Google Scholar]
- Froehlich J. P., et al. 2008Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry 47, 13 150–13 152 (doi:10.1021/bi801925p) [DOI] [PubMed] [Google Scholar]
- Hess D. T., Matsumoto A., Kim S. O., Marshall H. E., Stamler J. S.2005Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 6, 150–166 (doi:10.1038/nrm1569) [DOI] [PubMed] [Google Scholar]
- Icardo J. M., Imbrogno S., Gattuso A., Colvee E., Tota B.2005The heart of Sparus auratus: a reappraisal of cardiac functional morphology in teleosts. J. Exp. Zoolog. A Comp. Exp. Biol. 303, 665–675 (doi:10.1002/jez.a.195) [DOI] [PubMed] [Google Scholar]
- Imbrogno S., De Iuri L., Mazza R., Tota B.2001Nitric oxide modulates cardiac performance in the heart of Anguilla anguilla. J. Exp. Biol. 204, 1719–1727 [DOI] [PubMed] [Google Scholar]
- Imbrogno S., Cerra M. C., Tota B.2003Angiotensin II-induced inotropism requires an endocardial endothelium-nitric oxide mechanism in the in-vitro heart of Anguilla anguilla. J. Exp. Biol. 206, 2675–2684 (doi:10.1242/jeb.00468) [DOI] [PubMed] [Google Scholar]
- Imbrogno S., Angelone T., Corti A., Adamo C., Helle K. B., Tota B.2004Influence of vasostatins, the chromogranin A-derived peptides, on the working heart of the eel (Anguilla anguilla): negative inotropy and mechanism of action. Gen. Comp. Endocrinol. 139, 20–28 (doi:10.1016/j.ygcen.2004.07.008) [DOI] [PubMed] [Google Scholar]
- Imbrogno S., Angelone T., Adamo C., Pulerà E., Tota B., Cerra M. C.2006Beta3-Adrenoceptor in the eel (Anguilla anguilla) heart: negative inotropy and NO-cGMP-dependent mechanism. J. Exp. Biol. 209, 4966–4973 (doi:10.1242/jeb.02595) [DOI] [PubMed] [Google Scholar]
- Jaffrey S. R., Snyder S. H.2001The biotin switch method for the detection of S-nitrosylated proteins. Sci. STKE 86, PL1. [DOI] [PubMed] [Google Scholar]
- Katz A. M.2002Ernest Henry Starling, his predecessors, and the ‘Law of the Heart’. Circulation 106, 2986–2992 (doi:10.1161/01.CIR.0000040594.96123.55) [DOI] [PubMed] [Google Scholar]
- Kimura Y., Kurzydlowski K., Tada M., MacLennan D. H.1997Phospholamban inhibitory function is activated by depolymerization. J. Biol. Chem. 272, 15 061–15 064 (doi:10.1074/jbc.272.24.15061) [DOI] [PubMed] [Google Scholar]
- Layland J., Li J. M., Shah A. M.2002Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J. Physiol. 540, 457–467 (doi:10.1113/jphysiol.2001.014126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion P. B., Pelat M., Belge C., Balligand J. L.2005Regulation of the mammalian heart function by nitric oxide. Comp. Biochem. Physiol. 142A, 144–150 [DOI] [PubMed] [Google Scholar]
- McCabe T. J., Fulton D., Roman L. J., Sessa W. C.2000Enhanced electron flux and reduced calmodulin dissociation may explain ‘calcium-independent’ eNOS activation by phosphorylation. J. Biol. Chem. 275, 6123–6128 (doi:10.1074/jbc.275.9.6123) [DOI] [PubMed] [Google Scholar]
- Moroz L. L., Kohn A. B.2007On the comparative biology of Nitric oxide (NO) synthetic pathways: parallel evolution of NO-mediated signaling. In Nitric oxide (eds Tota B., Trimmer B.), pp. 1–44 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- Olson R. K.1998The cardiovascular system. In The physiology of fishes (ed. Evans H. D.), pp. 129–154 Boca Raton, NY: CRC Press [Google Scholar]
- Paolocci N., Ekelund U. E. G., Isoda T., Ozaki M., Vandegaer K., Georgakopoulos D., Harrison R., Kass D. A., Hare M. J.2000Cyclic nucleotide independent positive inotropic effects of nitric oxide donors: potential role for nitrosylation. Am. J. Physiol. Heart Circ. Physiol. 279, H1982–H1988 [DOI] [PubMed] [Google Scholar]
- Petroff M. G. V., Kim S. H., Pepe S., Dessy C., Marban E., Balligand J. L., Sollott S. J.2001Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat. Cell Biol. 3, 867–873 (doi:10.1038/ncb1001-867) [DOI] [PubMed] [Google Scholar]
- Pinsky D. J., Patton S., Mesaros S., Brovkovych V., Kubaszewski E., Grunfeld S., Malinski T.1997Mechanical transduction of nitric oxide synthesis in the beating heart. Circ. Res. 81, 372–379 [DOI] [PubMed] [Google Scholar]
- Preckel B., Kojda G., Schlack W., Ebel D., Kottenberg K., Noack E., Thamer V.1997Inotropic effects of glyceryl trinitrate and spontaneous NO donors in the dog heart. Circulation 96, 2675–2682 [DOI] [PubMed] [Google Scholar]
- Prendergast B. D., Sagach V. F., Shah A. M.1997Basal release of nitric oxide augments the Frank–Starling response in the isolated heart. Circulation 96, 1320–1329 [DOI] [PubMed] [Google Scholar]
- Reddy L. G., Autry J. M., Jones L. R., Thomas D. D.1999Co-reconstitution of phospholamban mutants with Ca-ATPase reveals dependence of inhibitory function on phospholamban structure. J. Biol. Chem. 274, 7649–7655 (doi:10.1074/jbc.274.12.7649) [DOI] [PubMed] [Google Scholar]
- Schmidt A. G., Edes I., Kranias E. G.2001Phospholamban: a promising therapeutic target in heart failure? Cardiovasc. Drugs. Ther 15, 387–396 (doi:10.1023/A:1013381204658) [DOI] [PubMed] [Google Scholar]
- Sears C. E., Bryant S. M., Ashley E. A., Lygate C. A., Rakovic S., Wallis H. L., Neubauer S., Terrar D. A., Casadei B.2003Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ. Res. 92, e52–e59 (doi:10.1161/01.RES.0000064585.95749.6D) [DOI] [PubMed] [Google Scholar]
- Sears C. E., Ashley E. A., Casadei B.2004Nitric oxide control of cardiac function: is neuronal nitric oxide synthase a key component? Phil. Trans. R. Soc. Lond. B 359, 1021–1044 (doi:10.1098/rstb.2004.1477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon M., Shah A. M., Casadei B.2007Cardiomyocytes as effectors of nitric oxide signalling. Cardiovasc. Res. 75, 315–326 (doi:10.1016/j.cardiores.2007.04.031) [DOI] [PubMed] [Google Scholar]
- Shah A. M.1996Paracrine modulation of heart cell function by endothelial cells. Cardiovasc. Res. 31, 847–867 [PubMed] [Google Scholar]
- Shaul P. W.2002Regulation of endothelial nitric oxide synthase: location, location, location. Annu. Rev. Physiol. 64, 749–774 (doi:10.1146/annurev.physiol.64.081501.155952) [DOI] [PubMed] [Google Scholar]
- Shiels H. A., White E.2008The Frank–Starling mechanism in vertebrate cardiac myocytes. J. Exp. Biol. 211, 2005–2013 (doi:10.1242/jeb.003145) [DOI] [PubMed] [Google Scholar]
- Sun J., Picht E., Ginsburg K. S., Bers D. M., Steenbergen C., Murphy E.2006Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel α1 subunit and reduced ischemia/reperfusion injury. Circ. Res. 98, 403–411 (doi:10.1161/01.RES.0000202707.79018.0a) [DOI] [PubMed] [Google Scholar]
- Sys S. U., Pellegrino D., Mazza R., Gattuso A., Andries L. J., Tota B.1997Endocardial endothelium in the avascular heart of the frog: morphology and role of nitric oxide. J. Exp. Biol. 200, 3109–3118 [DOI] [PubMed] [Google Scholar]
- Tota B., Gattuso A.1996Heart ventricle pumps in teleosts and elasmobranchs: a morphodynamic approach. J. Exp. Zool. 275, 162–171 (doi:10.1002/(SICI)1097-010X(19960601/15)275:2/3<162::AID-JEZ8>3.0.CO;2-B) [Google Scholar]
- Tota B., Cimini V., Salvatore G., Zummo G.1983Comparative study of the arterial and lacunary systems of the ventricular myocardium of elasmobranch and teleost fishes. Am. J. Anat. 167, 15–32 (doi:10.1002/aja.1001670103) [DOI] [PubMed] [Google Scholar]
- Tota B., Acierno R., Agnisola C.1991Mechanical performance of the isolated and perfused heart of the haemoglobinless Antarctic icefish Chionodraco hamatus (Lonnberg): effects of loading conditions and temperature. Phil. Trans. R. Soc. Lond. B 332, 191–198 (doi:10.1098/rstb.1991.0049) [Google Scholar]
- Tota B., Amelio D., Pellegrino D., Ip Y. K., Cerra M. C.2005NO modulation of myocardial performance in fish hearts. Comp. Biochem. Physiol. 142, 164–177 (doi:10.1016/j.cbpb.2005.04.019) [DOI] [PubMed] [Google Scholar]
- Will H., Küttner I., Kemsies C., Vetter R., Schubert E.1985Comparative analysis of phospholamban phosphorylation in crude membranes of vertebrate hearts. Experientia 41, 1052–1054 (doi:10.1007/BF01952139) [DOI] [PubMed] [Google Scholar]
- Williams J. C., et al. 2006The sarcolemmal calcium pump, a-1 syntrophin, and neuronal nitric-oxide synthase are parts of a macromolecular protein complex. J. Biol. Chem. 281, 23 341–23 348 (doi:10.1074/jbc.M513341200) [DOI] [PubMed] [Google Scholar]
- Xu K. Y., Huso D. L., Dawson T. M., Bredt D. S., Becker L. C.1999Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc. Natl Acad. Sci. USA 96, 657–662 (doi:10.1073/pnas.96.2.657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. H., Zhang M. H., Sears C. E., Emanuel K., Redwood C., El-Armouche A., Kranias E. G., Casadei B.2008Reduced Phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ. Res. 102, 242–249 (doi:10.1161/CIRCRESAHA.107.164798) [DOI] [PubMed] [Google Scholar]
- Zhou L., et al. 2002Lack of nitric oxide synthase depresses ion transporting enzyme function in cardiac muscle. Biochem. Biophys. Res. Commun. 294, 1030–1035 (doi:10.1016/S0006-291X(02)00599-5) [DOI] [PubMed] [Google Scholar]