Abstract
Aims
This paper examines trends in age-specific mortality in a rural South African population from 1992 to 2003, a decade spanning major sociopolitical change and emergence of the HIV/AIDS pandemic. Changing mortality patterns are discussed within a health-transition framework.
Methods
Data on population size, structure, and deaths, obtained from the Agincourt health and demographic surveillance system, were used to calculate person-years at risk and death rates. Life tables were computed by age, sex and calendar year. Mortality rates for the early period 1992–93 and a decade later, 2002–03, were compared.
Results
Findings demonstrate significant increases in mortality for both sexes since the mid-1990s, with a rapid decline in life expectancy of 12 years in females and 14 years in males. The increases are most prominent in children (0–4) and young adult (20–49) age groups, in which increases of two- and fivefold respectively have been observed in the past decade. Sex differences in mortality patterns are evident with increases more marked in females in most adult age groups.
Conclusions
Empirical data demonstrate a marked “counter transition” with mortality increasing in children and young adults, “epidemiologic polarization” with vulnerable subgroups experiencing a higher mortality burden, and a “protracted transition” with simultaneous emergence of HIV/AIDS together with increasing non-communicable disease in older adults. The health transition in rural South Africa is unlikely to predict patterns elsewhere; hence the need to examine trends in as many contexts as have the data to support such analyses.
Keywords: Age-specific mortality, Agincourt, demographic surveillance system, health transition, mortality trends, rural areas, South Africa
Background
Demographic transition theory describes marked changes from high mortality and fertility to those of lower levels, with mortality rates falling initially and leading to a subsequent fertility decline [1]. Limitations in this theory stimulated development of epidemiologic transition theory, which attempted to incorporate a multidisciplinary approach involving biomedical and socioeconomic, as well as demographic, aspects of the transitional process [2,3]. In his original model, Omran proposed three stages of epidemiologic transition: the era of “pestilence and famine” characterized by very high mortality, especially at young ages, due largely to malnutrition, infectious disease, and complications of childbirth and pregnancy, lower life expectancy in females than males, and no sustained population growth; the era of “receding pandemics” during which mortality declines steadily and population growth is sustained; and the era of “man-made or degenerative disease” with lower overall mortality peaking at older ages and dominated by non-communicable diseases and injuries, life expectancy higher in females than males, and low fertility. Omran also described three models of the epidemiologic transition, each differentiated by variations in the rate, the pattern, the determinants, and the impact of population change. The Western or Classical model, which started earliest and lasted some 100 years, describes the progressive mortality and fertility declines that occurred in most Western European societies following socioeconomic improvements associated with “modernization”. The Accelerated model describes change in Japan and Eastern Europe, which started later but proceeded more rapidly, and was due to public health and biomedical interventions. The Delayed or Contemporary model attempts to describe the transition in many developing countries, which started even later, is not yet complete, and which is also driven in part by medical and public health advances [3,4].
The theory appeals in both documenting and describing historical patterns of mortality, but particularly in its potential to predict future mortality change in specific populations. In so doing, social, medical, and public health interventions could be introduced to direct the transition along a positive course. The original model falls short in its predictive potential, however, provoking critique, extension, and refinement by Omran [5] and other scientists over the past three decades. Omran’s defined stages were deemed too restrictive and a fourth stage, that of “delayed degenerative disease”, was added to describe the shift to older ages of mortality from non-communicable diseases consequent on sharply declining death rates and a change in population structure [6]. The proposed sequential relationship of these stages has been challenged as change does not necessarily occur progressively – stages may overlap – or in a single direction [1,7]. Unanticipated “counter transitions” with reversals in morbidity and mortality gains have been seen in some African countries [8,9], in countries severely affected by HIV/AIDS [10] and in Russia and parts of Eastern Europe [11], demonstrating the effect of emerging diseases and failure of public health systems. Partial change in morbidity and mortality patterns results in a “prolonged or protracted” model in which different types of disease coexist – resurgence of “old” infectious disease and emergence of new ones together with non-communicable diseases [1,4,6,12]. The concept of “epidemiological polarization” illustrates social class inequalities with higher death rates among the poorest consequent on the unfinished agenda of infectious and nutritional diseases [12-14]. Mortality declines are not necessarily accompanied by improvements in morbidity or disability, hence policies and programmes to reduce mortality may not impact positively on disease rates [15]. Nevertheless, the positive contribution of public health interventions such as water purification and sanitation, together with advances in biomedical prevention and treatment, is insufficiently recognized relative to the impact of socioeconomic progress [16].
Despite theoretical and conceptual advances from demographic to epidemiologic transition models – and more recently to a broader health transition framework which includes social and behavioural changes that propel the epidemiologic transition – the theories remain limited in their ability to predict the rate, pattern and determinants of mortality in populations. Further research is needed to adjust and strengthen these frameworks to accurately anticipate negative consequences [1,2,4,17].
Aims
This paper examines trends in age-specific mortality in a rural South African population from 1992 to 2003, a decade spanning major sociopolitical change – the last years of apartheid and initial period of democracy – as well as emergence of the HIV/AIDS pandemic and resurging pulmonary tuberculosis (PTB). The changing mortality patterns documented are discussed and located within a health transition framework.
Material and methods
Study setting
The Agincourt study site, located in the rural northeast of South Africa close to its border with Mozambique, was located within one of the country’s so-called “bantustans” – inhospitable areas into which black South Africans were resettled as part of the apartheid regime’s strategy of “separate development”. Today the field site encompasses 21 villages with a population of some 70,000 people living in 11,500 households. Development efforts over the past several years are evident, with electrification of most villages and tarring of larger gravel roads. However, the area lacks any running water or sanitation system other than pit latrines. The area is dry much of the year and cannot sustain subsistence agriculture; employment opportunities are limited with labour migration still a major source of cash income for households in the area. While most children reach secondary school, few obtain any form of tertiary qualification.
Agincourt Health and Demographic Surveillance System
In 1992, the boundaries of the study site were demarcated and a household census conducted. This became the baseline for a health and demographic surveillance system (HDSS), which involves comprehensive annual updating of the household roster together with recording of all vital events – births, deaths, in- and out-migrations – that occurred during the preceding year. A verbal autopsy, to establish probable cause of death, is conducted on every death, with additional survey modules included in each round to support particular analyses (e.g. an asset survey permits equity analyses) and investigations. Details of the Agincourt HDSS are presented elsewhere [18-20].
Ethical clearance for the Agincourt HDSS has been granted by the University of the Witwatersrand’s Committee for Research on Human Subjects (Medical) (No. M960720). Informed consent is obtained at individual and household level and community consent was obtained from both civic and traditional leadership at the initiation of the study. On a regular basis the research team and fieldworkers present findings at community meetings held in each village.
Calculation of mortality rates
Data on population size, structure, and deaths were obtained from the Agincourt HDSS. Precise person-years at risk by age, sex, and period were calculated using a custom-designed computer program. The principle is to consider the dates (day, month, year) of entry and exit in each cell of the Lexis diagram by age and period for each sex separately, and to compute the person-days lived in each cell for every individual registered in the HDSS. Person-days are then cumulated for the whole population and converted into person-years. Corresponding death rates were derived, and life tables were computed for males and females by age and calendar year. Rates for the early period (1992–93) and a decade later (2002–03) were compared. Testing of mortality change was done using classic formulae of relative risk. Where appropriate, the slopes of trend lines were tested for significance by using a linear-logistic model. Changes over the 10-year period were so marked that statistical significance was not an issue, except in the few age groups where there was more limited change.
Results
Over the 11-year period 1992 to 2003, a total of 5,161 deaths were reported in the Agincourt study site and mortality steadily worsened. Life expectancy was relatively stable for both males and females until the mid-1990s, at 66 years for males and 72 years for females. Over the next seven years, however, male life expectancy dropped to 52 years and female life expectancy to 60 years, a loss of 14 and 12 years of life respectively (Figure 1).
Figure 1.
Trends in life expectancy at birth, Agincourt, 1992–2003.
Comparing age-specific mortality rates at the end of the surveillance period (2002–03) with those a decade earlier (1992–93) indicates differential increases by age and sex (Figure 2). We calculated the relative change in age-specific mortality rates during the two years prior to the political transition in South Africa and a decade thereafter during which period the HIV/AIDS epidemic had escalated. These rate ratios by age and sex are given in Table I. Deaths rates increased significantly between 1992–93 and 2002–03 (rate ratio > 1) for all age groups other than 5–19 and over 65 years, age groups usually considered free of AIDS in African settings. The increase in rates was greatest in the 20–34 year age group in both males and females.
Figure 2.
Relative increase in mortality, Agincourt 2002–03 compared with baseline 1992–93.
Table I.
Change in mortality rates by age and sex, Agincourt 1992–2003 (life table probability of dying, in age groups).
| Period |
Change 2002–03 / 1992–93 |
||||||
|---|---|---|---|---|---|---|---|
| 1992–93 |
2002–03 |
||||||
| Gender and age group | Death rate | Deaths | Death rate | Deaths | Rate ratio | p-value | Signif. |
| Males | |||||||
| 0–4 | 0.0365 | 52 | 0.0836 | 138 | 2.29 | 0.000 | * |
| 5–19 | 0.0185 | 23 | 0.0182 | 32 | 0.98 | 0.951 | |
| 20–34 | 0.0346 | 23 | 0.1401 | 156 | 4.04 | 0.000 | * |
| 35–49 | 0.1001 | 36 | 0.2667 | 173 | 2.66 | 0.000 | * |
| 50–64 | 0.2775 | 51 | 0.4272 | 144 | 1.54 | 0.001 | * |
| 65–79 | 0.5039 | 54 | 0.6169 | 97 | 1.22 | 0.234 | |
| Females | |||||||
| 0–4 | 0.0409 | 57 | 0.0714 | 120 | 1.75 | 0.000 | * |
| 5–19 | 0.0158 | 19 | 0.0195 | 34 | 1.23 | 0.465 | |
| 20–34 | 0.0279 | 19 | 0.1369 | 165 | 4.90 | 0.000 | * |
| 35–49 | 0.0478 | 20 | 0.1727 | 128 | 3.61 | 0.000 | * |
| 50–64 | 0.1025 | 25 | 0.2376 | 88 | 2.32 | 0.000 | * |
| 65–79 | 0.4044 | 51 | 0.3356 | 85 | 0.83 | 0.292 | |
Significant change, p<0.05.
Gender differences are apparent: female rate ratios were greater than those for males in all age groups of younger adulthood (20–64), and were significant when all groups were combined (p<0.009). There was no sex difference in rate of increase among children under 5 (p=0.233), or among youth 5–19 years (p=0.568), or among the elderly (p=0.113).
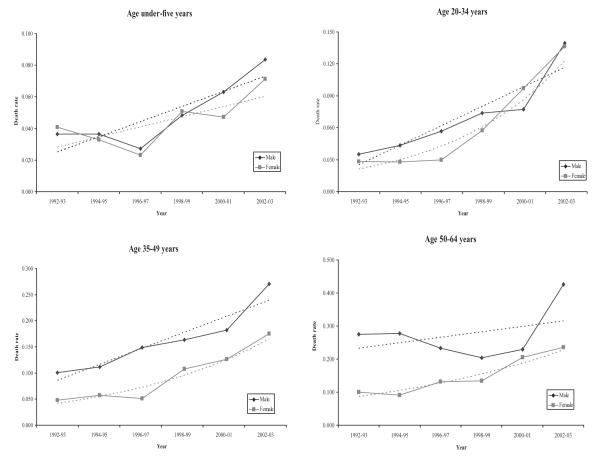
Under-5 mortality was declining until the mid-1990s and then increased markedly for both sexes, from 37/1,000 to 84/1,000 in boys, and 41/1,000 to 71/1,000 in girls (39/1,000 to 77/1,000 for both sexes together) (Figure 3). In contrast, mortality for children and adolescents aged 5–19 years has remained constant in males and increased slightly in females, and is at a low level of 18/1,000 for both sexes combined.
Figure 3.
Trends in child and younger adult mortality, Agincourt 1992–2003.
Figure 3 shows mortality steadily increasing in adults 20–34 and 35–49 years with a more rapid increase in females 20–34 years. Female mortality in this younger age group increases from 28/1,000 to 138/1,000, while male mortality increases from 35/1,000 to 141/1,000 (an increase of almost fivefold for females and fourfold for males). In adults 35–49 years, male mortality is at a higher level than female mortality and increases from 100/1,000 to 268/1,000, while female mortality increases from 48/1,000 to 174/1,000 (increases of 2.7- and 3.6-fold respectively).
Mortality trends in the 50–64 year age group from 1992 to 2000 reveal increasing female mortality in contrast to declining male mortality (Figure 3). Mortality of women converges with that of men over this period, their levels being almost the same in 2000; however, mortality in the three years following to 2003 reflects divergence by sex as male mortality reverses to levels worse than in 1992–93. The increase in female mortality in this age group is more rapid than that in males, with a 2.3-fold increase over the decade, compared with a 1.5-fold increase in males (p=0.142).
Discussion
The data on which these analyses have been performed were collected as part of routine health and demographic surveillance first introduced into the rural Agincourt study site in 1992, and updated annually since then. Relationships with study communities and households are carefully nurtured with high participation in the study. Much attention is given to completeness of data collection and to its quality, at both the field and data-entry levels. An annual household visit, however, limits recording of pregnancies and probably results in an undercount of infant deaths.
Relating mortality trends in Agincourt to South African and African patterns
The findings presented demonstrate significant increases in mortality for both sexes since the mid-1990s, reflected in a rapid decline in life expectancy over a very short period of time. The increases are most prominent in children (0–4) and younger adult (20–49) age groups, in which increases of two- and fivefold respectively have been observed in the past decade. Female mortality increased significantly more than male mortality for the combined adult age group 20–64 years. Sex differences in the mortality patterns of those 50–64 years are evident with the female mortality increase more prominent through the 1990s.
Agincourt findings are generally in line with South African mortality patterns based on national data, which show a consistent increase in the number of adult deaths between 1998 and 2003, even after accounting for the contribution of population growth and improved registration [21]. Death certification in South Africa has improved over the past decade – estimates of adult coverage have reached 90% although registration of childhood deaths is lower [22]. However, the reliability of South African vital registration and census data remains problematic due to coverage errors (duplication or omissions), lack of completeness (missing information on specific questions), and content errors (incorrect recording of individual or household characteristics during data collection or data processing) [23]. Factors limiting the accuracy of data from South African death notification forms include under-registration of deaths – particularly of children and in rural areas – leading to lower estimates of number and certain causes of death, and misreporting of causes due to both the capability and diligence of certifying officials [24]. Best estimates of adult mortality, based on methods that take into account and correct for errors where possible, are consistent with the Agincourt findings and demonstrate a rapid increase in mortality since the mid-1990s that is more marked in younger adults where it is increasing faster in women than in men. The 2001 national census data were deemed too inadequate, however, to derive any meaningful estimates of childhood mortality [23].
Perversely, the HIV/AIDS pandemic emerged concurrent with democratic change in South Africa and its promise of socioeconomic improvements for those previously most disenfranchised. Health gains, as reflected by mortality, have not been achieved post-1994. National mortality trends saw rates falling to the early 1980s, and then levelling off at least for females before increasing from the mid-1990s [23]. In Agincourt, female mortality was declining steadily to 1997 after which a reversal was documented [10].
While mortality is decreasing steadily, albeit slowly, in some African countries, those affected by HIV/AIDS are experiencing an increase in adult and child mortality, primarily due to the epidemic [9,25]. South Africa is one such example, with the Southern African region severely affected. A marked increase in adult mortality due to HIV/AIDS has been documented in northern KwaZulu-Natal Province [26] and in Zimbabwe [27]. Compared with many other African settings, mortality in Agincourt has increased very rapidly since the mid-1990s with a particularly high relative increase due to a baseline mortality that was lower than that for much of the rest of the continent [28,29]. The rising mortality of women aged 50–64 years in Agincourt has not been documented, as far as we know, anywhere else in Africa.
The health transition in rural South Africa
Modifications to the original epidemiologic transition model have been proposed that better accommodate some of the complex changes observed in countries undergoing rapid social change, those with marked socioeconomic disparities, and those subject to new or re-emerging disease epidemics. While few developing countries have adequate data with which to examine the transitions underway, a middle-income country such as Mexico provides an example of a “protracted transition” with “epidemiological polarization”. Crude mortality in Mexico has declined since the beginning of the twentieth century, with reductions concentrated in children, particularly in the middle of the century. By the 1950s, chronic and degenerative diseases coexisted with infectious diseases; the latter were then seen to decline, and diseases such as ischaemic heart disease, diabetes, motor vehicle accidents, and tobacco-related cancers, as well as HIV/AIDS, to increase. There was uneven expression in these changes among different social classes, due largely to the varying pace of mortality change and marked social class disparities [12].
Another striking example is the case of Russia, where life expectancy had been showing a slow but steadily declining trend since 1965 following a period of major improvement between 1925 and 1964. During the mid-1980s, however, life expectancy was seen to increase after major campaigns against alcohol abuse, but resumed its decline thereafter. This reversal has been attributed to a range of causes, above all increasing alcohol consumption and its consequences on cardiovascular disease, trauma and violence [11].
As with Mexico and Russia, mortality trends in rural South Africa over the past decade, based on empirical data from the Agincourt subdistrict, do not follow sequential stages as laid out in the original epidemiologic transition model. Certainly there is evidence of socioeconomic development in the area, and an impressive fertility decline has been documented dating back nearly three decades [30]. Classic epidemiological transition theory would have predicted a mortality decline associated with these favourable changes; instead we observe a mortality “incline” with a reversal in earlier mortality gains.
While detailed examination of cause-specific mortality is the subject of another paper there is some evidence that Agincourt is experiencing a “protracted or prolonged transition” in which change is incomplete and stages overlap, resulting in the coexistence of different types of disease. By the mid-1990s, a dual epidemic of persisting infectious and nutritional disease in children, concurrent with emerging non-communicable disease in adults, had been documented [31-33]. Since then, HIV/AIDS has become established as a major and escalating problem in the area. Estimates of HIV seroprevalence based on anonymous testing of pregnant women at antenatal clinics in the two provinces adjacent to the study site indicate an increase for the area from about 1.7% in 1992 to 25% in 2003 (average for Mpumalanga and Limpopo Provinces) [34]. Our finding that young adult mortality continues to increase unabated through 2003 extends earlier reports of increasing young adult mortality in Agincourt between 1992 and 1995, which was largely attributed to HIV/AIDS and tuberculosis [10].
“Epidemiological polarization” incorporates two concepts: differential mortality and morbidity rates based on social class, as well as differences in the nature and types of disease experienced [12]. The former inequity is apparent in Agincourt with the poorest experiencing higher infant and child mortality and lower life expectancy [35,36]. The relationship between social class and cause of death is more complicated. Early work reveals a negative relationship between socioeconomic status and infectious and parasitic diseases excluding HIV/AIDS (that is, higher mortality associated with lower wealth quintiles). This pattern did not hold, however, for HIV/AIDS specifically, or for accidents and violence, or for non-communicable diseases. Several national demographic and health surveys have documented a positive relationship between HIV sero-prevalence and socioeconomic status. For instance, the Tanzania 2003–04 HIV/AIDS Indicator Survey showed an increase in HIV sero-prevalence among women aged 15–49 from 2.8% in the lowest quintile to 11.4% in the highest quintile [37]. Work on the changing relationship between socioeconomic status and mortality is needed as the AIDS pandemic progresses.
Conclusion
While health transition models are an advance on the original formulation of epidemiologic transition, they require further development and refinement, particularly if they are to help us anticipate changes and not simply react to them after they have occurred [1,4,17]. Even without further theoretical advances, it is important to build our understanding of actual transition experiences through empirical research [17]; however, data are lacking for much of the developing world and most of Africa. Mortality findings from the Agincourt study site in rural South Africa are a contribution. These demonstrate a marked “counter transition”, with mortality increasing in children and most adult age groups since the mid-1990s. This has occurred in the face of democratic political change and associated socioeconomic advances. There is some evidence of “epidemiologic polarization” with the poorest experiencing a higher mortality burden, and a “protracted transition” that relates to the simultaneous emergence of a new infectious disease affecting children and young adults – HIV/AIDS – together with increasing non-communicable disease in older adults, with hypertension an important risk factor [10,31,38]. Analyses that examine patterns in causes of death, risk factors, and socioeconomic differentials by disease type will be presented elsewhere.
The impetus to pursue an understanding of changing mortality levels, and the causes and determinants thereof, is driven by its potential to inform development of health policies and programmes that are targeted to diseases and risk factors impacting most adversely on particular population subgroups. Given the level of socioeconomic development in South Africa relative to other African countries, can lessons on the health transition in rural South Africa be used to predict likely transition patterns in other African settings? In the absence of further theoretical development of the health transition model, the answer is probably not [17]. While hypotheses are very likely to be generated, and some insight may be gained, there are likely to be “as many models as there are societies” [16]. Hence it is necessary to examine the health transition in as many contexts as have data to support such analyses. Sources of longitudinal data, such as those generated by health and demographic surveillance sites, can make a particular contribution. In general, these sites are located in defined geographic areas, have strong links to the public health sector, and maintain an ongoing research infrastructure. Hence they are ideally placed to map the transition prospectively, conduct lines of research to test hypotheses generated, and propose necessary health interventions.
Acknowledgments
This work has been funded by the Wellcome Trust, UK (Grant no. 058893/Z/99/A), the Andrew W. Mellon Foundation, USA, and the University of the Witwatersrand and Medical Research Council, South Africa. The authors acknowledge with appreciation the contribution of community leaders, study communities, field team supervisors, and field workers, without whom such research could not be conducted. During the writing of this paper Kahn and Tollman were both supported by a grant from the Swedish Council for Working Life and Social Research (FAS) (Grant no. 370440104) and hosted by Epidemiology and Public Health Sciences, Umeå University, Sweden.
Footnotes
This paper has been independently peer-reviewed according to the usual Scand J Public Health practice and accepted as an original article.
References
- [1].Beaglehole R, Bonita R. Public health at the crossroads: Achievements and Prospects. 2nd ed Cambridge University Press; Cambridge: 2004. [Google Scholar]
- [2].Murray CJL, Chen LC. Dynamics and patterns of mortality change. In: Chen LC, Kleinman A, Ware NC, editors. Health and social change in international perspective. Harvard University Press; Boston, MA: 1994. pp. 3–23. [Google Scholar]
- [3].Omran AR. The epidemiologic transition: A theory of the epidemiology of population change. Milbank Memorial Fund Q. 1971;49:509–38. [PubMed] [Google Scholar]
- [4].Mackenbach JP. The epidemiologic transition theory. J Epidemiol Community Health. 1994;48:329–31. doi: 10.1136/jech.48.4.329-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Omran AR. Epidemiologic transition. In: Ross JA, editor. International encyclopaedia of population. Free Press; London: 1982. pp. 172–83. [Google Scholar]
- [6].Olshansky SJ, Ault EB. The fourth stage of the epidemiologic transition: The age of delayed degenerative diseases. Milbank Memorial Fund Q. 1986;64:355–91. [PubMed] [Google Scholar]
- [7].Gribble JN, Preston SH, editors. The epidemiological transition: Policy and planning implications for developing countries. National Academy Press; Washington, DC: 1993. [PubMed] [Google Scholar]
- [8].Garenne M, Gakusi E. Health transitions in sub-Saharan Africa: Overview of mortality trends in children under 5 years old (1950–2000) Bull World Health Organ. 2006;84:470–8. doi: 10.2471/blt.05.029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Garenne M, Gakusi E. DHS Working Papers No. 26. IRD-Macro; Calverton, MD: 2004. Reconstructing under-five mortality trends in Africa from demographic sample surveys. [Google Scholar]
- [10].Tollman SM, Kahn K, Garenne M, Gear JSS. Reversal in mortality trends: Evidence from the Agincourt field site, South Africa, 1992–1995. AIDS. 1999;13:1091–7. doi: 10.1097/00002030-199906180-00013. [DOI] [PubMed] [Google Scholar]
- [11].Leon DA, Chenet L, Shkolnikov VM, et al. Huge variation in Russian mortality rates 1984–94: Artefact, alcohol, or what? Lancet. 1997;350:383–8. doi: 10.1016/S0140-6736(97)03360-6. [DOI] [PubMed] [Google Scholar]
- [12].Frenk J, Bobadilla JL, Sepulveda J, Cervantes ML. Health transition in middle-income countries: New challenges for health care. Health Policy and Planning. 1989;4:29–39. [Google Scholar]
- [13].Heuveline P, Guillot M, Gwatkin DR. The uneven tides of the health transition. Soc Sci Med. 2002;55:313–22. doi: 10.1016/s0277-9536(01)00172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gwatkin DR. Distributional implications of alternative strategic responses to the demographic–epidemiological transition – an initial enquiry. In: Gribble JN, Preston SH, editors. The epidemiological transition: Policy and planning implications for developing countries. Workshop proceedings. National Academic Press; Washington, DC: 1993. pp. 192–228. [PubMed] [Google Scholar]
- [15].Riley JC. Why sickness and death rates do not move parallel to one another over time. Soc History Med. 1999;12:101–24. doi: 10.1093/shm/12.1.101. [DOI] [PubMed] [Google Scholar]
- [16].Caldwell JC. Population health in transition. Bull World Health Organ. 2001;79(2):159–60. [PMC free article] [PubMed] [Google Scholar]
- [17].Frenk J, Bobadilla JL, Stern C, Frejka T, Lozano R. Elements for a theory of the health transition. In: Chen LC, Kleinman A, Ware NC, editors. Health and social change in international perspective. Harvard University Press; Boston, MA: 1994. pp. 25–49. [Google Scholar]
- [18].Collinson MA, Mokoena O, Mgiba N, et al. Agincourt Demographic Surveillance System. In: Sankoh O, Kahn K, Mwageni E, Ngom P, Nyarko P, editors. Population and health in developing countries. Vol 1: Population, health, and survival at INDEPTH Sites. International Development Research Centre; Ottawa: 2002. pp. 197–206. [Google Scholar]
- [19].Tollman SM, Herbst K, Garenne M, Gear JSS, Kahn K. The Agincourt Demographic and Health Study: Site description, baseline findings and implications. S Afr Med J. 1999;89:858–64. [PubMed] [Google Scholar]
- [20].Kahn K, Tollman SM, Collinson MA, Clark SJ, Twine R, Clark BD, Gómez-Olivé FX, Shabangu M, Mokoena O, Garenne ML. Research into health, population and social transitions in rural South Africa: Data and methods of the Agincourt Health and Demographic Surveillance System. Scand J Public Health. 2007;35(Suppl 69):8–20. doi: 10.1080/14034950701505031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bradshaw D, Laubscher R, Dorrington RE, Bourne D, Timaeus IM. Unabated rise in number of adult deaths in South Africa. S Afr Med J. 2004;94:278–9. [PubMed] [Google Scholar]
- [22].Dorrington RE, Bourne D, Bradshaw D, Laubscher R, Timaeus IM. Technical report. Burden of Disease Unit, Medical Research Council; Cape Town: 2001. The impact of HIV/AIDS on adult mortality in South Africa. [Google Scholar]
- [23].Dorrington RE, Moutrie TA, Timaeus IM. Estimation of mortality using the South African Census 2001 data. Centre for Actuarial Research, University of Cape Town; Cape Town: 2004. pp. 1–87. Available at: http://www.commerce.uct.ac.za/care/Monographs/Monographs/Mono11.pdf, 2004. [Google Scholar]
- [24].Statistics South Africa . Mortality and causes of death in South Africa, 1997–2003: Findings from death notification. (Statistical release P0309.3) Statistics South Africa; Pretoria: 2005. pp. 1–107. [Google Scholar]
- [25].Garenne M. Mortality in sub-Saharan Africa: Trends and prospects. In: Lutz W, editor. The future population of the world: What can we assume today? Earthscan Publications and IASSA; Laxenburg: 1996. pp. 149–69. [Google Scholar]
- [26].Hosegood V, Vanneste A, Timaeus IM. Levels and causes of adult mortality in rural South Africa: The impact of AIDS. AIDS. 2004;18:663–71. doi: 10.1097/00002030-200403050-00011. [DOI] [PubMed] [Google Scholar]
- [27].Feeney G. The impact of HIV/AIDS on adult mortality in Zimbabwe. Population and Development Rev. 2001;27:771–80. [Google Scholar]
- [28].INDEPTH Network . INDEPTH model life tables for sub-Saharan Africa. Ashgate; Aldershot: 2004. [Google Scholar]
- [29].Clark SJ, Ngom P, INDEPTH Network . Population and health in developing countries. Vol 1: Population, health, and survival at INDEPTH sites. International Development Research Centre; Ottawa: 2002. Comparing mortality patterns at INDEPTH sites; pp. 51–82. [Google Scholar]
- [30].Garenne M, Tollman SM, Kahn K, Collinson MA. Fertility trends and net reproduction in rural South Africa, 1992–2004. Scand J Public Health. 2007;35(Suppl 69):68–76. doi: 10.1080/14034950701355650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kahn K, Tollman SM, Garenne M, Gear JSS. Who dies from what? Determining cause of death in South Africa’s rural north-east. Trop Med Int Health. 1999;4:433–41. doi: 10.1046/j.1365-3156.1999.00415.x. [DOI] [PubMed] [Google Scholar]
- [32].SASPI Project Team Prevalence of stroke survivors in rural South Africa: Results from the Southern Africa Stroke Prevention Initiative (SASPI) Agincourt field site. Stroke. 2004;35:627–32. doi: 10.1161/01.STR.0000117096.61838.C7. [DOI] [PubMed] [Google Scholar]
- [33].Kahn K, Tollman SM. Stroke in rural South Africa – contributing to the little known about a big problem. S Afr Med J. 1999;89:63–5. [PubMed] [Google Scholar]
- [34].Department of Health . Summary report: National HIV and Syphilis Antenatal Seroprevalence Survey in South Africa 2003. Directorate Health Systems Research, Research Coordination and Epidemiology, Department of Health; Pretoria: 2004. [Google Scholar]
- [35].Hargreaves JR, Collinson MA, Kahn K, Clark SJ, Tollman SM. Childhood mortality among former Mozambican refugees and their hosts in rural South Africa. Int J Epidemiol. 2004;33:1271–8. doi: 10.1093/ije/dyh257. [DOI] [PubMed] [Google Scholar]
- [36].Kahn K, Collinson MA, Hargreaves JR, Clark SJ, Tollman SM. Socio-economic status and child mortality in a rural sub-district of South Africa. In: de Savigny D, Debpuur C, Mwageni E, Nathan R, Razzaque A, Setel PW, editors. Measuring health equity in small areas – Findings from demographic surveillance systems. Ashgate; Aldershot: 2005. pp. 67–85. [Google Scholar]
- [37].Tanzania Commission for AIDS (TACAIDS) National Bureau of Statistics (NBS), ORC Macro, Tanzania HIV/AIDS Indicator Survey 2003–04. TACAIDS, NBS, and ORC Macro; Calverton, MD: 2005. [Google Scholar]
- [38].SASPI Team Secondary prevention of stroke: Results from the Southern Africa Stroke Prevention Initiative (SASPI), Agincourt field site. Bull World Health Organ. 2004;82:503–8. [PMC free article] [PubMed] [Google Scholar]