Abstract
Toll-like receptors (TLRs) present in innate immune cells recognize pathogen molecular patterns and influence immunity to control the host-parasite interaction. The objective of this study was to characterize the involvement of TLR4 in the innate and adaptive immunity to Paracoccidioides brasiliensis, the most important primary fungal pathogen of Latin America. We compared the responses of C3H/HeJ mice, which are naturally defective in TLR4 signaling, with those of C3H/HePas mice, which express functional receptors, after in vitro and in vivo infection with P. brasiliensis. Unexpectedly, we verified that TLR4-defective macrophages infected in vitro with P. brasiliensis presented decreased fungal loads associated with impaired synthesis of nitric oxide, interleukin-12 (IL-12), and macrophage chemotactic protein 1 (MCP-1). After intratracheal infection with 1 million yeasts, TLR4-defective mice developed reduced fungal burdens and decreased levels of pulmonary nitric oxide, proinflammatory cytokines, and antibodies. TLR4-competent mice produced elevated levels of IL-12 and tumor necrosis factor alpha (TNF-α), besides cytokines of the Th17 pattern, indicating a proinflammatory role for TLR4 signaling. The more severe infection of TLR4-normal mice resulted in increased influx of activated macrophages and T cells to the lungs and progressive control of fungal burdens but impaired expansion of regulatory T cells (Treg cells). In contrast, TLR4-defective mice were not able to clear their diminished fungal burdens totally, a defect associated with deficient activation of T-cell immunity and enhanced development of Treg cells. These divergent patterns of immunity, however, resulted in equivalent mortality rates, indicating that control of elevated fungal growth mediated by vigorous inflammatory reactions is as deleterious to the hosts as low fungal loads inefficiently controlled by limited inflammatory reactions.
Pathogen recognition receptors (PRRs) are a group of receptors present in the membrane and cytoplasm of innate immunity cells that recognize the presence of invading microbes by interacting with conserved pathogen structures, the so called “pathogen-associated molecular patterns” (PAMPs). This initial event of innate immunity is crucial for the control of pathogen growth and the subsequent activation of adaptive immunity. Toll like receptors (TLRs) constitute a major family of pattern recognition molecules and, like other PRRs, are able to respond to different structural homologies conserved in many microorganisms (2, 62). Activation of the TLRs is crucial for many aspects of microbe elimination, including microbial killing, recruitment of phagocytes to the site of infection, and activation of dendritic cells (DCs) (52). Early TLR activation results in the production of several inflammatory mediators, and the final balance among pro- and anti-inflammatory components regulates the type of adaptive immune response. Recent findings have shown that direct recognition of PAMPs by DCs is critical for priming appropriate T-cell responses, resulting in T helper 1 (Th1), Th2, or Th17 immunity (25, 31, 33, 60).
TLR4 is the key receptor that recognizes bacterial lipopolysaccharides (LPS), whereas TLR2 is involved in the interaction with bacterial peptidoglycans and lipoproteins (66). As reported for other microorganisms, TLRs have been shown to be involved in host defense against different fungal pathogens. In vivo and in vitro studies have demonstrated that Cryptococcus neoformans (7, 67), Candida albicans (43, 45), and Aspergillus fumigatus (24, 41) may signal through members of the TLR family, mainly TLR2 and TLR4. Different components of a certain pathogen can be used to stimulate the immune system. Thus, C. albicans phospholipomannan is sensed by TLR2 (34), while O-linked mannans are recognized by TLR4 (44). The contribution of individual TLRs to the immune response against pathogenic fungi depends on several factors, such as the fungal morphotype, fungal species, and route of infection. Activation signals mediated by innate immunity receptors, however, are not always beneficial to the host, and TLR activation can be used by pathogenic fungi to promote more-severe infections (6, 53).
Paracoccidioidomycosis (PCM) is a systemic granulomatous disease caused by the dimorphic fungus Paracoccidioides brasiliensis and constitutes the most prevalent deep mycosis in Latin America (28). The alveolar macrophages are the first host cells that interact with P. brasiliensis cells, and their activation is fundamental to the control of fungal growth. The molecular mechanisms controlling the initial steps of the interaction between P. brasiliensis and phagocytes are not well understood. It is known, however, that normal macrophages are permissive to P. brasiliensis growth, while cytokine-activated macrophages are able to restrain P. brasiliensis multiplication (12). Complement receptor 3 (CR3) and mannose receptor have been shown to play important roles in the initial interaction between P. brasiliensis cells and mouse peritoneal macrophages (14, 32, 50). Interestingly, recent work from our laboratory demonstrated that alveolar macrophages from susceptible mice (B10.A) are easily activated by P. brasiliensis infection and show efficient fungal killing associated with nitric oxide production, while pulmonary macrophages from resistant mice (A/Sn) are poorly activated and present inefficient killing activity associated with increased levels of transforming growth factor β (TGF-β) (49). Despite their inefficient innate immunity, A/Sn mice develop a balanced Th1/Th2 immune response that controls fungal growth without intense tissue pathology.
In previous work, our group demonstrated the influence of TLR2 on pulmonary PCM (15, 38). Using TLR2-normal and TLR2-deficient mice, we were able to show that the presence of TLR2 increases the severity of in vitro and in vivo P. brasiliensis infections. In addition, TLR2 deficiency results in increased Th17 immunity associated with diminished expansion of regulatory T cells (Treg cells) and increased lung pathology due to unrestrained inflammatory reactions (38). Characterizing the behavior of dendritic cells in murine PCM, Ferreira et al. observed increased expression of TLR2 by dendritic cells of susceptible, but not resistant, mice (26). Moreover, it has been suggested that TLR2, TLR-4, and dectin-1 are involved in the recognition and internalization of P. brasiliensis by human monocytes and neutrophils, indicating important roles for these pathogen receptors in the immune response against the fungus (10).
We decided to investigate the role of TLR4 in murine PCM by using in vitro and in vivo models of infection. Using TLR-competent C3H/HePas mice and C3H/HeJ mice, which possess a missense mutation in the TLR4 gene, we were able to demonstrate that both in vitro and in vivo, TLR4 signaling increases the severity of infection in association with increased activation of innate and adaptive immunity but decreased expansion of Treg cells. This pattern of immunity, however, was not beneficial to the hosts, due to the increased lung injury mediated by inefficient control of inflammatory reactions by Treg cells.
MATERIALS AND METHODS
Fungus.
P. brasiliensis Pb18, a highly virulent isolate, was used throughout this investigation (36). Pb18 yeast cells were maintained by weekly subcultivation in semisolid culture medium. Washed yeast cells were adjusted to 20 × 106/ml (for in vivo infection) and 4 × 104/ml (for in vitro infection) based on hemocytometer counts. Viability was determined with Janus Green B vital dye (Merck) and was always higher than 85%.
Mice and i.t. infection.
C3H/HeJ and C3H/HePas mice were obtained from our Isogenic Breeding Unit (Departmento de Imunologia, Instituto de Ciências Biomédicas, Universidade de São Paulo, São Paulo, Brazil) and were used at 8 to 12 weeks of age. The C3H/HeJ strain has a point mutation in the TLR4 gene, and the C3H/HePas strain has a functional TLR4 gene. In selected experiments, C57BL/6 mice that are genetically deficient in TLR4 (TLR4 knockout [KO]) and their normal C57BL/6 counterparts were used. Mice were anesthetized and subjected to intratracheal (i.t.) P. brasiliensis infection as previously described (18). Briefly, after intraperitoneal anesthesia, the animals were i.t. infected with 106 P. brasiliensis yeast cells, contained in 50 μl of phosphate-buffered saline (PBS). Mice were studied at 96 h, 2 weeks, and 11 weeks postinfection. The experiments were approved by the ethics committee on animal experiments of our institution.
Phagocytic and fungicidal assays.
Thioglycolate-induced peritoneal macrophages were either isolated by adherence (2 h at 37°C under 5% CO2) to plastic-bottom tissue culture plates (1 × 106 cells/well in 24-well plates for fungicidal assays) or plated onto 13-mm-diameter round glass coverslips (1 × 106 cells/well in 24-well plates) for phagocytosis. Macrophages were washed to remove nonadherent cells and were cultivated overnight with fresh complete medium (Dulbecco's modified Eagle's medium [DMEM; Sigma], containing 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin) in the presence or absence of recombinant gamma interferon (IFN-γ; 20 ng/ml in culture medium; BD Pharmingen). Nonadherent cells were counted in order to evaluate the number of remaining adherent cells used in phagocytic and killing assays. For phagocytic assays, macrophage cultures were infected with P. brasiliensis yeasts at a macrophage/yeast ratio of 25:1. This ratio was previously determined and was shown not to be deleterious to macrophage cultures and to be adequate for phagocytosis and killing assays (18, 49). The cells were cocultivated for 4 h at 37°C under 5% CO2 to allow adhesion and ingestion of fungi. Cells were washed twice with PBS to remove any noningested or nonadhered yeasts, and samples were processed for microscopy. Cells were fixed with methanol and stained with Giemsa stain (Sigma). Experimental conditions were performed in triplicate, and the number of phagocytosed or adhered yeasts per 1,000 macrophages was evaluated on at least three separate slides. For fungicidal assays, IFN-γ-primed and unprimed macrophage cultures were infected with P. brasiliensis yeasts as described above. After 48 h of culture at 37°C in a CO2 incubator, plates were centrifuged (400 × g, 10 min, 4°C), and supernatants were stored at −70°C and were further analyzed for the presence of nitrite and cytokines. The wells were washed with distilled water to lyse macrophages, and suspensions were collected in individual tubes. One hundred microliters of cell homogenates was assayed for the presence of viable yeasts. All assays were done with five wells per condition in more than three independent experiments.
CFU assay and histological and morphometric analyses.
The numbers of viable microorganisms in cell cultures and infected organs (lungs, liver, and spleen) from experimental and control mice were determined by counting the number of CFU. Animals from each group were sacrificed, and the enumeration of viable organisms was done as previously described (59). The numbers (log10) of viable P. brasiliensis organisms per gram of tissue (in vivo) or per milliliter of cell homogenate (in vitro) are expressed as means ± standard errors. For histological examinations, the left lung of the infected mouse was removed, fixed in 10% formalin, and embedded in paraffin. Five-micrometer-thick sections were stained with hematoxylin and eosin (H&E) for analysis of the lesions and were silver stained for fungal evaluation. Pathological changes were analyzed based on the size, morphology, and cell composition of granulomatous lesions, the presence of fungi, and the intensity of the inflammatory infiltrates. Morphometric analysis was performed using a Nikon DXM 1200c digital camera (magnification, ×10), and Nikon NIS Elements AR 2.30 software. The areas of lesions were measured (in square micrometers) in 10 microscopic fields per slide for 10 animals per group. Results were expressed as the mean total area of lesions for each animal ± the standard error.
Measurement of cytokines and NO.
Supernatants from lung homogenates or cell cultures were separated and stored at −70°C. The levels of interleukin-2 (IL-2), IL-4, IL-5, IL-23, IL-17, IL-12, IL-10, tumor necrosis factor alpha (TNF-α), IFN-γ, and the chemokine MCP-1 (monocyte chemoattractant protein 1) were measured by a capture enzyme-linked immunosorbent assay (ELISA) with antibody pairs purchased from BD Pharmingen. The ELISA procedure was performed according to the manufacturer's protocol, and absorbances were measured with a Versa Max microplate reader (Molecular Devices). NO production was quantified by the accumulation of nitrite in the supernatants from in vitro and in vivo protocols by a standard Griess reaction. All determinations were performed in duplicate, and results were expressed as micromolar concentrations of NO.
Measurement of serum P. brasiliensis-specific isotypes.
Levels of specific isotypes (total IgG, IgM, IgA, IgG1, IgG2a, IgG2b, and IgG3) were measured by a previously described ELISA (18) employing a cell-free antigen prepared by using a pool of different P. brasiliensis isolates (Pb339, Pb265, and Pb18). The average of the optical densities obtained with sera from control mice (PBS inoculated), diluted 1:20, was considered the cutoff for each respective isotype. Optical densities for each dilution of experimental sera were compared to control values. The titer for each sample was expressed as the reciprocal of the highest dilution that presented an absorbance higher than the cutoff.
Assessment of leukocyte subpopulations in lung inflammatory exudates.
After 2 and 11 weeks of infection, lungs from each mouse were digested enzymatically for 30 min with collagenase (1 mg/ml) and DNase (30 μg/ml) in culture medium (Sigma). Lung cell suspensions were centrifuged in the presence of 20% Percoll (Sigma) to separate leukocytes from cell debris. Total lung leukocyte numbers were assessed in the presence of trypan blue using a hemocytometer; viability was >85%. For differential leukocyte counts, samples of lung cell suspensions were cytospun (Shandon Cytospin) onto glass slides and stained with the Diff-Quik blood stain (Baxter Scientific). A total of 200 to 400 cells were counted from each sample. For flow cytofluorometry, lung leukocytes were resuspended at 106/ml in staining buffer (PBS with 0.1% NaN3 and 1% fetal calf serum). Fc receptors were blocked by unlabeled anti-CD16/32 antibodies (BD Biosciences), and cells were stained for 20 min at 4°C. Phycoerythrin (PE)-labeled monoclonal antibodies against CD40, CD86, CD11b, CD25, and CD69 and fluorescein isothiocyanate (FITC)-labeled monoclonal antibodies against IAk, CD80, CD4, and CD8 (BD Biosciences) were used. Treg cells were characterized by intracellular staining for Foxp3, using the Treg staining kit of BD Bioscience. Surface staining of CD25+ and intracellular FoxP3 expression were backgated on the CD4+ T-cell population. Cells were fixed with 1% paraformaldehyde (Sigma) and were stored in the dark at 4°C until analysis in a flow cytometer. The acquisition and analysis gates were restricted to the lymphocytes or macrophages. The expression of leukocyte markers on the cell surface and the intracellular expression of FoxP3 in lung-infiltrating leukocytes were analyzed in a FACScalibur flow cytometer (BD Pharmingen) using FlowJo software (Tristar).
Limulus amoebocyte lysate activity assay.
Solutions used for the preparation of yeast cell suspensions and the cultivation of macrophages were tested for the presence of LPS using the Limulus amoebocyte lysate chromogenic assay (E-TOXATE; Sigma) and always showed LPS levels lower than 0.015 endotoxin unit (EU)/ml.
Statistical analyses.
Data were analyzed by Student's t test or two-way analysis of variance depending on the number of experimental groups. P values under 0.05 were considered significant.
RESULTS
TLR4 deficiency leads to less-severe fungal infection of macrophages, associated with decreased synthesis of NO, IL-12, and MCP-1.
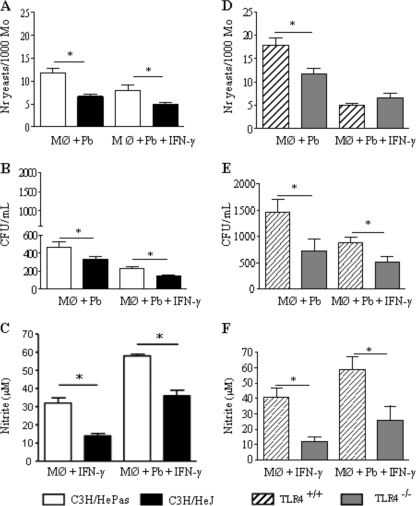
Before performing fungicidal studies, we asked whether the initial interactions between P. brasiliensis yeasts and peritoneal macrophages from C3H/HeJ and C3H/HePas mice were equivalent. Macrophage cultures (1 × 106 cells/well), performed in round glass coverslips, were preactivated with IFN-γ (20 ng/ml) or not and were infected with 4 × 104 viable yeasts (fungus/macrophage ratio, 1:25). After 4 h of incubation, supernatants were aspirated, the monolayers were gently washed with PBS, and the cells were stained with Giemsa stain. An average of 1,000 macrophages were counted, and the number of ingested and/or adherent yeasts was determined. As shown in Fig. 1A, TLR4-deficient macrophages presented a lower number of associated (ingested/adhered) yeasts than normal macrophages.
FIG. 1.
TLR4 deficiency leads to less-severe fungal infection of macrophages (Mφ) associated with decreased synthesis of NO. The phagocytic and fungicidal abilities of macrophages from mice with defective TLR4 signaling (C3H/HeJ) or defective TLR4 expression (C57BL/6 TLR4−/−) were compared with those of their TLR4-normal controls (C3H/HePas and C57BL/6 TLR4+/+, respectively). (A and D) For phagocytic assays, IFN-γ-primed (20 ng/ml, overnight) and unprimed macrophage cultures were infected with P. brasiliensis yeasts at a macrophage/yeast ratio of 25:1. The cells were cocultivated for 4 h at 37°C under 5% CO2 to allow adhesion and ingestion of fungi. Cells were washed, fixed, and stained with Giemsa stain; an average of 1,000 macrophages were analyzed, and the number of macrophages with adhered or ingested yeasts was determined. (B and E) For fungicidal assays, IFN-γ-primed and unprimed macrophages were infected with yeast cells as described for panel A. After 48 h at 37°C under 5% CO2, plates were centrifuged, and supernatants were used to determine levels of nitrite and cytokines. The monolayers were washed with distilled water to lyse macrophages, and 100 μl of cell homogenates was assayed for the presence of viable yeasts by a CFU assay. (C and F) Supernatants from fungicidal assays were used to determine the levels of nitrites using the Griess reagent. Data are means ± standard errors of the means for quintuplicate samples from one experiment representative of three independent determinations. *, P < 0.05.
Macrophages were cultivated with P. brasiliensis yeasts for an additional 48 h. Supernatants were removed and assayed for the presence of nitric oxide and cytokines, and cell homogenates were plated for CFU determinations. As can be seen in Fig. 1B, TLR4 signaling resulted in increased recovery of viable yeasts from untreated and IFN-γ-primed macrophages. In addition, higher levels of NO were produced by IFN-γ-activated macrophages from TLR4-normal mice than by those of TLR4-defective mice (Fig. 1C). P. brasiliensis infection of unprimed macrophages did not induce NO production, which was detected only in IFN-γ-treated macrophages.
We further asked if TLR4 expression or signaling was involved in the decreased fungal loads observed with TLR4-defective macrophages. Since C3H/HeJ mice have a defect in the intracellular signaling domain but a normal extracellular moiety, we comparatively assessed the behavior of macrophages genetically deficient for TLR4 expression. Thus, TLR4−/− macrophages from C57BL/6 mice were infected and compared with their TLR4+/+ counterparts. TLR4-deleted macrophages showed decreased phagocytic ability (Fig. 1D), and decreased numbers of viable yeasts were recovered from killing assays (Fig. 1E). As with C3H/HeJ macrophages, TLR4−/− cells produced diminished levels of NO. This result suggested that TLR4 signaling influenced the endocytic and killing ability of P. brasiliensis-infected macrophages.
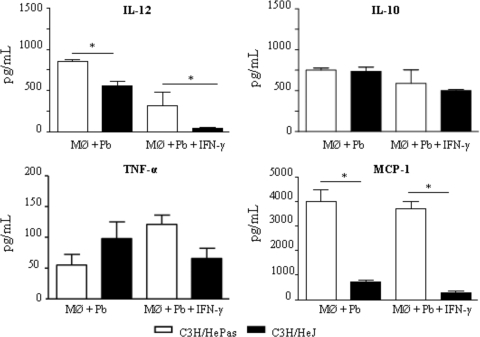
To further characterize the role of TLR4 in P. brasiliensis infection of C3H macrophages, culture supernatants obtained from killing assays were tested for the presence of some macrophage-activating cytokines (IL-12 and TNF-α), a deactivating cytokine (IL-10), and a chemokine involved in mononuclear cell chemotaxis, MCP-1. As depicted in Fig. 2, IFN-γ-treated and untreated macrophages from TLR4-defective mice secreted lower levels of IL-12 and MCP-1 than those of TLR4-normal mice. IL-10 and TNF-α, however, appeared at similar levels.
FIG. 2.
Macrophages (Mφ) from TLR4-defective mice secrete diminished levels of IL-12 and MCP-1. IFN-γ-treated (20.00 ng/ml) or untreated macrophages of TLR4-competent (C3H/HePas) and TLR4-defective (C3H/HeJ) mice were challenged with viable P. brasiliensis yeasts (fungus/macrophage ratio, 1:25) for 4 h, washed, and further cultivated for 48 h at 37°C under 5% CO2. Plates were then centrifuged, and supernatants were used for cytokine measurements by ELISA. Data are means ± standard errors of the means for triplicate samples from one experiment representative of three independent determinations. *, P < 0.05.
In vivo, the absence of TLR4 signaling induces lower fungal loads and diminished NO production.
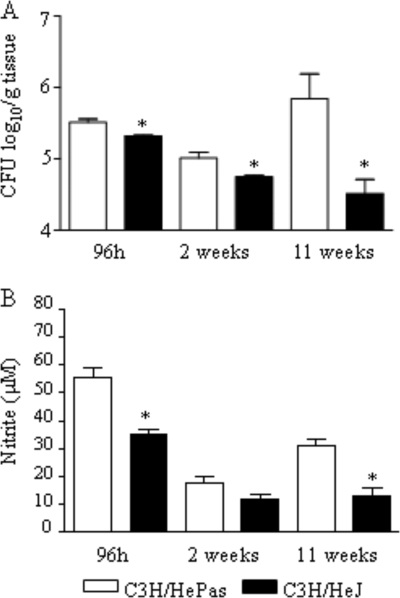
To study the in vivo role of TLR4, groups of C3H/HeJ and C3H/HePas mice (six to eight animals per group) were infected i.t. with 1 million P. brasiliensis yeast cells and were evaluated in the course of infection. Diminished fungal burdens were detected in the lung tissues of mice lacking functional TLR4 at all postinfection times (96 h and 2 and 11 weeks) assayed, as can be seen in Fig. 3A. In both strains, no fungal growth was observed in liver and spleen tissues (data not shown). Decreased NO levels were detected at 96 h and at week 11 after infection, although by week 2 similar levels were observed (Fig. 3B).
FIG. 3.
In vivo, TLR4 dysfunction leads to less-severe fungal infection. (A) Recovery of CFU from the lungs of TLR4-defective and TLR4-normal control mice infected i.t. with 1 × 106 yeasts. (B) Lung homogenates were used to determine the levels of nitrites using the Griess reagent. Data are means ± standard errors of the means for groups of six to eight mice at 96 h, 2 weeks, and 11 weeks after infection. The results are representative of three independent experiments. *, P < 0.05.
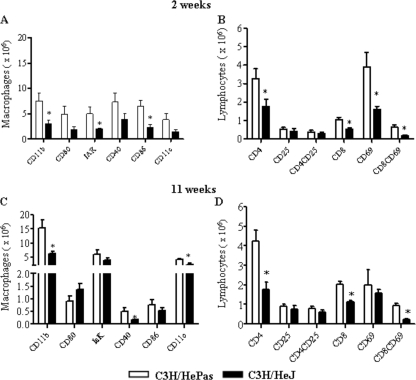
Defective TLR4 signaling determines decreased inflammatory reactions characterized by lower numbers of activated macrophages and T cells.
We further analyzed the phenotype and activation of lung inflammatory cells at weeks 2 and 11 of P. brasiliensis infection (Fig. 4). To determine the activation profile of pulmonary macrophages, the expression of CD11b, major histocompatibility complex (MHC) class II (IAK), CD80, CD86, and CD40 antigens was assessed by flow cytometry. As can be seen in Fig. 4A, all activation markers were expressed at lower levels by deficient macrophages, although significant differences were noticed with CD11b, the MHC class II antigen, and CD86. To determine the lymphocyte influx and the activation profile of CD4+ and CD8+ T cells in the lungs of P. brasiliensis-infected mice, we determined the expression of CD69 and CD25 by T cells freshly isolated from the lungs. The marker CD69 is a very early activation antigen (70), as well as CD25, the α-chain of the interleukin-2 receptor (56), which is rapidly upregulated on activated T cells. Compared with the control group, at week 2 of infection, TLR4-deficient mice presented significantly reduced recruitment of CD4+ and CD8+ T cells to the lungs, and the latter subpopulation also showed decreased expression of CD69 (Fig. 4B). Studies at week 11 postinfection confirmed those of week 2. TLR4-normal mice presented increased numbers of CD11b+, CD11c+, and CD40+ macrophages (Fig. 4C), besides augmented numbers of CD4+, CD8+, and CD8+ CD69+ T lymphocytes, in the inflammatory exudates of lungs (Fig. 4D).
FIG. 4.
Increased numbers of activated macrophages, CD4+ T lymphocytes, and CD8+ T lymphocytes were detected in the lungs of TLR4-competent mice at weeks 2 and 11 of infection. Leukocyte subsets in the lung-infiltrating leukocytes (LIL) from TLR4-defective and TLR4-normal mice inoculated i.t. with 1 million P. brasiliensis yeast cells were characterized by flow cytometry. Lungs of C3H/HePas and C3H/HeJ mice (six to eight mice per group) were excised, washed in PBS, minced, and digested enzymatically. At weeks 2 and 11 after infection, lung cell suspensions were obtained and stained as described in Materials and Methods. The acquisition and analysis gates were restricted to lymphocytes or macrophages. The data are mean results from six to eight mice per group ± standard errors of the means and are representative of two independent experiments. *, P < 0.05.
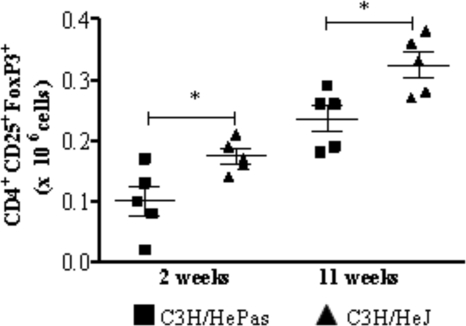
The limited inflammatory reaction of TLR4-deficient mice was associated with increased numbers of Treg cells.
Because Treg cells control the expansion of effector T cells, and because the number and function of these cells have been shown to be influenced by TLR4 activation (33), we investigated the presence of CD4+ CD25+ FoxP3+ T cells in the lung cell infiltrates of TLR4-defective and normal mice (Fig. 5). At both postinfection periods studied, TLR4-defective mice showed increased numbers of CD4+ CD25+ FoxP3+ Treg cells in their lungs (Fig. 5).
FIG. 5.
TLR4-defective mice presented increased numbers of Treg cells in the lungs. FoxP3 expression by lung lymphocytes from TLR4-defective (C3H/HeJ) and normal (C3H/HePas) mice inoculated i.t. with 1 million P. brasiliensis yeast cells was determined by flow cytometric analysis. Lungs of six to eight mice per group were excised, washed in PBS, minced, and digested enzymatically; at 2 and 11 weeks after infection, cell suspensions were obtained and stained as described in Materials and Methods. The expression of leukocyte markers on the cell surface, as well as intracellular FoxP3 expression in lung-infiltrating leukocytes, was analyzed by flow cytometry. Surface staining of CD25+ cells and intracellular FoxP3 expression were backgated on the CD4+ T-cell population. The data are numbers of CD4+ CD25+ FoxP3+ cells for individual mice (five or six per group) and are representative of two independent experiments.
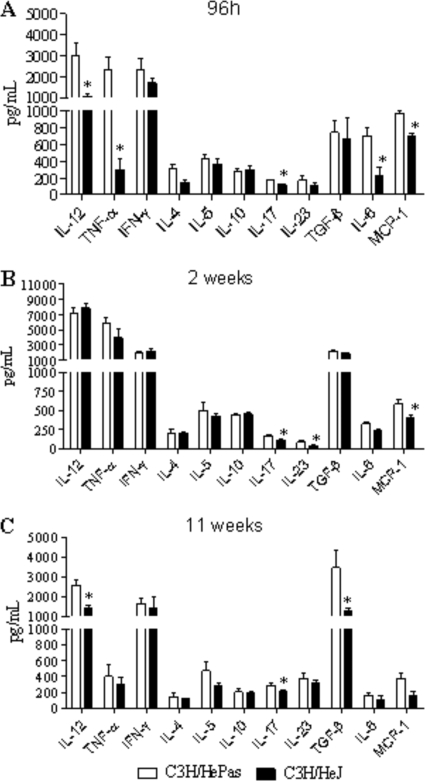
TLR4 dysfunction leads to diminished production of proinflammatory and Th17 cytokines.
Levels of cytokines associated with Th1, Th2, and Th17 cells were assessed in lung homogenates obtained at different periods of infection. The production of type 1 (IL-12, TNF-α, and IFN-γ) and type 2 (IL-4, IL-5, and IL-10) cytokines, as well as that of the Th17-associated (IL-17, IL-6, TGF-β, and IL-23) cytokines, was studied 96 h, 2 weeks, and 11 weeks after infection. Mice lacking the ability to signal through TLR4 showed early (96 h) deficient production of IL-12, TNF-α, IL-17, and IL-6 (Fig. 6A). By week 2, IL-17 and IL-23 appeared at lower levels in the lungs of TLR4-defective mice (Fig. 6B). This decreased production of cytokines was confirmed at week 11, when these mice presented decreased amounts of IL-12, IL-17, and TGF-β (Fig. 6C). Interestingly, IL-17 and MCP-1 were constantly produced at higher levels by TLR4-normal mice (Fig. 6).
FIG. 6.
Lung homogenates of TLR4-competent mice presented increased levels of proinflammatory cytokines. At 96 h, 2 weeks, and 11 weeks after i.t. infection with 106 P. brasiliensis yeast cells, lungs from TLR4-defective and TLR4-competent mice were collected and disrupted in 5.0 ml of PBS, and supernatants were analyzed for cytokine contents by capture ELISAs. Data are mean cytokine levels ± standard errors of the means (six to eight animals per group). The results are representative of three independent experiments. *, P < 0.05.
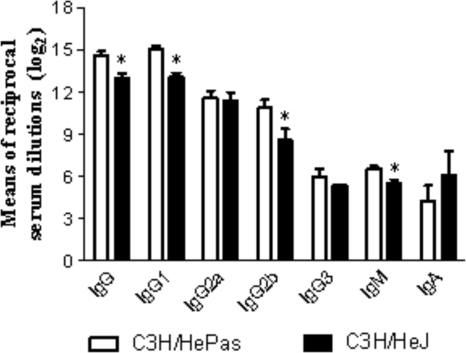
TLR4-defective mice produced lower levels of P. brasiliensis-specific antibodies.
Although in some fungal infections specific antibodies may have a protective role (19), in human and experimental PCM antibody production is a marker of disease severity (13, 17). Since expression of TLRs has been shown to influence B-cell activation (30, 40), we decided to characterize the humoral immunity of TLR4-deficient and normal mice at week 11 postinfection. The less-severe infection of TLR4-deficient mice was associated with decreased production of IgG1-, IgG2b-, and IgM-specific isotypes (Fig. 7).
FIG. 7.
TLR4 deficiency determines impaired humoral immunity. Levels of P. brasiliensis-specific antibodies in the sera of TLR4-defective (C3H/HeJ) and normal (C3H/HePas) mice at week 11 after i.t. infection with 1 × 106 yeast cells are shown. Sera were assayed for total IgG, IgM, IgA, IgG1, IgG2a, IgG2b, and IgG3 by using an isotype-specific ELISA as detailed in Materials and Methods. Data are mean serum titers (log2) ± standard errors (six to eight mice per group). *, P < 0.05 for comparison with controls.
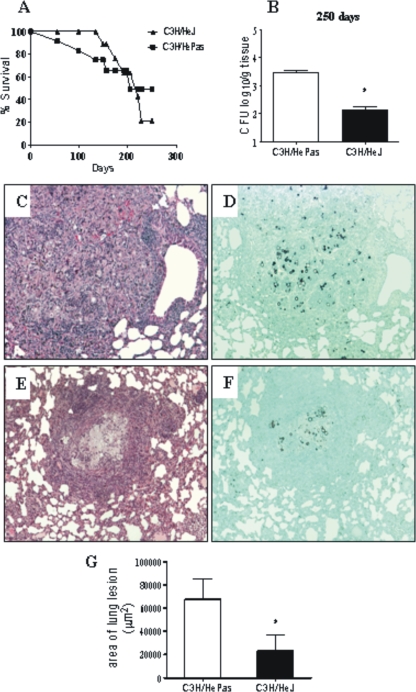
The absence of TLR4 signaling does not increase mortality rates but results in less-severe inflammatory reactions in the lungs.
To verify the influence of TLR4 deficiency in the disease outcome, the mortality of P. brasiliensis-infected C3H/HeJ and C3H/HePas mice was registered daily for a 250-day period, and the median survival time was calculated for each strain. Surprisingly, despite the significant differences in fungal burdens, no differences between mortality data (P = 0.9) were detected (Fig. 8A). The mean survival times of C3H/HeJ and C3H/HePas mice were 221 and 203 days, respectively. At day 250 (35 weeks postinfection), the remaining survivors were sacrificed, and the numbers of CFU in organs were determined. Compared with those at other postinfection periods assayed, lower fungal burdens were detected in both mouse strains, but TLR4-normal mice still had higher pulmonary fungal loads than TLR4-defective mice (Fig. 8B). To further characterize the severity of P. brasiliensis infection, histopathological examination of lungs was conducted at week 11 of infection. As can be seen in Fig. 8C and E, similar patterns of inflammatory reactions were detected in the lungs of the two mouse strains, but better-preserved lung tissue was seen in TLR4-defective mice. The pulmonary tissue presented several confluent or isolated granulomas of various sizes containing yeast cells with preserved morphology (Fig. 8D and F). Large aggregates of macrophages, rare epithelioid cells, and a poor mantle of lymphocytes made up the granulomas, which were usually in the interlobular septa. Plasma cells, eosinophils, and multinucleated cells were scarcely seen. The total areas of lesions were quantified in histological sections, and the results are shown in Fig. 8G. At week 11, the areas of lesions of TLR4-normal mice were significantly larger than those presented by TLR4-deficient mice. Thus, the higher influx of inflammatory cells observed in the lungs of TLR4-normal mice was concomitant with increased pathology of lung tissue.
FIG. 8.
Compared with TLR4-normal mice, TLR4-defective mice present decreased fungal loads and lung pathology but equivalent survival times. (A) Survival times of TLR4-defective and control mice after i.t. infection with 1 × 106 P. brasiliensis yeast cells were determined for a period of 250 days. No significant differences were seen between the median survival times of the two mouse strains. The results are representative of two independent experiments. (B) By 250 days after infection, survivor mice (three to six per group) were sacrificed, and CFU counts in tissues were determined. No viable yeasts were recovered from livers and spleens. (C to F) Photomicrographs of pulmonary lesions of TLR4-competent C3H/HePas mice (C and D) and TLR4-defective C3H/HeJ mice (E and F) at week 11 of infection with 1 million P. brasiliensis yeasts. At this period, the morphology of lesions was equivalent in the two mouse strains; fungal cells were surrounded by confluent or isolated granulomas scattered through the lung tissue. Lesions were stained with H&E (C and E) or with Grocott stain (D and F); magnification, ×100. (G) Total areas of lesions in the lungs of mice (n = 10) at week 11 after infection. *, P < 0.05.
DISCUSSION
The innate immune mechanisms of hosts infected with P. brasiliensis are poorly defined, but macrophages and their pathogen recognition receptors are thought to play a crucial role in the initial interaction of this fungus with the immune system (16, 26, 29, 49, 50). Despite several studies with diverse fungal pathogens (16, 43), the role played by TLRs in paracoccidioidomycosis is still unclear. In a previous report we were able to show the dual role played by TLR2 in the immunity to P. brasiliensis infection. TLR2 activation prevented uncontrolled inflammatory reactions in pulmonary paracoccidioidomycosis associated with increased expansion of Th17 cells and diminished function of Treg cells (38).
Initially we characterized the influence of TLR4 on the phagocytic and fungicidal abilities of macrophages. Both the absence of TLR4 expression by TLR4−/− C57BL/6 mice and defective TLR4 signaling (C3H/HeJ mice) resulted in deficient P. brasiliensis ingestion/adherence and lower fungal loads recovered 48 h after cocultivation. In both deficient mouse strains, lower levels of nitric oxide (and cytokines with C3H/HeJ cells) were detected, indicating that the lower CFU counts recovered were not due to increased activation of phagocytes and enhanced fungal killing but probably were due to decreased endocytosis of P. brasiliensis yeasts. TLRs usually do not act as phagocytic receptors, but their engagement by pathogen components results in strong activation of inflammatory responses (8, 9). There are, however, several examples demonstrating that cell signaling can influence endocytosis and vice versa (20, 23). Indeed, TLR4 was shown to actively participate in bacterial phagocytosis (4) and to be rapidly internalized by human monocytes after in vitro interaction with P. brasiliensis yeasts or A. fumigatus conidia (10, 21). In addition, a recent paper has clearly demonstrated that TLR4 and TLR2 synergize with class A scavenger receptor to mediate phagocytosis of Gram-negative and Gram-positive bacteria, respectively (3). Thus, we can suppose that TLR signaling facilitated the endocytosis of P. brasiliensis and further induced the activation of proinflammatory pathways, which, however, were not sufficient to control the early increased fungal loads. Since equivalent results were obtained with macrophages lacking TLR4 expression, we believe that TLR4 signaling could have influenced phagocytosis mediated by another pathogen receptor. Although our experiments have not identified the main PRR involved in initial P. brasiliensis recognition (particularly due to the number and complexity of components that comprise fungal cell walls), we have clearly demonstrated that TLR4 participates in the activation of innate immune cells required for the initial interaction with P. brasiliensis yeasts. Our in vitro findings were validated by in vivo experiments, which demonstrated that early in infection, TLR4-normal mice presented higher fungal loads than their TLR4-defective counterparts and that this was accompanied by increased activation of the immune system. Additional experiments with PRR agonists and antagonists using TLR4-normal and -deficient cells are needed, however, to further clarify the role of TLRs in pulmonary PCM.
Our in vivo data showed that mice expressing defective TLR4 developed a less-severe infection associated with lower production of nitric oxide and cytokines and less migration of inflammatory cells to the site of infection. The decreased presence of activated macrophages expressing CD11b, CD86, CD40, and MHC class II molecules was concomitant with reduced synthesis of MCP-1. In addition, the diminished presence of CD4+ T cells and recently activated CD69+ CD8+ T cells in the lungs of TLR4-defective mice demonstrates that TLR4 signaling is necessary to the proper activation of adaptive immunity to P. brasiliensis and enhanced migration of inflammatory cells into the lungs. Consistent with these observations, several reports have demonstrated that TLR4 signaling is needed for the activation and maturation of dendritic cells, which acquire the competent phenotype to preferentially differentiate naïve T cells to the Th1 or Th17 pattern (57, 64, 65). No differences in Th1 and Th2 cytokines, however, were detected in lung homogenates. The increased production of IL-12 and TNF-α concomitant with unaltered synthesis of Th2 cytokines (IL-4, IL-5, and IL-10) indicated, however, that TLR4 signaling promoted a cytokine milieu biased toward a proinflammatory balance. This cytokine response could have protected C3H/HePas mice from high fungal burdens due to the enhanced fungicidal mechanisms of activated phagocytes. Indeed, in experimental and human PCM, cytokine-activated phagocytes (activated mainly by IFN-γ, IL-12, and TNF-α) were shown to be the most important effector cells against P. brasiliensis infection (36, 39, 46, 55). Our data on cytokine production showed an additional fact not previously reported in PCM. The expression of TLR4 facilitated the expansion of IL-17-producing cells, since IL-17 and other Th17-associated cytokines (IL-6 and IL-23) appeared at higher levels in the lungs of TLR4-competent mice. In our previous report, we could verify that the absence of TLR2 signaling induced enhanced expansion of Th17 cells and that both CD4+ and CD8+ T cells displayed intracellular IL-17 (38). Further studies of the TLR4-deficient model will help us to characterize the phenotype of cells involved in IL-17 production.
TLR4 ligation is important for the activation of Th1 or Th17 responses (65), while TLR4-deficiency can lead to increased expansion of CD4+ CD25+ regulatory T cells (47, 48). When the presence of Treg cells in the lungs of Toll-deficient and control mice at weeks 2 and 11 of infection was assessed, increased numbers of CD4+ CD25+ FoxP3+ cells were found in the lungs of TLR4-defective mice. This finding was associated with decreased fungal loads and diminished influx of inflammatory cells to the site of infection. Since Treg cells have been shown to control the inductive and effector phases of immunity against pathogens (5), we can suppose that Treg cells could have negatively controlled the expansion and migration of P. brasiliensis-specific T cells to the lungs. Thus, the advantage of low fungal loads conferred by TLR4 deficiency appeared to be negatively compensated for by deficient T-cell immunity and increased numbers of Treg cells, which appear to hamper the total clearance of fungal cells from the lungs.
At week 11 of infection, decreased levels of IL-12 were detected, probably due to the decreased migration of macrophages to the lungs. Interestingly, in C3H/HeJ mice, decreased levels of IL-17 were concomitant with diminished levels of TGF-β, indicating that another cytokine or costimulatory signal could have participated in the increased expansion of Treg cells (27).
Since the expression of TLRs has been shown to influence B-cell activation (40), we decided to analyze the levels of anti-P. brasiliensis isotypes in our model and observed an impaired humoral immune response in TLR4-defective mice. At week 11 of infection (Fig. 7), TLR4-deficient mice produced lower levels of IgG1-, IgG2b-, and IgM-specific antibodies. This could be due to the diminished fungal loads or the decreased production of cytokines observed in this mouse strain. Alternatively, since almost all TLR ligands were recently shown to induce the expansion and differentiation of B cells (30), we can suppose that TLR4 agonists present in P. brasiliensis yeasts could have exerted a stimulatory effect on B cells of TLR4-normal mice, resulting in increased humoral immunity. Independently of the mechanisms used, this is the first demonstration on the stimulatory role of TLR4 in the humoral immunity of P. brasiliensis-infected hosts.
TLR4 recognizes LPS of Gram-negative bacteria and favors Th1immunity due to the increased ability of LPS-stimulated DCs to produce IL-12 and TNF-α (51). In some fungal infections, however, cell wall polysaccharides have been reported to function as TLR agonists (42, 63). To our knowledge, no studies of paracoccidioidomycosis have addressed the characterization of TLR agonists. Although LPS or LPS-like components have not been characterized in P. brasiliensis, a few investigations have described the presence of polysaccharides, lipids, and glycolipids in P. brasiliensis cell walls (35, 61). The alkali-soluble fraction of P. brasiliensis cell walls has been shown to contain a high proportion of galactomannan (35), and it is tempting to suppose that this component could play a role in TLR4 activation.
Dectin and TLR4 signaling by microbial agonists has been reported to induce prevalent expansion of Th17 cells (1, 22, 34, 37). In our model, mice that possessed functional TLR4 were shown to have increased levels of IL-17 and other Th17-associated cytokines in their lungs. Although not investigated in the present work, IL-17-mediated immunity has been shown to exert deleterious or protective effects in infectious processes (54, 58). Actually, Th17 immunity can protect hosts due to its proinflammatory and chemotactic effect on polymorphonuclear (PMN) cells. Conversely, the enhanced oxidative metabolism and increased synthesis of metalloproteinases can result in tissue pathology and a detrimental effect on the hosts (11, 68, 69). In our previous work on the role of TLR2 in pulmonary PCM, we could demonstrate the dual role of Th17 immunity. The increased presence of inflammatory neutrophils conferred immune protection by reducing fungal loads but also resulted in tissue pathology equivalent to that induced by higher fungal burdens (38).
Mortality studies, unexpectedly, demonstrated that TLR4 signaling does not influence disease outcome, since TLR4-competent and -deficient mice presented equivalent survival times. In the course of the disease, both mouse strains were able to control fungal growth and to develop granulomatous reactions. However, the higher fungal loads, the enhanced Tcell immunity, and the lower expansion of Treg cells resulted in more-extensive inflammatory lesions, which exerted a deleterious effect on the lungs of TLR4-normal mice. On the other hand, the inefficient T-cell immunity of TLR4-deficient mice, tightly controlled by Treg cells, was not sufficient to totally clear the diminished fungal loads of TLR4-defective mice, abolishing the initial advantage conferred by their defective phagocytic ability. In sum, our findings indicate that high fungal loads accompanied by enhanced inflammatory responses mediated by uncontrolled T-cell immunity are equivalent to low fungal loads poorly controlled by a deficient T-cell response. Both mechanisms of immunity result in the chronic evolution of infection and equivalent mortality rates.
Acknowledgments
This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp) e Conselho Nacional de Pesquisas (CNPq).
We are grateful to Tania A. Costa and Paulo Albee for invaluable technical assistance.
Editor: G. S. Deepe, Jr.
Footnotes
Published ahead of print on 14 December 2009.
REFERENCES
- 1.Abdollahi-Roodsaz, S., L. A. Joosten, M. I. Koenders, I. Devesa, M. F. Roelofs, T. R. Radstake, M. Heuvelmans-Jacobs, S. Akira, M. J. Nicklin, F. Ribeiro-Dias, and W. B. van den Berg. 2008. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118:205-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira, A. 2006. TLR signaling. Curr. Top. Microbiol. Immunol. 311:1-16. [DOI] [PubMed] [Google Scholar]
- 3.Amiel, E., A. Alonso, S. Uematsu, S. Akira, M. E. Poynter, and B. Berwin. 2009. Toll-like receptor regulation of scavenger receptor-A-mediated phagocytosis. J. Leukoc. Biol. 85:595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand, R. J., J. W. Kohler, J. A. Cavallo, J. Li, T. Dubowski, and D. J. Hackam. 2007. Toll-like receptor 4 plays a role in macrophage phagocytosis during peritoneal sepsis. J. Pediatr. Surg. 42:927-932. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid, Y., and B. T. Rouse. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353-360. [DOI] [PubMed] [Google Scholar]
- 6.Bellocchio, S., C. Montagnoli, S. Bozza, R. Gaziano, G. Rossi, S. S. Mambula, A. Vecchi, A. Mantovani, S. M. Levitz, and L. Romani. 2004. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 172:3059-3069. [DOI] [PubMed] [Google Scholar]
- 7.Biondo, C., A. Midiri, L. Messina, F. Tomasello, G. Garufi, M. R. Catania, M. Bombaci, C. Beninati, G. Teti, and G. Mancuso. 2005. MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur. J. Immunol. 35:870-878. [DOI] [PubMed] [Google Scholar]
- 8.Blander, J. M. 2007. Signalling and phagocytosis in the orchestration of host defence. Cell. Microbiol. 9:290-299. [DOI] [PubMed] [Google Scholar]
- 9.Blander, J. M., and R. Medzhitov. 2004. Regulation of phagosome maturation by signals from toll-like receptors. Science 304:1014-1018. [DOI] [PubMed] [Google Scholar]
- 10.Bonfim, C. V., R. L. Mamoni, M. H. Souza, and L. Blotta. 2009. TLR-2, TLR-4 and dectin-1 expression in human monocytes and neutrophils stimulated by Paracoccidioides brasiliensis. Med. Mycol. 7:722-733. [DOI] [PubMed] [Google Scholar]
- 11.Bozza, S., T. Zelante, S. Moretti, P. Bonifazi, A. De Luca, C. D'Angelo, G. Giovannini, C. Garlanda, L. Boon, F. Bistoni, P. Puccetti, A. Mantovani, and L. Romani. 2008. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J. Immunol. 180:4022-4031. [DOI] [PubMed] [Google Scholar]
- 12.Brummer, E. 1994. Interaction of Paracoccidioides brasiliensis with host defense cells. .In M. Franco, C. S. Lacaz, A. Restrepo-Moreno, and G. Del Negro (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, FL.
- 13.Calich, V. L. G., and M. H. S. L. Blotta. 2005. Pulmonary paracoccidioidomycosis, p. 201-208. In P. L. Fidel and G. B. Huffnagle (ed.), Fungal immunology: from an organ perspective. Springer Press, New York, NY.
- 14.Calich, V. L. G., T. L. Kipnis, M. Mariano, C. F. Neto, and W. D. Dias da Silva. 1979. The activation of the complement system by Paracoccidioides brasiliensis in vitro: its opsonic effect and possible significance for an in vivo model of infection. Clin. Immunol. Immunopathol. 12:21-30. [DOI] [PubMed] [Google Scholar]
- 15.Calich, V. L., T. A. da Costa, M. Felonato, C. Arruda, S. Bernardino, F. V. Loures, L. R. Ribeiro, R. de Cássia Valente-Ferreira, and A. Pina. 2008. Innate immunity to Paracoccidioides brasiliensis infection. Mycopathologia 165:223-236. [DOI] [PubMed] [Google Scholar]
- 16.Calich, V. L. G., A. Pina, M. Felonato, S. Bernardino, T. A. Costa, and F. V. Loures. 2008. Toll-like receptors and fungal infections: the role of TLR2, TLR4 and MyD88 in paracoccidioidomycosis. FEMS Immunol. Med. Microbiol. 53:1-7. [DOI] [PubMed] [Google Scholar]
- 17.Camargo, Z. P., and L. E. Cano. 1994. Humoral immunity, p. 187-197. In M. Franco, C. S. Lacaz, A. Restrepo-Moreno, and G. Del Negro (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, FL.
- 18.Cano, L. E., L. M. Singer-Vermes, C. A. C. Vaz, M. Russo, and V. L. G. Calich. 1995. Pulmonary paracoccidioidomycosis in resistant and susceptible mice: relationship among progression of infection, bronchoalveolar cell activation, cellular immune response, and specific isotype patterns. Infect. Immun. 63:1777-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casadevall, A., M. Feldmesser, and L. A. Pirofski. 2002. Induced humoral immunity and vaccination against major human fungal pathogens. Curr. Opin. Microbiol. 5:386-391. [DOI] [PubMed] [Google Scholar]
- 20.Cavalli, V., M. Corti, and J. Gruenberg. 2001. Endocytosis and signaling cascades: a close encounter. FEBS Lett. 498:190-196. [DOI] [PubMed] [Google Scholar]
- 21.Chai, L. Y., B. J. Kullberg, A. G. Vonk, A. Warris, A. Cambi, J. P. Latgé, L. A. Joosten, J. W. van der Meer, and M. G. Netea. 2009. Modulation of Toll-like receptor 2 (TLR2) and TLR4 responses by Aspergillus fumigatus. Infect. Immun. 77:2184-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennehy, K. M., J. A. Willment, D. L. Williams, and G. D. Brown. 2009. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur. J. Immunol. 39:1379-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Fiore, P. P., and P. De Camilli. 2001. Endocytosis and signaling. an inseparable partnership. Cell 106:1-4. [DOI] [PubMed] [Google Scholar]
- 24.Dubourdeau, M., R. Athman, V. Balloy, M. Huerre, M. Chignard, D. J. Philpott, J. P. Latgé, and O. Ibrahim-Granet. 2006. Aspergillus fumigatus induces innate immune responses in alveolar macrophages through the MAPK pathway independently of TLR2 and TLR4. J. Immunol. 177:3994-4001. [DOI] [PubMed] [Google Scholar]
- 25.Fedele, G., M. Nasso, F. Spensieri, R. Palazzo, L. Frasca, M. Watanabe, and C. M. Ausiello. 2008. Lipopolysaccharides from Bordetella pertussis and Bordetella parapertussis differently modulate human dendritic cell functions resulting in divergent prevalence of Th17-polarized responses. J. Immunol. 181:208-216. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira, K. S., K. R. Bastos, M. Russo, and S. R. Almeida. 2007. Interaction between Paracoccidioides brasiliensis and pulmonary dendritic cells induces interleukin-10 production and toll-like receptor-2 expression: possible mechanisms of susceptibility. J. Infect. Dis. 196:1108-1115. [DOI] [PubMed] [Google Scholar]
- 27.Feuerer, M., J. A. Hill, D. Mathis, and C. Benoist. 2009. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat. Immunol. 10:689-695. [DOI] [PubMed] [Google Scholar]
- 28.Franco, M. 1987. Host-parasite relationships in paracoccidioidomycosis. J. Med. Vet. Mycol. 25:5-18. [DOI] [PubMed] [Google Scholar]
- 29.González, A., A. Yáρez, D. Gozalbo, and M. L. Gil. 2008. MyD88 is dispensable for resistance to Paracoccidioides brasiliensis in a murine model of blood-borne disseminated infection. FEMS Immunol. Med. Microbiol. 54:365-374. [DOI] [PubMed] [Google Scholar]
- 30.Gururajan, M., J. Jacob, and B. Pulendran. 2007. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PloS One 2(9):e863. [DOI] [PMC free article] [PubMed]
- 31.Higgins, S. C., A. G. Jarnicki, E. C. Lavelle, and K. H. Mills. 2006. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 177:7980-7989. [DOI] [PubMed] [Google Scholar]
- 32.Jiménez, M. D. P., A. Restrepo, D. Radzioch, L. E. Cano, and L. F. Garcia. 2006. Importance of complement 3 and mannose receptors in phagocytosis of Paracoccidioides brasiliensis conidia by Nramp1 congenic macrophages lines. FEMS Immunol. Med. Microbiol. 47:56-66. [DOI] [PubMed] [Google Scholar]
- 33.Jordan, J. M., M. E. Woods, J. Olano, and D. H. Walker. 2008. Absence of TLR4 signaling in C3H/HeJ mice predisposes to overwhelming rickettsial infection and decreased protective Th1 responses. Infect. Immun. 76:3717-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jouault, T., S. Ibata-Ombetta, O. Takeuchi, P. A. Trinel, P. Sacchetti, P. Lefebvre, S. Akira, and D. Poulain. 2003. Candida albicans phospholipomannan is sensed through toll-like receptors. J. Infect. Dis. 188:165-172. [DOI] [PubMed] [Google Scholar]
- 35.Kanetsuna, F., L. M. Carbonell, R. E. Moreno, and J. Rodriguez. 1969. Cell wall composition of the yeast and mycelial forms of Paracoccidioides brasiliensis. J. Bacteriol. 97:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kashino, S. S., R. A. Fazioli, C. Cafalli-Favati, L. H. Meloni-Bruneri, C. A. Vaz, E. Burger, L. M. Singer, and V. L. G. Calich. 2000. Resistance to Paracoccidioides brasiliensis infection is linked to a preferential Th1 immune response, whereas susceptibility is associated with absence of IFN-gamma production. J. Interferon Cytokine Res. 20:89-97. [DOI] [PubMed] [Google Scholar]
- 37.Leibundgut-Landmann, S., F. Osorio, G. D. Brown, and C. Reis e Sousa. 2008. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood 112:4971-4980. [DOI] [PubMed] [Google Scholar]
- 38.Loures, F. V., A. Pina, M. Felonato, and V. L. G. Calich. 2009. TLR2 is a negative regulator of Th17 cells and tissue pathology in a pulmonary model of fungal infection. J. Immunol. 183:1279-1290. [DOI] [PubMed] [Google Scholar]
- 39.Mamoni, R. L., and M. H. Blotta. 2006. Flow-cytometric analysis of cytokine production in human paracoccidioidomycosis. Cytokine 35:207-216. [DOI] [PubMed] [Google Scholar]
- 40.Manicassamy, S., and B. Pulendran. 2009. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 21:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meier, A., C. J. Kirschning, T. Nikolaus, H. Wagner, J. Heesemann, and F. Ebel. 2003. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell. Microbiol. 5:561-570. [DOI] [PubMed] [Google Scholar]
- 42.Monari, C., E. Pericolini, G. Bistoni, A. Casadevall, T. R. Kozel, and A. Vecchiarelli. 2005. Cryptococcus neoformans capsular glucuronoxylomannan induces expression of Fas ligand in macrophages. J. Immunol. 174:3461-3468. [DOI] [PubMed] [Google Scholar]
- 43.Netea, M. G., G. Ferwerda, C. A. van der Graaf, J. W. van der Meer, and B. J. Kullberg. 2006. Recognition of fungal pathogens by toll-like receptors. Curr. Pharm. Des. 12:4195-4201. [DOI] [PubMed] [Google Scholar]
- 44.Netea, M. G., N. A. Gow, C. A. Munro, S. Bates, C. Collins, G. Ferwerda, R. P. Hobson, G. Bertram, H. B. Hughes, T. Jansen, L. Jacobs, E. T. Buurman, K. Gijzen, D. L. Williams, R. Torensma, A. McKinnon, D. M. MacCallum, F. C. Odds, J. W. Van der Meer, A. J. Brown, and B. J. Kullberg. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Invest. 116:1642-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Netea, M. G., C. A. Van Der Graaf, A. G. Vonk, I. Verschueren, J. W. Van Der Meer, and B. J. Kullberg. 2002. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J. Infect. Dis. 185:1483-1489. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira, S. J., R. L. Mamoni, C. C. Musatti, P. M. Papaiordanou, and M. H. Blotta. 2002. Cytokines and lymphocyte proliferation in juvenile and adult forms of paracoccidioidomycosis: comparison with infected and non-infected controls. Microbes Infect. 4:139-144. [DOI] [PubMed] [Google Scholar]
- 47.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299:1033-1036. [DOI] [PubMed] [Google Scholar]
- 48.Pasare, C., and R. Medzhitov. 2004. Toll-dependent control mechanisms of CD4 T cell activation. Immunity 21:733-741. [DOI] [PubMed] [Google Scholar]
- 49.Pina, A., S. Bernardino, and V. L. G. Calich. 2008. Alveolar macrophages from susceptible mice are more competent than those of resistant mice to control initial Paracoccidioides brasiliensis infection. J. Leukoc. Biol. 83:1088-1099. [DOI] [PubMed] [Google Scholar]
- 50.Popi, A. F., J. D. Lopes, and M. Mariano. 2002. gp43 from Paracoccidioides brasiliensis inhibits macrophage functions. An evasion mechanism of the fungus. Cell. Immunol. 218:87-94. [DOI] [PubMed] [Google Scholar]
- 51.Qi, H., T. L. Denning, and L. Soong. 2003. Differential induction of interleukin-10 and interleukin-12 in dendritic cells by microbial toll-like receptor activators and skewing of T-cell cytokine profiles. Infect. Immun. 71:3337-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reis e Sousa, C. 2004. Activation of dendritic cells: translating innate into adaptative immunity. Curr. Opin. Immunol. 16:21-25. [DOI] [PubMed] [Google Scholar]
- 53.Romani, L. 2004. Immunity to fungal infections. Nat. Rev. Immunol. 4:1-23. [DOI] [PubMed] [Google Scholar]
- 54.Romani, L., T. Zelante, A. De Luca, F. Fallarino, and P. Puccetti. 2008. IL-17 and therapeutic kynurenines in pathogenic inflammation to fungi. J. Immunol. 180:5157-5162. [DOI] [PubMed] [Google Scholar]
- 55.Romano, C. C., M. J. Mendes-Giannini, A. J. Duarte, and G. Benard. 2002. IL-12 and neutralization of endogenous IL-10 revert the in vitro antigen-specific cellular immunosuppression of paracoccidioidomycosis patients. Cytokine 18:149-157. [DOI] [PubMed] [Google Scholar]
- 56.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151-1164. [PubMed] [Google Scholar]
- 57.Shen, H., B. M. Tesar, W. E. Walker, and D. R. Goldstein. 2008. Dual signaling of MyD88 and TRIF is critical for maximal TLR4-induced dendritic cell maturation. J. Immunol. 181:1849-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sieve, A. N., K. D. Meeks, S. Bodhankar, S. Lee, J. K. Kolls, J. W. Simecka, and R. E. Berg. 2009. A novel IL-17-dependent mechanism of cross protection: respiratory infection with mycoplasma protects against a secondary listeria infection. Eur. J. Immunol. 39:426-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singer-Vermes, L. M., M. C. Ciavaglia, S. S. Kashino, E. Burguer, and V. L. G. Calich. 1992. The source of the growth-promoting factor(s) affects the plating efficiency of Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 30:261-264. [DOI] [PubMed] [Google Scholar]
- 60.Spörri, R., and C. Reis e Sousa. 2005. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat. Immunol. 6:163-170. [DOI] [PubMed] [Google Scholar]
- 61.Tada, H., E. Nemoto, H. Shimauchi, T. Watanabe, T. Mikami, T. Matsumoto, N. Ohno, H. Tamura, K. Shibata, S. Akashi, K. Miyake, S. Sugawara, and H. Takada. 2002. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol. Immunol. 46:503-512. [DOI] [PubMed] [Google Scholar]
- 62.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 63.Toledo, M. S., E. Suzuki, A. H. Straus, and H. K. Takahashi. 1995. Glycolipids from Paracoccidioides brasiliensis. Isolation of a galactofuranose-containing glycolipid reactive with sera of patients with paracoccidioidomycosis. J. Med. Vet. Mycol. 33:247-251. [DOI] [PubMed] [Google Scholar]
- 64.Weighardt, H., G. Jusek, J. Mages, R. Lang, K. Hoebe, B. Beutler, and B. Holzmann. 2004. Identification of a TLR4- and TRIF-dependent activation program of dendritic cells. Eur. J. Immunol. 34:558-564. [DOI] [PubMed] [Google Scholar]
- 65.Wynn, T. A. 2005. T(H)-17: a giant step from T(H)1 and T(H)2. Nat. Immunol. 6:1069-1070. [DOI] [PubMed] [Google Scholar]
- 66.Yang, R. B., M. R. Mark, A. Gray, A. Huang, N. H. Xie, and M. Zhang. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signaling. Nature 395:284-288. [DOI] [PubMed] [Google Scholar]
- 67.Yauch, L. E., M. K. Mansour, S. Shoham, J. B. Rottman, and S. M. Levitz. 2004. Involvement of CD14, toll-like receptors 2 and 4, and MyD88 in the host response to the fungal pathogen Cryptococcus neoformans in vivo. Infect. Immun. 72:5373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zelante, T., A. De Luca, P. Bonifazi, C. Montagnoli, S. Bozza, S. Moretti, M. L. Belladonna, C. Vacca, C. Conte, P. Mosci, F. Bistoni, P. Puccetti, R. A. Kastelein, M. Kopf, and L. Romani. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 37:2695-2706. [DOI] [PubMed] [Google Scholar]
- 69.Zelante, T., A. De Luca, C. D'Angelo, S. Moretti, and L. Romani. 2009. IL-17/Th17 in anti-fungal immunity: what's new? Eur. J. Immunol. 39:645-648. [DOI] [PubMed] [Google Scholar]
- 70.Ziegler, S. F., S. D. Levin, L. Johnson, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, E. Baker, G. R. Sutherland, A. L. Feldhaus, and F. Ramsdell. 1994. The mouse CD69 gene. Structure, expression, and mapping to the NK gene complex. J. Immunol. 152:1228-1236. [PubMed] [Google Scholar]