Abstract
Paired immunoglobulin-like type 2 receptors (PILRs) inhibitory PILRα and activating PILRβ are predominantly expressed on myeloid cells. Their functions in host defense and inflammation are largely unknown, and in this study, we evaluated their roles in an acute Staphylococcus aureus pneumonia model. Compared to their respective controls, Pilrb−/− mice or mice in which PILRα was activated with an agonistic antibody showed improved clearance of pulmonary staphylococci and improved survival. These mice had reduced serum or bronchoalveolar lavage fluid levels of interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-6 and elevated levels of gamma interferon (IFN-γ), IL-12, and IL-10. In contrast, mice in which PILRβ was activated had increased lung bacterial burdens and higher mortality coupled with an intense proinflammatory response with highly elevated levels of IL-1β, TNF-α, and IL-6. Treatment groups with reduced bacterial burdens had higher levels of Keratinocyte-derived chemokine (KC), macrophage inflammatory protein 2 (MIP-2), and MIP-1α in bronchoalveolar lavage fluid and an increased influx of neutrophils and macrophages to the lungs. Consistent with our in vivo findings, bone marrow-derived macrophages from Pilrb−/− mice released significantly less IL-1β and TNF-α and more IFN-γ and IL-12 than did the wild-type macrophages when directly stimulated with heat-killed S. aureus. To our knowledge, this is the first evidence that S. aureus directly interacts with PILRβ. It provides a mechanism by which manipulating the balance in favor of an inhibitory PILR signal, by activation of PILRα or deletion of PILRβ, helps to control acute S. aureus-mediated pneumonia and attenuate the inflammatory response. These results highlight the importance of PILRs in innate immunity and the control of inflammation.
Infection of the lung parenchyma by pathogenic bacteria can result in bacterial invasion of the epithelial linings of the alveoli, ultimately leading to pneumonia. Staphylococcus aureus, a Gram-positive extracellular bacterium, accounts for up to 20% of nosocomial pneumonia and 2% of community-associated pneumonia and is also a major cause of sepsis (14, 23). Recent studies have indicated a high prevalence of community-acquired methicillin-resistant S. aureus in otherwise healthy individuals (12, 18). This growing resistance of S. aureus to β-lactam antibiotics warrants the search for new therapeutic targets to combat infections, including pneumonia, caused by this pathogen.
Neutrophils, monocytes, and macrophages constitute a major fraction of blood and tissue leukocytes and are responsible for mounting a rapid innate immune response as well as initiating and directing adaptive immunity (29). Upon activation, these cells migrate to sites of infection, where they phagocytose and eradicate invading pathogens by using an arsenal of cytotoxic agents in preformed granules and by releasing reactive oxygen species. They also release inflammatory cytokines and chemokines, including tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-8, that attract and activate additional neutrophils and monocytes. Phagocytes play a role in both destroying and healing tissue and are potential targets for pharmacological intervention to regulate inflammation (10). In the lung, the local inflammatory response to a bacterial pathogen such as S. aureus is mediated through a tight regulation of, and interaction between, pattern recognition receptors and various stimulatory innate immunoreceptors present on cells of the myeloid lineage (38). Previous reports have shown that effective defense against S. aureus infection in the lungs of immunocompetent mice is primarily accomplished by the ability of the host to evoke a strong innate immune response through neutrophil and macrophage sequestration (35). However, the precise functions of many immune regulatory receptors present on these cells and their involvement in the molecular and cellular mechanisms of host defense against pulmonary S. aureus infection remain to be understood.
Neutrophils and macrophages express a number of paired immune regulatory receptors of either the C-type lectin or Ig superfamilies. Paired receptors have similar ectodomains and frequently interact with the same ligand, but function to produce opposing signals (22, 31). This fine balance between the activation and inhibitory signals is viewed as critical to avoid an inappropriate and detrimental inflammatory response. The paired immunoglobulin-like type 2 receptor (PILR) family comprises two isoforms, inhibitory PILRα (also known as inhibitory FDF03) and activating PILRβ (also known as activating FDF03), and is well conserved among mammals (15, 34). These paired receptors belong to the v-type immunoglobulin superfamily and are mapped to chromosome 7q22 in humans. PILRα has two immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in its cytoplasmic domain and delivers inhibitory signals through the recruitment of SHP-1 via its amino-terminal Src homology 2 (SH2) domain (27). Conversely, PILRβ, which does not contain an ITIM, associates with the adaptor molecule DAP12 and transduces an activating signal by means of the DAP12 immunoreceptor tyrosine-based activation motif (ITAM) (34). Both isoforms are expressed on the cell surface of neutrophils, monocytes, macrophages, and dendritic cells (DCs). PILRβ is also present on NK cells and a small population of T cells in both mice and humans (15, 34). A CD99-like molecule was initially reported to be a ligand for both PILR isoforms in mice (34), while more recently, it was observed that the O-glycan sugar chain on CD99 is involved in receptor recognition (39). Recent studies have also demonstrated that glycoprotein B of herpes simplex virus type 1 is a ligand for PILRα (33), signifying an alternative route of viral entry into infected cells.
Although PILRα and PILRβ are abundantly expressed on myeloid cells, very little is known about their role in host defense against extracellular bacterial infection. In this study, we investigated the biological relevance of PILRα and PILRβ in S. aureus-mediated acute lung infection. We hypothesized that modulation of the two receptors can independently either blunt or exacerbate the inflammatory response during the early phase of infection. To address this, we utilized agonistic monoclonal antibodies to PILRα and PILRβ, as well as mice deficient in PILRβ. Our results demonstrate that absence of PILRβ or activation of PILRα signaling helps to control acute S. aureus infection in the lung and identify a critical role for neutrophils and macrophages in combating acute staphylococcal infection in the lungs of Pilrb−/− mice.
MATERIALS AND METHODS
Antibodies.
Monoclonal antibodies against mouse inhibitory PILRα and activating PILRβ were generated as described previously (15). Briefly, female Lewis rats were immunized at 2- to 3-week intervals with a fusion protein consisting of the extracellular domain of either PILRα or PILRβ fused to the Fc domain of human immunoglobulin (hIg) (40). Hybridomas that recognized PILRα-Ig or PILRβ-Ig (but not the control Ig) were initially selected by indirect enzyme-linked immunosorbent assay (ELISA). Hybridomas were then further selected based on their ability to recognize stably transfected mast cell lines DT755 and DT754 expressing PILRα and PILRβ, respectively. Antibodies specific for PILRα (DX276) or PILRβ (DX266) were characterized as agonistic based on their ability to inhibit or activate degranulation (measured by β-hexosaminidase release) in a second pair of mast cell transfectants, DT866 and DT865, expressing PILRα and PILRβ, respectively (41).
Mice.
Animal experiments were approved by the Institutional Animal Care and Use Committee at Schering-Plough Biopharma. Pathogen-free C57BL/6J mice, 6 to 8 weeks old, were purchased from Jackson Laboratories, Sacramento, CA. Pilrb−/− mice were generated as described elsewhere (see Fig. S1 in the supplemental material) and maintained and bred in the Schering-Plough Biopharma animal facility.
Cell isolation.
Whole-blood samples were obtained from 6- to 8-week-old mice by cardiac puncture and mixed with five times the volume of lysis buffer (168 mM ammonium chloride, 10 mM potassium bicarbonate, 2 mM EDTA [pH 7.3]) for 5 min to lyse red blood cells. The mixture was centrifuged, and the pellet containing the leukocytes was resuspended in 1 ml of phosphate-buffered saline (PBS).
Phenotypic characterization of Pilrb−/− mice.
The lack of cell surface expression of PILRβ was confirmed by fluorescence-activated cell sorter (FACS) staining of mouse leukocytes purified from 6- to 8-week-old male or female Pilrb−/− mice and their corresponding C57BL/6J age-matched wild-type (WT) controls. Cells were purified as described above and incubated with anti-PILRα or anti-PILRβ monoclonal antibodies for 1 h at 4°C. Cells were washed twice in staining buffer (PBS containing 2 mM EDTA and 0.5% bovine serum albumin [BSA]) and then incubated for 30 min with phycoerythrin (PE)-conjugated goat anti-rat secondary antibody. Expression levels of PILRα and PILRβ in WT and Pilrb−/− mice were determined by flow cytometric analysis using FACSCalibur (BD Biosciences, Mountain View, CA). Furthermore, a complete blood count was obtained for these mice by use of the Advia system 120 (Siemens). In order to evaluate the knockdown of the Pilrb gene at the mRNA level, the heart, lung, liver, kidney, and spleen were harvested and subjected to real-time quantitative reverse transcription-PCR (qRT-PCR) analysis.
Bacterial strain and culture.
The S. aureus strain ATCC 27271 was used for the mouse lung infections. A 1:50 dilution of an overnight culture was made into fresh tryptic soy broth. Staphylococci were grown with shaking at 37°C to an optical density of 0.9 at 600 nm (corresponding to ∼1 × 109 CFU/ml). A 40-ml aliquot of the culture was sedimented by centrifugation at 3,000 rpm for 15 min, and the bacteria were resuspended in 10 ml Hanks' balanced salt solution (HBSS) buffer at 1 × 108 CFU per 25 μl. Heat-killed S. aureus strains were prepared as described previously (25), with minor modifications. Briefly, a 1:50 dilution of the overnight culture was grown to log phase in fresh tryptic soy broth to an optical density of 0.9 at 600 nm. Approximately 40 ml of this bacterial culture was washed in HBSS (pH 7.0) and resuspended in 4 ml of HBSS to obtain a 10-fold concentrated suspension of S. aureus (1010 CFU/ml) and then heat-killed at 80°C for 10 min. A 10-μl aliquot of the heat-killed bacterial suspension was plated onto tryptic soy agar plates, and no bacterial colonies were found after overnight incubation at 37°C.
Intranasal lung infection.
To induce pulmonary infection, 6- to 8-week-old female C57BL/6J and Pilrb−/− mice were anesthetized with isoflurane and inoculated with 25 μl of the S. aureus slurry into the left nare, as described previously (8). Animals were held upright for 1 min after inoculation, placed in a supine position during recovery, and observed for 48 to 72 h. About 10% of animals routinely died in the first 6 h following infection, probably from the additive effects of anesthesia and aspiration, and were thus not included in statistical analyses. In antibody experiments, 6- to 8-week-old female C57BL/6J mice were dosed with 1 mg/mouse of anti-PILRα, anti-PILRβ, or rat IgG1 isotype control antibody either subcutaneously 24 h prior to infection or intravenously 2 h postinfection.
Lung bacterial burden.
To evaluate the bacterial burden of infected mice, lungs were harvested in ice-cold PBS at 6, 24, and 48 h postinfection and homogenized. Tenfold serial dilutions (in PBS) of lung homogenates were plated onto tryptic soy agar plates. Colonies were counted after 24 h of incubation at 37°C and presented as log10 CFU per lung. A portion of the homogenate was processed with STAT-60 (Tel-Test, Friendswood, TX) and analyzed by qRT-PCR.
Cytokine and chemokine analyses in BAL fluid and blood samples.
Lungs of euthanized mice were lavaged with 1 ml PBS through a polyethylene tube cannulated into the trachea as previously described (11). Bronchoalveolar lavage (BAL) fluid specimens were centrifuged and supernatants collected for analysis. Blood samples were collected by cardiac puncture. The mouse cytokine/chemokine Milliplex kit was used for all cytokine and chemokine measurements (Millipore, Billerica, MA).
Preparation of lung cell suspension.
Upon collection of the BAL fluid samples, as described above, whole lungs were perfused with 10 ml of cold PBS and dissected. Briefly, the isolated lung was shred into several small pieces and incubated with 15 ml RPMI containing 250 μg/ml of Liberase R1 purified enzyme blend (Roche Diagnostics, Indianapolis, IN) and 100 μg/ml penicillin-streptomycin at 37°C for 1 h. The enzymatic reaction was stopped by adding 10 ml ice-cold PBS-2 mM EDTA, and the tissue suspension was incubated on ice for an additional 10 min. The digested lungs were further disrupted by pipetting the mixture through a 10-ml pipette several times and gently pushing the tissue suspension through a nylon screen. The resulting single-cell suspension was washed and centrifuged at 1,300 rpm. Contaminating red blood cells were lysed by incubating the cell pellet for 5 min at room temperature in red blood cell lysis buffer (Sigma). Cells were washed with complete RPMI and resuspended in 2 ml complete RPMI, and total cell counts were obtained using the Vi-cell Coulter counter (19).
Flow cytometry.
To determine the cell differential, single-cell suspensions obtained from the lungs of infected WT and Pilrb−/− mice by enzymatic digestion were washed and incubated in staining buffer (PBS containing 2% fetal bovine serum, 0.1% sodium azide, and 2 mM EDTA) containing Fc block CD32/CD16 (clone 2.4G2). Cells were stained for 1 h at 4°C with directly conjugated antibodies. Cells were stained for the following cell surface markers: Gr-1, CD11b, CD11c, F4/80, CD3e, CD4, CD8, CD45, and NK1.1. Cell acquisition was performed using FACSCalibur (BD Biosciences, Mountain View, CA), and the data were analyzed using CellQuest software (BD Biosciences). In addition, single-cell suspensions prepared from the bone marrow and spleens were labeled with PE-conjugated antibodies against GR-1, major histocompatibility complex class II (MHC-II), CD3, and NK1.1; fluorescein isothiocyanate (FITC)-conjugated antibodies against CD45, CD11c, CD8, and CD25; and allophycocyanin (APC)-conjugated antibodies against CD11b, B220, and CD4 (all from BD Biosciences).
Myeloperoxidase assay.
In order to determine the levels of myeloperoxidase (MPO) in the lungs of infected mice, whole lungs were harvested as indicated above at 6 and 24 h postinfection and weighed. The lung tissues were homogenized in 1 ml PBS and centrifuged at 10,000 rpm for 10 min at 4°C. After the supernatant was aspirated, the pellet was resuspended in 1 ml of CTAB buffer (6.8 g KH2PO4, 5.0 g cetyl trimethyl ammonium bromide, 50 mM acetic acid [pH 6.0]) followed by the addition of 100 μl of 0.5% sodium deoxycholate. The samples were mixed thoroughly and incubated on ice for 30 min. The samples were then centrifuged at 10,000 rpm for 30 min at 4°C. The supernatant was collected and incubated at 60°C for 2 h. To measure MPO levels, 50 μl of the supernatant was mixed with 90 μl of TNB solution, followed by the addition of 90 μl of stop solution. An MPO standard (20 μg/ml) was appropriately diluted and used as the control. The samples were read at 450 nm using a microplate reader (Molecular Devices). The absorbance values obtained for each sample were normalized to their respective lung weights and the MPO concentrations were presented as μg MPO/g of lung tissue.
Isolation and in vitro stimulation of bone marrow-derived macrophages.
Bone marrow cells were isolated from hind femurs and tibiae of WT and Pilrb−/− mice and washed once with ice-cold RPMI 1640 medium containing 100 U/ml of penicillin-streptomycin, 10% fetal calf serum, 2 mM l-glutamine, sodium pyruvate, and 2-beta-mercaptoethanol. For the generation of bone marrow-derived macrophages, bone marrow cells were cultured in complete RPMI containing 50 ng/ml of recombinant mouse M-CSF (Escherichia coli-derived; R&D Systems, Minneapolis, MN) for 7 days. Fresh medium was added on days 4 and 6. On day 7, cells were harvested using 2 mM EDTA in PBS, centrifuged at 1,200 rpm for 15 min and plated at 2 × 105 cells/100 μl onto 96-well flat-bottom plates. Cells were stimulated with heat-inactivated S. aureus at a multiplicity of infection of 100:1 in complete RPMI without antibiotics for 6 h or 24 h prior to removal of supernatants for cytokine analysis.
RNA isolation.
Total RNA was extracted from STAT-60-treated lung homogenates according to the manufacturer's instructions. After isopropanol precipitation, total RNA was reextracted with phenol:chloroform:isoamyl alcohol (25:24:1) (Sigma Chemicals) by the use of phase-lock light tubes (Eppendorf).
Real-time qRT-PCR for gene expression.
DNase-treated total RNA was reverse transcribed using Superscript II (Invitrogen) according to manufacturer's instructions. Primers were designed using Primer Express (PE Biosystems, Foster City, CA) or obtained commercially from Applied Biosystems (Foster City, CA). Real-time quantitative PCR on 10 ng of cDNA from each sample was performed using either of two methods. In the first method, two gene-specific unlabeled primers were utilized at 400 nM in an Applied Biosystems SYBR green qRT-PCR assay utilizing an ABI 7000, 7300, or 7900 instrument. In the second method, two unlabeled primers at 900 nM each were used with 250 nM 6-carboxyfluorescein (FAM)-labeled probe (Applied Biosystems, Foster City, CA) in a TaqMan qRT-PCR on an ABI 7000, 7300, or 7700 sequence detection system. The absence of genomic DNA contamination was confirmed using primers that recognize genomic regions of the CD4 promoter. Ubiquitin levels were measured in a separate reaction and used to normalize the data by the threshold cycle (ΔΔCT) method.
Statistical analysis.
Survival curves were compared using the log rank test (GraphPad Prism 4.0). Bacterial burden, cytokine production, MPO levels, and lung cell differential counts were analyzed using one-way analysis of variance (ANOVA), the Mann-Whitney U test, and Student's t test. A P value of ≤0.05 was considered significant.
RESULTS
Specificity and agonist activity of anti-PILRα and anti-PILRβ antibodies.
Despite the great degree of similarity between the extracellular domains of the PILR proteins, antibodies that bound specifically to PILRα- and PILRβ-expressing mouse mast cell transfectants DT755 and DT754, respectively, were generated (Fig. 1A). In a functional assay with a different mast cell transfectant line, DT865 (expressing PILRβ), anti-PILRβ (DX266) triggered degranulation in a concentration-dependent manner (Fig. 1B). Neither isotype control rat IgG1 (rIgG1) nor anti-PILRα (DX276) triggered degranulation in this cell line. In contrast, treatment with anti-PILRα inhibited degranulation in mast cell transfectant line DT866 (expressing PILRα) that was stimulated with DX87, an antibody specific for the activating receptor CD200RLa (Fig. 1C). These results confirm the specificity of the antibodies and identify them as agonistic for their cognate receptors. When given subcutaneously at 1 mg/mouse, neither DX266 nor DX276 significantly altered the numbers of PILR-expressing myeloid cells compared to those in isotype control-treated mice, based on differential blood counts 48 h after dosing (data not shown).
FIG. 1.
Specificity and agonist activity of anti-PILR antibodies. (A) Anti-PILRβ (DX266) bound only to the mouse mast cell transfectant DT754, which expresses PILRβ, and not to DT755, which expresses PILRα. Conversely, anti-PILRα (DX276) bound only to DT755 and not to DT754. (B) DX266, but not DX276 or the isotype control, triggered degranulation in a concentration-dependent manner in mast cell transfectant DT865, which also expresses only PILRβ. (C) DX276, but not the isotype control, blocked anti-CD200RLa-triggered degranulation in DT866, which expresses only PILRα. OD, optical density; conc, concentration.
Triggering PILRβ results in increased bacterial burden, mortality, and an inflammatory cytokine response.
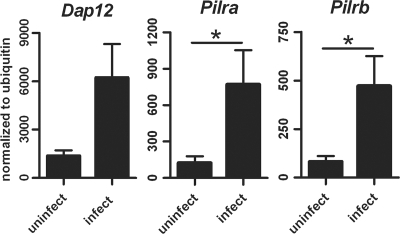
Forty-eight hours after S. aureus infection, lung mRNA levels for Dap12, Pilra, and Pilrb (Fig. 2) had all significantly increased compared to the constitutive levels in uninfected mice. This increase in expression hinted at a possible role for these proteins during acute S. aureus infection. To assess the direct involvement of PILRα and PILRβ in response to bacterial infections, we used agonist PILRα and PILRβ antibodies in a recently developed model of S. aureus-induced pneumonia that closely mimics the clinicopathological features of human disease. In most experiments, we adopted a therapeutic approach in which the agonist antibodies were administered intravenously 2 h after intranasal infection. The anti-PILRβ-treated mice displayed a significantly increased lung bacterial burden (P = 0.036) (Fig. 3A) and decreased survival, with 75% mortality at 48 h after infection (Fig. 3B), compared to those for isotype control-treated mice. In contrast, anti-PILRα-treated mice displayed significantly reduced numbers of staphylococci in the lungs (P = 0.031) (Fig. 3A) and survival similar to that of control mice (Fig. 3B). Prophylactic treatment with these antibodies 24 h and 6 h before bacterial infection gave results similar to the therapeutic treatment (data not shown). Thus, tipping the balance in favor of PILR activation (with anti-PILRβ) made the mice more susceptible to bacterial infection, while tipping the balance toward a PILR inhibitory signal (with anti-PILRα) enabled mice to clear the bacteria more efficiently.
FIG. 2.
Expression levels of Dap12, Pilra, and Pilrb in lungs from control and S. aureus-infected mice. Transcription levels of Dap12, Pilra, and Pilrb in lungs from infected and uninfected WT mice were analyzed by qRT-PCR. Infected lungs were removed 48 h postinoculation. Levels are expressed relative to that of ubiquitin mRNA. *, P ≤ 0.05.
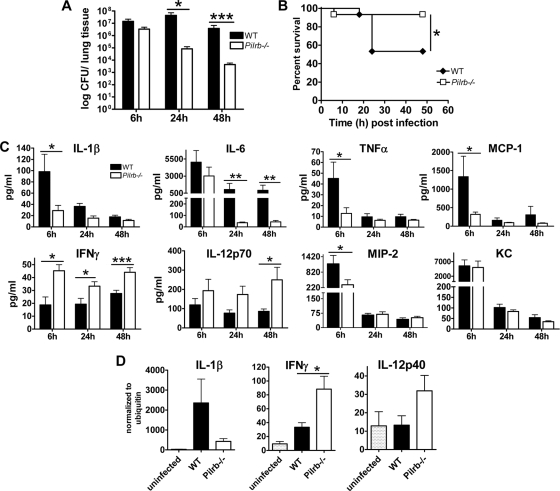
FIG. 3.
Effect of anti-PILRα or anti-PILRβ treatment on S. aureus-mediated pneumonia. C57BL/6J mice were challenged intranasally with 1 × 108 CFU of S. aureus followed by an intravenous dose of anti-PILRα, anti-PILRβ, or isotype control antibody 2 h postinfection. Mice were monitored for lung bacterial burden (A), mortality (B), MPO levels in the lung at 6 and 24 h postinfection (C), and serum cytokine levels at 24 and 48 h (D). Data points in panel A represent the bacterial load of lungs harvested from individual mice at 48 h postinfection. Data for each of the panels are representative of at least two individual experiments, with 8 to 12 mice per group in each experiment. *, P ≤ 0.05; **, P ≤ 0.002.
An appropriate recruitment of neutrophils is required for an effective clearance of bacterial infection from the lungs. In order to determine neutrophil recruitment, total MPO activity was measured. At 6 h after infection, MPO levels were similar among groups, but by 24 h, lung tissue from mice treated with anti-PILRβ had significantly reduced levels of MPO compared to those from isotype control-treated mice. In mice treated with anti-PILRα, MPO levels were considerably higher than those in control mice (Fig. 3C), suggesting continued recruitment of phagocytes to the lungs of these mice. At both 24 and 48 h after infection, increased systemic levels of proinflammatory cytokines IL-1β, TNF-α, and IL-6 in anti-PILRβ-treated mice (Fig. 3D) corresponded with their increased susceptibility to S. aureus infection. In contrast, in anti-PILRα-treated mice, IL-1β was significantly lower, and TNF-α and IL-6 were as low as those in the isotype control-treated animals. Anti-PILRα-treated mice also had increased levels of IFN-γ and increased levels (without reaching significance) of IL-12p70 compared to those for control animals (Fig. 3D). IL-1β, TNF-α, and IL-6 are critical proinflammatory mediators during an S. aureus pulmonary infection. However, since IFN-γ and IL-12 are cytokines that promote phagocytic uptake and killing of S. aureus, these cytokines may be considered less inflammatory and more antimicrobial in the context of an S. aureus lung infection (2, 42).
Deletion of the Pilrb gene does not result in gross phenotypic changes.
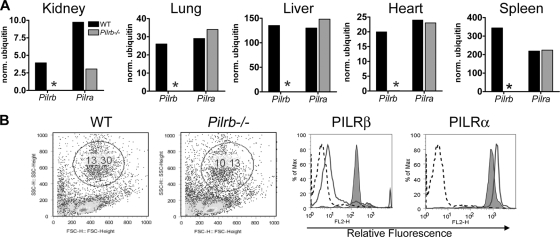
Having established a homozygous C57BL/6 Pilrb−/− strain, we sought to determine if the deletion of the Pilrb gene resulted in any critical phenotypic alterations. Extensive analyses were performed with both female and male knockout mice and with age- and sex-matched control WT animals in each experiment. qRT-PCR analysis of various organs (Fig. 4A) confirmed the complete absence of the Pilrb message and showed that the expression levels of the inhibitory Pilra remained largely unaltered. Furthermore, the mRNA expression levels of Dap12, Toll-like receptors (TLRs), and several proinflammatory cytokine and chemokine genes associated with acute bacterial infections remained unaffected as a result of the Pilrb deletion (data not shown).
FIG. 4.
Phenotypic characterization of Pilrb−/− mice. (A) Transcription of Pilra and Pilrb genes was analyzed by qRT-PCR in various organs of WT mice (black bars) and Pilrb−/− mice (gray bars). An asterisk indicates a lack of Pilrb expression in the knockout mice. (B) Following depletion of red blood cells, the remaining blood cells were stained with anti-PILRβ or anti-PILRα antibodies followed by PE-conjugated secondary antibody, as described in the text. Cells from WT and Pilrb−/− mice were gated on the granulocyte population as shown in the dot plots. The histograms show cellular expression of PILRα and PILRβ in leukocytes from WT (shaded) and Pilrb−/− (solid line) mice. The isotype control rIgG1 (anti-hIL-4) is indicated by dashed lines for both WT and Pilrb−/− cells.
We next evaluated the cell surface expression of PILRα and PILRβ in WT and Pilrb−/− peripheral blood leukocytes. Isolated peripheral blood leukocytes were gated on the granulocyte population, as displayed in the FSC-versus-SSC plot of Fig. 4B. Cell surface staining of the WT granulocyte population with anti-PILRα and anti-PILRβ antibodies produced a 2- to 3-log shift in mean fluorescence intensity compared to that of the isotype control. As would be predicted, cell surface staining was observed only for PILRα in cells from Pilrb−/− mice (Fig. 4B).
A complete blood count analysis revealed no major differences between WT and Pilrb−/− mice in the number of total white blood cells, lymphocytes, monocytes, neutrophils, eosinophils, and basophils (see Fig. S2A in the supplemental material). We also analyzed the hematopoietic compartment in bone marrow and splenic leukocytes isolated from Pilrb−/− and WT control littermates for expression of cell lineage markers by flow cytometry. No significant differences in the B cells, T cells, DCs, NK cells, or myeloid cell lineage were observed (see Fig. S2B in the supplemental material). These results indicate that the deletion of the Pilrb gene had little impact on PILRα at either the mRNA or protein level and that PILRβ is not required for proper development of the murine hematopoietic compartment.
Absence of PILRβ protects against acute S. aureus lung infection.
To further evaluate the role of PILRβ during S. aureus infection, WT and Pilrb−/− mice were infected intranasally with 1 × 108 CFU of bacteria, and the severity of infection was monitored. Pilrb−/− mice were more resistant to S. aureus infection than were WT mice, with improved bacterial clearance at 24 and 48 h postinfection (P = 0.012 and P = 0.001, respectively) (Fig. 5A) and reduced mortality (P = 0.023) (Fig. 5B). As shown in Fig. 5C, S. aureus infection of Pilrb−/− mice resulted in lower serum levels of IL-1β (P = 0.037), IL-6 (P = 0.04), TNF-α, and monocyte chemoattractant protein 1 (MCP-1; P = 0.034) at 6, 24, or 48 h postinfection than in WT controls. Consistent with the reduced bacterial load and improved survival, serum samples from Pilrb−/− mice had higher levels of IFN-γ and IL-12p70, and the serum concentrations of these two cytokines remained elevated throughout the study period. This is in agreement with previously reports regarding IFN-γ and IL-12p70 during an intranasal S. aureus infection, suggesting a critical role for them in mobilizing the immune response and clearing the infection. Serum levels of Keratinocyte-derived chemokine (KC) (GROα) were elevated in both groups at 6 h, followed by a significant decrease by 24 h, while levels of macrophage inflammatory protein 2 (MIP-2) were significantly lower in Pilrb−/− mice at 6 h postinfection than in the WT controls (Fig. 5C). These results are consistent with their neutrophil-attracting roles and suggest that these chemokines become elevated at the onset of infection and help attract neutrophils from the peripheral blood and bone marrow to the site of infection. Furthermore, as shown in Fig. 5D, expression of IL-1β transcripts was considerably lower in lung tissue from Pilrb−/− mice. In contrast, IFN-γ and IL-12p40 transcripts were elevated in the lungs of knockout mice at 48 h postinfection (Fig. 5D), consistent with the serum protein levels of these cytokines (Fig. 5C).
FIG. 5.
Deletion of PILRβ protects mice from S. aureus-mediated pneumonia. C57BL/6J WT and Pilrb−/− mice were infected intranasally with 1 × 108 CFU of S. aureus. Mice were monitored for lung bacterial burden at 6, 24, and 48 h postinfection (n = 8 mice/group in three different experiments) (A); survival (cumulative data from three experiments) (B); serum cytokine levels (mean ± standard error of the mean [SEM] from three experiments each with five to six mice per group (C); and lung mRNA levels for IL-1β, IFN-γ, and IL-12p40 at 48 h postinfection (D). *, P ≤ 0.05; **, P ≤ 0.002; ***, P ≤ 0.001.
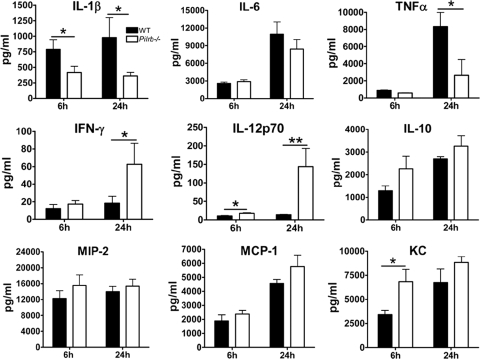
As this study focuses on S. aureus lung infection, we next measured cytokines and chemokines in the BAL fluid of infected mice (Fig. 6). Notably, Pilrb−/− mice had lower levels of IL-1β and TNF-α (at 6 and 24 h postinoculation) as well as IL-6 and MCP-1 (at 6 h only) than did WT mice. Significantly higher levels of IFN-γ and IL-10 (P values of ≤0.0008 and ≤0.035, respectively) were observed in BAL fluid of the knockout mice at 24 h postinfection, likely to counteract the damaging effect of the proinflammatory cytokines. In contrast to serum values, levels of KC, MIP-2, and MIP-1α in the BAL fluid of infected mice were significantly elevated at 6 and 24 h after infection in the Pilrb−/− mice compared to those of WT control animals (Fig. 6). These elevated chemokine levels in the local environment of the lungs of the Pilrb−/− mice could imply more efficient attraction and sequestration of monocytes/macrophages and neutrophils to control the infection. On the other hand, the lower levels observed in the BAL fluid of WT mice could lead to inadequate neutrophil numbers in the local environment and the need to attract more neutrophils from the bone marrow, consistent with the higher levels of these chemokines in the sera of WT mice.
FIG. 6.
Chemokine and cytokine levels in BAL fluid of WT and Pilrb−/− mice following pulmonary infection with S. aureus. Levels of various cytokines and chemokines in BAL fluid samples collected by lavage at 6, 24, and 48 h postinfection were measured as described in the text. Data are expressed as means ± SEM of five to six mice per time point from at least three different experiments. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
Effective and early recruitment of neutrophils into the bronchoalveolar space protects Pilrb−/− mice from S. aureus pneumonia.
To better understand the cause of the protective phenotype observed in Pilrb−/− mice during intranasal S. aureus infection, we used flow cytometry to define the influx of cells into the lungs during the acute phase of infection. Cells in the lungs of naïve and challenged mice were initially analyzed according to their FSC and SSC characteristics (Fig. 7A). Cells that clustered as FSCloSSClo were gated as “R2” and were comprised mainly of lymphocytes and monocytes, while those that clustered as FSChiSSChi were gated as “R3” and included mainly macrophages and granulocytes. The R2 population was almost twice the size of the R3 population in both naïve WT and Pilrb−/− mice. However, a dramatic increase in the percentages of cells in the R3 population was observed after bacterial challenge, as shown in Fig. 7A.
FIG. 7.
Dynamics of neutrophil and macrophage infiltration during acute S. aureus pulmonary infection. (A) Cells isolated from the lungs of naïve and S. aureus-infected WT and Pilrb−/− mice were divided into two main populations. Cells defined as FSCloSSClo were gated as R2 (primarily comprising the lymphocyte and monocyte populations), and those defined as FSCmid-hiSSCmid-hi were gated as R3 (primarily comprising the granulocyte population). The R3 population was significantly increased in infected mice compared to that in naïve mice. (B and D) Plots showing a significant increase in the CD11b+Gr1lo-int (macrophage) population in the Pilrb−/− mice (white squares) compared to that in the WT mice (gray squares) at 24 h in the R2 gate and at both 24 and 48 h in the R3 gate. (C and E) Plots showing cellular infiltration in R2 and R3 gates, with a significant increase in the CD11b+Gr1+hi (neutrophil) population in Pilrb−/− mice at 24 h postinfection. The plots are representative of three to four separate experiments with five to six mice per group at each time point. Data are expressed as means ± SEM. *, P ≤ 0.05; **, P ≤ 0.001.
Further analysis showed that cells in the R2 gate were mainly CD3+, CD11b+Gr-1lo-int (small macrophages), CD11b+CD11clo (monocytes and small macrophages) and CD11b+/F4/80int (alveolar macrophages) in naïve mice (see Fig. S3A in the supplemental material). Analysis of the R3 gate was primarily dominated by resident alveolar macrophages defined as CD11b+/F4/80int and CD11b−CD11c+ (35%) and a lower percentage of CD11b+Gr1+ (18%) (see Fig. S3C in the supplemental material). Previous reports have also characterized the CD11b+/F/480int and CD11b−CD11c+ cells as small macrophages and alveolar macrophages, respectively (19). During S. aureus infection, a significant increase was observed in CD11b+GR-1lo-int cells (Fig. 7B and D) and CD11b+GR-1hi cells (Fig. 7C and E; see also Fig. S3B and D in the supplemental material) in both the R2 and R3 gates at 24 h. Both of these populations were significantly higher in the Pilrb−/− mice than in the WT mice (Fig. 7B to E). Furthermore, a sustained increase in the number of CD11b+GR-1lo-int cells (macrophages) in Pilrb−/− mice was observed in the R3 population even at 48 h postinfection (Fig. 7D). Another striking observation was a predominant increase in CD11b+GR-1hi cells in the R2 and R3 gates and a significant decrease in the CD11b−CD11c+ and CD11b+F4/80int cells in the knockout mice (data not shown). Taken together, these results suggest that upon infection, the Pilrb−/− mice were better able to initiate and maintain an influx of neutrophils and macrophages to the lung for more effective clearance of S. aureus, thus adding further credence to the importance of these cells in combating acute pulmonary bacterial infection. In addition, cells were stained with anti-CD3, anti-CD8, anti-CD4, and anti-NK1.1 monoclonal antibodies, but no apparent difference in cell number was observed between the two groups under naive or infected conditions (data not shown).
Evidence for a direct interaction between macrophages and S. aureus.
In order to define more precisely the mechanism by which PILRβ regulates the response to S. aureus, we determined if the absence of the receptor alters the direct in vitro response of macrophages to staphylococci. As shown in Fig. 8, when Pilrb−/− bone marrow-derived macrophages were directly stimulated with heat-killed S. aureus, they produced levels of TNF-α and IL-1β that were 2- to 3-fold lower and levels of IFN-γ, IL-12p70, and KC that were 2- to 6-fold higher than those produced by WT macrophages. These results are consistent with the serum and BAL fluid cytokine levels obtained from anti-PILRα-treated and Pilrb−/− mice and strongly support the in vivo observations. They also provide the first evidence, to our knowledge, for a direct interaction between PILRβ and S. aureus.
FIG. 8.
Cytokine and chemokine release from bone marrow-derived macrophages stimulated with heat-killed S. aureus. Bone marrow-derived macrophages from WT and Pilrb−/− mice were stimulated for 6 h or 24 h with heat-killed S. aureus, and the supernatants were assayed for various key cytokines and chemokines. Results represent the means ± standard deviation (SD) of three experiments. *, P ≤ 0.05; **, P ≤ 0.001.
DISCUSSION
Cells of the innate immune system provide the first line of cellular defense against S. aureus infection (23), a process mediated in part by signaling through immunoreceptors. More than 20 pairs of immune regulatory receptors, consisting of highly related activating and inhibitory isoforms, have been identified so far, suggesting that receptor pairing is critical to the regulation of an innate immune response (22, 36). PILRα and PILRβ are one such pair of receptors with opposing signaling capabilities (34), and little is known about their involvement in host responses to bacterial infection. Here we demonstrate for the first time that modulation of the PILR pathway, by triggering PILRα with an agonist antibody or by deleting PILRβ, attenuates pulmonary and systemic inflammation and promotes improved survival of S. aureus-infected mice. We also provide evidence for a direct interaction of S. aureus with PILRβ.
Receptors of the Ig superfamily (32) and the C-type lectin family (3) are involved in pathogen recognition and fine-tuning the regulatory mechanism during a bacterial infection. A recent study showed that paired Ig-like receptors (PIR) comprising activating PIR-A and inhibitory PIR-B were able to recognize S. aureus and regulate TLR-mediated cytokine production (28). In another study, it was shown that PIR-B-deficient mice were susceptible to Salmonella infection (36). Additionally, the activating receptors MDL-1 (4) and the TREMs (6), which associate with DAP12, were implicated in mycobacterial infections and in the diagnosis of lung infections. Owing to the high expression of PILRα and PILRβ in the myeloid cell lineage (34), we hypothesized that one or both of the PILRs could be important in the pathogenesis of acute S. aureus lung infection.
The constitutive expression levels of the PILRs in lungs of uninfected mice were significantly lower than that of DAP12. However, after S. aureus infection, a dramatic increase in expression of all three molecules was observed. It is likely that this amplification in expression of DAP12, PILRα, and PILRβ is primarily due to the enhanced number of neutrophils and macrophages that infiltrate the lungs in response to infection. The overall expression of these receptors can, in part, determine the balance between immune activation and inhibition. We therefore independently activated the PILRs with agonist antibodies and were able to induce opposite immune responses during S. aureus infection. While agonist anti-PILRα-treated mice were better able to clear the infection, anti-PILRβ-treated animals were highly susceptible to the pathogen and displayed an increased bacterial burden accompanied by a high mortality rate. Furthermore, in accordance with our findings in antibody experiments, Pilrb−/− mice were more resistant to infection than were WT mice and exhibited decreased bacterial burden and greater survival. A similar protective phenotype was observed during Pseudomonas aeruginosa induced-pneumonia in rats wherein blocking TREM-1 by using LP17 peptide appeared to be beneficial (16). In a lipopolysaccharide (LPS)-induced septic shock model, 100% mortality was observed in mice injected with an agonist TREM-1 antibody. Treatment with a TREM-1 fusion protein protected these mice against LPS-induced shock (7, 17). Previous studies have also demonstrated the role of DAP12 in the development of inflammation by showing that Dap12−/− mice were protected from LPS-mediated septic shock and displayed reduced plasma cytokine concentrations and a lower mortality rate (37). Contrary to these findings, Hamerman et al. reported that DAP12-deficient macrophages produced higher concentrations of inflammatory cytokines in response to various TLR stimuli (21). The findings of Hamerman et al. suggest that one or more DAP12-pairing receptors negatively regulate signaling through TLRs and also that many DAP12-pairing receptors have a related inhibitory receptor (21). Thus, the overactivation of the DAP12 pathway could be partly mediated by the relative expression of the DAP12 associating molecules. Consistent with the association of PILRβ with DAP12, we observed that activation of PILRβ with an agonist antibody augments susceptibility to infection, possibly resulting from an overactivation of the PILRβ/DAP12 pathway and ultimately leading to an excessive inflammatory response. Conversely, absence of PILRβ or triggering of PILRα with an agonist antibody inhibits the DAP12 activation pathway, resulting in improved survival and effective clearance of the pathogen.
S. aureus infection was also responsible for the development of a profound inflammatory response in mice that displayed a high bacterial burden and high mortality. Both locally, as assessed by BAL fluid cytokine levels, and systemically, these mice displayed increased concentrations of IL-1β, IL-6, and TNF-α and significantly reduced amounts of IFN-γ and IL-10. Interestingly, in Pilrb−/− mice and in agonist anti-PILRα-treated mice, the production of these proinflammatory immune mediators was notably reduced, while levels of IFN-γ, IL-12, and IL-10 were elevated. As IL-1β is considered the hallmark of acute lung injury (9), it is important to note that although IL-1β in the knockout and anti-PILRα-treated animals was significantly reduced, it was not completely absent. This is in agreement with the role of IL-1β in recruiting immune cells to the site of infection (20). However, very high levels of this cytokine can predispose mice to acute lung injury and enhanced systemic inflammation (20). Likewise, TNF-α can stimulate the production of other cytokines, and requisite amounts of this cytokine are also important for preventing tissue damage (24). In contrast, IL-10 inhibits cytokine production by macrophages (13), and IFN-γ and IL-12 can promote phagocytic uptake and killing of S. aureus by immune cells (2, 42). IL-10, IL-12, and IFN-γ levels were elevated among the Pilrb−/− and anti-PILRα-treated mice. Consistent with these in vivo observations, Pilrb−/− bone marrow-derived macrophages stimulated directly with heat-inactivated S. aureus produced levels of TNF-α and IL-1β that were 2- to 3-fold lower and levels of IFN-γ and IL-12 that were 3- to 6-fold higher than those produced by WT macrophages. These data provide good evidence for a direct interaction between PILRβ and S. aureus. They also provide an explanation for why manipulating the balance in favor of an inhibitory PILR signal, by activation of PILRα or deletion of PILRβ, helps to control acute S. aureus-mediated pneumonia and attenuate the inflammatory response.
As would be expected, soon after infection significant cellular recruitment to the lungs was observed (30, 32). Importantly, a further increase in neutrophils, macrophages, and chemokines was observed in the lungs and BAL fluid of Pilrb−/− and agonist anti-PILRα-treated mice. Thus, efficient phagocytosis and clearance of the infection in these mice could be attributed to a suitable cytokine and chemokine response leading to efficient migration of neutrophils and macrophages. While neutrophil influx (CD11bloGR1+hi) peaked at 24 h after infection, the infiltrating macrophage population defined by CD11b+Gr1lo remained significantly higher in the infected knockout mice even at 48 h, suggesting that macrophages play a critical role in clearing bacteria. In support of this observation, levels of IFN-γ in these mice were also notably elevated, further emphasizing the association between IFN-γ and increased phagocytic uptake and killing of S. aureus (42). Conversely, mice that were severely affected and unable to clear the infection displayed reduced neutrophil numbers (and MPO levels), suggesting an impaired neutrophil migration to the site of infection. Previous studies have shown that severe inflammation and infection can lead to impairment in neutrophil migration, coupled with increased bacterial burden and mortality (1). McLoughlin et al. (26) recently found that IFN-γ correlated with increased CXC chemokine levels that, in turn, directly recruited neutrophils during S. aureus infection. Our data are compatible with this, but in the McLoughlin et al. paper, as well as in the paper by Bartlett et al. (5), increased chemokines and neutrophils were associated with worse outcomes. We found that increased levels of IFN-γ, chemokines, and neutrophils in S. aureus-infected lungs from Pilrb−/− mice were associated with a significantly better outcome. The likely explanation for this difference is that in the recruited cells in our study, the balance had been shifted in favor of an inhibitory PILR signal, by activation of PILRα or deletion of PILRβ. Our in vitro data in Fig. 8 demonstrate that this shift can significantly alter the cytokines released from macrophages, which in turn appear to help better control the infection and attenuate the inflammatory response.
Taken together, our data show that an exacerbated proinflammatory response can impair adequate neutrophil or macrophage recruitment and lead to reduced antibacterial activity and increased tissue damage. Additionally, the data also suggest that elevated levels of PILRβ and DAP12 during infection can result in overactivation of the PILRβ/DAP12 pathway, likely through a direct interaction between S. aureus and PILRβ, and have deleterious consequences, as observed with the WT and anti-PILRβ-treated mice. Tipping the balance in favor of an inhibitory PILR signal, through activation of PILRα or deletion of PILRβ, helps to control acute S. aureus-mediated pneumonia and attenuate the systemic inflammatory response. These results are intriguing and reveal an important role for the PILR proteins in innate immunity and the control of inflammation.
Supplementary Material
Acknowledgments
We thank Jennifer Louten and Andrew Rankin for their useful suggestions, constructive criticism, and careful review of the manuscript.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 11 January 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Alves-Filho, J. C., A. de Freitas, F. Spiller, F. O. Souto, and F. Q. Cunha. 2008. The role of neutrophils in severe sepsis. Shock 30(Suppl. 1):3-9. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, N., and Z. Xing. 2004. Use of cytokines in infection. Expert Opin. Emerg. Drugs 9:223-236. [DOI] [PubMed] [Google Scholar]
- 3.Aoki, N., A. Zganiacz, P. Margetts, and Z. Xing. 2004. Differential regulation of DAP12 and molecules associated with DAP12 during host responses to mycobacterial infection. Infect. Immun. 72:2477-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker, A. B., E. Baker, G. R. Sutherland, J. H. Phillips, and L. L. Lanier. 1999. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells. Proc. Natl. Acad. Sci. U. S. A. 96:9792-9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett, A. H., T. J. Foster, A. Hayashida, and P. W. Park. 2008. Alpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J. Infect. Dis. 198:1529-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchon, A., J. Dietrich, and M. Colonna. 2000. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164:4991-4995. [DOI] [PubMed] [Google Scholar]
- 7.Bouchon, A., F. Facchetti, M. A. Weigand, and M. Colonna. 2001. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410:1103-1107. [DOI] [PubMed] [Google Scholar]
- 8.Bubeck Wardenburg, J., R. J. Patel, and O. Schneewind. 2007. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect. Immun. 75:1040-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bubeck Wardenburg, J., and O. Schneewind. 2008. Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 205:287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig, A., J. Mai, S. Cai, and S. Jeyaseelan. 2009. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect. Immun. 77:568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Divangahi, M., T. Yang, K. Kugathasan, S. McCormick, S. Takenaka, G. Gaschler, A. Ashkar, M. Stampfli, J. Gauldie, J. Bramson, T. Takai, E. Brown, W. M. Yokoyama, N. Aoki, and Z. Xing. 2007. Critical negative regulation of type 1 T cell immunity and immunopathology by signaling adaptor DAP12 during intracellular infection. J. Immunol. 179:4015-4026. [DOI] [PubMed] [Google Scholar]
- 12.Dufour, P., Y. Gillet, M. Bes, G. Lina, F. Vandenesch, D. Floret, J. Etienne, and H. Richet. 2002. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin. Infect. Dis. 35:819-824. [DOI] [PubMed] [Google Scholar]
- 13.Fiorentino, D. F., A. Zlotnik, T. R. Mosmann, M. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815-3822. [PubMed] [Google Scholar]
- 14.Fournier, B., and D. J. Philpott. 2005. Recognition of Staphylococcus aureus by the innate immune system. Clin. Microbiol. Rev. 18:521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fournier, N., L. Chalus, I. Durand, E. Garcia, J. J. Pin, T. Churakova, S. Patel, C. Zlot, D. Gorman, S. Zurawski, J. Abrams, E. E. Bates, and P. Garrone. 2000. FDF03, a novel inhibitory receptor of the immunoglobulin superfamily, is expressed by human dendritic and myeloid cells. J. Immunol. 165:1197-1209. [DOI] [PubMed] [Google Scholar]
- 16.Gibot, S., C. Alauzet, F. Massin, N. Sennoune, G. C. Faure, M. C. Bene, A. Lozniewski, P. E. Bollaert, and B. Levy. 2006. Modulation of the triggering receptor expressed on myeloid cells-1 pathway during pneumonia in rats. J. Infect. Dis. 194:975-981. [DOI] [PubMed] [Google Scholar]
- 17.Gibot, S., M. N. Kolopp-Sarda, M. C. Bene, P. E. Bollaert, A. Lozniewski, F. Mory, B. Levy, and G. C. Faure. 2004. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J. Exp. Med. 200:1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillet, Y., B. Issartel, P. Vanhems, J. C. Fournet, G. Lina, M. Bes, F. Vandenesch, Y. Piemont, N. Brousse, D. Floret, and J. Etienne. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753-759. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Juarrero, M., T. S. Shim, A. Kipnis, A. P. Junqueira-Kipnis, and I. M. Orme. 2003. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J. Immunol. 171:3128-3135. [DOI] [PubMed] [Google Scholar]
- 20.Goodman, R. B., J. Pugin, J. S. Lee, and M. A. Matthay. 2003. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 14:523-535. [DOI] [PubMed] [Google Scholar]
- 21.Hamerman, J. A., N. K. Tchao, C. A. Lowell, and L. L. Lanier. 2005. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat. Immunol. 6:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanier, L. L. 2001. Face off—the interplay between activating and inhibitory immune receptors. Curr. Opin. Immunol. 13:326-331. [DOI] [PubMed] [Google Scholar]
- 23.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 24.Marks, J. D., C. B. Marks, J. M. Luce, A. B. Montgomery, J. Turner, C. A. Metz, and J. F. Murray. 1990. Plasma tumor necrosis factor in patients with septic shock. Mortality rate, incidence of adult respiratory distress syndrome, and effects of methylprednisolone administration. Am. Rev. Respir. Dis. 141:94-97. [DOI] [PubMed] [Google Scholar]
- 25.Marsh, C. B., H. A. Pope, and M. D. Wewers. 1994. Fc gamma receptor cross-linking down-regulates IL-1 receptor antagonist and induces IL-1 beta in mononuclear phagocytes stimulated with endotoxin or Staphylococcus aureus. J. Immunol. 152:4604-4611. [PubMed] [Google Scholar]
- 26.McLoughlin, R. M., J. C. Lee, D. L. Kasper, and A. O. Tzianabos. 2008. IFN-gamma regulated chemokine production determines the outcome of Staphylococcus aureus infection. J. Immunol. 181:1323-1332. [DOI] [PubMed] [Google Scholar]
- 27.Mousseau, D. D., D. Banville, D. L'Abbe, P. Bouchard, and S. H. Shen. 2000. PILRalpha, a novel immunoreceptor tyrosine-based inhibitory motif-bearing protein, recruits SHP-1 upon tyrosine phosphorylation and is paired with the truncated counterpart PILRbeta. J. Biol. Chem. 275:4467-4474. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama, M., D. M. Underhill, T. W. Petersen, B. Li, T. Kitamura, T. Takai, and A. Aderem. 2007. Paired Ig-like receptors bind to bacteria and shape TLR-mediated cytokine production. J. Immunol. 178:4250-4259. [DOI] [PubMed] [Google Scholar]
- 29.Nathan, C. 2006. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6:173-182. [DOI] [PubMed] [Google Scholar]
- 30.Nizet, V. 2007. Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J. Allergy Clin. Immunol. 120:13-22. [DOI] [PubMed] [Google Scholar]
- 31.Ravetch, J. V., and L. L. Lanier. 2000. Immune inhibitory receptors. Science 290:84-89. [DOI] [PubMed] [Google Scholar]
- 32.Richeldi, L., M. Mariani, M. Losi, F. Maselli, L. Corbetta, C. Buonsanti, M. Colonna, F. Sinigaglia, P. Panina-Bordignon, and L. M. Fabbri. 2004. Triggering receptor expressed on myeloid cells: role in the diagnosis of lung infections. Eur. Respir. J. 24:247-250. [DOI] [PubMed] [Google Scholar]
- 33.Satoh, T., J. Arii, T. Suenaga, J. Wang, A. Kogure, J. Uehori, N. Arase, I. Shiratori, S. Tanaka, Y. Kawaguchi, P. G. Spear, L. L. Lanier, and H. Arase. 2008. PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell 132:935-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiratori, I., K. Ogasawara, T. Saito, L. L. Lanier, and H. Arase. 2004. Activation of natural killer cells and dendritic cells upon recognition of a novel CD99-like ligand by paired immunoglobulin-like type 2 receptor. J. Exp. Med. 199:525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Small, C. L., S. McCormick, N. Gill, K. Kugathasan, M. Santosuosso, N. Donaldson, D. E. Heinrichs, A. Ashkar, and Z. Xing. 2008. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J. Immunol. 180:5558-5568. [DOI] [PubMed] [Google Scholar]
- 36.Torii, I., S. Oka, M. Hotomi, W. H. Benjamin, Jr., T. Takai, J. F. Kearney, D. E. Briles, and H. Kubagawa. 2008. PIR-B-deficient mice are susceptible to Salmonella infection. J. Immunol. 181:4229-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turnbull, I. R., J. E. McDunn, T. Takai, R. R. Townsend, J. P. Cobb, and M. Colonna. 2005. DAP12 (KARAP) amplifies inflammation and increases mortality from endotoxemia and septic peritonitis. J. Exp. Med. 202:363-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Underhill, D. M., and B. Gantner. 2004. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect. 6:1368-1373. [DOI] [PubMed] [Google Scholar]
- 39.Wang, J., I. Shiratori, T. Satoh, L. L. Lanier, and H. Arase. 2008. An essential role of sialylated O-linked sugar chains in the recognition of mouse CD99 by paired Ig-like type 2 receptor (PILR). J. Immunol. 180:1686-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright, G. J., H. Cherwinski, M. Foster-Cuevas, G. Brooke, M. J. Puklavec, M. Bigler, Y. Song, M. Jenmalm, D. Gorman, T. McClanahan, M. R. Liu, M. H. Brown, J. D. Sedgwick, J. H. Phillips, and A. N. Barclay. 2003. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol. 171:3034-3046. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, S., H. Cherwinski, J. D. Sedgwick, and J. H. Phillips. 2004. Molecular mechanisms of CD200 inhibition of mast cell activation. J. Immunol. 173:6786-6793. [DOI] [PubMed] [Google Scholar]
- 42.Zhao, Y. X., I. M. Nilsson, and A. Tarkowski. 1998. The dual role of interferon-gamma in experimental Staphylococcus aureus septicaemia versus arthritis. Immunology 93:80-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.