Abstract
Maternally derived yolk antibodies provide neonates with immune protection in early life at negligible cost to mothers. However, developmental effects on the neonate's future immunity are potentially costly and thus could limit yolk antibody deposition. The benefits to neonatal immunity must be balanced against costs, which may depend on neonate vulnerability to pathogens, developmental trajectories and the immunological strategies best suited to a species' pace of life. We measured yolk antibodies and life-history features of 23 species of small Neotropical birds and assessed the evidence for each of several hypotheses for life history and ecological effects on the evolution of yolk antibody levels. Developmental period and yolk antibodies are negatively related, which possibly reflect the importance of humoral immune priming through antigen exposure, and selection to avoid autoimmunity, in species with a slower pace of life. There is also a strong relationship between body size and yolk antibody concentration, suggesting that larger species are architecturally equipped to produce and transfer higher concentrations of antibodies. These results suggest that developmental effects of maternally derived antibodies, such as imprinting effects on B-cell diversity or autoimmune effects, are important and deserve more consideration in future research.
Keywords: disease ecology, immune development, maternal effects, life-history physiology, parasites, yolk antibodies
1. Introduction
Maternal influences on offspring phenotype, termed maternal effects (Mousseau & Fox 1998), have received special attention as an indirect genetic effect influencing an individual's life-history traits. The recent literature has focused on maternal allocation of hormones that direct resources to influence offspring behaviour, growth and development (Schwabl 1996; Gil 2003). However, few studies to date have investigated maternal provisioning of antibodies, which protect offspring from pathogens, influencing resource allocation and development of immunity (Grindstaff et al. 2003; Boulinier & Staszewski 2008; Hasselquist & Nilsson 2009).
Maternally derived yolk antibodies protect neonates against environmental pathogens during early life (Graczyk et al. 1994; Baintner 2007; Pravieux et al. 2007). Yolk antibodies are deposited during yolk formation by the active transport of circulating immunoglobulins across the follicle (Linden & Roth 1978; West et al. 2004). The primary antibody present in avian eggs is immunoglobulin Y (IgY is for yolk, Rose et al. 1974; Tizard 2002), which is usually secreted by B-cells in direct response to an immune challenge. IgA and IgM appear at much lower concentrations and are primarily in the albumen of the egg. Chicken embryos undergo a period of rapid IgY uptake from their residual yolk, starting approximately 3 days prior to hatch and continuing for approximately 2 days post-hatch (Rose & Orlans 1981). Because most IgY is produced in a specific response to an immune challenge, neonates receive protection against pathogens likely to be relevant within their lifetime, and thus their passive immunity is shaped by their mothers' previous disease exposure. Maternally derived antibodies benefit chicks through two documented routes; first, they directly interfere with pathogenicity by coating pathogens (Carlier & Truyens 1995; Leuridan & Van Damme 2007; Nemeth et al. 2008), and second, they reduce the demands on the neonate immune system, and thus free resources to be directed towards other growth or maintenance needs (Pihlaja et al. 2006; Gallizzi et al. 2008; Grindstaff 2008). There has also been a proposed imprinting effect of maternal antibodies on immune development of offspring (Lemke & Lange 1999), where the B-cell repertoire is proposed to be modelled after the antibodies present during development. This is usually considered a potential benefit as there is some evidence that ‘naive’ offspring of non-naive mothers have higher responses to an antigen long after maternal antibodies have dissipated (Reid et al. 2006), though it can also have negative effects if mothers transfer autoantibodies (Greeley et al. 2002) or if imprinting mechanisms interfere with other immune system arms (Lemke & Lange 1999).
Despite their protective function, yolk antibodies make up only a small proportion of the total protein in the yolk. Presumably, maternal provisioning of antibodies also imposes costs that limit the amount allocated to eggs. These costs could be imposed on the hen during egg formation, or they may be imposed on the developing chick. The direct cost to the hen of producing antibodies is relatively low and is not likely to be higher than producing other egg proteins (Klasing 1998). Physiological factors might also constrain antibody synthesis by egg producing females. During reproduction, the immune system may be generally suppressed or reproductive resource use prioritized. For instance, during egg production in waterfowl, antibody synthesis changes to an alternate isotype (called IgYΔFc), which reduces the inflammatory response but is also not transferred to yolks (Humphrey et al. 2004). Two distinct developmental costs to chicks of maternal antibodies have been proposed. During B-cell development, lineages of pre-B-cells are positively selected if they are functional and bind to environmental antigens presented in the bursa or bind very loosely to self-antigens that might have molecular patterns similar to pathogenic organisms (Baumgarth et al. 2005). Pre-B-cells that are not functional or that bind tightly to self-antigens are negatively selected. The resulting set of B-cells recognizes a diverse set of antigens, but should not inflict damage via autoimmunity. However, large volumes of maternally derived antibodies could coat, and effectively hide, antigens that are important to B-cell development (Carlier & Truyens 1995). There is evidence that maternal antibodies coat antigens, inhibiting the juvenile immune response (Lung et al. 1996; Staszewski et al. 2007). If environmental and self-antigens were hidden from the B-cell selection process, antibody diversity in later life might be compromised by a failure of positive selection at the pre-B-cell stage. The hiding of self-antigens could also cause autoimmune disorders through a failure of the negative selection process. The influence of maternal antibodies on diversification of the B-cell repertoire (Fink et al. 2008) or autoimmune disease has a relatively short history in the medical literature (Zinkernagel 2001; Von Herrath & Bach 2002; Agarwal & Agarwal 2006; Victora et al. 2007). Although the costs remain poorly understood, we can assume that these costs do influence yolk antibody allocation decisions.
Many ecological and life-history factors influence total and specific maternal antibody allocation to eggs (Grindstaff et al. 2003). Antibody allocation should match the exposure of offspring to pathogens, which will be determined by characteristics of the rearing habitat, the nest type and the amount of time spent in the nest exposed to parasites. The developmental strategy will not only influence exposure period, but could also alter the relative importance of allocation of resources to immune responses or maturation (Lee 2006). We investigated ecological and life-history patterns of maternal antibody allocation to eggs in a variety of lowland tropical bird species. If mothers adaptively allocated antibodies in response to ecological or life-history factors, we would expect support for two non-mutually exclusive hypotheses: (i) developmental life-history hypothesis: a fast life-history strategy, where growth is rapid and adaptive immunity is less important (Lee 2006), will select for higher yolk antibody levels because this will help divert resources towards growth, despite costs to adaptive immune development; and (ii) nestling ecology hypothesis: open nests and long nestling periods, where offspring are relatively exposed to environmental pathogens and vectors of disease, will select for higher yolk antibody levels relative to enclosed nests or short nestling periods. Alternatively, enclosed nests have been suggested to harbour more ectoparasites and so may select for higher yolk antibodies compared with open nests.
2. Material and methods
We located nests of passerine birds in and around Gamboa, Panama, from 15 March to 30 June 2006 and from 27 February to 25 May 2007. Gamboa is adjacent to Neotropical lowland dry forest in the Panama Canal Zone. The study period from February to May covers the dry-to-wet season transition (usually around mid-April) during the onset of the breeding season for most species in the area. Nests located during the building stage, or with only one egg, were used in the present study. Nests were checked daily, and first eggs were collected on the day the second egg was laid to prevent nest abandonment (except in clay-coloured robins Turdus grayi, where whole clutches were collected for another study). Collected eggs were stored at 4°C for up to 24 h until IgY extraction.
(a). Life-history and ecological traits
We included the following life history and ecological variables in the analyses: clutch size; incubation and nestling periods; nest type (open or enclosed) and adult body mass. We collected life-history data by locating nests and weighing adult birds captured during the breeding season. We found nests of most species during the nest construction and egg-laying phases of the breeding cycle. Nests were visited daily during egg laying and near expected hatch and fledging dates. Eggs were numbered individually as they were laid, and chicks were marked with non-toxic markers to facilitate individual identification. In this study, clutch size is the mean number of eggs per nest. Incubation period is the time from onset of incubation to hatching of the first egg; onset of incubation in most species was on the evening the penultimate egg of a clutch was laid. Nestling period is the time between hatching of the first egg and fledging of the first hatched chick. We categorized nests as open when they were open cups, platforms or scrapes on the ground. If nests were burrows, tree cavities, gourd- or pendant-shaped nests or cavities in termitaria, we categorized them as enclosed. Body masses were obtained from mist-netted birds at and near Gamboa.
(b). Yolk immunoglobulin Y extraction and measurement
Eggs were weighed whole and then broken open, and the yolks separated and weighed to the nearest 0.001 g. For extraction, we aliquoted 0.1 g of yolk into a 1.5 ml microcentrifuge tube and removed the lipid by vortexing with 400 µl of 3.5 per cent PEG-6000 in 0.9 per cent NaCl, incubating overnight at 4°C and centrifuging for 30 min at 8000g, 4°C. The yolk pellet was discarded, and the supernatant reserved at 4°C for analysis. For yolk samples processed fresh, extraction efficiency is close to 100 per cent, as validated with a stripped yolk pool spiked with known quantities of chicken IgY (B. Addison 2007, unpublished data).
Yolk IgY was quantified by SDS–PAGE on 5 per cent gels run at 90 V for 30 min. Chicken IgY (purified polyclonal, Sigma-Aldrich, St Louis, MO, USA) standard dilutions were run simultaneously to generate a standard curve. Protein was stained with gelcode blue (Coomassie) stain (Pierce Biotechnology, Rockford, IL, USA) for 1 h and washed in distilled water overnight. Gels were then photographed on a white light table, and the images were analysed for band density using the gel tool in ImageJ (NIH, figure S1, electronic supplementary material). Integrated density values were converted to concentration, in mg ml−1, using the chicken IgY standard curve, and the values were multiplied by 4 to account for dilution during extraction. We used yolk IgY concentration in the analysis because our species varied greatly in egg size.
Martinez et al. (2003) raise concerns about IgY overlap with other serum proteins, making SDS–PAGE methods for comparing among species difficult. However, to some degree, these concerns do not apply to our methods. First, we measure antibodies in yolk, which has significantly fewer proteins than plasma, and so concerns about protein overlap are to a large degree alleviated by this. Moreover, we use an extraction technique that separates protein from the plasma component of yolk and probably also selects to some degree for antibody-like proteins. This excludes a significant number of proteins from the SDS–PAGE analysis. To more thoroughly address these concerns, we calculated the amount of protein overlapping the IgY band of yolk plasma extracts from chicken yolk based on the table in Mann & Mann (2008). The emPAI (a measure of protein concentration) of IgY in the plasma portion only was reported as 1394. We calculated the emPAI values for all yolk plasma proteins overlapping the IgY band to total 53.3 (an overestimate, since some of these proteins were spread over regions beyond the IgY band). While the degree to which these proteins overlap and the proportions of total yolk protein are likely to vary among species, we expect this would only introduce noise or dampen relationships.
(c). Statistical analysis
For all species, we calculated the mean value of IgY concentration for our comparative analysis (response variable). We selected nine statistical models from the parameters we measured, including a null model and a global model for AICc model ranking and multimodel inference (Burnham & Anderson 2002). Our candidate model set included a global model with all parameters, a nestling ecology model, a development model, the bivariate models for each of the individual parameters and a null model (table 1).
Table 1.
List of models included in the AICc analysis of life-history effects on yolk IgY deposition. The parameters included are clutch size (species mean and clutch), incubation period (species mean in days, incp), nestling period (species mean in days, nestlp), adult body mass (mean in g, mass), nest type (open or closed, nesttype) and an evolutionary model (evolmodel).
| ID | model |
|---|---|
| global 1 | clutch, incp, nestlp, mass, nesttype, nestlp*nesttype, incp*nestlp, evolmodel |
| nestling ecology 2 | clutch, nestlp, mass, nesttype, nestlp*nesttype, evolmodel |
| development 3 | incp, nestlp, mass, incp*nestlp, evolmodel |
| 4 | clutch, evolmodel |
| 5 | incp, evolmodel |
| 6 | nestlp, evolmodel |
| 7 | mass, evolmodel |
| 8 | nesttype, evolmodel |
| null | evolmodel |
We used a tree pruned from Cohen et al. (2008) to test for the effect of phylogeny. Using the regressionv2.m package (Lavin et al. 2008) in Matlab (R2007a), we first fitted the best model of evolution for our traits of interest and then selected a set of candidate models for explaining life history and ecology effects on yolk IgY, accounting for phylogeny and our model of evolution. The regressionv2.m package selects the best model of evolution for the traits of interest by fitting the saturated model for each of several models of evolution, including species values (ordinary least squares, no phylogenetic signal), Brownian motion (phylogenetic generalized least squares, equivalent to Felsenstein's contrasts), Ornstein–Uhlenbeck process (OU, drift about a fitness peak) or with branch lengths transformed using Pagel's λ parameter. The OU and Pagel's λ fitting procedures calculate branch length transformation parameters, d for the OU process that is a function of time and λ for Pagel's transformation, which is constant across the tree, by restricted maximum likelihood (REML). Values close to 1 indicate that evolution is close to Brownian motion, whereas values close to 0 approximate a star phylogeny.
3. Results
We obtained life history and yolk antibody values for 23 species including dove, suboscine and oscine species (figure S2, electronic supplementary material). All species included have an altricial developmental mode, with incubation periods ranging from 12 to 23 days, nestling periods from 9 to 28 days, clutch size from two to four eggs, mass from 7.3 to 68 g and yolk antibody concentrations from 0.10 to 0.75 mg ml−1. The within-species variability in IgY varied greatly by species.
In AICc evaluation of the evolutionary models for phylogenetic signal, the OLS star phylogeny was the best fit to the data for the global model with an AICc weight of 0.9 (table 2). To be conservative, we also evaluated evolutionary models for the null model. Both the OU and OLS trait evolution models had ΔAICc values under 2 and can be considered to have support. Furthermore, the REML estimates of phylogenetic signal were λ = 0.75 (Pagel's lambda evolution model) and d = 0.35 (OU evolution model) for the null model and λ = 0.39 and d = 0.15 for the global model, such that the λ- and d-transformed trees had shorter interior branches for the traits of interest. We report AICc scores for all life-history ecology models of yolk IgY for both OLS and OU evolutionary models.
Table 2.
Evaluation of the best-fit evolutionary model for the global model and the null model. Non-phylogenetic analysis (OLS) was best fit for the global model, but second to models of a weak phylogenetic signal (OU) in the null model.
| model | K | n | LgL | AICc | ΔAICc | LΔAICc | AICcW | evidence ratio | r2 |
|---|---|---|---|---|---|---|---|---|---|
| global = clutch, incp, nestlp, mass, nesttype, nestlp*nesttype, incp*nestlp, evolmodel | |||||||||
| OLS | 9 | 23 | 12.358 | 7.130 | 0.000 | 1.000 | 0.920 | 1.000 | 0.707 |
| OU | 10 | 23 | 12.500 | 13.333 | 6.203 | 0.045 | 0.041 | 22.229 | 0.667 |
| λ | 10 | 23 | 12.358 | 13.617 | 6.487 | 0.039 | 0.036 | 25.626 | 0.669 |
| PGLS | 9 | 23 | 6.4207 | 19.005 | 11.875 | 0.003 | 0.002 | 379.001 | 0.573 |
| null = evolmodel | |||||||||
| OU | 2 | 23 | −0.872 | 9.008 | 0.000 | 1.000 | 0.440 | 1.000 | 0 |
| OLS | 2 | 23 | −2.584 | 9.768 | 0.761 | 0.684 | 0.301 | 1.463 | 0 |
| λ | 3 | 23 | −1.842 | 10.947 | 1.939 | 0.379 | 0.167 | 2.637 | 0 |
| PGLS | 3 | 23 | −3.768 | 12.136 | 3.128 | 0.209 | 0.092 | 4.779 | 0 |
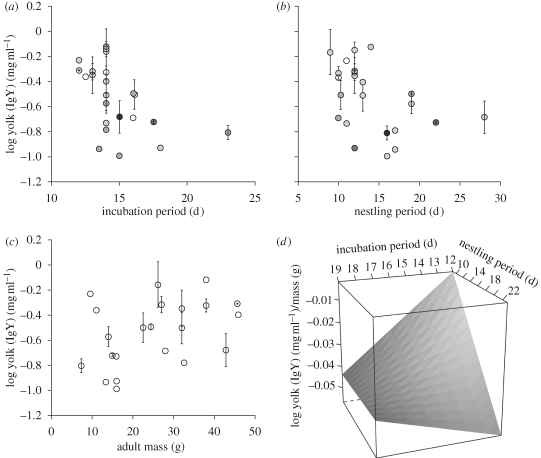
The strongest model for both the OLS and OU analyses was the development model of yolk IgY (AICc weights of 0.95 and 0.81, respectively, table 3). The models that followed in support were the bivariate models of the parameters contained within the development model. Because the results for the OLS and OU analyses were qualitatively similar and because the AICc model weight for the top OLS model was greater than 0.95, we report only the OLS parameter estimates for the top model (table 4). The relationships between yolk IgY and both incubation and nestling periods were negative, but the interaction term was positive, indicating that the slopes are not equal. For species with longer incubation periods, the relationship with nestling period was weaker (figure 1). These patterns hold even with the exclusion of the common tody-flycatcher (Todirostrum cinereum) and grey-breasted martin (Progne chalybea), the species with the longest incubation and nestling periods, respectively, in our dataset. There was also a strong positive relationship between yolk IgY and adult body mass (figure 1).
Table 3.
AICc ranking, delta values, likelihoods and weights and model r2 for models of life-history effects on yolk IgY deposition. Analysis was done assuming a star phylogeny (OLS) and assuming stabilizing selection with drift (OU) using a tree compiled from the literature and presented in Cohen et al. (2008).
| model | K | n | LgL | AICc | ΔAICc | LΔAICc | AICcW | evidence ratio | r2 |
|---|---|---|---|---|---|---|---|---|---|
| evolmodel = OLS | |||||||||
| 3 | 6 | 23 | 10.517 | −3.784 | 0.000 | 1.000 | 0.953 | 1.000 | 0.656 |
| 7 | 3 | 23 | 1.648 | 3.968 | 7.752 | 0.021 | 0.020 | 48.220 | 0.297 |
| 5 | 3 | 23 | 0.933 | 5.398 | 9.181 | 0.010 | 0.010 | 98.558 | 0.254 |
| 6 | 3 | 23 | 0.623 | 6.018 | 9.801 | 0.007 | 0.007 | 134.380 | 0.187 |
| 1 | 9 | 23 | 12.358 | 7.130 | 10.913 | 0.004 | 0.004 | 234.317 | 0.707 |
| 8 | 3 | 23 | −0.255 | 7.773 | 11.557 | 0.003 | 0.003 | 323.223 | 0.176 |
| 2 | 7 | 23 | 6.326 | 8.814 | 12.598 | 0.002 | 0.002 | 543.915 | 0.505 |
| null | 2 | 23 | −2.584 | 9.768 | 13.552 | 0.001 | 0.001 | 876.573 | 0.000 |
| 4 | 3 | 23 | −2.043 | 11.350 | 15.133 | 0.001 | 0.000 | 1932.556 | 0.044 |
| evolmodel = OU | |||||||||
| 3 | 7 | 23 | 10.658 | 0.151 | 0.000 | 1.000 | 0.814 | 1.000 | 0.624 |
| 7 | 4 | 23 | 3.093 | 4.037 | 3.886 | 0.143 | 0.117 | 6.980 | 0.297 |
| 5 | 4 | 23 | 1.406 | 7.411 | 7.261 | 0.027 | 0.022 | 37.723 | 0.171 |
| 6 | 4 | 23 | 1.503 | 7.216 | 7.066 | 0.029 | 0.024 | 34.220 | 0.131 |
| null | 3 | 23 | −0.872 | 9.008 | 8.857 | 0.012 | 0.010 | 83.799 | 0.000 |
| 8 | 4 | 23 | 0.171 | 9.880 | 9.729 | 0.008 | 0.006 | 129.632 | 0.078 |
| 4 | 4 | 23 | −0.651 | 11.524 | 11.373 | 0.003 | 0.003 | 294.864 | 0.019 |
| 2 | 8 | 23 | 7.645 | 10.996 | 10.846 | 0.004 | 0.004 | 226.507 | 0.492 |
| 1 | 10 | 23 | 12.500 | 13.333 | 13.182 | 0.001 | 0.001 | 728.460 | 0.667 |
Table 4.
Parameter estimates of effect on yolk IgY deposition based on the top OLS model (assuming star phylogeny) that had an AICc weight greater than 0.95.
| estimate | (±s.e.) | |
|---|---|---|
| intercept | 3.723478 | 1.298166 |
| incp | −0.2828359 | 0.08787441 |
| nestlp | −0.2780414 | −0.08941521 |
| mass | 0.008275306 | 0.002668124 |
| incp*nestlp | 0.0171757 | 0.005923048 |
Figure 1.
Life-history predictors of yolk IgY deposition. Points are species means±s.e. (a) Incubation period is strongly negatively related to yolk IgY deposition, even if the common tody-flycatcher (T. cinereum, incubation period of 23 days) is excluded. Marker shade is coded by the nestling period, darker colours correspond to longer nestling periods. (b) Nestling period is also negatively related to yolk IgY deposition, again, even with the exclusion of the extreme value of the grey-breasted martin (P. chalybea, nestling period of 28 days). Marker shade corresponds to incubation period. (c) Adult body mass is positively related to yolk IgY deposition. (d) Predictive surface showing the relationship between incubation period, nestling period and mass corrected yolk [IgY], generated without the two extreme value species.
We found no support for the effects of clutch size or nest type on yolk IgY, and the relationship with nestling period was opposite to the direction expected for the nestling ecology hypothesis.
4. Discussion
We evaluated models representing two hypotheses for the evolution of yolk IgY deposition strategies. Both in AICc model evaluation and in multimodel inference, factors related to life history and development weighted substantially more important than factors related to nestling ecology. Life history, specifically development period, has a strong effect on yolk IgY allocation in the resident Neotropical birds sampled in our study. Moreover, the effect of phylogenetic history on this trait was weak, suggesting that it is highly labile. The effect of development on yolk IgY could be operating through several pathways, which are not mutually exclusive.
Larger adults deposit higher concentrations of yolk IgY in their eggs, which could reflect a greater capacity for antibody synthesis in larger animals (Davis et al. 1999; Magnadóttir et al. 1999; Lee et al. 2008) or some other allometric reason (Hasselquist 2007). Alternatively, the offspring of larger species may have a longer development period (Gillooly et al. 2008); however, there was no relationship between body mass and developmental period in our species set. The effect of body size appears to reflect the architectural costs of yolk antibody deposition to the hen. It would be interesting to investigate whether these differences are indeed driven largely by plasma cell density and productivity or whether there are also differences in IgY receptor expression on the yolk follicle.
The strong negative relationship with incubation period most likely derives from incubation period serving as an index of development rate and/or pace of life. Since yolk IgY remains relatively inert until uptake by the embryo a short time before hatching (Tressler & Roth 1987; Kaspers et al. 1991), incubation period per se should not be related to yolk IgY deposition. If degradation of IgY over time prior to embryonic uptake were of concern, we would predict a positive relationship between incubation period and yolk IgY, which is the opposite of what we found. Thus, the relationship between IgY and this index of pace of life fits well with a developmental cost to maternal antibody allocation (discussed subsequently). There is little information on comparative rates of immune development in avian species, and what is known is largely restricted to precocial species (Apanius 1998).
Although species with longer nestling periods also deposit less IgY into their eggs, the relationship was weaker for species with long incubation periods, generating a positive interaction between incubation and nestling periods in their effect on yolk IgY. This could reflect a compromise between long development periods and long exposure periods, suggesting weak effects of nestling ecology. For instance, species with long incubation periods tend to have correspondingly long nestling periods (Lack 1968), which make the nestling more vulnerable to parasites normally found in high densities in the nest, such as philornid flies and mites. Nestling period is a poor index of the developmental period in the species in our dataset because many tropical species leave the nest at an early developmental state (Ricklefs 1976; Remes & Martin 2002). However, it might be a good indication of developmental priorities in very early life, as species leaving the nest early probably expend more resources prior to nest departure on traits such as feather growth and muscle maturation, required to achieve that early nest departure. As such, the benefits of maternal antibodies could be of greater importance, while the potential developmental costs are diminished, for these species.
The idea that maternally derived antibodies have a blocking effect on offspring immunity has been important for the development of vaccination schedules in human and veterinary medicine (Block et al. 2007; Siegrist 2007). Although the effect of maternal antibodies on immune responses is well documented, it is not clear whether this blocking effect is exclusive to T-cell-mediated responses or whether it might also affect lymphocyte development (Carlier & Truyens 1995). The recognition of antigen is an important component of B-cell development (Tizard 2002; Baumgarth et al. 2005), presenting a potential mechanism for a negative effect of maternal antibodies on immune system development. However, some evidence also supports a positive effect via immunological imprinting (Gasparini et al. 2006; Grindstaff et al. 2006; Reid et al. 2006). The exact mechanism is unclear, but the B-cell repertoire may somehow model itself after antibodies in circulation (Fink et al. 2008).
The relationship between maternal antibodies, development time and immunity is probably complex and dependent on the arm of the immune system of interest and possibly even the route of response to a specific antigen. The evidence for an effect of developmental period, or pace of life in general, on immunity is mixed, and much more needs to be done to understand what predictions should be made for different arms of the immune system. Tella et al. (2002) found that slow pace of life species have higher cell-mediated immunity assayed by phytohaemagglutinin (PHA) wing web swelling, whereas Palacios & Martin (2006) found the opposite relationship using the same measure of immunity. Tieleman et al. (2005) found that slow pace of life species had higher innate immunity measured by bactericidal activity of blood. Lee et al. (2008) found that slow pace of life species had higher natural antibodies (relationship with incubation period) and lower complement activity (relationship with clutch size) in a set of species that includes many of the same species as this study. Different arms of the immune system could evolve independently, and the exact directional predictions probably depend a great deal on what aspect of immunity is measured (Blount et al. 2003). Perhaps, one of the best illustrations of this is a study of immune function in high (fast pace of life) and low latitude (slow pace of life) populations of house sparrows (Passer domesticus, Martin et al. 2006), which found that the slow pace of life population had a higher speed of humoral response measured as secondary antibody response to wing web injections of keyhole limpet haemocyanin and a higher magnitude of cell-mediated response measured by PHA wing web injection, but a lower magnitude peak of humoral response and lower T-cell response to KLH challenge compared with the fast pace of life population. It has been suggested that long developmental periods, and a slow pace of life, permit and perhaps necessitate the development of a stronger adaptive immune system (Lee et al. 2008). Species with long incubation periods have previously been shown to have lower parasite-induced nestling mortality (Møller 2005) and lower adult stage hematozoan infection prevalence (Ricklefs 1992), suggesting potentially important effects of pace of life on immune development.
Yolk IgY concentrations measured in this study were lower than levels measured in chickens (1.15–2.26 mg ml−1, Hamal et al. 2006) or quail Coturnix japonica (3.27–4.35 mg ml−1, Grindstaff et al. 2005) and comparable to estimates reported in Ficedula albicollis (0.63 mg ml−1 relative to chicken standard, Hargitai et al. 2006). Several factors could contribute to the low levels measured in wild altricial birds. Domesticated fowls have better nutrition and thus more easily produce antibodies for allocation to eggs. Alternatively, the species studied in the wild are generally of small body size, and this appears to be a very important factor. Also, the altricial developmental mode, where much of the immune development probably occurs after hatch when maternal antibodies are active, may contribute to lower levels. Many of the studies of maternal antibodies in wild species have not attempted to quantify actual concentrations and have instead used relative values measured in immunoassays. This inhibits further comparative analysis, and researchers are encouraged to quantify actual values alongside relative values whenever possible.
Previous studies have found that hen plasma antibody levels and egg yolk antibodies are correlated (Hamal et al. 2006). For specific antigens, the slope of the correlation between hen plasma and yolk antibodies differs for different idiotypes (Tizard 2002), which could indicate a degree of selectivity for the movement of antibodies across the follicle membrane. There are two possible ways for adjustment of yolk antibody levels. Adjustment of circulating plasma antibodies allows control both of the total antibodies deposited in yolks as the molecules will tend to move down a concentration gradient and of the proportion of different antibody idiotypes in the yolk. The second way to modify yolk antibodies is by adjustment of yolk follicle receptor density. It is not clear to what degree different species employ these two possible strategies.
Much of the ecoimmunology literature has addressed almost exclusively benefits of maternal antibody allocation (Boulinier & Staszewski 2008; Hasselquist & Nilsson 2009), but the low levels of yolk IgY in wild species strongly indicate important costs. We suggest three physiological avenues of investigation that remain unexplored in a comparative context. First, we need to understand the role of maternally derived antibodies in B-cell development, in particular during pre-B-cell selection in the bursa, and in T-cell development. Studies should consider the different modes of immune response, T-cell dependent versus independent, for different antigen patterns. Second, we need more information on the time course of immune development for different arms of the immune system and among different life-history strategies. For instance, species investing little in humoral immunity should prioritize development of the innate arms of the immune system, with B-cell development occurring later and more rapidly. Third, we need to understand the long-term consequences of maternal antibodies for B-cell and T-cell repertoires, immunologic responsiveness and memory, immunosenescence, and autoimmunity.
Overall, our analysis suggests a developmental cost limiting yolk IgY deposition, at least in small Neotropical birds. Although life history appears to be an important determinant of the evolution of this maternal effect, outstanding questions remain about the causes of inter-individual variability of this trait and the physiological activity of maternal antibodies, especially with respect to costs.
Acknowledgements
All collections were under permit from Panamanian ANAM authorities, University of Missouri IACUC and the Smithsonian Tropical Research Institute.
We would like to thank members of the Ricklefs Lab at UMSL and the Klasing Lab at UC Davis for discussion, especially Kelly Lee and Kim Livingston. Field and laboratory work was supported by the life history physiology nexus project (NSF IBN-0212587) and grants from Sigma Xi, the St Louis Audubon Society and the Whitney R. Harris World Ecology Center. An NSERC PGS D supported B.A. We would also like to thank the Smithsonian Tropical Research Institute in Panamá for assistance with field logistics. The nests would not have been found without the keen eyes of Jorge Herrera, Kathania Herrera, Lisa Miller, Betzi Perez and Ruby Zambrano, among others who have worked on the Robinson lab life history project. Finally, we would like to acknowledge the input of two anonymous reviewers that significantly improved the manuscript.
References
- Agarwal K., Agarwal R.2006Effects of maternal autoimmune diseases on fetus and neonates. J. Neonatol. 20, 172–175 [Google Scholar]
- Apanius V.1998The immune system. In Avian growth and development: evolution within the altricial–precocial spectrum (eds Starck J. M., Ricklefs R. E.), pp. 203–222 Oxford, UK: Oxford University Press [Google Scholar]
- Baintner K.2007Transmission of antibodies from mother to young: evolutionary strategies in a proteolytic environment. Vet. Immunol. Immunopathol. 117, 153–161 (doi:10.1016/j.vetimm.2007.03.001) [DOI] [PubMed] [Google Scholar]
- Baumgarth N., Tung J. W., Herzenberg L. A.2005Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Semin. Immunopathol. 26, 347–362 (doi:10.1007/s00281-004-0182-2) [DOI] [PubMed] [Google Scholar]
- Block H., Meyer-Block K., Rebeski D. E., Scharr H., de Wit S., Rohn K., Rautenschlein S.2007A field study on the significance of vaccination against infectious bursal disease virus (IBDV) at the optimal time point in broiler flocks with maternally derived IBDV antibodies. Avian Pathol. 36, 401–485 (doi:10.1080/03079450701589175) [DOI] [PubMed] [Google Scholar]
- Blount J. D., Houston D. C., Møller A. P., Wright J.2003Do individual branches of immune defence correlate? A comparative case study of scavenging and non-scavenging birds. Oikos 102, 340–350 (doi:10.1034/j.1600-0706.2003.12413.x) [Google Scholar]
- Boulinier T., Staszewski V.2008Maternal transfer of antibodies: raising immuno-ecology issues. Trends Ecol. Evol. 23, 282–288 (doi:10.1016/j.tree.2007.12.006) [DOI] [PubMed] [Google Scholar]
- Burnham K. P., Anderson D. R.2002Model selection and multimodel inference: a practical information-theoretic approach New York, NY: Springer-Verlag [Google Scholar]
- Carlier Y., Truyens C.1995Influence of maternal infection on offspring resistance towards parasites. Parasitol. Today 11, 94–99 (doi:10.1016/0169-4758(95)80165-0) [DOI] [PubMed] [Google Scholar]
- Cohen A. A., McGraw K. J., Wiersma P., Williams J. B., Robinson W. D., Robinson T. R., Brawn J. D., Ricklefs R. E.2008Interspecific associations between circulating antioxidant levels and life-history variation in birds. Am. Nat. 172, 178–193 [DOI] [PubMed] [Google Scholar]
- Davis C. R., Marty G. D., Adkison M. A., Freiberg E. F., Hedrick R. P.1999Association of plasma IgM with body size, histopathologic changes, and plasma chemistries in adult Pacific herring. Clupea pallasi. Dis. Aquat. Organ. 38, 125–133 (doi:10.3354/dao038125) [DOI] [PubMed] [Google Scholar]
- Fink K., Zellweger R., Weber J., Manjarrez-Orduno N., Holdener M., Senn B. M., Hengartner H., Zinkernagel R. M., Macpherson A. J.2008Long-term maternal imprinting of the specific B cell repertoire by maternal antibodies. Eur. J. Immunol. 38, 90–101 (doi:10.1002/eji.200737872) [DOI] [PubMed] [Google Scholar]
- Gallizzi K., Guenon B., Richner H.2008Maternally transmitted parasite defence can be beneficial in the absence of parasites. Oikos 117, 223–230 (doi:10.1111/j.2007.0030-1299.16172.x) [Google Scholar]
- Gasparini J., McCoy K. D., Staszewski V., Haussy C., Boulinier T.2006Dynamics of anti-Borrelia antibodies in blacklegged kittiwake (Rissa tridactyla) chicks suggest a maternal educational effect. Can. J. Zool.-Revue Canadienne De Zoologie 84, 623–627 (doi:10.1139/Z06-024) [Google Scholar]
- Gil D.2003Golden eggs: maternal manipulation of offspring phenotype by egg androgen in birds. Ardeola 50, 281–294 [Google Scholar]
- Gillooly J. F., Londono G. A., Allen A. P.2008Energetic constraints on an early developmental stage: a comparative view. Biol. Lett. 4, 123–126 (doi:10.1098/rsbl.2007.0460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graczyk T. K., Cranfield M. R., Shaw M. L., Craig L. E.1994Maternal antibodies against Plasmodium spp. in African black-footed penguin (Spheniscus demersus) chicks. J. Wildl. Dis. 30, 365–371 [DOI] [PubMed] [Google Scholar]
- Greeley S. A. W., Katsumata M., Yu L., Eisenbarth G. S., Moore D. J., Goodarzi H., Barker C. F., Naji A., Noorchashm H.2002Elimination of maternally transmitted autoantibodies prevents diabetes in nonobese diabetic mice. Nat. Med. 8, 399–402 (doi:10.1038/nm0402-399) [DOI] [PubMed] [Google Scholar]
- Grindstaff J. L.2008Maternal antibodies reduce costs of an immune response during development. J. Exp. Biol. 211, 654–660 (doi:10.1242/jeb.012344) [DOI] [PubMed] [Google Scholar]
- Grindstaff J. L., Brodie E. D., Ketterson E. D.2003Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. R. Soc. Lond. B 270, 2309–2319 (doi:10.1098/rspb.2003.2485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff J. L., Demas G. E., Ketterson E. D.2005Diet quality affects egg size and number but does not reduce maternal antibody transmission in Japanese quail Coturnix japonica. J. Anim. Ecol. 74, 1051–1058 (doi:10.1111/j.1365-2656.2005.01002.x) [Google Scholar]
- Grindstaff J. L., Hasselquist D., Nilsson J. A., Sandell M., Smith H. G., Stjernman M.2006Transgenerational priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc. R. Soc. B 273, 2551–2557 (doi:10.1098/rspb.2006.3608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamal K. R., Burgess S. C., Pevzner I. Y., Erf G. F.2006Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poult. Sci. 85, 1364–1372 [DOI] [PubMed] [Google Scholar]
- Hargitai R., Prechl J., Torok J.2006Maternal immunoglobulin concentration in collared flycatcher (Ficedula albicollis) eggs in relation to parental quality and laying order. Funct. Ecol. 20, 829–838 (doi:10.1111/j.1365-2435.2006.01171.x) [Google Scholar]
- Hasselquist D.2007Comparative immunoecology in birds: hypotheses and tests. J. Ornithol. 148, 571–582 (doi:10.1007/s10336-007-0201-x) [Google Scholar]
- Hasselquist D., Nilsson J. A.2009Maternal transfer of antibodies in vertebrates: trans-generational effects on offspring immunity. Phil. Trans. R. Soc. B 364, 51–60 (doi:10.1098/rstb.2008.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey B. D., Calvert C. C., Klasing K. C.2004The ratio of full length IgY to truncated IgY in immune complexes affects macrophage phagocytosis and the acute phase response of mallard ducks (Anas platyrhynchos). Dev. Comp. Immunol. 28, 665–672 (doi:10.1016/j.dci.2003.11.003) [DOI] [PubMed] [Google Scholar]
- Kaspers B., Schranner I., Losch U.1991Distribution of immunoglobulins during embryogenesis in the chicken. J. Vet. Med. A Physiol. Pathol. Clin. Med. 38, 73–79 [DOI] [PubMed] [Google Scholar]
- Klasing K. C.1998Nutritional modulation of resistance to infectious diseases. Poult. Sci. 77, 1119–1125 [DOI] [PubMed] [Google Scholar]
- Lack D. L.1968Ecological adaptations for breeding in birds. London, UK: Methuen [Google Scholar]
- Lavin S. R., Karasov W. H., Ives A. R., Middleton K. M., Garland T.2008Morphometrics of the avian small intestine compared with that of nonflying mammals: a phylogenetic approach. Physiol. Biochem. Zool. 81, 526–550 (doi:10.1086/590395) [DOI] [PubMed] [Google Scholar]
- Lee K. A.2006Linking immune defenses and life history at the levels of the individual and the species. Integr. Comp. Biol. 46, 1000–1015 (doi:10.1093/icb/icl049) [DOI] [PubMed] [Google Scholar]
- Lee K. A., Wikelski M., Robinson W. D., Robinson T. R., Klasing K. C.2008Constitutive immune defences correlate with life-history variables in tropical birds. J. Anim. Ecol. 77, 356–363 (doi:10.1111/j.1365-2656.2007.01347.x) [DOI] [PubMed] [Google Scholar]
- Lemke H., Lange H.1999Is there a maternally induced immunological imprinting phase a la Konrad Lorenz? Scand. J. Immunol. 50, 348–354 (doi:10.1046/j.1365-3083.1999.00620.x) [DOI] [PubMed] [Google Scholar]
- Leuridan E., Van Damme P.2007Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine 25, 6296–6304 (doi:10.1016/j.vaccine.2007.06.020) [DOI] [PubMed] [Google Scholar]
- Linden C. D., Roth T. F.1978IgG receptors on foetal chick yolk sac. J. Cell Sci. 33, 317–328 [DOI] [PubMed] [Google Scholar]
- Lung N. P., Thompson J. P., Kollias G. V., Olsen J. H., Zdziarski J. M., Klein P. A.1996Maternal immunoglobulin G antibody transfer and development of immunoglobulin G antibody responses in blue and gold macaw (Ara ararauna) chicks. Am. J. Vet. Res. 57, 1162–1167 [PubMed] [Google Scholar]
- Magnadóttir B., Jónsdóttir H., Helgason S., Björnsson B., Jøgensen T. Ø., Pilström L.1999Humoral immune parameters in Atlantic cod (Gadus morhua L.). II. The effects of size and gender under different environmental conditions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 122, 181–188 (doi:10.1016/S0305-0491(98)10157-8) [DOI] [PubMed] [Google Scholar]
- Mann K., Mann M.2008The chicken egg yolk plasma and granule proteomes. Proteomics 8, 178–191 (doi:10.1002/pmic.200700790) [DOI] [PubMed] [Google Scholar]
- Martin L. B., II, Hasselquist D., Wikelski M.2006Investment in immune defense is linked to pace of life in house sparrows. Oecologia 147, 565–575 (doi:10.1007/s00442-005-0314-y) [DOI] [PubMed] [Google Scholar]
- Martinez J., Tomas G., Merino S., Arriero E., Moreno J.2003Detection of serum immunoglobulins in wild birds by direct ELISA: a methodological study to validate the technique in different species using antichicken antibodies. Funct. Ecol. 17, 700–706 (doi:10.1046/j.1365-2435.2003.00771.x) [Google Scholar]
- Møller A. P.2005Parasites, predators and the duration of developmental periods. Oikos 111, 291–301 (doi:10.1111/j.0030-1299.2005.14005.x) [Google Scholar]
- Mousseau T. A., Fox C. W.1998The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407 (doi:10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- Nemeth N. M., Oesterle P. T., Bowen R. A.2008Passive immunity to West Nile virus provides limited protection in a common passerine species. Am. J. Trop. Med. Hyg. 79, 283–290 [PubMed] [Google Scholar]
- Palacios M. G., Martin T. E.2006Incubation period and immune function: a comparative field study among coexisting birds. Oecologia 146, 505–512 (doi:10.1007/s00442-005-0220-3) [DOI] [PubMed] [Google Scholar]
- Pihlaja M., Siitari H., Alatalo R. V.2006Maternal antibodies in a wild altricial bird: effects on offspring immunity, growth and survival. J. Anim. Ecol. 75, 1154–1164 (doi:10.1111/j.1365-2656.2006.01136.x) [DOI] [PubMed] [Google Scholar]
- Pravieux J. J., Poulet H., Charreyre C., Juillard V.2007Protection of newborn animals through maternal immunization. J. Comp. Pathol. 137, S32–S34 (doi:10.1016/j.jcpa.2007.04.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J. M., Arcese P., Keller L. F., Hasselquist D.2006Long-term maternal effect on offspring immune response in song sparrows Melospiza melodia. Biol. Lett. 2, 573–576 (doi:10.1098/rsbl.2006.0544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remes V., Martin T. E.2002Environmental influences on the evolution of growth and developmental rates in passerines. Evolution 56, 2505–2518 [DOI] [PubMed] [Google Scholar]
- Ricklefs R. E.1976Growth rate of birds in the humid New World tropics. Ibis 118, 176–207 (doi:10.1111/j.1474-919X.1976.tb03065.x) [Google Scholar]
- Ricklefs R. E.1992Embryonic development period and the prevalence of avian blood parasites. Proc. Natl Acad. Sci. USA 89, 4722–4725 (doi:10.1073/pnas.89.10.4722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Orlans E.1981Immunoglobulins in the egg, embryo and young chick. Dev. Comp. Immunol. 5, 15–20 (doi:10.1016/S0145-305X(81)80003-1) [DOI] [PubMed] [Google Scholar]
- Rose M. E., Orlans E., Buttress N.1974Immunoglobulin classes in hens egg—their segregation in yolk and white. Eur. J. Immunol. 4, 521–523 (doi:10.1002/eji.1830040715) [DOI] [PubMed] [Google Scholar]
- Schwabl H.1996Maternal testosterone in the avian egg enhances postnatal growth. Comp. Biochem. Physiol. A Physiol. 114, 271–276 (doi:10.1016/0300-9629(96)00009-6) [DOI] [PubMed] [Google Scholar]
- Siegrist C. A.2007The challenges of vaccine responses in early life: selected examples. J. Comp. Pathol. 137, S4–S9 (doi:10.1016/j.jcpa.2007.04.004) [DOI] [PubMed] [Google Scholar]
- Staszewski V., Gasparini J., McCoy K. D., Tveraa T., Boulinier T.2007Evidence of an interannual effect of maternal immunization on the immune response of juveniles in a long-lived colonial bird. J. Anim. Ecol. 76, 1215–1223 (doi:10.1111/j.1365-2656.2007.01293.x) [DOI] [PubMed] [Google Scholar]
- Tella J. L., Scheuerlein A., Ricklefs R. E.2002Is cell-mediated immunity related to the evolution of life-history strategies in birds? Proc. R. Soc. Lond. B 269, 1059–1066 (doi:10.1098/rspb.2001.1951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieleman B. I., Williams J. B., Ricklefs R. E., Klasing K. C.2005Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proc. R. Soc. B 272, 1715–1720 (doi:10.1098/rspb.2005.3155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizard I.2002The avian antibody response. Semin. Avian Exot. Pet Med. 11, 2–14 (doi:10.1053/saep.2002.28216) [Google Scholar]
- Tressler R. L., Roth T. F.1987IgG receptors on the embryonic chick yolk-sac. J. Biol. Chem. 262, 15 406–15 412 [PubMed] [Google Scholar]
- Victora G. D., Bilate A. M. B., Socorro-Silva A., Caldas C., Lima R. C., Kalil J., Coelho V., Victora C. G.2007Mother–child immunological interactions in early life affect long-term humoral autoreactivity to heat shock protein 60 at age 18 years. J. Autoimmun. 29, 38–43 (doi:10.1016/j.jaut.2007.02.018) [DOI] [PubMed] [Google Scholar]
- Von Herrath M., Bach J. F.2002Juvenile autoimmune diabetes: a pathogenic role for maternal antibodies? Nat. Med. 8, 331–333 (doi:10.1038/nm0402-331) [DOI] [PubMed] [Google Scholar]
- West A. P., Herr A. B., Bjorkman P. J.2004The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC-related Fc receptor, is a phospholipase A(2) receptor homolog. Immunity 20, 601–610 [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M.2001Maternal antibodies, childhood infections, and autoimmune diseases. N. Engl. J. Med. 345, 1331–1335 (doi:10.1056/NEJMra012493) [DOI] [PubMed] [Google Scholar]