Abstract
Myco-heterotrophy is one of the longest-studied aspects of the mycorrhizal symbiosis, but there remain many critical, unanswered questions regarding the ecology and physiology of myco-heterotrophic plants and their associated fungi. The vast majority of all myco-heterotrophs studied to date have exhibited specificity towards narrow lineages of fungi, but it is unclear whether the loss of photosynthesis in these plants is contingent upon fungal specialization. Here, we examine the fungal associates of the myco-heterotroph Pyrola aphylla (Ericaceae) and its closest green relative Pyrola picta to determine the pattern of mycorrhizal specialization. Our findings show that both plant species associate with a range of root-inhabiting fungi, the majority of which are ectomycorrhizal taxa. This study provides the first example of a eudicotyledonous myco-heterotroph that is a mycorrhizal generalist, indicating that the loss of photosynthesis in myco-heterotrophs is not contingent upon fungal specialization.
Keywords: Pyrola, Ericaceae, myco-heterotrophy, mycorrhiza, specialization
1. Introduction
The understorey maintains the highest diversity of vascular plants in temperate forests (Battles et al. 2001; Whigham 2004). However, light, one of the critical resources for plant growth and establishment, is most limited in the forest understorey. Understorey plants have evolved numerous traits in order to survive in these light-limited environments, including slow growth, clonal reproduction and evergreen leaves for year-round photosynthesis (Whigham 2004). Some plants have circumvented this light limitation altogether by becoming partially or fully dependent on associations with fungi to meet their demands for carbon and other essential elements. These plants are referred to as myco-heterotrophs (Leake 1994). Some plants are only myco-heterotrophic for a portion of their life cycle, such as during germination, while others remain entirely dependent on fungi throughout their growth and establishment (Leake 1994). In the latter group, there are some species that act as carbon sinks and gain carbon via mycorrhizal fungi that they share with surrounding autotrophic plants. This tripartite interaction between a myco-heterotrophic plant, an autotrophic plant and a shared mycorrhizal fungus is an epiparasitism where the potential fitness costs to the fungus are still unknown (Bidartondo 2005). All myco-heterotrophic epiparasitisms are thought to have evolved from initial tripartite associations between two autotrophic plants sharing at least one mycorrhizal fungus (Bidartondo 2005). However, the ordering of the steps that lead to one plant defaulting on the mycorrhizal mutualism remains the subject of debate. One possibility is that prior to the transition to full myco-heterotrophy, plants may first be capable of partial myco-heterotrophy where the plant's carbon demands are met through both photosynthesis and fungi (Bidartondo et al. 2004; Selosse et al. 2004; Julou et al. 2005; Abadie et al. 2006).
Our current knowledge of the extent of myco-heterotrophy in the plant kingdom includes over 10 families and 400 species of land plants and involves many independent origins of the habit (Leake 2004). Convergent traits of myco-heterotrophic plants across families include small dust-like seeds, reduction of leaves to scales, few or no stomata, loss of chlorophyll and modified root systems or rhizomes that are heavily colonized with mycorrhizal fungi (Leake 1994). A hallmark of almost all myco-heterotrophic plants examined thus far is extreme specificity throughout wide geographical ranges to particular families, genera or even species of fungi (Bidartondo & Bruns 2002; Taylor et al. 2002) that include both saprotrophic (Ogura-Tsujita et al. 2009) and a suite of mycorrhizal fungi in the Basidiomycota, Ascomycota and Glomeromycota (Bidartondo et al. 2002; Taylor et al. 2002; Bidartondo & Bruns 2005). This phylogenetic tracking of certain fungal lineages by myco-heterotrophic plants is so strongly coupled that when sister ericaceous myco-heterotrophs (such as Sarcodes sanguinea Torr. and Pterospora andromedea Nutt.) that associate with sister species of Rhizopogon (Fr.) Nordholm are found in sympatry, adult plants’ roots only harbour their respective Rhizopogon species (Bidartondo & Bruns 2002). There are even examples of this high level of specificity at the plant genotypic level between individuals in the myco-heterotrophic species Corallorhiza maculata (Raf.) Raf. where a particular genotype will only associate with specific subclades in the Russulaceae (Bidartondo & Bruns 2002; Taylor et al. 2004). The few known exceptions to this pattern of specificity are albino mutants of normally green orchids in the genera Cephalanthera and Epipactis that were found to associate with the same suite of ectomycorrhizal (EM) fungi as green individuals (Selosse et al. 2004; Julou et al. 2005; Abadie et al. 2006). However, there are emerging examples of adult non-photosynthetic orchids from the tropics that show a lack of fungal specificity (Roy et al. in press).
While the evolutionary processes leading to this level of fungal specificity are unclear, two possible mechanisms are as follows. (i) Similar to partner filtering (selection of the most beneficial mutualist from a community of potential symbionts) by the plant, the myco-heterotroph has ‘chosen’ from the existing fungal community the best partner to meet its nutrient demands. (ii) Similar to partner filtering by the fungi, the myco-heterotroph, owing to its parasitic-like interaction with fungi, has been ‘rejected’ by members of the fungal community until a fungus is ‘tricked’ into associating with the plant (Bruns et al. 2002; Egger & Hibbett 2004). These mechanisms are not necessarily mutually exclusive and could act in tandem to determine the mycobiont of adult myco-heterotrophic plants. In either case, the maintenance of a carbon supply is paramount for the survival of the myco-heterotroph, and it has been argued that once an appropriate fungal partner has been found, prior to the loss of photosynthesis, the plant fine-tunes its physiology to adapt to that particular fungus and is therefore incapable of broad host jumps (Bidartondo & Bruns 2002).
Within the plant family Ericaceae, the mainly myco-heterotrophic subfamily Monotropoideae currently contains three tribes: Pterosporeae, Monotropeae and Pyroleae (Kron & Johnson 1997; Kron et al. 2002). Most species within this subfamily produce large quantities of dust seeds that lack an endosperm and are therefore fully dependent in the earlier stages of development on myco-heterotrophic nutrition (Leake 1994). While all species in the tribes Pterosporeae and Monotropeae remain fully myco-heterotrophic throughout their life cycle, most members of Pyroleae form leaves and are capable of fixing carbon through photosynthesis. At least one species in Pyroleae (Ericaceae), Pyrola aphylla Sm., is fully myco-heterotrophic (Camp 1940; Haber 1987; Hynson et al. 2009), and there is some evidence that its green relatives may be partially myco-heterotrophic (Tedersoo et al. 2007; Zimmer et al. 2007; Hynson et al. 2009). Owing to the similarity of Pyrola aphylla's geographical distribution and floral morphology to P. picta, until recently they were not considered separate species; rather P. aphylla was thought to be a rare variety of P. picta. However, molecularly based phylogenies have separated the two as distinct species (D. Jolles 2009, personal communication).
All ericaceous myco-heterotrophs studied to date are epiparasites that exhibit specificity towards narrow phylogenetic lineages of EM fungi (Bidartondo & Bruns 2005). However, the fungal associates of P. aphylla have yet to be determined, and it remains unclear whether the loss of photosynthesis in this family is contingent on specializing on a particular group of ectomycorrhizal (EM) fungi (Bidartondo et al. 2004). From a previous study conducted by Zimmer et al. (2007), there is some evidence that the green sister species to P. aphylla, P. picta, associates with a broad range of EM fungi, but these data were collected from only a few plants at a single site in northern California. In the current study, we hypothesize that if the loss or reduction of photosynthesis first involves specializing on a particular group of EM fungi, then adults of both the potentially partially myco-heterotrophic P. picta and its sister taxon, the myco-heterotrophic P. aphylla, should associate with a phylogenetically narrow range of fungi throughout a broad geographical area. To address this hypothesis, we collected root systems from P. picta and P. aphylla plants from throughout their geographical range in northern California and southern Oregon, and used DNA sequence analysis to identify the fungi associated with both species.
2. Material and methods
(a). Sample collection and locations
Root systems from 12 Pyrola aphylla and 11 P. picta plants were collected over a period of approximately one and a half years from seven forest sites throughout northern California and southern Oregon. All forests are dominated by second-growth conifer species. Collected plants and their locations are summarized in the electronic supplementary material, table S1. In the field, entire plants were dug up, loose soil was removed and the remaining above- and below-ground plant parts were then placed in plastic bags on ice and transported back to the University of California Berkeley where they were washed and examined under a microscope for colonized roots. Root colonization was identified based on the presence of at least one of the following features known in pyroloid mycorrhizas: presence of a Hartig net, presence of intracellular hyphae and/or coils in epidermal root cells, and/or presence of a fungal sheath (Largent et al. 1980; Read 1983; Robertson & Robertson 1985; Massicotte et al. 2008). Fungal colonization was mainly found on second- and third-order roots (figure 1). All colonized root fragments from a single plant were washed thoroughly in a series of de-ionized H2O baths, put in 300 µl CTAB buffer and stored at −20°C until molecular analysis. This procedure was repeated for each root system of every plant collected, resulting in 72 colonized root sections of P. aphylla and 70 of P. picta; each root fragment was approximately 5–6 mm in length. To examine root-level fungal specificity in coordination with root development, two colonized root pieces were sectioned from a single P. aphylla plant sampled in October of 2007 from El Dorado National Forest. These two root pieces were sectioned from the root tip back every 15 epidermal root cells (similar to Selosse et al. 2002). The root pieces varied in total length, not in diameter; hence every section contained approximately the same number of cells, but the total number of sections made per root differed depending on total colonized root length. In total, one root had a total of 10 sections (RT1) and the other four (RT2). In the molecular analyses, these samples were treated slightly differently from the other relatively larger root fragments and will be referred to as the root-scape samples.
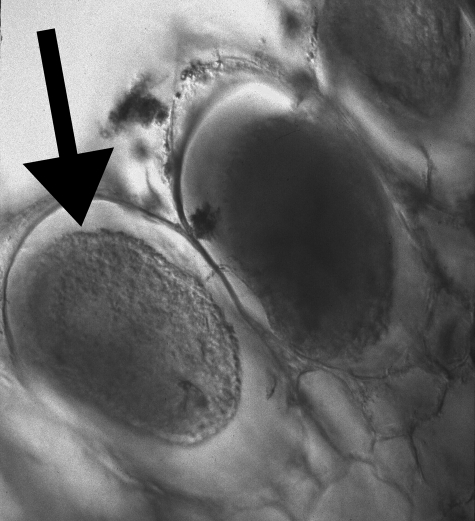
Figure 1.
A cross section at 40× magnification of a colonized root of P. aphylla showing hyphal coils within the plant's para-epidermal root cells. Photo courtesy of Martin I. Bidartondo.
(b). Molecular identification of Pyrola root fungi
Individual colonized root fragments suspended in CTAB buffer were thawed at 65°C and frozen in liquid nitrogen three times to soften the tissue before grinding with a micropestle. DNA was then extracted from each root fragment using the Qiagen DNeasy kit (Qiagen Inc., Valencia, CA, USA) following the manufacturer's instructions. Using polymerase chain reaction (PCR), the nuclear ribosomal internal transcribed spacer (ITS) region was amplified with the fungal-specific primer combination ITS1F (Gardes & Bruns 1993) and ITS4 (White et al. 1990) and PCR conditions described in Gardes & Bruns (1993). Despite our efforts to thoroughly wash root sections, it is possible that fungal hyphae attached to the surface of Pyrola roots that did not have an existing fungal mantle were detected in our PCR reactions and that these hyphae are not necessarily mycorrhizal with P. picta or P. aphylla. For all samples except the root-scape ones, positive PCR products from the root systems of individual P. picta or P. aphylla plants were then pooled. These pooled single-plant products were purified using the StrataPrep PCR Purification Kit (Stratagen, La Jolla, CA, USA) following the manufacturer's instructions and eluting the cleaned PCR products into 50 µl of Nuclease Free Water (ISC BioExpress, Kaysville, UT, USA). Pooled PCR products were cloned using TOPO TA Kit for sequencing (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. Positive transformants were screened using X-Gal (BioVectra dcl, Charlottetown, P.E.I., Canada), picked and then amplified with plasmid primers M13 forward and M13 reverse. Positive clones were then cleaned for sequencing using 0.5 µl of Exosap-IT (GE Healthcare, Piscataway, NJ, USA) and 1 µl of dH2O combined with 3.5 µl of PCR product. Clean PCR products were then unidirectionally sequenced using the plasmid primer T3. DNA sequencing was performed on an ABI3100 Genetic Analyser using BigDye v. 3.1 chemistry (Applied Biosystems, Foster City, CA, USA) and absolute ethanol/EDTA precipitation. All sequences from each single-plant clone library were aligned at 98 per cent similarity using Sequencher v. 4.2.2 (Gene Codes Corporation). Each 98 per cent minimum similarity clone pool was defined as a single fungal operational taxonomic unit (OTU). The pools of clone sequences were checked for chimeras by blasting the ITS1 and ITS2 regions separately, and any identified chimeric sequences were excluded from further analyses. The longest fragment of the dominant haplotype of each OTU was BLASTed in NCBI's GenBank to ascertain taxonomic affinity.
Genomic DNA was extracted, amplified, cloned and sequenced from the root-scape samples as described above, except that positive PCR products were not pooled and a larger fragment that included part of the nuclear large subunit rRNA gene was amplified from the original DNA extracts using the primer pair ITS 1F (Gardes & Bruns 1993) and TW13 (Taylor & Bruns 1999). Clone libraries for these samples represent individual root fragments rather than entire root systems. Clone sequences were edited in a similar fashion as above and once pools of clone sequences were resolved at 98 per cent or more similarity, we used Sequencher to compare them with sequences derived from other root sections to determine whether there was any OTU overlap among root sections. From all final OTU sequence pools, the longest fragment of the dominant haplotype was selected for submission to GenBank (accession numbers FJ440860–FJ440949), except for those matching the fungi of P. picta sampled in 2005, which were already submitted by Zimmer et al. (2007).
3. Results
(a). Morphology of pyroloid mycorrhizas
Both Pyrola picta and P. aphylla were found to associate with a suite of root-inhabiting and mycorrhizal fungi (table 1; electronic supplementary material, table S1) and, unlike all other ericaceous fully myco-heterotrophic plants studied to date, P. aphylla shows no specificity towards any particular EM fungus (table 1). The root systems of all 12 P. aphylla plants produced a total of 204 positive clones matching root-inhabiting fungi, resulting in 47 OTUs (figure 2; electronic supplementary material, table S1). Pyrola picta roots from 11 plants produced a total of 203 positive clones matching root-inhabiting fungi, resulting in 61 OTUs (figure 2; electronic supplementary material, table S1). The root morphology of both Pyrola species was basically identical, with colonized roots found either off the plants' long white rhizome or axial buds in the dark first-, second-and third-order lateral roots (Holm 1898; Massicotte et al. 2008). The overall root morphology of both species was similar to what Leake (1994) referred to as filiform—a root system that is poorly developed and lacks root hairs, which is typical of autotrophic herbs. Similar to other pyroloid mycorrhizas, colonized roots of both species had various morphotypes: some had fungal mantles or hyphal mats surrounding the roots; others had distinct Hartig nets, but lacked a mantle (Robertson & Robertson 1985; Massicotte et al. 2008; Vincenot et al. 2008). However, the clearest indicator of mycorrhization was the presence of intracellular fungal coils within the para-epidermal cells of the roots (Robertson & Robertson 1985; Massicotte et al. 2008; figure 1).
Table 1.
Summary of ectomycorrhizal (EM) associates of Pyrola aphylla and P. picta. Additional fungal associates of both species such as endophytic, ericoid, saprophytic and fungi of unknown trophic status can be the found in the electronic supplementary material, table S1.
| plant ID | EM associates |
|---|---|
| Pyrola aphylla | |
| 1 | Wilcoxina sp.a |
| 2 | Sebacina sp.2 |
| 3 | Rhizopogon sp., Russulaceae |
| 4 | Cortinarius sp.2,aLaccaria sp., Piloderma sp.2a |
| 5 | Melanogaster sp. |
| 6 | Hysterangium sp., Rhizopogon sp.2, Atheliaceae sp.2b |
| 7 | Lactarius sp.1, Piloderma sp.2, Russula sp.2, Sebacina sp.1,aSebacina sp.2 |
| 8 | Boletaceae, Cortinarius sp.2,aGymnomyces sp.,aSuillus sp.2, Thelephora sp.1, Tomentella sp.3 |
| 9 | Gomphales,bRhizopogon sp.1 |
| 10 | Gomphales,bTricholoma sp.2a |
| 11 | Cenococcum sp.1, Gomphalesb |
| 12 | Suillus sp.1 |
| P. picta | |
| 1 | Hebeloma sp., Piloderma sp.1, Piloderma sp.2,aR. salebrosus, Russula sp.1, Thelephoraceae sp.1, Tometella sp.1 |
| 2 | Russula sp.4 |
| 3 | Russula sp. 3, Thelephoraceae sp.2 |
| 4 | Piloderma sp.1, Wilcoxina sp.a |
| 5 | Cortinarius sp.2,aRhizopogon arctostaphyli, R. salebrosus, Tometella sp.2 |
| 6 | Inocybe sp.1, Piloderma sp.2,aRhizopogon arctostaphyli, Russula sp.4, Russula sp.6, Thelephora sp.2, Thelephoraceae sp.3, Tricholoma sp.1, Tricholoma sp.2,a Tricholomataceae sp.1b |
| 7 | Piloderma sp.2,aPiloderma sp.3, Piloderma sp.4, Russula sp.6, Thelephoraceae sp.4 |
| 8 | Gymnomyces sp.a |
| 9 | Russula sp.1, Russula sp.5, |
| 10 | Cenococcum sp.2, Lactarius sp.2, Sebacina sp.1a |
| 11 | Cenococcum sp.2, Cortinarius sp.1, Cortinarius sp.3, Gymnomyces sp.,aInocybe sp.2 |
aEM taxa shared between P. picta and P. aphylla.
bLineages known to contain EM taxa all others listed are known EM fungi.
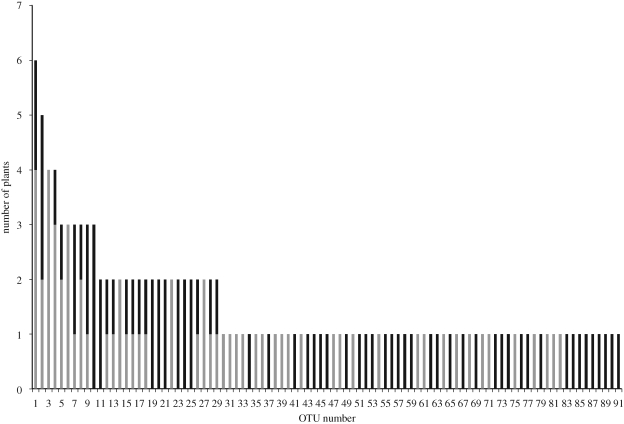
Figure 2.
Ranked abundance curve of fungal OTUs associated with individual Pyrola aphylla (grey bars) and P. picta (black bars) plants. OTU number identities can be found in electronic supplementary material, table S2.
(b). Identities of fungal associates of Pyrola aphylla and Pyrola picta
Of the 72 root fragments of Pyrola aphylla plants from which DNA was extracted, 72 per cent had positive PCR products, whereas 85 per cent of the 70 root fragments of P. picta had successful PCR reactions. From all clone sequences of both plant species, a total of 91 OTUs were determined; 31 were unique to P. aphylla roots, 44 to P. picta roots and 16 were shared between the two plant species (figure 2; electronic supplementary material, tables S1 and S2). The two most abundant fungal OTUs were a fungal endophyte in the genus Phialocephala and an EM fungus in the genus Piloderma (figure 2; electronic supplementary material, tables S1 and S2). Based on best BLAST matches, 44 per cent of the OTUs from all P. aphylla roots are fungi known to form ectomycorrhizas, 6 per cent are fungi traditionally thought to strictly form ericoid mycorrhizas, 6 per cent are saprotrophic or necrotrophic fungi, 19 per cent are root endophytes and 24 per cent are fungi of unknown trophic status. Of all the fungal OTUs from the root systems of P. picta plants, a total of 56 per cent are known EM fungi, 5 per cent are ericoid mycorrhizal fungi, 3 per cent are saprotrophic or necrotrophic fungi, 19 per cent are root endophytes and 17 per cent are fungi of unknown trophic status. In one P. aphylla plant, we failed to detect any mycorrhizal fungi from the pooled PCR products of 12 colonized root fragments. However, this happened to be the same plant that was sampled for the root-scape study, and from these root fragments EM fungi were detected (electronic supplementary material, table S3), indicating that there may have been some bias for non-mycorrhizal taxa such as ascomycetous endophytes in the pooling/cloning technique. The mycorrhizal OTUs identified from the roots of P. aphylla and P. picta fell into a total of 20 fungal families, 10 with each plant species, and the largest percentage (19% in P. aphylla and 35% in P. picta) belonged to the Russulaceae. There was little intra- or interspecific overlap between the observed fungi from Pyrola roots (even from the same site and sampling time), reflecting the high diversity of fungi associated with both species in our limited sampling (figure 2).
(c). Pyrola aphylla root-scape fungi
The two root pieces collected from a single root system of P. aphylla resulted in ten root sections from RT1 that produced nine positive PCR products, while RT2 produced three from the four total root sections. When each of these products was cloned in individual cloning reactions, RT1 produced 98 positive clone sequences matching root-inhabiting fungi and RT2 produced 30. At 98 per cent sequence similarity, RT1 contained 15 distinct OTUs: two were EM fungi, seven were root endophytes, three were plant pathogens, two were saprophytes and one was an ascomycete of unknown trophic status (electronic supplementary material, table S3). Five distinct OTUs were found in RT2: one endophyte, three saprophytes and one Atheliaceae of unknown trophic status (electronic supplementary material, table S3). No OTUs were shared between the two roots. Interestingly, RT1 contained two fungal endophyte OTUs (Phialocephala species 4 and 5) that matched those of other P. aphylla roots from the same plant and from a neighbouring P. picta plant collected during the same sampling period. RT2 contained one OTU (Cadophora species 2) that matched P. aphylla roots collected in Umpqua National Forest (electronic supplementary material, table S3).
4. Discussion
Pyrola aphylla represents the first ericaceous myco-heterotroph to lack adult fungal specificity. In all previous studies on the mycorrhizal fungi associated with myco-heterotrophs, the only other plants that have been found to associate with multiple families of EM fungi are albino orchids in the otherwise mainly green genera Cephalanthera and Epipactis (Selosse et al. 2004; Julou et al. 2005; Abadie et al. 2006). The lack of fungal specificity in P. aphylla indicates that it is not strictly necessary for a myco-heterotroph to specialize on a particular group of fungi to meet its carbon demands, nor is the loss of photosynthesis contingent upon mycorrhizal specialization. Both P. picta and P. aphylla were found to associate with mainly EM fungi from a diversity of fungal families that also form ectomycorrhizae with overstorey trees (table 1). The association with EM fungi in both Pyrola species provides further evidence for potential epiparasitism in this group, especially in the case of P. aphylla owing to its lack of photosynthetic tissues and the similarity of its carbon stable isotope signature to other ericaceous myco-heterotrophic epiparasites (Hynson et al. 2009). However, it remains unknown which of these EM fungi are functionally responsible for carbon transfer from overstorey host trees to P. aphylla. Owing to the dust-seed morphology of pyroloids, it is clear that they require fungal nutrition in their early stages of development but, which, if any, of the fungi identified from adult plants are responsible for stimulating seed germination remains unknown. However, there is some indication that seedlings of green Pyrola species harbour sebacinoid fungi (Smith & Read 2008). It is possible that pyroloids exhibit higher fungal specificity at germination than adult plants, and this could explain the rarity of such species as P. aphylla (Bidartondo & Read 2008). This has been somewhat demonstrated through an ongoing seed burial experiment by the authors where small packets containing hundreds of P. aphylla seeds buried in situ have not germinated after 4 years (data not included).
Previous studies support our findings that Pyroleae associate with a suite of endophytic, ericoid and EM species belonging to both the Basidiomycota and Ascomycota (Largent et al. 1980; Read 1983; Robertson & Robertson 1985; Tedersoo et al. 2007; Zimmer et al. 2007; Massicotte et al. 2008; Vincenot et al. 2008). Even though adult pyroloid plants have shown little to no specialization towards particular groups of EM fungi, some fungal genera such as Tomentella (Tedersoo et al. 2007; Zimmer et al. 2007; Massicotte et al. 2008; Vincenot et al. 2008; this study), Sebacina, Wilcoxina and Inocybe (Tedersoo et al. 2007; Zimmer et al. 2007; Vincenot et al. 2008; this study) appear to be common symbionts of Pyroleae species. Fungal endophytes in the genus Phialocephala and the order Helotiales, which may contain some EM species (Vrålstad et al. 2002), also appear to be common associates of Pyroleae, though their functional role within the plant roots is unclear. Pyroloids also associate with EM fungal genera and species such as Russula (Zimmer et al. 2007; Vincenot et al. 2008 and this study), Tricholoma (Tedersoo et al. 2007; Vincenot et al. 2008) and Rhizopogon salebrosus (this study) that are known to form mycorrhizas with other ericaceous myco-heterotrophs (Bidartondo 2005).
Despite the small sample sizes, the results from the root-scape root fragments of P. aphylla provide further insight into pyroloid mycorrhizas. In the root-scape samples, both root apices harboured multiple EM species and different fungi were found in both tips. This result indicates that even at the fine spatial scale of a few root cells, there is no evidence of fungal specialization in P. aphylla. For example, within the first 30 epidermal root cells back from the actively growing meristem of RT1, we identified a species of Hysterangium, which has been previously recorded from morphological studies of Pyrola secunda (=Orthilia secunda) roots by Robertson & Robertson (1985); also found in the same root section was a species of Rhizopogon and an unknown ascomycete. A fungus in the family Atheliaceae, which is known to contain some EM taxa (Plamboeck et al. 2007), was found in the first 15 epidermal root cells of RT2, the shorter root piece collected from the same plant.
The lack of fungal specificity in P. aphylla reveals new information on the ordering of the steps from autotrophy to full myco-heterotrophy. Excepting two potentially fully myco-heterotrophic orchids in the genus Aphyllorchis (Roy et al. in press), all myco-heterotrophic plants that have not specialized on a set of EM fungi either exhibit some normally green individuals, as in the albino orchids within the tribe Neottieae, or some individuals with small basal leaves and green flowering stalks, as in P. aphylla (Hynson et al. 2009). This phenotypic plasticity could potentially be due to more recent losses of photosynthetic abilities among these species compared with the consistently achlorophyllous myco-heterotrophic species in the Monotropoideae and Neottieae. Therefore, though the loss of photosynthetic abilities may not be contingent on fungal specialization, once photosynthesis is lost, there may be strong selective pressures to specialize on a particular fungal group (Bronstein 2001; Bidartondo & Bruns 2002). The tribe Pyroleae provides a unique group among the Ericaceae for studying the transition from autotrophy to myco-heterotrophy owing to the range of trophic strategies among closely related species. Now that the identities of the fungal associates of P. aphylla have been surveyed, what is of pressing interest is which of these fungi are responsible for nutrient exchange between overstorey trees P. aphylla and possibly P. picta at various stages of plant growth and establishment.
Acknowledgements
The authors would like to thank Rachel Adams for field assistance, Shannon Schechter for laboratory assistance, and Martin Bidartondo, Kabir Peay, Peter Kennedy and David Read for many valuable conversations regarding this project, as well as Marc-Andre Selosse and one anonymous reviewer for valuable comments on an earlier version of this manuscript.
References
- Abadie J. C., Puttsepp U., Gebauer G., Faccio A., Bonfante P., Selosse M. A.2006Cephalanthera longifolia (Neottieae, Orchidaceae) is mixotrophic: a comparative study between green and nonphotosynthetic individuals. Can. J. Bot. 84, 1462–1477 (doi:10.1139/B06-101) [Google Scholar]
- Battles J. J., Shlisky A. J., Barrett R. H., Heald R. C., Allen-Diaz B. H.2001The effects of forest management on plant species diversity in a Sierran conifer forest. Forest Ecol. Manage. 146, 211–222 (doi:10.1016/S0378-1127(00)00463-1) [Google Scholar]
- Bidartondo M. I.2005The evolutionary ecology of myco-heterotrophy. New Phytol. 167, 335–352 (doi:10.1111/j.1469-8137.2005.01429.x) [DOI] [PubMed] [Google Scholar]
- Bidartondo M. I., Bruns T. D.2002Fine-level mycorrhizal specificity in the Monotropoideae (Ericaceae): specificity for fungal species groups. Mol. Ecol. 11, 557–569 (doi:10.1046/j.0962-1083.2001.01443.x) [DOI] [PubMed] [Google Scholar]
- Bidartondo M. I., Bruns T. D.2005On the origins of extreme mycorrhizal specificity in the Monotropoideae (Ericaceae): performance trade-offs during seed germination and seedling development. Mol. Ecol. 14, 1549–1560 (doi:10.1111/j.1365-294X.2005.02503.x) [DOI] [PubMed] [Google Scholar]
- Bidartondo M. I., Read D. J.2008Fungal specificity bottlenecks during orchid germination and development. Mol. Ecol. 17, 3707–3716 [DOI] [PubMed] [Google Scholar]
- Bidartondo M. I., Redecker D., Hijri I., Wiemken A., Bruns T. D., Dominguez L., Sersic A., Leake J. R., Read D. J.2002Epiparasitic plants specialized on arbuscular mycorrhizal fungi. Nature 419, 389–392 (doi:10.1038/nature01054) [DOI] [PubMed] [Google Scholar]
- Bidartondo M. I., Burghardt B., Gebauer G., Bruns T. D., Read D. J.2004Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc. R. Soc. Lond. B 271, 1799–1806 (doi:10.1098/rspb.2004.2807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein J. L.2001The exploitation of mutualisms. Ecol. Lett. 4, 277–287 (doi:10.1046/j.1461-0248.2001.00218.x) [Google Scholar]
- Bruns T. D., Bidartondo M. I., Taylor D. L.2002Host specificity in ectomycorrhizal communities: what do the exceptions tell us? Integr. Comp. Biol. 42, 352–359 (doi:10.1093/icb/42.2.352) [DOI] [PubMed] [Google Scholar]
- Camp W. H.1940Aphyllous forms in Pyrola. Bull. Torrey Bot. Club 67, 453–465 (doi:10.2307/2480967) [Google Scholar]
- Egger K. N., Hibbett D. S.2004The evolutionary implications of exploitation in mycorrhizas. Can. J. Bot. 82, 1110–1121 (doi:10.1139/b04-056) [Google Scholar]
- Gardes M., Bruns T. D.1993ITS primers with enhanced specificity for Basidiomycetes—application to the identification of mycorrhizae and rusts. Mol. Ecol. 2, 113–118 (doi:10.1111/j.1365-294X.1993.tb00005.x) [DOI] [PubMed] [Google Scholar]
- Haber E.1987Variability distribution and systematics of Pyrola picta sensu-lato Ericaceae in western North America. Syst. Bot. 12, 324–335 (doi:10.2307/2419328) [Google Scholar]
- Holm T.1898Pyrola aphylla: a morphological study. Bot. Gazette 25, 246–254 (doi:10.1086/327666) [Google Scholar]
- Hynson N. A., Preiss K., Gebauer G., Bruns T. D.2009Isotopic evidence of full and partial myco-heterotrophy in the plant tribe Pyroleae (Ericaceae). New Phytol. 182, 719–726 (doi:10.1111/j.1469-8137.2009.02781.x) [DOI] [PubMed] [Google Scholar]
- Julou T., Burghardt B., Gebauer G., Berveiller D., Damesin C., Selosse A.2005Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytol. 166, 639–653 (doi:10.1111/j.1469-8137.2005.01364.x) [DOI] [PubMed] [Google Scholar]
- Kron K. A., Johnson S. L.1997Phylogenetic analysis of the monotropoids and pyroloids (Ericaceae) using nrITS and 18s sequence data. Am. J. Bot. 84, 205–206 [Google Scholar]
- Kron K. A., Judd W. S., Stevens P. F., Crayn D. M., Anderberg A. A., Gadek P. A., Quinn C. J., Luteyn J. L.2002Phylogenetic classification of Ericaceae: molecular and morphological evidence. Bot. Rev. 68, 335–423 (doi:10.1663/0006-8101(2002)068[0335:PCOEMA]2.0.CO;2) [Google Scholar]
- Largent D. L., Sugihara N., Wishner C.1980Occurrence of mycorrhizae on ericaceous and pyrolaceous plants in northern California. Can. J. Bot. 58, 2274–2279 (doi:10.1139/b80-262) [Google Scholar]
- Leake J. R.1994Tansley review No. 69. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytol. 127, 171–216 (doi:10.1111/j.1469-8137.1994.tb04272.x) [DOI] [PubMed] [Google Scholar]
- Leake J. R.2004Myco-heterotroph/epiparasitic plant interactions with ectomycorrhizal and arbuscular mycorrhizal fungi. Curr. Opin. Plant Biol. 7, 422–428 (doi:10.1016/j.pbi.2004.04.004) [DOI] [PubMed] [Google Scholar]
- Massicotte H. B., Melville L. H., Tackaberry L. E., Peterson R. L.2008A comparative study of mycorrhizas in several genera of Pyroleae (Ericaceae) from western Canada. Botany 86, 610–622 (doi:10.1139/B08-027) [Google Scholar]
- Ogura-Tsujita Y., Gebauer G., Hashimoto T., Umata H., Yukawa T.2009Evidence for novel and specialized mycorrhizal parasitism: the orchid Gastrodia confusa gains carbon from saprotrophic Mycena. Proc. R. Soc. B 276, 761–767 (doi:10.1098/rspb.2008.1225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamboeck A. H., Dawson T. E., Egerton-Warburton L. M., North M., Bruns T. D., Querejeta J. I.2007Water transfer via ectomycorrhizal fungal hyphae to conifer seedlings. Mycorrhiza 17, 439–447 (doi:10.1007/s00572-007-0119-4) [DOI] [PubMed] [Google Scholar]
- Read D. J.1983The biology of mycorrhiza in the Ericales. Can. J. Bot. 61, 985–1004 (doi:10.1139/b83-107) [Google Scholar]
- Robertson D., Robertson J.1985Ultrastructural aspects of Pyrola mycorrhizae. Can. J. Bot. 63, 1089–1098 (doi:10.1139/b85-150) [Google Scholar]
- Roy M., Watthana S., Stier A., Richard F., Vessabutr S., Selosse M.-A.In press Two mycoheterotrophic orchids from Thailand tropical dipterocarpacean forests associate with a broad diversity of ectomycorrhizal fungi. BMC Ecology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selosse M.-A., Weiss M., Jany J.-L., Tillier A.2002Communities and populations of sebacinoid basidiomycetes associated with the achlorophyllous orchid Neottia nidus-avis (L.) L.C.M. Rich. and neighbouring tree ectomycorrhizae. Mol. Ecol. 11, 1831–1844 (doi:10.1046/j.1365-294X.2002.01553.x) [DOI] [PubMed] [Google Scholar]
- Selosse M. A., Faccio A., Scappaticci G., Bonfante P.2004Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal Septomycetes, including truffles. Microb. Ecol. 47, 416–426 (doi:10.1007/s00248-003-2034-3) [DOI] [PubMed] [Google Scholar]
- Smith S. E., Read D. J.2008Mycorrhizal symbiosis, 3rd edn London, UK: Academic Press [Google Scholar]
- Taylor D. L., Bruns T. D.1999Community structure of ectomycorrhizal fungi in a Pinus muricata forest: minimal overlap between the mature forest and resistant propagule communities. Mol. Ecol. 8, 1837–1850 (doi:10.1046/j.1365-294x.1999.00773.x) [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Bruns T. D., Leake J. R., Read D. J.2002Mycorrhizal specificity and function in myco-heterotrophic plants. In Ecological studies. Mycorrhizal ecology (eds Sanders I. R., van der Heijden M.), pp. 375–414 Berlin, Germany: Springer Verlag [Google Scholar]
- Taylor D. L., Bruns T. D., Hodges S. A.2004Evidence for mycorrhizal races in a cheating orchid. Proc. R. Soc. Lond. B 271, 35–43 (doi:10.1098/rspb.2003.2557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedersoo L., Pellet P., Koljalg U., Selosse M. A.2007Parallel evolutionary paths to mycoheterotrophy in understorey Ericaceae and Orchidaceae: ecological evidence for mixotrophy in Pyroleae. Oecologia 151, 206–217 (doi:10.1007/s00442-006-0581-2) [DOI] [PubMed] [Google Scholar]
- Vincenot L., Tedersoo L., Richard F., Horcine H., Kõljalg U., Selosse A.2008Fungal associates of Pyrola rotundifolia, a mixotrophic Ericaceae, from two Estonian boreal forests. Mycorrhiza 19, 15–25 (doi:10.1007/s00572-008-0199-9) [DOI] [PubMed] [Google Scholar]
- Vrålstad T., Schumacher T., Taylor A. F. S.2002Mycorrhizal synthesis between fungal strains of the Hymenoscyphus ericae aggregate and potential ectomycorrhizal and ericoid hosts. New Phytol. 153, 143–152 (doi:10.1046/j.0028-646X.2001.00290.x) [Google Scholar]
- Whigham D.2004Ecology of woodland herbs in temperate deciduous forests. Annu. Rev. Ecol. Evol. Syst. 35, 583–621 (doi:10.1146/annurev.ecolsys.35.021103.105708) [Google Scholar]
- White T., Bruns T., Lee S., Taylor J.1990Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols—a guide to methods and applications (eds Innis M., Gelfand D., Sninsky J., White T.), pp. 315–322 New York, NY: Academic Press [Google Scholar]
- Zimmer K., Hynson N. A., Gebauer G., Allen E. B., Allen M. F., Read D. J.2007Wide geographical and ecological distribution of nitrogen and carbon gains from fungi in pyroloids and monotropoids (Ericaceae) and in orchids. New Phytol. 175, 166–175 (doi:10.1111/j.1469-8137.2007.02065.x) [DOI] [PubMed] [Google Scholar]