Abstract
The primary goal of virtually all organisms is to produce genetic offspring, thereby passing on their genes to future generations. Offspring production, however, is limited by available resources within an environment. Moreover, distributing sufficient energy among competing physiological systems is challenging and can result in trade-offs between self-maintenance and offspring investment when resources are limited. In the current study, we tested the hypothesis that the adipose hormone leptin is involved in mediating energetic trade-offs between competing physiological systems. Specifically, we tested the effects of elevated maternal leptin on investment into offspring production versus self maintenance (immune function), in the Siberian hamster (Phodopus sungorus). The current study provides the first evidence that leptin serves as a signal to mothers of available energy resulting in epigenetic effects. Therefore, elevated leptin allows females to retain more embryos to parturition, and rear more offspring to weaning via reduced maternal infanticide. Innate immune response was suppressed seemingly as a result of these enlarged litters, suggesting that the observed fitness increase is not without costs to the mother. Collectively, these findings suggest that leptin plays a critical role in allowing mothers to determine how much energy to invest in the production and care of young versus self-maintenance.
Keywords: energy, immunity, reproduction, trade-offs
1. Introduction
The production of offspring is an energetically costly process in all organisms and can thus limit the concurrent allocation of energy resources to other important physiological functions within the mother. Likewise, it is vital for organisms to maintain adequate health in order to survive and reproduce. For example, studies have shown significant elevations in metabolic expenditure during pregnancy and lactation as well as during specific immune responses (Demas et al. 1997; Mauget et al. 1997; Nilsson & Raberg 2001; Martin et al. 2003). Therefore, allocating sufficient energy resources to multiple energetically costly functions is a challenge, and trade-offs among these functions often result when energy is limited. Yet, the resulting trade-offs and their underlying physiological mechanisms remain unclear.
Immune function is suppressed during breeding stages across a wide range of species, concurrent with increased energy expenditure required for courtship, territorial defence or pregnancy (Cichon et al. 2001; Drazen et al. 2003; for review see Zuk & Stoehr 2002). For example, immune function is suppressed during pregnancy and lactation in the Siberian hamster (Phodopus sungorus) (Drazen et al. 2003). Moreover, changes in sex steroid hormones, such as testosterone and oestradiol, that occur during reproduction can exert pronounced effects on various components of the immune system (Saino et al. 1995; Casto et al. 2000; Greives et al. 2006). For example, elevated testosterone in breeding dark-eyed juncos (Junco hyemalis) suppresses cell-mediated immunity (Casto et al. 2000). In addition, exogenous treatment with oestradiol or testosterone in gonadectomized Siberian hamsters results in enhanced cell-mediated immune function (Bilbo & Nelson 2001). It remains unclear, however, whether these results are due directly to changes in hormonal signalling or indirectly via changes in energetic expenditure and investment (Owen-Ashley et al. 2004).
Pregnancy and lactation in particular are times of an extreme energetic bottleneck and result in significant behavioural, hormonal and energetic changes within an animal. These changes include marked increases in resting metabolic rate (Mauget et al. 1997; Nilsson & Raberg 2001) and decreased fat reserves (Day et al. 2002). Many studies demonstrate that specific immune components are suppressed during these reproductive stages. For example, cell-mediated immunity is suppressed during pregnancy across a variety of mammalian species (Weinberg 1984). Furthermore, experimentally enlarging brood size impairs parental immune function in collared flycatchers (Ficedula albicollis) and nestling immune function in barn swallows (Hirundo rustica) (Cichon et al. 2001; Saino et al. 2002). Pregnant females, however, must also maintain a sufficient level of antibody production for allocation to offspring. Thus far, it is unclear what physiological mechanisms underlie these energy allocation decisions.
Leptin presents a likely candidate for regulating reproductive/immune trade-offs. Leptin is a protein hormone secreted primarily by adipose tissue that serves as an indicator of fat reserves, and is also involved in immunoregulation (Drazen et al. 2000, 2001; Demas & Sakaria 2005; Fantuzzi 2006). In mammals there is a well-established link between leptin, fat stores and organismal immunocompetence (Lord et al. 1998), making it a likely candidate to mediate physiological trade-offs between the immune and other systems. Specifically, treatment of mice with leptin reverses the immunosuppressive effects of food restriction (Lord et al. 1998). Leptin also fluctuates according to photoperiod and season (Rousseau et al. 2002; Gaspar-López et al. 2009). Siberian hamsters exposed to short, winter-like days typically exhibit decreased body fat and immunity; however, treatment with leptin attenuates this seasonal immunosuppression (Drazen et al. 2001). Likewise, leptin treatment reverses the immunosuppressive effects of experimental lipectomy on humoural immunity (Demas & Sakaria 2005). In addition, leptin is a permissive regulator of reproduction in mammals (Schneider et al. 2000), whereby it allows reproduction to occur when resources are limited, but does not necessarily enhance it above normal function (Casanueva & Dieguez 1999; Baldelli et al. 2002; Margetic et al. 2002; Zieba et al. 2005). Therefore, leptin may act as a physiological signal for the observed resource-based trade-offs that naturally occur between the immune system and other physiological systems in many organisms.
In the present study, we investigated the effects of altered leptin signalling on investment in offspring, versus investment in humoural and innate immunity in the Siberian hamster model. Thus far, most of the research in this area has focused on adult animals, while very little is known about maternal or trans-generational effects. Therefore, we focused on specific effects in offspring. We also examined passive maternal immunity, which involves the transfer of maternal antibodies to offspring via maternal lactation. This transfer can act as an additional energetic sink for the mothers, which is critical to offspring survival; however, this energetic cost is often overlooked. Siberian hamsters present an important model to examine these phenomena. Specifically, they display seasonal immunosuppression during the winter which is associated with decreased body mass and available body fat stores, but is not concurrent with breeding stage. As a result, hamsters breed in long summer-like days when resources are readily available, and they have large fat stores. Laboratory rats and mice are bred for their relative unresponsiveness to environmental variation, whereas hamsters are highly plastic, and display marked responses to even modest environmental fluctuations. Because, hamsters are less buffered from environmental perturbations, they are a good model to examine how animals modulate responses such as physiological trade-offs to energetic challenges. Studies have also demonstrated a suppressed antibody response in pregnant hamsters during the reproductive season (Drazen et al. 2003), making them an ideal candidate to assess whether leptin serves as a mediator attenuating the observed immunosuppression.
To alter the maternal energetic signal, and examine whether leptin serves as a mediator of resource investment into offspring versus self, we experimentally elevated leptin in females paired with either intact or castrated male hamsters, via osmotic mini-pumps containing murine leptin, and all animals then underwent an immune challenge. We also sampled all resulting offspring for passive maternal immune investment, specifically maternal antibodies. We hypothesized that pregnant females would interpret elevated leptin levels as greater energy stores and thus generate antibody responses comparable to non-pregnant females, and above those of pregnant control females. Alternatively, elevated leptin may increase investment in offspring relative to self-maintenance, thereby exacerbating any pregnancy-associated immunosuppression.
2. Material and methods
(a). Study system
Adult female Siberian hamsters (n = 40) were obtained from our breeding colony at Indiana University. All animals were initially group-housed (two to four per cage with same sex siblings upon weaning at 21 days of age), and maintained in long summer-like day lengths (light : dark, 16∶8). Temperature (20 ± 2°C) and humidity (50 ± 10%) were maintained constant. Ten days before the start of the experiment, animals were housed individually in polypropylene cages (28 × 17 × 12 cm), weighed and then randomly assigned to one of the two treatment groups: paired with an intact adult male or paired with a castrated adult male (control). Five days after pairing, females were separated from males and housed individually. All animals were given ad libitum access to food (Purina rat chow, St Louis, MO, USA) and water throughout the study. Food intake was monitored daily and female body mass was measured weekly throughout the study except for the 5 days when females were paired with males. Food intake was also measured for 5 days prior to pairing with a male to assess baseline pre-pregnancy, pre-hormone, intake levels (starting 5 days post-individual housing). Finally, after the 18-day gestation period, litter size and number were measured on the initial birth date and daily thereafter.
Animals that were paired with intact males, but failed to become pregnant were included in the control group for analyses to increase statistical power; however, their inclusion did not significantly alter the analyses. Of these animals, two leptin-treated and two vehicle-treated animals paired with intact males did not become pregnant and were included in the control group. This resulted in the following sample sizes: pregnant leptin, n = 10; pregnant vehicle, n = 10; control leptin, n = 10; control vehicle, n = 10.
(b). Leptin manipulations and immunizations
After the five-day pairing, the pairs were separated and all animals were implanted with osmotic minipumps under isoflurane anaesthesia (Alzet 2002; 200 µl volume; 0.5 µl h−1 delivery rate; 14 days; Alzet Scientific, Mountain View, CA, USA). Half of the females paired with intact males and half of the females paired with castrated males were randomly assigned to receive minipumps containing recombinant murine leptin (n = 20; 2.6 µg µl−1 leptin; Peprotech Inc., Rocky Hill, NJ, USA) dissolved in 0.5 M Tris buffer (Drazen et al. 2001). The remaining animals received minipumps containing vehicle (n = 20; 0.5 M Tris buffer) (Drazen et al. 2001).
Three days after implantation of the minipumps (day 0), all hamsters received a single 100 µg subcutaneous injection of the antigen keyhole limpet haemocyanin (KLH), to which all animals were previously naive, suspended in 0.1 ml sterile saline. All animals were then returned to the colony room. KLH is an innocuous respiratory protein derived from the giant keyhole limpet (Megathura crenulata). KLH was used because it generates a robust antigenic response in rodents, but does not make the animals sick (e.g. prolonged inflammation or fever). Body mass was measured initially upon individual housing, prior to pairing and implantation treatments, and then weekly throughout the experiment. Body mass was important both to track pregnancy, and assess the health and condition of animals within a treatment.
(c). Blood sampling and adipose measurements
On days 5 and 10 post-KLH injection, a blood sample was taken to measure serum concentrations of leptin (day 10) and KLH-specific antibodies (days 5 and 10). Days 5 and 10 incorporate the peak rises in immunoglobulins IgM and IgG, respectively. IgM is the initial immunoglobulin secreted following an immune challenge (measured from day 5 sample), and IgG is the principal circulating immunoglobulin present throughout an immune response (Demas et al. 1997; Drazen et al. 2000). IgG is also transferred to the offspring during pregnancy and lactation. On the day of sampling, animals were brought into the surgery room, lightly anaesthetized with isoflurane vapours (Sigma Chemical), and blood samples were drawn from the retro-orbital sinus between 1000 h and 1200 h. Samples were allowed to clot for 1 h, the clots were removed and the samples centrifuged (at 4°C) for 30 min at 2500 r.p.m. Serum aliquots were aspirated and stored in sealable polypropylene microcentrifuge tubes at −80°C until assayed for IgM and IgG.
On day 16 post-implantation, after all pregnant females had given birth, animals were euthanized and necropsies were performed to assess uterine and fat pad masses. Uterine mass was important to assess reproductive function, whereas fat stores are also an important indicator of reproductive condition. We removed PWAT, RWAT and inguinal WAT (IWAT) pads from all hamsters. All tissues were cleaned of connective tissue and weighed to the nearest 0.1 mg blind to experimental treatment.
Finally, litters were euthanized via rapid decapitation and trunk blood was collected and pooled within a litter to attain sufficient sample volume. All samples from the offspring in a given litter were pooled into a single sample for analysis. Offspring blood samples were collected prior to offspring immune system development (i.e. prior to post-natal day 7). Therefore, immune components measured from offspring samples were representative of maternally transferred immune components.
(d). Circulating leptin
Serum leptin levels were assayed from samples using LINCO (Millipore, St Charles, MO, USA) mouse leptin enzyme-linked immunosorbent assay (ELISA) kits. Due to a large sample size, serum leptin concentrations were assayed within two separate ELISAs using a 1∶2 dilution, performed according to assay instructions. The intra-assay variability for each plate was 1.0 per cent and 5.3 per cent, and the inter-assay coefficient of variation was 11 per cent.
(e). Humoural immunity
To assess humoural immunity, microtitre plates were coated with antigen by incubating overnight at 4°C with 0.5 mg ml−1 KLH in sodium bicarbonate buffer (pH 9.6). Plates were then washed with phosphate-buffered saline (PBS; pH 7.4) containing 0.05 per cent Tween 20 (PBS-T; pH 7.4), and blocked with 5 per cent non-fat dry milk in PBS-T overnight at 4°C to reduce non-specific binding, and then washed again with PBS-T. Thawed serum samples were diluted 1∶20 with PBS-T, and 150 µl of each serum dilution was added in duplicate to the wells of the antigen-coated plates. Positive control samples (pooled sera from hamsters previously determined to have high levels of anti-KLH antibodies, similarly diluted with PBS-T) and negative control samples (pooled sera from KLH-naive hamsters, similarly diluted with PBS-T) were also added in duplicate to each plate; plates were sealed, incubated at 37°C for 3 h, then washed with PBS-T. A secondary antibody (alkaline phosphatase-conjugated anti-hamster IgG (Rockland, Gilbertsville, PA, USA); and anti-mouse IgM diluted 1∶500 with PBS-T (Cappel, Durham, NC, USA)) was added to the wells, and the plates were sealed and incubated for 1 h at 37°C. Plates were washed again with PBS-T and 150 µl of the enzyme substrate p-nitrophenyl phosphate (Sigma Chemical; 1 mg ml−1 in diethanolamine substrate buffer) was added to each well. Plates were protected from light during the enzyme–substrate reaction. The optical density (OD) of each well was determined using a plate reader (Bio-Rad, Benchmark; Richmond, CA, USA) equipped with a 405 nm wavelength filter and the mean OD for each set of duplicate wells was calculated. To minimize intra-assay variability, the mean OD for each sample was expressed as a per cent of its plate positive control OD for statistical analyses.
(f). Innate immunity
The bactericidal assay was performed in a sterile laminar flow hood. This assay is advantageous because it measures a functional response by the animal's innate immune system against a relevant pathogen, Escherichia coli (Irene Tieleman et al. 2005). In short, a bacterial stock solution was prepared by adding one pellet of lyophilized E. coli (EpowerTM Microorganisms no. 0483E7, ATCC 8739, MicroBioLogics, St Cloud, MN, USA) to 40 ml of 1 M sterile PBS. The solution was activated via incubation at 37°C for 30 min. Immediately following incubation, the bacterial working solution was prepared by diluting 2 ml of the stock solution into 8 ml 1 M PBS. Meanwhile, serum samples were diluted 1∶20 in CO2-independent media (Gibco no. 18045, Carlsbad, GA, USA) containing 2.34 mg of l-glutamine (Sigma-Aldrich). Twenty microlitres of the bacterial working solution was added to each diluted sample and the mixture was allowed to incubate at 37°C for 30 min to induce bacterial killing. After incubation, 50 µl of each sample was added to tryptic soy agar plates in duplicate. All plates were covered and left to incubate upside down (prevents condensation from collecting on developing colonies) overnight at 37°C. After 24 h, colony numbers were counted and bactericidal capacity was calculated as 100 per cent minus the mean number of colonies for each sample divided by the mean of colonies for the positive controls (containing only media and bacterial solution), i.e. percentage of bacteria killed relative to the positive control.
(g). Statistical analyses
Differences among all dependent measures were determined using separate two-way (pregnancy × hormone treatment) analyses of variance (ANOVA) (JMP 7.0.1 SAS Institute Inc., Cary, NC, USA). When significant interactions were present, separate one-way ANOVAs were used to test main effects. Differences among litters of pregnant females were determined using one-way ANOVA. The changes in body mass and food intake over time were compared using two-way and one-way repeated measures ANOVAs, respectively (JMP 7.0.1 SAS Institute). Within-subject comparisons violated assumptions of sphericity, and were therefore Greenhouse–Geisser (GG)-corrected. Again, when significant interactions were present, separate one-way repeated measures ANOVAs were used to test main effects. In all cases, differences between group means were considered statistically significant if p ≤ 0.05.
3. Results
(a). Leptin
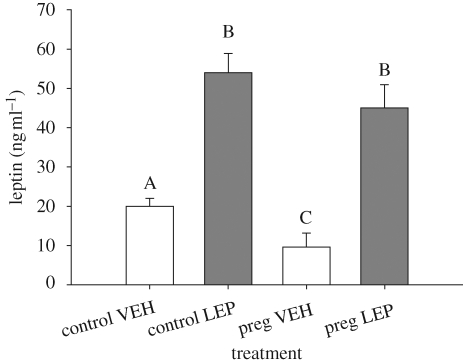
The results on leptin treatment of pregnant and control females showed a significant interaction, such that pregnant and control animals responded differently to the hormone treatments (Fhormone × pregnancy = 5.50; d.f. = 1,35; p = 0.025; figure 1). Separate one-way ANOVAs, revealed that pregnant vehicle-treated females had lower leptin levels than pregnant leptin-treated females (F = 19.10; d.f. = 1,18; p < 0.01; figure 1). Similarly, control vehicle-treated females had lower leptin levels than control leptin-treated females (F = 9.76; d.f. = 1,18; p < 0.01; figure 1).
Figure 1.
Circulating leptin concentrations. Leptin is significantly elevated in leptin-treated individuals compared with vehicle-treated individuals. Leptin is also significantly lower in pregnant individuals relative to control individuals. Different letters denote groups that differ significantly (α = 0.05 level). Error bars represent ±1 s.e.
Circulating leptin levels did not differ significantly between pregnant and control leptin-treated females (F = 0.33; d.f. = 1,18; p = 0.57; figure 1). Within the vehicle-treated group, however, pregnant females had significantly higher leptin levels than control females (F = 10.61; d.f. = 1,18; p < 0.01; figure 1).
(b). Maternal investment
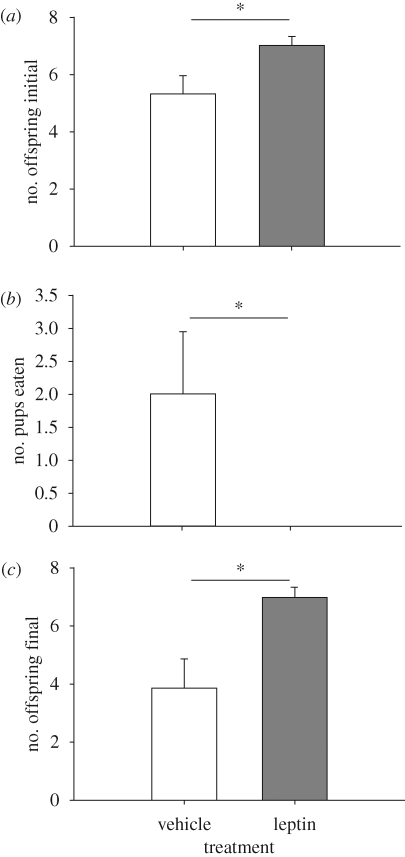
Pregnant leptin-treated animals had significantly more pups than vehicle-treated females (F = 4.86; d.f. = 1,17; p = 0.04; figure 2a). There was also a trend, although not significant, for litters from leptin-treated mothers to initially weigh more (F = 3.70; d.f. = 1,17; p = 0.07). Finally, 40 per cent of vehicle-treated females engaged in maternal cannibalism, whereas none of the leptin-treated animals cannibalized their offspring (Fisher's exact test p = 0.08; figure 2b). This maternal culling of litter size is a common practice in many small rodent species (McClure 1981; Bronson & Marsteller 1985; Schneider & Wade 1989). Owing to maternal infanticide, the mean difference in final litter size (both number and mass) was even greater between groups (all F > 5.3, all p < 0.04; figure 2c).
Figure 2.
Effects of leptin treatment on reproductive output. Females treated with leptin had significantly (a) more pups, (b) maintained larger litters via suppressed maternal infanticide and (c) larger final litter size (i.e. at the termination of study) than vehicle-treated control females. Asterisks denote statistically significant differences (α = 0.05 level). Error bars represent ±1 s.e.
(c). Body mass and food intake
Individual repeated measures ANOVAs for pregnant and control groups revealed that, in pregnant animals, body mass increased over time (Ftime = 35.33; d.f. = 1,23; p < 0.01; GG-corrected) but not according to hormone treatment or time × hormone treatment interaction (all F < 0.26; all p > 0.62; interaction GG-corrected). These results suggest that the treatments do not differ from one another over time. The results are similar for control animals, where body mass changed over time (Ftime = 8.95; d.f. = 1,31; p < 0.01; GG-corrected) but not according to treatment or time × treatment interaction (all F < 0.55, all p > 0.56; GG-corrected).
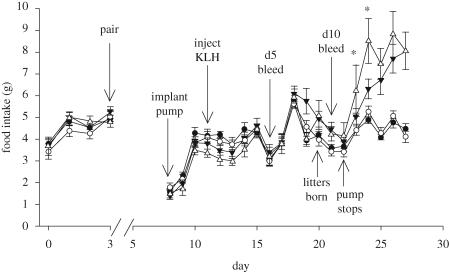
Food intake was subdivided into five different time periods: (i) baseline (pre-pairing with males); (ii) pre-KLH injection (post-pairing with males); (iii) pre-day 5 bleed (post-KLH injection); (iv) pre-day 10 bleed (post KLH injection); and (v) post-pump function (i.e. no longer receiving exogenous leptin). Pregnancy states were analysed separately using repeated measures ANOVAs. For both pregnant and control females, food intake changed over time (all F > 49.42, all p < 0.01; GG-corrected), but not according to hormone treatment or a hormone treatment × time interaction (all F < 2.25, all p > 0.14). Paired t-tests within pregnant and control groups revealed that leptin-treated animals in both groups increased their food intake to a greater degree than vehicle-treated animals after the pumps stop delivering leptin (all t > 4.29, all p < 0.05; figure 3).
Figure 3.
Food intake over time. There was a significant difference in food intake among treatments for ‘post-pump’ intake and a significant time × treatment interaction for ‘pre-day 10 bleed’ and ‘post-pump’ intake periods. Food intake changed significantly over time in all the analyses. Leptin-treated animals in both groups increase their food intake to a greater degree than vehicle-treated animals after the pumps stop delivering leptin, suggesting that leptin treatment was suppressing intake. Filled circles, control vehicle; open circles, control leptin; filled triangles, pregnant vehicle; open triangles, pregnant leptin. Asterisks denote statistically significant differences (α = 0.05 level). Error bars represent ±1 s.e.
(d). Uterine and adipose mass
Final uterine mass and PWAT pads were significantly affected by pregnancy state (Futerine = 4.92; d.f. = 1,32; p = 0.03; FPWAT = 18.58; d.f. = 1,30; p < 0.01; table 1), such that pregnant animals had larger overall uterine and smaller PWAT masses. There was no effect or interaction with leptin treatment for either uterine or PWAT mass (all F < 2.16; p > 0.15).
Table 1.
Size and mass data. Initial body mass, change in body mass, paired uterine and fat pad masses in different treatment groups (±1 s.e.m.)
| group | initial body mass (g) | change body mass* (g) | uterine mass* (g) | PWAT* (g) | RWAT* (g) | IWAT (g) |
|---|---|---|---|---|---|---|
| pregnant leptin | 37.30 ± 1.61 | 6.79 ± 2.36 | 0.13 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 1.52 ± 0.14 |
| pregnant vehicle | 38.63 ± 2.23 | 7.12 ± 1.55 | 0.14 ± 0.01 | 0.14 ± 0.05 | 0.09 ± 0.03 | 1.97 ± 0.24 |
| control leptin | 36.60 ± 1.49 | −0.22 ± 0.56 | 0.09 ± 0.01 | 0.24 ± 0.04 | 0.18 ± 0.03 | 1.94 ± 0.18 |
| control vehicle | 36.61 ± 1.74 | −1.04 ± 0.77 | 0.12 ± 0.02 | 0.28 ± 0.04 | 0.18 ± 0.02 | 2.14 ± 0.19 |
*Denote significant differences among treatment groups (α = 0.05).
Final RWAT also varied significantly according to pregnancy state (FRWAT = 25.99; d.f. = 1,30; p < 0.01; table 1), where pregnant animals, regardless of leptin treatment, had significantly smaller RWAT pads than their non-pregnant control counterparts. There was no effect or interaction with leptin treatment on RWAT (all F < 1.49, all p > 0.23). There was also no significant effect of pregnancy state, leptin treatment or interaction, for final IWAT pads (all F < 2.16, all p > 0.15; table 1).
(e). Humoural immunity
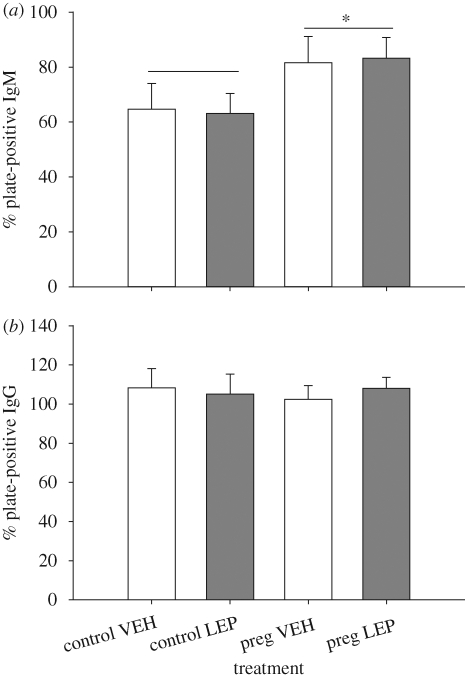
Circulating IgM levels varied significantly according to pregnancy state (F = 4.27; d.f. = 1,32; p = 0.04; figure 4a), such that pregnant animals had significantly higher IgM levels than control animals regardless of leptin treatment. There was no effect or interaction with leptin treatment on IgM levels (all F < 0.10, all p > 0.75). There was also no effect of pregnancy state, leptin treatment or interaction on IgG levels (all F < 0.25, all p > 0.62; figure 4b).
Figure 4.
Effects of leptin treatment and pregnancy on maternal antibody production. Pregnant females had significantly elevated anti-KLH IgM (a) production compared with control animals. There was no significant difference in anti-KLH IgG (b) among groups; however, IgG antibodies are delivered passively to the offspring via lactation which could explain the absence of a difference among pregnant and control females. In addition, pups from leptin-treated mothers trended towards having decreased anti-KLH IgG when compared with pups from vehicle-treated mothers (p = 0.06). Asterisks denote statistically significant differences (α = 0.05 level). Error bars represent ±1 s.e.
(f). Passive immunity
Serum samples from individuals within a litter were combined to achieve sufficient volume to run the assay. We found a trend towards pups from leptin-treated mothers (91.8 ± 7.2% plate positive) having lower anti-KLH IgG antibodies (t = 1.62, p = 0.06), relative to pups from vehicle-treated mothers (104.9 ± 7.2% plate positive).
(g). Innate immunity
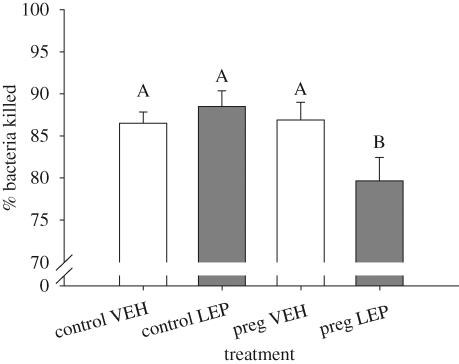
There was a significant interaction between pregnancy state and leptin treatment on bacterial killing (F = 4.82; d.f. = 1,35; p = 0.03; figure 5). Separate one-way ANOVAs revealed that there was no difference between leptin- and vehicle-treated control females (F = 0.73; d.f. = 1,19; p = 0.40), while there was a difference between leptin- and vehicle-treated pregnant females (F = 5.32; d.f. = 1,17; p = 0.03). There was no difference between pregnant and non-pregnant vehicle-treated females (F = 0.43; d.f. = 1,18; p = 0.52), while there was a difference between pregnant and non-pregnant leptin-treated females (F = 6.31; d.f. = 1,18; p = 0.02; figure 5).
Figure 5.
Effects of leptin treatment on bactericidal capacity. Pregnant females treated with leptin had significantly lower bacterial killing ability than all other treatments. Differing letters denote groups that differ significantly (α = 0.05 level). Error bars represent ±1 s.e.
4. Discussion
The number of successfully weaned or fledged offspring probably predicts lifetime reproductive success in small vertebrate species (Lack 1947, 1948; Williams 1966). Thus, selection should favour mechanisms that allow females to maximize litter size given sufficient resources. In nature, however, energetic resources are often limited, thus resulting in the abortion or resorption of embryos or the maternal reduction of litters (McClure 1981; Bronson 1985; Bronson & Marsteller 1985; Schneider & Wade 1989). In order to make these key fitness decisions, females must be able to reliably evaluate their energy status. The current study provides the first evidence that leptin serves as a signal to mothers of available energy stores, thereby allowing individuals to retain more embryos to parturition and rear more offspring to weaning or fledging. The observed fitness increase is not without costs to the mothers; one particular immune component was suppressed seemingly as a result of these larger litters.
Overall, the results of this study support the hypothesis that leptin serves as a hormonal signal-mediating resource among physiological systems, such as the reproductive and immune systems. Leptin-treated females had significantly larger litters than vehicle-treated females, suggesting that leptin signalled greater resource (fat) stores in these females permitting them to produce larger litters, perhaps via decreased resorption of embryos. While there were no clear adverse effects to the humoural immune system, the innate branch (measured by bacterial killing ability), was suppressed in leptin-treated mothers with larger litters.
Interestingly, leptin treatment also prevented post-partum maternal litter reduction or infanticide, which is often observed in females. In many rodent species, including hamsters, females cannibalize one or more pups, especially right after birth (McClure 1981; Bronson & Marsteller 1985; Schneider & Wade 1989). This may provide the required energy to compromised females, while simultaneously decreasing the energy demand by larger litters for milk production. Presumably, high leptin levels communicate sufficient resources to treated mothers, deterring these females from cannibalizing offspring. Studies have shown reduced resource availability and maternal body weight composition can lead to maternal infanticide (Schneider & Wade 1989). The pregnant females in the current study had significantly reduced fat stores (i.e. PWAT and RWAT). RWAT has been shown to be an important fat store that varies seasonally, relative to their control counterparts. Therefore, leptin may provide a false signal of enhanced body condition to leptin-treated mothers, preventing them from cannibalizing their offspring. In comparison, 40 per cent vehicle-treated females consumed at least one pup.
The energy required to produce and sustain larger litters is substantial; however, there were no differences in food intake or fat stores between leptin- and vehicle-treated mothers. This is not an uncommon result in Siberian hamsters, where other multiple studies find no effect of leptin on body mass or food intake in long-day animals (i.e. similar to the ones in this study), but do find an effect on other physiological systems (i.e. fat stores, immunity) (Drazen et al. 2001; Rousseau et al. 2002; Demas & Sakaria 2005). Although control and induced levels of leptin were comparable to previous studies in long-day housed hamsters (Rousseau et al. 2002; Demas & Sakaria 2005), some of which demonstrated leptin-induced immunoenhancement in energetically challenged individuals (Drazen et al. 2001; Demas & Sakaria 2005), no effect on humoural immunity was observed in the present study. Previous studies were conducted in males and ovariectomized females, and the treatments used in these studies differed (i.e. using lipectomies or photoperiodic decreases in fat stores and intake versus pregnancy), which could partially account for differing results (Drazen et al. 2001; Demas & Sakaria 2005). Temporal differences in study design, including leptin treatment and antigen challenge, may also account for these differences. In addition, alternative measures of humoural immunity (e.g. total immunoglobulin counts, alternative antigen challenge) may reveal different results.
One potential explanation for the disparate humoural immune results in the current study is that animals are foregoing the leptin-induced immune enhancement that has been observed in previous studies. For example, leptin treatment has been shown to increase both the production of proinflammatory cytokines and phagocytosis (Loffreda et al. 1998). Exogenous leptin treatment has also been shown to ameliorate the immunosuppressive effects of experimentally reduced body fat (i.e. experimental excision of body fat), as well as naturally occurring seasonal reductions in immune function (Drazen et al. 2001; Demas & Sakaria 2005). Across these studies leptin plays a permissive role without additional immune enhancement occurring in immunocompetent control animals (Drazen et al. 2001; Demas & Sakaria 2005). In the present study leptin treatment did result in similar changes to fat stores as observed in previous studies. Leptin, however, did not enhance immune function in either pregnant or control females, as might be expected. Presumably, the reduced bacterial killing observed in leptin-treated pregnant females may have been due to the energetic demands needed to gestate and nurse a larger than average litter. In fact, the innate immune response is suppressed in pregnant leptin-treated females, further suggesting energy allocation away from other systems towards the production of offspring.
Although all animals in this study were fed ad libitum, there was no significant effect of leptin treatment on food intake while the leptin pumps were active. Whereas pregnant females ate significantly more food than controls during the post-pump time period, there were no detectable differences earlier in the study. There were also no overall differences between leptin- and vehicle-treated animals in food intake, which is a common result in this species. Previous studies in long-day housed hamsters do not find an effect of leptin on food intake, but do demonstrate leptin-related immunological changes (Drazen et al. 2001). Therefore, even though leptin-treated females were producing larger litters, they did not compensate by increasing their intake, as might be expected. Leptin-treated mothers significantly increased food intake relative to vehicle-treated mothers, when the osmotic mini-pumps were depleted (14 days), suggesting that they are in an energy deficit and this deficit is masked by leptin treatment. Both pregnant groups also increased food intake during the post-pump period, probably due to the high costs of lactation (Cripps & Williams 1975). In fact, many studies show that leptin treatment decreases food intake, signalling to animals that they have sufficient energy reserves, regardless of their actual energy state (Mistry et al. 1997; Henry et al. 1999). The observation that leptin females did not decrease food intake, combined with the lack of an equivalent increase in food intake in females with larger litters, may be due to exogenous leptin treatment, thereby resulting in the necessary suppression of innate immune function.
Recent evidence suggests that maintenance of innate immune function requires significant energy investment (Lee & Klasing 2004). It would therefore make sense that this energetically costly process is suppressed in pregnant leptin-treated females in response to increased reproductive investment. Most studies across mammalian species demonstrate decreased immunity, including production of certain antibodies, during pregnancy (Weinberg 1984; Medina et al. 1993). In fact, previous studies in Siberian hamsters show decreased anti-KLH IgM response during pregnancy (Drazen et al. 2003). We neither observed any pregnancy-related decrease, nor any change in response to increased litter size in leptin-treated females (either IgG or IgM; figure 4). In fact, IgM production was enhanced in all pregnant females. This finding is not surprising given that pregnant females passively deliver antibodies to their offspring via both the placenta during pregnancy and milk during lactation (Butler 1969). Although IgM cannot be transferred to offspring, it is a precursor to IgG production, which may be transferred to offspring (Brambell 1958; Freda 1962; Klaus et al. 1969). Therefore, the lack of a difference in IgG levels among adult females may be, in part, because maternal IgG is being sequestered in the offspring. Antibody production, in this context, can therefore also be considered a form of reproductive/parental investment. The observed increase in IgM levels that is converted to IgG in order to allow transfer to offspring, may also allow females to avoid a substantial personal deficit during periods of passive transfer (i.e. lactation). Instead, leptin-treated pregnant females who have more pups have suppressed bacterial killing ability (innate immune response) relative to vehicle-treated pregnant females (figure 4). Vehicle-treated pregnant females did not differ significantly in their bacterial killing ability from non-pregnant females.
Collectively, these results suggest that leptin serves as a proximate endocrine signal of available energy. Instead of attenuating previously observed immunosuppression, it enhances investment into reproduction, thereby resulting in the suppression of certain components of self-maintenance (i.e. innate immunity). These results provide the first evidence that leptin serves as a signal to mothers, thus influencing the allocation of available energy. Leptin exerts trans-generational effects, increasing overall litter size and blocking maternal infanticide, resulting in a larger number of weaned offspring and thereby increasing maternal reproductive success. These results further emphasise the context-dependent nature of physiological trade-offs; they are dynamic and adjusting to current environmental conditions, including energetic signals (e.g. leptin). In the current study we masked this honest energetic signal by supplying mothers with exogenous leptin. Perhaps, the most significant finding is that the physiological mechanisms in place have evolved to protect investment in reproduction, thereby resulting in a potential reduction in self-maintenance. Future studies will examine long-term generational effects of leptin-induced reproductive enhancement.
Acknowledgements
We thank Nick Garcia for valuable help measuring food intake and body mass throughout the study. We thank Edmund Brodie Jr. for valuable commentary and feedback on this manuscript. We also thank three anonymous reviewers and a statistician for valuable commentary on this manuscript. This work was supported by Common Themes in Reproductive Diversity Training grant, NIH no. HD 049336-04. This work was reviewed and approved by the Institutional Animal Care and Use Committee at Indiana University under protocol no. 07-097.
References
- Baldelli R., Dieguez C., Casanueva F. F.2002The role of leptin in reproduction: experimental and clinical aspects. Ann. Med. 34, 5–18 (doi:10.1080/078538902317338599) [DOI] [PubMed] [Google Scholar]
- Bilbo S. D., Nelson R. J.2001Sex steroid hormones enhance immune function in male and female Siberian hamsters. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 280, R207–R213 [DOI] [PubMed] [Google Scholar]
- Brambell F. W. R.1958The passive immunity of the young mammal. Biol. Rev. 33, 488–531 (doi:10.1111/j.1469-185X.1958.tb01412.x) [Google Scholar]
- Bronson F. H.1985Mammalian reproduction: an ecological perspective. Biol. Reprod. 32, 1–26 (doi:10.1095/biolreprod32.1.1) [DOI] [PubMed] [Google Scholar]
- Bronson F. H., Marsteller F. A.1985Effect of short-term food deprivation on reproduction in female mice. Biol. Reprod. 33, 660–667 (doi:10.1095/biolreprod33.3.660) [DOI] [PubMed] [Google Scholar]
- Butler J. E.1969Bovine immunoglobulins: a review. J. Dairy Sci. 52, 1895–1909 [Google Scholar]
- Casanueva F. F., Dieguez C.1999Neuroendocrine regulation and actions of leptin. Front. Neuroendocrinol. 20, 317–363 (doi:10.1006/frne.1999.0187) [DOI] [PubMed] [Google Scholar]
- Casto J. M., Parker-Renga I. M., Ketterson E. D., Nolan V., Jr2000Experimentally elevated testosterone in male dark-eyed juncos suppresses cell-mediated immune function of social mates and offspring. Am. Zool. 40, 967–967 [Google Scholar]
- Cichon M., Dubiec A., Chadzinska M.2001The effect of elevated reproductive effort on humoral immune function in collared flycatcher females. Acta Oecol. Int. J. Ecol. 22, 71–76 (doi:10.1016/S1146-609X(00)01094-8) [Google Scholar]
- Cripps A. W., Williams V. J.1975The effect of pregnancy and lactation on food intake, gastrointestinal anatomy and the absorptive capacity of the small intestine in the albino rat. Br. J. Nutr. 33, 17–32 [DOI] [PubMed] [Google Scholar]
- Day D. E., Mintz E. M., Bartness T. J.2002Diet choice exaggerates food hoarding, intake and pup survival across reproduction. Physiol. Behav. 75, 143–157 (doi:10.1016/S0031-9384(01)00655-2) [DOI] [PubMed] [Google Scholar]
- Demas G. E., Sakaria S.2005Leptin regulates energetic tradeoffs between body fat and humoural immunity. Proc. R. Soc. B 272, 1845–1850 (doi:10.1098/rspb.2005.3126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas G. E., Chefer V., Talan M. I., Nelson R. J.1997Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 42, R1631–R1637 [DOI] [PubMed] [Google Scholar]
- Drazen D. L., Kriegsfeld L. J., Schneider J. E., Nelson R. J.2000Leptin, but not immune function, is linked to reproductive responsiveness to photoperiod. Am. J. Physiol.-Regul. Int. Comp. Physiol. 278, R1401–R1407 [DOI] [PubMed] [Google Scholar]
- Drazen D. L., Demas G. E., Nelson R. J.2001Leptin effects on immune function and energy balance are photoperiod dependent in Siberian hamsters (Phodopus sungorus). Endocrinology 142, 2768–2775 (doi:10.1210/en.142.7.2768) [DOI] [PubMed] [Google Scholar]
- Drazen D. L., Trasy A., Nelson R. J.2003Photoperiod differentially affects energetics of immunity in pregnant and lactating Siberian hamsters (Phodopus sungorus). Can. J. Zool.-Rev. Can. Zool. 81, 1406–1413 (doi:10.1139/z03-120) [Google Scholar]
- Fantuzzi G.2006Leptin: Nourishment for the immune system. Eur. J. Immunol. 36, 3101–3104 (doi:10.1002/eji.200636770) [DOI] [PubMed] [Google Scholar]
- Freda V. J.1962Placental transfer of antibodies in man. Am. J. Obstet. Gynecol. 84, 1756–1777 [DOI] [PubMed] [Google Scholar]
- Gaspar-López E., Casabiell J., Estevez J., Landete-Castillejos T., De La Cruz L., Gallego L., García A.2009Seasonal changes in plasma leptin concentration related to antler cycle in Iberian red deer stags. J. Comp. Physiol. B-Biochem. Syst. Environ. Physiol. 179, 617–622 (doi:10.1007/s00360-009-0343-7) [DOI] [PubMed] [Google Scholar]
- Greives T. J., McGlothlin J. W., Jawor J. M., Demas G. E., Ketterson E. D.2006Testosterone and innate immune function inversely covary in a wild population of breeding dark-eyed juncos (Junco hyemalis). Funct. Ecol. 20, 812–818 (doi:10.1111/j.1365-2435.2006.01167.x) [Google Scholar]
- Henry B. A., Goding J. W., Alexander W. S., Tilbrook A. J., Canny B. J., Dunshea F., Rao A., Mansell A., Clarke I. J.1999Central administration of leptin to ovariectomized ewes inhibits food intake without affecting the secretion of hormones from the pituitary gland: evidence for a dissociation of effects on appetite and neuroendocrine function. Endocrinology 140, 1175–1182 (doi:10.1210/en.140.3.1175) [DOI] [PubMed] [Google Scholar]
- Irene Tieleman B., Williams J. B., Ricklefs R. E., Klasing K. C.2005Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proc. R. Soc. B 272, 1715–1720 (doi:10.1098/rspb.2005.3155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus G. G., Bennett A., Jones E. W.1969A quantitative study of the transfer of colostral immunoglobulins to the newborn calf. Immunology 16, 293–299 [PMC free article] [PubMed] [Google Scholar]
- Lack D.1947The significance of clutch-size. Ibis 89, 302–352 (doi:10.1111/j.1474-919X.1947.tb04155.x) [Google Scholar]
- Lack D.1948The significance of clutch-size. III. Some interspecific comparisons. Ibis 90, 25–45 (doi:10.1111/j.1474-919X.1948.tb01399.x) [Google Scholar]
- Lee K. A., Klasing K. C.2004A role for immunology in invasion biology. TREE 19, 523–529 [DOI] [PubMed] [Google Scholar]
- Loffreda S., et al. 1998Leptin regulates proinflammatory immune responses. FASEB J. 12, 57–65 [PubMed] [Google Scholar]
- Lord G. M., Matarese G., Howard J. K., Baker R. J., Bloom S. R., Lechler R. I.1998Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394, 897–901 (doi:10.1038/29795) [DOI] [PubMed] [Google Scholar]
- Margetic S., Gazzola C., Pegg G. G., Hill R. A.2002Leptin: a review of its peripheral actions and interactions. Int. J. Obes. 26, 1407–1433 (doi:10.1038/sj.ijo.0802142) [DOI] [PubMed] [Google Scholar]
- Martin L. B., Scheuerlein A., Wikelski M.2003Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc. R. Soc. Lond. B 270, 153–158 (doi:10.1098/rspb.2002.2185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauget C., Mauget R., Sempere A.1997Metabolic rate in female European roe deer (Capreolus capreolus): incidence of reproduction. Can. J. Zool.-Rev. Can. Zool. 75, 731–739 (doi:10.1139/z97-094) [Google Scholar]
- McClure P. A.1981Sex-biased litter reduction in food-restricted wood rats (Neotoma floridana). Science 211, 1058–1060 (doi:10.1126/science.211.4486.1058) [DOI] [PubMed] [Google Scholar]
- Medina K., Smithson G., Kincade P.1993Suppression of B lymphopoiesis during normal pregnancy. J. Exp. Med. 178, 1507–1515 (doi:10.1084/jem.178.5.1507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry A. M., Swick A. G., Romsos D. R.1997Leptin rapidly lowers food intake and elevates metabolic rates in lean and ob/ob mice. J. Nutr. 127, 2065–2072 [DOI] [PubMed] [Google Scholar]
- Nilsson J. A., Raberg L.2001The resting metabolic cost of egg laying and nestling feeding in great tits. Oecologia 128, 187–192 (doi:10.1007/s004420100653) [DOI] [PubMed] [Google Scholar]
- Owen-Ashley N. T., Hasselquist D., Wingfield J. C.2004Androgens and the immunocompetence handicap hypothesis: unraveling direct and indirect pathways of immunosuppression in song sparrows. Am. Nat. 164, 490–505 (doi:10.1086/423714) [DOI] [PubMed] [Google Scholar]
- Rousseau K., Atcha Z., Cagampang F. R. A., Le Rouzic P., Stirland J. A., Ivanov T. R., Ebling F. J. P., Klingenspor M., Loudon A. S. I.2002Photoperiodic regulation of leptin resistance in the seasonally breeding siberian hamster (Phodopus sungorus). Endocrinology 143, 3083–3095 (doi:10.1210/en.143.8.3083) [DOI] [PubMed] [Google Scholar]
- Saino N., Møller A. P., Bolzern A. M.1995Testosterone effects on the immune system and parasite infestations in the barn swallow (Hirundo rustica): an experimental test of the immunocompetence hypothesis. Behav. Ecol. 6, 397–404 (doi:10.1093/beheco/6.4.397) [Google Scholar]
- Saino N., Ferrari R. P., Romano M., Ambrosini R., Møller A. P.2002Ectoparasites and reproductive trade-offs in the barn swallow (Hirundo rustica). Oecologia 133, 139–145 (doi:10.1007/s00442-002-1015-4) [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Wade G. N.1989Effects of maternal diet, body weight and body composition on infanticide in Syrian hamsters. Physiol. Behav. 46, 815–821 (doi:10.1016/0031-9384(89)90042-5) [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Zhou D., Blum R. M.2000Leptin and metabolic control of reproduction. Horm. Behav. 37, 306–326 (doi:10.1006/hbeh.2000.1590) [DOI] [PubMed] [Google Scholar]
- Weinberg E. D.1984Pregnancy-associated depression of cell-mediated immunity. Rev. Infect. Dis. 6, 814–831 [DOI] [PubMed] [Google Scholar]
- Williams G. C.1966Natural selection costs of reproduction and a refinement of Lack's principle. Am. Nat. 100, 687–690 (doi:10.1086/282461) [Google Scholar]
- Zieba D. A., Amstalden M., Williams G. L.2005Regulatory roles of leptin in reproduction and metabolism: a comparative review. Domest. Anim. Endocrinol. 29, 166–185 (doi:10.1016/j.domaniend.2005.02.019) [DOI] [PubMed] [Google Scholar]
- Zuk M., Stoehr A. M.2002Immune defense and host life history. Am. Nat. 160, S9–S22 (doi:10.1086/342131) [DOI] [PubMed] [Google Scholar]