Abstract
Wilson disease ATPase (ATP7B) has been implicated in the resistance of cancer cells to cisplatin. Using a simple in vivo assay in bacterial culture, in the present study we demonstrate that ATP7B can confer resistance to cisplatin by sequestering the drug in its N-terminal metal-binding domain without active drug extrusion from the cell. Expression of a protein fragment containing four N-terminal MBRs (metal-binding repeats) of ATP7B (MBR1–4) protects cells from the toxic effects of cisplatin. One MBR1–4 molecule binds up to three cisplatin molecules at the copper-binding sites in the MBRs. The findings of the present study suggest that suppressing enzymatic activity of ATP7B may not be an effective way of combating cisplatin resistance. Rather, the efforts should be directed at preventing cisplatin binding to the protein.
Keywords: ATPase, chemotherapy, cisplatin, drug resistance, Wilson disease
INTRODUCTION
Cisplatin [cis-diammineplatinum(II) dichloride] is one of the most potent and widely used anticancer agents effective against several common types of solid tumours. The biological activity of cisplatin is believed to be associated with platination of nuclear DNA at guanine sites, formation of intrastrand cross-links and consequent induction of DNA bending that prevents normal replication [1]. DNA damage eventually triggers apoptosis through a complex cascade of biochemical reactions [2]. Previously, cisplatin has also been shown to activate apoptotic caspases through the ER (endoplasmic reticulum) stress pathway [3].
The effectiveness of cisplatin-based chemotherapy can be undermined by several resistance mechanisms, including reduced uptake by the cell, rapid detoxification inside the cell, increased drug extrusion from the cell, DNA repair, decreased mismatch repair and defective apoptosis [4,5]. Copper transporters appear to be involved both in cisplatin uptake and efflux [6,7]. The uptake of cisplatin is mediated by the copper transporter Ctr1 [8], whereas the copper-transporting P-type adenosine triphosphatases ATP7B and ATP7A are suggested to be involved in cisplatin efflux [5].
The physiological role of the copper ATPases is to deliver copper from the cytoplasm to the secretory pathway for subsequent biosynthetic incorporation into various copper-dependent enzymes and to export the excess of copper from the cell. Mutations disrupting the function of ATP7B and ATP7A lead to severe hereditary disorders of copper metabolism, Wilson disease and Menkes disease respectively [9,10]. ATP7B and ATP7A are large membrane proteins with significant primary sequence homology (50–60% identity). Both enzymes belong to the subgroup of P1B-ATPases within the P-type ATPase family and share the same mechanism of ATP hydrolysis with the well-studied SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase) and Na+/K+-ATPase. A characteristic feature of the copper-transporting ATPases is the presence of six repetitive sequences of approx. 70 amino acid residues at the N-terminus of the molecule. Each repeat contains an invariant GMxCxxC motif and binds a single copper ion in the reduced Cu(I) form via two cysteine residues [11].
Initially, copper-transporting ATPases were implicated in cisplatin resistance because the latter is often associated with overexpression of ATP7B and ATP7A [12,13]. ATP7B and ATP7A appear to facilitate cisplatin accumulation in the intracellular vesicles, thereby increasing the whole-cell cisplatin concentration, while at the same time rendering cells resistant to the drug [14,15]. Moderate overexpression of ATP7A in an ovarian carcinoma cell line resulted in the increased resistance to cisplatin without a concomitant decrease in intracellular platinum concentration [16]. These results clearly indicate that the copper-transporting ATPases are involved in the compartmentalization of cisplatin inside the cell, but leave open the question about the molecular mechanism of this process. ATP-dependent transport of cisplatin or its derivative has been demonstrated in vitro using ATP7B expressed in insect cells [17]. The extremely low rates of transport (less than 50 pmol·mg−1 of protein·min−1) and the requirement for an acidic pH raises the question as to whether this process can account for cisplatin resistance in tumour cells. An alternative mechanism of cis-platin resistance mediated by copper ATPases could involve drug sequestration through binding to the multiple GMxCxxC metal-binding sites in the N-terminal domains of ATP7B and ATP7A. Since cisplatin is known to produce complexes with glutathione and metallothionein reacting with cysteine residues [18–20], cisplatin binding to the copper-binding domain of ATP7B may be easier to reconcile with the properties of P1B-ATPases than active transport across the lipid membrane.
To test this hypothesis, in the present study we have developed a simple in vivo assay and used it to investigate the involvement of the metal-binding domain of ATP7B in cisplatin resistance. The biological activity of platinum compounds was discovered by Rosenberg et al. [21] who observed that products of electrolysis of the platinum electrode caused Escherichia coli cells to form long filaments, up to 300 times the length of a normal cell. This effect turned out to be a common property of many platinum complexes including cisplatin [22]. In the present study we used E. coli filamentation caused by submicromolar concentrations of cisplatin as an indicator of the concentration of active species of platinum in cell cytoplasm, and demonstrated that expression of a protein fragment containing four MBRs (metal-binding repeats) of ATP7B (MBR1–4) protects cells from the toxic effects of cisplatin. We have shown that cisplatin binds to the purified MBR1–4 at a ratio of up to three cisplatin molecules per MBR1–4, consistent with its protective effect in cell culture.
EXPERIMENTAL
Expression and purification of the metal-binding domain MBR1–4
The pTYB12 vector (New England Biolabs) encoding the N-terminal MBR1–4 of ATP7B fused with the chitin-binding domain and an intein under the control of an IPTG (isopropyl β-D-thiogalactoside)-inducible lac promoter was used for the in vivo expression of MBR1–4 in BL21(DE3) E. coli cells and for the isolation of pure MBR1–4 for MS. For control experiments, the same vector containing the sequence of either the ATP7B nucleotide-binding domain (termed the N-domain) [23], or of the MBR1–4 mutant with the four conserved CxxC motifs replaced with SxxS motifs [24] instead of the wild-type MBR1–4 was used.
The wild-type and mutant variants of MBR1–4 were purified essentially as described for the N-domain of ATP7B [23], except that cells were transferred into M63 minimal medium [25] before induction of protein expression. A typical yield of the MBR1–4 domain was approx. 1 mg of protein per 1 litre of cell culture. Purified protein was frozen in liquid nitrogen with 5% glycerol and 0.5 M NaCl added and stored at −80°C. The protein concentration was measured according to the method of Lowry et al. [26]. The expression levels of the MBR1–4 and of the N-domain were analysed by SDS/PAGE and Western blot analysis with the commercially available antibodies against the chitin-binding domain (New England Biolabs).
Analysis of cisplatin binding by MS
Purified MBR1–4 was incubated on ice for 30 min with 10 mM EDTA at a protein concentration of 50 μM, and then dialysed against 50 mM Hepes (pH 7.5), 150 mM NaCl and 0.6 mM TCEP [tris-(2-carboxyethyl)phosphine] to remove bound metals and reduce cysteine residues. To remove TCEP, the sample was dialysed twice against 50 mM Hepes (pH 7.5) and 150 mM NaCl. After dialysis, the protein was incubated for 30 min at 20°C with a 10-fold molar excess of cisplatin. The samples were then dialysed three times against 20 mM ammonium acetate (pH 7.5) to remove free cisplatin and prepare the protein for MS.
For tryptic digestion, the wild-type MBR1–4 was treated with EDTA and TCEP as described above and incubated with an 8-fold molar excess of cisplatin or an 8-fold molar excess of Cu(I) for 30 min at 20°C. Protein samples were digested with Poroszyme®-immobilized trypsin (Applied Biosystems) for 30 min at 20°C. Digested peptides were immobilized on a C18 reverse-phase matrix in a Zip Tip (Millipore), washed with 0.1% TFA (trifluoroacetic acid) and eluted with 75%acetonitrile containing 0.1% TFA prior to MS. Samples were analysed on a 4800 MALDI–TOF (matrix-assisted laser-desorption ionization–time-of-flight)/TOF mass spectrometer (Applied Biosystems) operated in positive-ion linear mode.
Differential interference contrast microscopy
Overnight E. coli cultures (1 ml) were pelleted, washed twice with M63 minimal medium [25] and resuspended in 100 ml of M63 medium. Cisplatin, at a final concentration of 0.25 μM or 0.5 μM, and IPTG were added to the cultures, and cells were incubated for 6 h at 37°C on a shaking incubator until the D600 of the cell suspension reached 0.6. For DIC (differential interference contrast) imaging, 2 μl of the cell culture was mixed with 2 μl of 2%(w/v) low-melting-point agarose and immediately applied to a microscope slide. The immobilized cells were viewed with an Olympus BX60 fluorescence microscope with a 60× oil-immersion objective. The images were captured with a Photometrics CoolSnap fx CCD (charge-coupled device) camera and InVitro 3 software (Media Cybernetics). At least 400 cells from at least three individual trials were measured using Image Pro Plus software (Media Cybernetics). The effect of cisplatin was quantified by measuring the average cell length and the percentage of the filamented cells in the culture. Results were analysed using ANOVA statistics, or a Student’s t test where indicated.
RESULTS
MBR1–4 expression decreases filamentation of E. coli induced by cisplatin
The E. coli cells form long filaments, up to 300 times the normal cell length, upon treatment with platinum compounds [21]. This observation suggested to us a simple in vivo assay for protective effects of proteins against cisplatin. If expression of a certain protein in the E. coli cell culture prevents filamentation caused by a normally effective concentration of cisplatin, the protein must detoxify cisplatin. The half-maximal concentration for E. coli BL21(DE3) growth inhibition by cisplatin (IC50) was determined to be 6 ± 1 μM. For the assay we used 0.25 and 0.5 μM cisplatin, concentrations that did not inhibit bacterial growth during at least 6 h of incubation at 37°C on M63 minimal medium. The results reported are based on measurements of approx. 18000 cells in total.
We hypothesized that the ATP7B-mediated resistance to cisplatin is a result of drug sequestration by the metal-binding domain of ATP7B, a process that does not require active transport across the cell membrane. To test this hypothesis, we compared the extent of cisplatin-induced filamentation in the control E. coli cells and in the cells expressing the MBR1–4 domain of ATP7B. To control for possible non-specific effects of protein overexpression, we used E. coli cells expressing the N-domain of ATP7B from the same vector as MBR1–4. The maximum expression level of the N-domain observed at 0.5 mM IPTG was much higher than that of MBR1–4 (Supplementary Figure S1 at http://www.BiochemJ.org/bj/419/bj4190051add.htm). Consequently, the IPTG concentration was adjusted to 30 μM for the N-domain to decrease its level of expression. At this IPTG concentration, the expression level of the N-domain was approx. 1.5-fold higher than that of MBR1–4 at 0.5 mM IPTG. An IPTG concentration of 20 μM, which resulted in an N-domain expression of approx. 50% of the MBR1–4 expression, was also tested in the control experiments with similar results (results not shown). The amount of MBR1–4 expressed under these conditions constituted less than 1% of the total cell protein as estimated from the Coomassie-Blue-stained SDS gels of the cell extracts (results not shown).
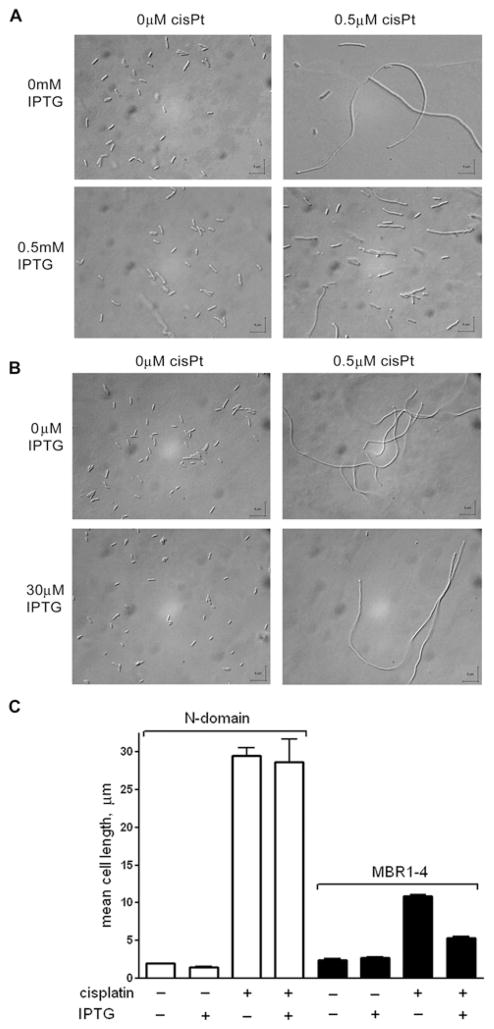
In the absence of cisplatin, the E. coli cells had a normal morphology. IPTG at concentrations of 0.1 mM and higher caused formation of a small number of filaments. (For statistical purposes we defined filaments as cells longer than 7.5 μm, i.e. approx. three times the length of a normal E. coli cell). Cisplatin at concentrations of 0.25–0.5 μM caused filamentation of more than 90% of cells in the cultures (Figure 1). Filamentation was significantly reduced by the expression of MBR1–4 (Figure 1A), but not by the N-domain (Figure 1B). In the presence of 0.5 μM cisplatin, the mean cell length was approx. five times smaller in the strain expressing MBR1–4 than in the strain expressing the N-domain (Figure 1C) or the background strain containing no plasmid (results not shown). Interestingly, even without IPTG, the extent of filamentation in the presence of cisplatin was noticeably lower in the cells carrying the MBR1–4 expression vector than in the cells transformed with the plasmid encoding the N-domain, or in the background strain. This is probably due to the basal expression of MBR1–4 without IPTG at a level that may be sufficient to cause a decrease in cisplatin-induced filamentation. In all cases, the difference was statistically highly significant (P < 0.0001).
Figure 1. Expression of the metal-binding domain of Wilson disease ATPase (MBR1–4) reduces filamentation of E. coli cells caused by cisplatin.
(A and B) Effect of cisplatin on the morphology of E. coli cells transformed with pTYB12-MBR1–4 (A) and pTYB12-N-domain (B) with IPTG-induced protein expression and without IPTG addition. CisPt, cisplatin. (C) Effects of IPTG and cisplatin on the mean cell length in the cultures of the BL21(DE3) strain transformed with pTYB12-N-domain or pTYB12-MBR1–4.
Purified MBR1–4 binds cisplatin
The protective effect of MBR1–4 against cisplatin-induced filamentation suggests that MBR1–4 binds cisplatin or a platinum species derived from cisplatin thus preventing the drug from reaching its target. To directly verify the ability of MBR1–4 to sequester cisplatin, purified MBR1–4 was pretreated with EDTA and TCEP to remove trace metals and reduce cysteine residues. The MBR1–4 was then incubated with a 10-fold molar excess of cisplatin, which corresponds to a 2.5 molar excess over the number of copper-binding sites in the protein. The cisplatin-treated protein was then analysed by MALDI–TOF MS. Whereas the untreated MBR1–4 sample showed a single major peak at 46235 Da consistent with the calculated molecular mass of the protein (Supplementary Figure 2A at http://www.BiochemJ.org/bj/419/bj4190051add.htm), the spectrum of MBR1–4 treated with cisplatin contained four poorly resolved peaks corresponding to the apparent molecular masses of 46289, 46592, 46868 and 47267 Da (Supplementary Figure S2B). A significant peak overlap precludes determination of the exact molecular masses of the reaction products. Consequently, the nature of the platinum derivative bound to the protein is difficult to determine. However, the observed mass differences between peaks are consistent with the molecular mass of cisplatin (molecular mass 300 Da) and/or the product of cisplatin aquation (molecular mass 265 Da). Therefore the latter three peaks probably represent the products of the MBR1–4 reaction with one, two and three molecules of platinum derivatives respectively.
Isolated metal-binding domains can tightly bind cisplatin or its derivatives, which explains the protective effect of MBR1–4 expression in cell culture against filamentation caused by cisplatin. Based on the well-established reactivity of cisplatin derivatives with glutathione [27], the interaction of cisplatin with the CxxC motif in the copper-binding sites appeared to be likely.
Cisplatin protects MBR1–4 from proteolytic cleavage in the proximity of the copper-binding sites
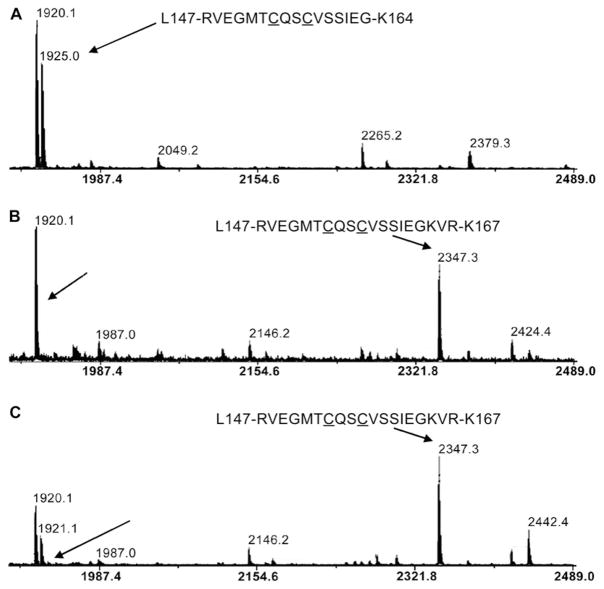
In order to locate cisplatin-binding sites in MBR1–4 we compared tryptic peptide fingerprints of the protein in the presence and absence of cisplatin. Based on the amino acid sequence of MBR1–4, trypsin digestion should produce the following peptides containing CxxC motifs belonging to MBRs 1–4 respectively: m/z 1182.6, m/z 1925.0, m/z 804.3 and m/z 6538.5. (The cleavage site in MBR3 is located inside the CxxC motif.) The first three peptides were indeed observed in the spectra of the untreated MBR1–4, but not in the spectrum of MBR1–4 treated with cisplatin (Figure 2 and Supplementary Figure S3 at http://www.BiochemJ.org/bj/419/bj4190051add.htm) consistent with protection against tryptic cleavage in the proximity of the copper-binding sites caused by cisplatin. The tryptic peptide containing MBR4 lies outside the analysed m/z range and was not observed. Treatment of the cisplatin-bound MBR1–4 with dithiothreitol, and then with the SH-specific reagent iodoacetamide, prior to trypsinolysis, abolished the protective effect of cisplatin and produced a peptide pattern identical with that of the untreated MBR1–4 (results not shown). These observations suggest that cysteine residues are involved in cisplatin binding.
Figure 2. Cisplatin protects MBR2 from tryptic digestion at Lys164.
MALDI–TOF mass spectra of trypsinolysis products of the untreated MBR1–4 (A), the protein treated with cisplatin (B) and Cu(I) (C). Peaks corresponding to the peptides Leu147–Lys164 and Leu147–Lys167 are shown by arrows. Cysteine residues in the copper-binding motif are underlined.
The peptides resulting from cisplatin protection of cleavage sites in MBR1 and MBR3 could not be identified unambiguously in the fingerprint spectra. However, cisplatin binding clearly prevented cleavage at Lys164 located next to the copper binding C154QSC156 sequence in MBR2. Consequently, a peptide comprising residues 147–167 (m/z 2347.3) replaced the Leu147–Lys164 peptide in the spectrum of cisplatin-treated MBR1–4 (Figures 2A and 2B). Importantly, copper binding also resulted in the protection of the Lys164 cleavage site in MBR2 and caused spectral changes very similar to cisplatin (Figure 2C). Neither cisplatin nor copper were bound to the Leu147–Lys167 peptide and were probably removed during the sample treatment prior to MALDI–TOF analysis. An identical pattern of cleavage protection is a strong indication that cisplatin binds at precisely the same site as copper.
MBR1–4 lacking the CxxC motifs does not protect E. coli against cisplatin toxicity
Unlike the wild-type, expression of the mutant variant of MBR1–4 where all four copper-binding CxxC motifs were replaced with SxxS did not prevent cisplatin-induced filamentation of E. coli cells (Supplementary Figure S4 at http://www.BiochemJ.org/bj/419/bj4190051add.htm). No significant binding of cisplatin to the purified mutant protein was detected by MS (Supplementary Figure S5 at http://www.BiochemJ.org/bj/419/bj4190051add.htm). Thus the cysteine residues in the conserved metal-binding motifs are required for biologically effective sequestration of cisplatin by MBR1–4.
DISCUSSION
Tumour resistance to platinum compounds mediated by copper-transporting ATPases can in principle result from active extrusion of the drug either directly out of the cell or into the intracellular vesicles, or from non-productive binding of the drug to the protein. Although Wilson-disease-associated protein expressed in insect cell culture was shown to drive active transport of platinum into the membrane vesicles [17], the observed transport rate was extremely low and probably insufficient to account for cisplatin resistance by active extrusion during chemotherapy.
The results of the present study demonstrate that the expression of the metal-binding domain of Wilson disease ATPase alone protects bacterial cells from the toxic effects of cisplatin. MBR1–4 is water-soluble, is not associated with the cell membrane and is found entirely in the cytosol during cell fractionation. Therefore the protective effect of the isolated MBR1–4 cannot involve active transport of the drug across the cell membranes and must be caused by binding of the active platinum compounds to the protein before they can reach their targets.
Drug sequestration in the metal-binding domain of ATP7B and possibly ATP7A may account for tumour resistance to cisplatin associated with overexpression of these two proteins. The full-length N-terminal copper-binding domain in the native ATP7B contains six MBRs (MBR1–6). In the present study, in experiments with MBR1–4 we have observed binding of up to three cisplatin molecules per MBR1–4. Consistent with these results, binding of 2.2 platinum atoms per MBR1–6 molecule was observed by ICP–MS (inductively coupled plasma–MS) (E. LeShane, M. Ralle and S. Lutsenko, unpublished work). Binding of several cisplatin molecules per ATP7B molecule would provide an effective way of detoxifying cisplatin in the cell. The subsequent fate of platinum complexes bound to the metal-binding domain of ATP7B remains to be investigated. Possibly, high-affinity sites in copper-binding repeats serve as kinetic traps that subsequently release platinum derivatives to glutathione or metallothionein in the cytoplasm.
Consistent with the in vivo observations, cisplatin, or its derivative, was found to bind to isolated MBR1–4 in vitro. Fingerprint analysis of tryptic digest peptides by MS suggests that platinum compounds react with the cysteine residues in the copper-binding sites in MBR1–4. The essential role of the CxxC motif in cisplatin binding is supported by two additional lines of evidence. First, modification of SH groups with iodoacetamide prevents cisplatin binding to the purified MBR1–4. Secondly, a mutant variant of MBR1–4 where a serine residue was substituted for a cysteine residue in all four of the CxxC motifs has no protective effect against cisplatin toxicity in E. coli cell culture and does not bind significant amounts of cisplatin in vitro. The chemistry of cisplatin binding to MBR1–4 may be similar to the well-documented interaction of cisplatin with glutathione, where two glutathione molecules chelate platinum through the side-chain sulfur of cysteine and backbone amide atoms [20]. A possible competition between cisplatin and copper for binding sites in the MBRs suggests that the copper status may be an important factor that determines the extent of tumour resistance to cisplatin associated with copper-transporting ATPases.
In conclusion, we have demonstrated that cisplatin or its derivative binds to the metal-binding domain of Wilson-disease-associated protein (ATP7B). Sequestration of cisplatin or its derivatives in the MBRs of ATP7B protects bacterial cells from the toxic effects of cisplatin and may account for the resistance of cancer cells to cisplatin associated with copper ATPase overexpression. These results suggest that down-regulating ATP7B and ATP7A expression, or preventing drug binding to these proteins may be more effective approaches to combating cancer resistance to cisplatin than suppressing transport activity of the enzymes.
Supplementary Material
Acknowledgments
We thank Dr Jo-Anne Dillon and Ms Monica Wang for advice and assistance with DIC microscopy, Ms Eva-Maria Uhlemann for excellent technical assistance, Dr Jeremy Lee for a helpful discussion and Dr Amy Rosenzweig (Department of Biochemistry, Molecular Biology and Cell Biology, Northwestern University, Evanston, IL, U.S.A.) for providing the SxxS mutant construct of ATP7B.
This work was supported by the Canadian Institutes of Health Research and the Saskatchewan Health Research Foundation (to O. Y. D.); the National Institutes of Health [grant number R01 DK071865 (to S. L.)]. N. D. is a recipient of Saskatchewan Health Research Foundation postdoctoral fellowship.
Abbreviations used
- DIC
differential interference contrast
- IPTG
isopropyl β-D-thiogalactoside
- MALDI–TOF
matrix-assisted laser-desorption ionization–time-of-flight
- MBR
metal-binding repeat
- N-domain
nucleotide-binding domain
- TCEP
tris-(2-carboxyethyl)phosphine
- TFA
trifluoroacetic acid
References
- 1.Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 2.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discovery. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 3.Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem. 2003;278:9100–9106. doi: 10.1074/jbc.M210284200. [DOI] [PubMed] [Google Scholar]
- 4.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 5.Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol. 2007;63:12–31. doi: 10.1016/j.critrevonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- 7.Safaei R, Howell SB. Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Crit Rev Oncol Hematol. 2005;53:13–23. doi: 10.1016/j.critrevonc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gitlin JD. Wilson disease. Gastroenterology. 2003;125:1868–1877. doi: 10.1053/j.gastro.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Kaler SG. Metabolic and molecular bases of Menkes disease and occipital horn syndrome. Pediatr Dev Pathol. 1998;1:85–98. doi: 10.1007/s100249900011. [DOI] [PubMed] [Google Scholar]
- 11.Lutsenko S, Petrukhin K, Cooper MJ, Gilliam CT, Kaplan JH. N-terminal domains of human copper-transporting adenosine triphosphatases (the Wilson’s and Menkes disease proteins) bind copper selectively in vivo and in vitro with stoichiometry of one copper per metal-binding repeat. J Biol Chem. 1997;272:18939–18944. doi: 10.1074/jbc.272.30.18939. [DOI] [PubMed] [Google Scholar]
- 12.Katano K, Kondo A, Safaei R, Holzer A, Samimi G, Mishima M, Kuo YM, Rochdi M, Howell SB. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res. 2002;62:6559–6565. [PubMed] [Google Scholar]
- 13.Komatsu M, Sumizawa T, Mutoh M, Chen ZS, Terada K, Furukawa T, Yang XL, Gao H, Miura N, Sugiyama T, Akiyama S. Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Cancer Res. 2000;60:1312–1316. [PubMed] [Google Scholar]
- 14.Samimi G, Katano K, Holzer AK, Safaei R, Howell SB. Modulation of the cellular pharmacology of cisplatin and its analogs by the copper exporters ATP7A and ATP7B. Mol Pharmacol. 2004;66:25–32. doi: 10.1124/mol.66.1.25. [DOI] [PubMed] [Google Scholar]
- 15.Samimi G, Howell SB. Modulation of the cellular pharmacology of JM118, the major metabolite of satraplatin, by copper influx and efflux transporters. Cancer Chemother Pharmacol. 2006;57:781–788. doi: 10.1007/s00280-005-0121-5. [DOI] [PubMed] [Google Scholar]
- 16.Samimi G, Safaei R, Katano K, Holzer AK, Rochdi M, Tomioka M, Goodman M, Howell SB. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin Cancer Res. 2004;10:4661–4669. doi: 10.1158/1078-0432.CCR-04-0137. [DOI] [PubMed] [Google Scholar]
- 17.Safaei R, Otani S, Larson BJ, Rasmussen ML, Howell SB. Transport of cisplatin by the copper efflux transporter ATP7B. Mol Pharmacol. 2008;73:461–468. doi: 10.1124/mol.107.040980. [DOI] [PubMed] [Google Scholar]
- 18.Hagrman D, Goodisman J, Dabrowiak JC, Souid AK. Kinetic study on the reaction of cisplatin with metallothionein. Drug Metab Dispos. 2003;31:916–923. doi: 10.1124/dmd.31.7.916. [DOI] [PubMed] [Google Scholar]
- 19.Hagrman D, Goodisman J, Souid AK. Kinetic study on the reactions of platinum drugs with glutathione. J Pharmacol Exp Ther. 2004;308:658–666. doi: 10.1124/jpet.103.059410. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa T, Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J Biol Chem. 1993;268:20116–20125. [PubMed] [Google Scholar]
- 21.Rosenberg B, VanCamp L, Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg B, Van Camp L, Grimley EB, Thomson AJ. The inhibition of growth or cell division in Escherichia coli by different ionic species of platinum(IV) complexes. J Biol Chem. 1967;242:1347–1352. [PubMed] [Google Scholar]
- 23.Morgan CT, Tsivkovskii R, Kosinsky YA, Efremov RG, Lutsenko S. The distinct functional properties of the nucleotide-binding domain of ATP7B, the human copper-transporting ATPase: analysis of the Wilson disease mutations E1064A, H1069Q, R1151H, and C1104F. J Biol Chem. 2004;279:36363–36371. doi: 10.1074/jbc.M404553200. [DOI] [PubMed] [Google Scholar]
- 24.Yatsunyk LA, Rosenzweig AC. Cu(I) binding and transfer by the N terminus of the Wilson disease protein. J Biol Chem. 2007;282:8622–8631. doi: 10.1074/jbc.M609533200. [DOI] [PubMed] [Google Scholar]
- 25.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1972. [Google Scholar]
- 26.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Bose RN. Biomolecular targets for platinum antitumor drugs. Mini Rev Med Chem. 2002;2:103–111. doi: 10.2174/1389557024605500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.