Abstract
It is well established that the average metabolism of many species of fish varies with group size. However, it is not clear whether all individuals respond in the same way. Here, we use a newly calibrated method of measuring the metabolic rate of fish from opercular (ventilatory) movements that allows for the first-time estimation of changes in resting metabolic rate (RMR) of each individual within different social groups and when alone. The presence of a conspecific had divergent effects on the RMR of juvenile Atlantic salmon Salmo salar, depending on its relative body size: the presence of a smaller fish caused a 40 per cent reduction, whereas the presence of a slightly larger fish approximately doubled RMR. These effects occurred in the absence of activity and were sustained at lower magnitude in the case of the relatively smaller conspecific even if a transparent barrier prevented any physical interactions between fish. Changes in RMR were mirrored by changes in eye colour that indicate they were linked to stress levels. These contrasting and strong responses show that even the nearby presence of a conspecific can have profound and variable effects on an individual's energy budget; they also highlight the complex trade-offs involved in social interactions.
Keywords: Salmo salar, resting metabolism, salmon, body size, social behaviour, stress
1. Introduction
The resting metabolic rate (RMR) of an animal reflects its rate of energy expenditure (usually measured as oxygen consumption rate) when at rest. For ectotherms, major components of RMR are the costs of maintaining cellular processes, protein turnover and repair (Hulbert & Else 2004). The rate of oxygen consumption can vary substantially (up to fivefold) among individuals of the same species, even after controlling for factors such as body size, developmental stage and environmental conditions (Metcalfe et al. 1995; Steyermark et al. 2005). This variation has been linked to both physiological (Bayne 2000; Steyermark et al. 2005) and behavioural differences between individuals (Metcalfe et al. 1995; Cutts et al. 2001; McCarthy 2001). RMR also varies within an individual depending on its state of nutrition (O'Connor et al. 2000; Fu et al. 2005) or torpor (Guppy & Withers 1999), but under constant conditions has been found to be relatively stable and repeatable over time (McCarthy 2000), as is found to be the case with the closely related basal metabolic rate (BMR) of endotherms (Bech et al. 1999; Labocha et al. 2004; Szafranska et al. 2007).
However, it has recently been shown that changes in the physical structure of the habitat can induce a substantial increase in RMR in the absence of any physiological changes and no measureable change in behaviour: for instance, Millidine et al. (2006) found that the RMR of juvenile Atlantic salmon Salmo salar was increased by 30 per cent if the habitat contained no refuge from predators. It is thought that such increases in resting metabolism are due to a heightened state of readiness in preparation for fast reaction escape movements (Fischer 2000; Millidine et al. 2006). This suggests that the animal's level of alertness may have a profound influence on metabolic costs—a hypothesis given circumstantial support by the findings that the presence of a predator more than doubled the metabolic rate of longnose killifish Fundulus majalis (Woodley & Peterson 2003), although the latter result is based on routine rather than resting metabolism and so could be due to changes in activity levels.
The presence of conspecifics may also cause changes in alertness and hence metabolic costs, but their effects are potentially more complex. Conspecifics may be beneficial, conferring the well-known advantages of grouping (e.g. reduced predation risk or ease of food finding), but they may also be disadvantageous through increased competition for resources (Krause & Ruxton 2002). This competition may lead to aggressive interactions; hence while conspecifics may effectively provide shelter from predators (sensu the selfish herd of Hamilton 1971), they can also be the aggressors from which flight may be required. Confinement in the presence of a more dominant and aggressive individual can lead to elevated levels of physiological stress, causing a subsequent increase in metabolic costs once the fish are isolated again (Sloman et al. 2000). It is therefore unclear whether the presence of conspecifics would be expected to cause a reduction or an increase in metabolic costs.
Most investigations of the impact of social structure on metabolism have not controlled for activity levels (which are likely to change in the presence of conspecifics) and so the results are ambiguous (Wirtz & Davenport 1976; Schleuter et al. 2007). Herskin (1999) found no effect of grouping on the mean RMR of sea bass but, by averaging across individuals, may have missed intraspecific variation because subjects may differ in their response to the presence of conspecifics, depending on their relative size: being in the proximity of smaller individuals may confer anti-predator benefits, whereas larger conspecifics may be perceived as a competitive or predatory threat. No information is available regarding among-individual variation in group effects on metabolism because studies hitherto have measured only the total oxygen consumption of groups rather than the individuals within them. However, Millidine et al. (2008) established that the rate at which a fish's operculae (gill covers) oscillate during ventilation of the gills correlates closely with the oxygen consumption of juvenile Atlantic salmon, providing a means of determining the metabolism of each individual within a group.
Establishing the effect of the social environment on metabolic rates is of fundamental importance for understanding both the energy budgets that link feeding, growth and activity of animals, and the costs and benefits of grouping. Using juvenile Atlantic salmon as a subject and the opercular method for the measurement of metabolism in individuals within a group, here we test for the first time the novel prediction that the energy costs and benefits of grouping depend on the relative size of the individuals concerned.
2. Material and methods
The experiments were carried out on yearling hatchery-reared juvenile Atlantic salmon parr derived from wild parents and reared at the Marine Scotland Freshwater Laboratory field station, Perthshire. In early April 2007, a sample of fish that would remain as parr in fresh water for a further year (on the basis of their size; Thorpe 1977) was transferred to Glasgow University, where they were held in a 1 m2 tank at 9°C in aerated, recirculated, copper-free water under an ambient photoperiod. They were fed to satiation on defrosted bloodworms (Chironomid larvae) once a day. While in the holding tank the fish had access to shelters in the form of large stones and lengths of semicircular cut piping (approx. 120 mm in diameter).
On 20 April 2007, 18 pairs of fish were selected, such that one fish was noticeably larger than the other (mean initial body mass of the smaller fish = 2.05 g ± 0.41 s.d., range 1.17–2.63 g; larger fish = 3.63 g ± 0.63, range 2.35–4.57 g; mean weight difference between pairs = 1.59 g ± 0.66, range 0.27–2.63 g). The experiment consisted of recording the RMR of each experimental fish during a sequence of treatments (presented in random order to control for sequence effects) that differed in terms of the social environment. It was conducted using replicate paired compartments in a series of glass-sided observation channels. The treatments were as follows:
solitary (unable to see the other member of the pair);
visual contact (able to see the other member of the pair in an adjacent compartment through glass dividing wall);
full contact (in same compartment as the other member of the pair).
This design was similar to that used by Huntingford et al. (1993). No other fish were visible to experimental fish during the trials. Compartments for single fish (treatments 1 and 2) measured 12.5 × 20 × 20 cm3, while those for the pair of fish (treatment 3) were double the size so as to maintain the same density. All compartments contained 3 cm of levelled natural-coloured standard aquarium gravel and one opaque tube per fish (provided as a refuge because salmon without access to a refuge have higher metabolic rates; Millidine et al. 2006); the water depth was 12 cm and the flow rate through the compartments was 20 l min−1. Water temperature in the compartments was kept constant at 9°C throughout the experimental period.
The experimental protocol was as follows. The first two days (after introduction into the new environment) constituted the period of settling and exploration, after which fish were generally inactive and resting on the bottom of the experimental tank. Data on ventilation rates (VRs) were collected on three occasions (8, 11 and 14 h) on day 3. Metabolism was estimated from the equations in Millidine et al. (2008) that use information on opercular beat rates, fish mass (W) and water temperature (T) to predict rates of oxygen consumption (mg O2 h−1). The regression equations used to predict RMR from VR were
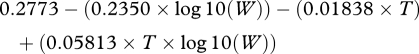 |
2.1 |
and
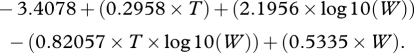 |
2.2 |
Equations (2.1) and (2.2) were then used to formulate a general relationship (RMR = m(VR) + c) in which VR is expressed as b.p.m., m is derived from equation (2.1) and c from equation (2.2). For further details, see Millidine et al. (2008).
This non-intrusive method has been shown to be highly successful on juvenile Atlantic salmon of this size range (the body mass of fish used ranged from 1.76 to 12.64 g, correlated between predicted and actual metabolic rates = 0.95; Millidine et al. 2008) and has the advantage over more intrusive methods of allowing individual measurements to be made on each fish within a social group. On each occasion, three replicate measurements were made (within 5 min of each other) of the number of ventilatory movements of the operculum per 20 s, and a mean value for the day was then calculated from the nine individual measurements. Activity was visually monitored every 30 min throughout the day of observations; however, movement was negligible during each scan (number of ventilation movements per 20 s recorded during each of the nine observation periods deviated between 0 and 3), indicating that the metabolic rates corresponded to RMR.
As the sclera (i.e. eye iris) colour of juvenile salmon has been shown to be related to their subordinate status and/or stress levels (O'Connor et al. 1999; Suter & Huntingford 2002), the sclera colour of each fish was also taken at the above times using the method described by Suter & Huntingford (2002). A score ranging from 1 (pale) to 5 (black) was assigned on each occasion, and the mean of these values was used in subsequent analyses. At the end of the observations on day 3, the fish were weighed and measured and returned to the same experimental treatment tank.
On days 4 and 5, the fish were fed ad libitum with defrosted bloodworms once per day. On day 6, each pair of fish was moved to another compartment, which was configured to be a different treatment. The following day (classed as day 1 of the protocol) constituted the settling period again, and this procedure (from day 1 through to day 6) was then followed repeatedly until all fish had been observed in each of the three treatments. If one member of the pair died during the experiment, its corresponding fish was removed from the experiment and none of the data from this pair were used in subsequent analyses.
On 18 May 2007, the experiment was repeated with a further 18 pairs of new fish (mean initial body mass of smaller fish = 2.81 g ± 0.68, range 1.66–4.51 g; larger = 4.25 g ± 0.65, range 3.31–5.74 g; mean weight difference between pairs = 1.44 g ± 0.53, range 0.58–2.12 g) and the same protocol was repeated. There was a significant difference between the initial weights of the first and second groups (independent samples t-test, t64 = 2.88, p = 0.005), as a consequence of growth while in the holding tanks. The effects of treatments on RMR and sclera colouration were analysed using repeated-measures ANOVAs, with the individual fish as a subject. Each fish was classified as being the larger or the smaller member of its pair (on the basis of body mass), and relative size (larger or smaller) was then used in the analysis as a between-subject factor.
3. Results
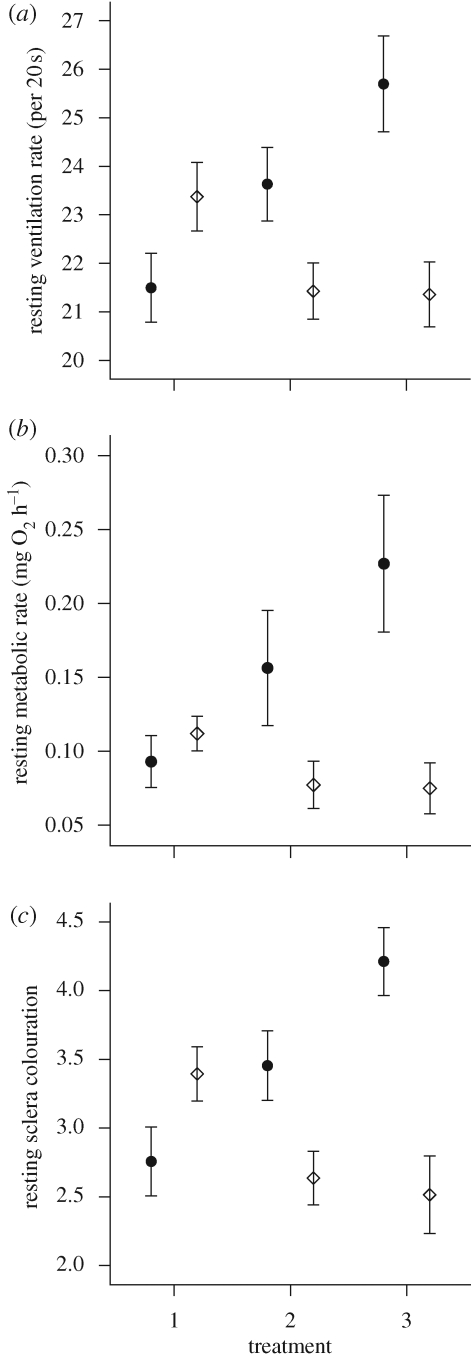
From the raw measurements of resting VR, there was a clear interaction between treatment and relative fish size (figure 1a). When these data were converted to RMR using equations (2.1) and (2.2) to account for fish mass, the interaction persisted (figure 1b). RMR of relatively small fish increased with the degree of exposure to a conspecific (one-way ANOVA, F2,31 = 23.95, p < 0.001), whereas RMR of relatively large fish decreased (one-way ANOVA, F2,31 = 8.74, p < 0.001).
Figure 1.
The effects of treatment on (a) VR, (b) calculated RMR (see text) and (c) index of eye sclera colouration (see text for details). Data presented as mean ± 95% confidence limits; open diamonds and closed circles represent the larger and the smaller member of a pair, respectively. N = 33 fish per size category. Treatments: 1, solitary fish; 2, visual contact only with a conspecific; 3, full contact with a conspecific (in the same compartment of a stream channel).
The extent of these changes depended on whether the other fish was actually in the same compartment or was simply visible through a transparent partition: a comparison of treatments 2 and 3 showed that the relatively smaller fish had a higher RMR when actually in the presence of a larger fish (paired t-test, t32 = 4.96, p < 0.001), whereas in the larger fish, RMR was unaffected by whether the smaller fish was in the same compartment or was only visible through a partition (paired t-tests, t32 = 0.39, p = 0.695).
Changes in the eye sclera colour of the fish showed similar trends to the changes in metabolic rate (figure 1c). Sclera colour became darker with the degree of exposure to conspecifics in relatively small fish (repeated-measures ANOVA, F2,31 = 29.94, p < 0.001) and lighter in relatively large fish (repeated-measures ANOVA, F2,31 = 15.85, p < 0.001).
As with RMR the sclera colour changed between treatments 2 and 3 in relatively small fish (paired t-test, t32 = 5.24, p < 0.001), but not relatively large fish (paired t-test, t32 = 1.00, p = 0.33).
4. Discussion
This study has demonstrated a group effect on RMR, with the oxygen consumption of a quiescent single fish altering when it was able to see a conspecific. More importantly, it showed that the direction of the effect varied among individuals depending on their relative size, and that the magnitude of the effect varied with the degree of exposure to conspecifics.
It is evident that identifying the basal maintenance metabolism of unstressed animals, termed standard or BMR, is far from simple and requires careful consideration of the costs and benefits of aggregating with conspecifics, as well as the physical habitat. These costs and benefits of aggregation will depend on the social interactions that take place among conspecifics. Juvenile Atlantic salmon can be territorial in riverine habitats (Grant et al. 1998; Klemetsen et al. 2003), and the negative group effects (i.e. increased metabolic rate when with others) observed in some contexts might be interpreted as reflecting a cost of territoriality, as in blennies (Wirtz & Davenport 1976). Such energy costs might be incurred even in the absence of actual aggression because they may be due to a heightened state of readiness to defend a territory or other ongoing metabolic costs of dominance (Bryant & Newton 1994). However, this seems unlikely in the present study because the larger fish of a pair (which would presumably win a dominance contest) actually showed a decrease in metabolic costs compared with when solitary. Aggregation may confer more benefits on relatively larger individuals because they gain the advantages of being in a group (e.g. reduced vulnerability to predators) while minimizing the costs of increased competition for food resources owing to their greater competitive ability or dominance status (Krause & Ruxton 2002). They are also much less likely to face aggression than the smaller members of the group. In the present study, the reduction in RMR of salmon when in the presence of smaller conspecifics was similar to their response to the presence of a physical shelter and may result from a similar physiological relaxation, or calming effect, associated with reduced predation risk (Millidine et al. 2006). The effect was evident despite the presence of a physical shelter, indicating the calming effects of social and physical habitat can be additive.
In contrast, fish showed an elevated RMR (and associated colouration indicative of subordinate status; O'Connor et al. 1999; Suter & Huntingford 2002) when in the presence of another larger salmon. This suggests that a metabolic cost is paid by subordinates, as has been found in other taxa (Senar et al. 2000). For these fish, while the presence of a larger conspecific is a dilution of the risk of predation, it is also a potential source of attack (Yue et al. 2006). Their response suggests that they were in a heightened state of physiological readiness to respond to an attack or were showing other signs of stress (Sloman et al. 2000). The elevation in RMR occurred when contact between fish was only visual, but was highest when direct physical contact was possible, suggesting that the fish were aware of variation in the level of risk.
While it has previously been shown that the RMR of animals can vary owing to factors such as diet composition (Cruz-Neto & Bozinovic 2004; Bozinovic et al. 2007), the implicit assumption is that such changes within individuals will be slow and gradual. In the present experiment, we recorded within-individual changes that were both rapid and dramatic: there was a twofold variation in RMR within the same fish owing to changes in the social environment. Energy budget calculations for free-living teleost fishes suggest that a twofold variation in RMR can result in up to 100 per cent variation in growth performance (Armstrong & Hawkins 2008). Such large metabolic costs will have substantial effects on growth rates across a range of energy intakes, as reflected perhaps in widely reported group effects on growth of fish (Blanckenhorn 1992; Marchand & Boisclair 1998). For juvenile salmonids, the differential effect of conspecifics on metabolic rate shown in this study will accentuate any differences in growth potential of high- and low-status fish, and may explain why dominance rank can have such a pronounced effect on the growth rate of similar-sized fish eating identical amounts of food (Abbott & Dill 1989). It may also help explain why fish prefer to shoal with same-size rather than larger conspecifics (Ranta et al. 1992; Ward & Krause 2001), and highlights the need to consider intraspecific variation when evaluating the costs and benefits of aggregation.
Acknowledgements
We thank M. S. Miles, S. Keay and J. Muir for rearing the fish at Almondbank, and J. Laurie and G. Law for tending them in Glasgow. K.J.M. was funded by an NERC CASE PhD studentship. The experiments were authorized by licenses from the UK Home Office and were approved by the University Ethics Committee.
References
- Abbott J. C., Dill L. M.1989The relative growth of dominant and subordinate juvenile steelhead trout (Salmo gairdneri) fed equal rations. Behaviour 108, 104–113 (doi:10.1163/156853989X00079) [Google Scholar]
- Armstrong J. D., Hawkins L. A.2008Standard metabolic rate of pike, Esox lucius: variation among studies and implications for energy flow modelling. Hydrobiologia 601, 83–90 (doi:10.1007/s10750-007-9268-x) [Google Scholar]
- Bayne B. L.2000Relations between variable rates of growth, metabolic costs and growth efficiencies in individual Sydney rock oysters (Saccostrea commercialis). J. Exp. Mar. Biol. Ecol. 251, 185–203 (doi:10.1016/S0022-0981(00)00211-2) [DOI] [PubMed] [Google Scholar]
- Bech C., Langseth I., Gabrielsen G. W.1999Repeatability of basal metabolism in breeding female kittiwakes Rissa tridactyla. Proc. R. Soc. Lond. B 266, 2161–2167 (doi:10.1098/rspb.1999.0903) [Google Scholar]
- Blanckenhorn W. U.1992Group size and the cost of agonistic behavior in pumpkinseed sunfish. Ethol. Ecol. Evol. 4, 255–271 [Google Scholar]
- Bozinovic F., Munoz J. L. P., Cruz-Neto A. P.2007Intraspecific variability in the basal metabolic rate: testing the food habits hypothesis. Physiol. Biochem. Zool. 80, 452–460 (doi:10.1086/518376) [DOI] [PubMed] [Google Scholar]
- Bryant D. M., Newton A. V.1994Metabolic costs of dominance in dippers, Cinclus cinclus. Anim. Behav. 48, 447–455 (doi:10.1006/anbe.1994.1258) [Google Scholar]
- Cruz-Neto A. P., Bozinovic F.2004The relationship between diet quality and basal metabolic rate in endotherms: insights from intraspecific analysis. Physiol. Biochem. Zool. 77, 877–889 (doi:10.1086/425187) [DOI] [PubMed] [Google Scholar]
- Cutts C. J., Adams C. E., Campbell A.2001Stability of physiological and behavioural determinants of performance in Arctic char (Salvelinus alpinus). Can. J. Fish. Aquat. Sci. 58, 961–968 (doi:10.1139/cjfas-58-5-961) [Google Scholar]
- Fischer P.2000An experimental test of metabolic and behavioural responses of benthic fish species to different types of substrate. Can. J. Fish. Aquat. Sci. 57, 2336–2344 (doi:10.1139/cjfas-57-11-2336) [Google Scholar]
- Fu S. J., Xie X. J., Cao Z. D.2005Effect of fasting on resting metabolic rate and postprandial metabolic response in Silurus meridionalis. J. Fish Biol. 67, 279–285 (doi:10.1111/j.0022-1112.2005.00723.x) [Google Scholar]
- Grant J. W. A., Steingrímsson S. O., Keeley E. R., Cunjak R. A.1998Implications of territory size for the measurement and prediction of salmonid abundance in streams. Can. J. Fish. Aquat. Sci. 55(Suppl. 1), 181–190 [Google Scholar]
- Guppy M., Withers P.1999Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biol. Rev. 74, 1–40 (doi:10.1017/S0006323198005258) [DOI] [PubMed] [Google Scholar]
- Hamilton W. D.1971Geometry for selfish herd. J. Theor. Biol. 31, 295–302 (doi:10.1016/0022-5193(71)90189-5) [DOI] [PubMed] [Google Scholar]
- Herskin J.1999Effects of social and visual contact on the oxygen consumption of juvenile sea bass measured by computerized intermittent respirometry. J. Fish Biol. 55, 1075–1085 (doi:10.1111/j.1095-8649.1999.tb00742.x) [Google Scholar]
- Hulbert A. J., Else P. L.2004Basal metabolic rate: history, composition, regulation, and usefulness. Physiol. Biochem. Zool. 77, 869–876 (doi:10.1086/422768) [DOI] [PubMed] [Google Scholar]
- Huntingford F. A., Metcalfe N. B., Thorpe J. E.1993Social status and feeding in Atlantic salmon Salmo salar parr: the effect of visual exposure to a dominant. Ethology 94, 201–206 [Google Scholar]
- Klemetsen A., Amundsen P. A., Dempson J. B., Jonsson B., Jonsson N., O'Connell M. F., Mortensen E.2003Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecol. Freshwater Fish 12, 1–59 (doi:10.1034/j.1600-0633.2003.00010.x) [Google Scholar]
- Krause J., Ruxton G. D.2002Living in groups, 1st edn Oxford, UK: Oxford University Press [Google Scholar]
- Labocha M. K., Sadowska E. T., Baliga K., Semer A. K., Koteja P.2004Individual variation and repeatability of basal metabolism in the bank vole, Clethrionomys glareolus. Proc. R. Soc. Lond. B 271, 367–372 (doi:10.1098/rspb.2003.2612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand F., Boisclair D.1998Influence of fish density on the energy allocation pattern of juvenile brook trout (Salvelinus fontinalis). Can. J. Fish. Aquat. Sci. 55, 796–805 (doi:10.1139/cjfas-55-4-796) [Google Scholar]
- McCarthy I. D.2000Temporal repeatability of relative standard metabolic rate in juvenile Atlantic salmon and its relation to life history variation. J. Fish Biol. 57, 224–238 (doi:10.1111/j.1095-8649.2000.tb00788.x) [Google Scholar]
- McCarthy I. D.2001Competitive ability is related to metabolic asymmetry in juvenile rainbow trout. J. Fish Biol. 59, 1002–1014 (doi:10.1111/j.1095-8649.2001.tb00167.x) [Google Scholar]
- Metcalfe N. B., Taylor A. C., Thorpe J. E.1995Metabolic rate, social status and life-history strategies in Atlantic salmon. Anim. Behav. 49, 431–436 (doi:10.1006/anbe.1995.0056) [Google Scholar]
- Millidine K. J., Armstrong J. D., Metcalfe N. B.2006Presence of shelter reduces maintenance metabolism of juvenile salmon. Funct. Ecol. 20, 839–845 (doi:10.1111/j.1365-2435.2006.01166.x) [Google Scholar]
- Millidine K. J., Metcalfe N. B., Armstrong J. D.2008The use of ventilation frequency as an accurate indicator of metabolic rate in juvenile Atlantic salmon. Can. J. Fish. Aquat. Sci. 65, 2081–2087 (doi:10.1139/F08-118) [Google Scholar]
- O'Connor K. I., Metcalfe N. B., Taylor A. C.1999Does darkening signal submission in territorial contests between juvenile Atlantic salmon, Salmo salar? Anim. Behav. 58, 1269–1276 (doi:10.1006/anbe.1999.1260) [DOI] [PubMed] [Google Scholar]
- O'Connor K. I., Taylor A. C., Metcalfe N. B.2000The stability of standard metabolic rate during a period of food deprivation in juvenile Atlantic salmon. J. Fish Biol. 57, 41–51 (doi:10.1111/j.1095-8649.2000.tb00774.x) [Google Scholar]
- Ranta E., Lindstrom K., Peuhkuri N.1992Size matters when three-spined sticklebacks go to school. Anim. Behav. 43, 160–162 (doi:10.1016/S0003-3472(05)80082-X) [Google Scholar]
- Schleuter D., Haertel-Borer S., Fischer P., Eckmann R.2007Respiration rates of Eurasian perch Perca fluviatilis and ruffe: lower energy costs in groups. Trans. Am. Fish. Soc. 136, 43–55 (doi:10.1577/T06-123.1) [Google Scholar]
- Senar J. C., Polo V., Uribe F., Camerino M.2000Status signalling, metabolic rate and body mass in the siskin: the cost of being a subordinate. Anim. Behav. 59, 103–110 (doi:10.1006/anbe.1999.1281) [DOI] [PubMed] [Google Scholar]
- Sloman K. A., Motherwell G., O'Connor K. I., Taylor A. C.2000The effect of social stress on the standard metabolic rate (SMR) of brown trout, Salmo trutta. Fish Physiol. Biochem. 23, 49–53 (doi:10.1023/A:1007855100185) [Google Scholar]
- Steyermark A. C., Miamen A. G., Feghahati H. S., Lewno A. W.2005Physiological and morphological correlates of among-individual variation in standard metabolic rate in the leopard frog Rana pipiens. J. Exp. Biol. 208, 1201–1208 (doi:10.1242/jeb.01492) [DOI] [PubMed] [Google Scholar]
- Suter H. C., Huntingford F. A.2002Eye colour in juvenile Atlantic salmon: effects of social status, aggression and foraging success. J. Fish Biol. 61, 606–614 (doi:10.1111/j.1095-8649.2002.tb00899.x) [Google Scholar]
- Szafranska P. A., Zub K., Konarzewski M.2007Long-term repeatability of body mass and resting metabolic rate in free-living weasels, Mustela nivalis. Funct. Ecol. 21, 731–737 (doi:10.1111/j.1365-2435.2007.01273.x) [Google Scholar]
- Thorpe J. E.1977Biomodal distribution of length of juvenile Atlantic salmon (Salmo salar L.) under artificial rearing conditions. J. Fish Biol. 12, 541–548 (doi:10.1111/j.1095-8649.1977.tb04111.x) [Google Scholar]
- Ward A. J. W., Krause J.2001Body length assortative shoaling in the European minnow, Phoxinus phoxinus. Anim. Behav. 62, 617–621 (doi:10.1006/anbe.2001.1785) [Google Scholar]
- Wirtz P., Davenport J.1976Increased oxygen consumption in blennies (Blennius pholis L.) exposed to their mirror images. J. Fish Biol. 9, 67–74 (doi:10.1111/j.1095-8649.1976.tb04662.x) [Google Scholar]
- Woodley C. M., Peterson M. S.2003Measuring responses to simulated predation threat using behavioral and physiological metrics: the role of aquatic vegetation. Oecologia 136, 155–160 (doi:10.1007/s00442-003-1236-1) [DOI] [PubMed] [Google Scholar]
- Yue S., Duncan I. J. H., Moccia R. D.2006Do differences in conspecific body size induce social stress in domestic rainbow trout? Env. Biol. Fish. 76, 425–431 (doi:10.1007/s10641-006-9015-6) [Google Scholar]