Abstract
Campylobacter infection in humans is accompanied by severe inflammation of the intestinal mucosa, in contrast to colonization of chicken. The basis for the differential host response is unknown. Toll-like receptors (TLRs) sense and respond to microbes in the body and participate in the induction of an inflammatory response. Thus far, the interaction of Campylobacter with chicken TLRs has not been studied. Here, we investigated the potential of four Campylobacter strains to activate human TLR1/2/6, TLR4, TLR5, and TLR9 and chicken TLR2t2/16, TLR4, TLR5, and TLR21. Live bacteria showed no or very limited potential to activate TLR2, TLR4, and TLR5 of both the human and chicken species, with minor but significant differences between Campylobacter strains. In contrast, lysed bacteria induced strong NF-κB activation through human TLR1/2/6 and TLR4 and chicken TLR2t2/16 and TLR4 but not via TLR5 of either species. Interestingly, C. jejuni induced TLR4-mediated beta interferon in human but not chicken cells. Furthermore, isolated chromosomal Campylobacter DNA was unable to activate human TLR9 in our system, whereas chicken TLR21 was activated by DNA from all of the campylobacters tested. Our data are the first comparison of TLR-induced immune responses in humans and chickens. The results suggest that differences in bacterial cell wall integrity and in TLR responses to Campylobacter LOS and/or DNA may contribute to the distinct clinical manifestation between the species.
Campylobacter jejuni, a Gram-negative highly motile bacterium, is a major cause of intestinal enteritis in humans (2). The infection of the gut is accompanied by high numbers of infiltrating neutrophils, loss of epithelial barrier integrity, and a watery or bloody diarrhea (7, 57). The inflammatory pathology suggests a strong induction of innate immune responses. Indeed, experimental infection of several types of human tissue culture cells has demonstrated the potential of Campylobacter to initiate a range of immune responses. For instance, intestinal INT407 and T84 cells respond to C. jejuni by producing cytokines interleukin-8 (IL-8) and/or tumor necrosis factor alpha (TNF-α) (4, 8, 20, 25, 58, 60), whereas infected THP-1 monocytes and dendritic cells secrete an even broader range of cytokines (23, 26). Although some reports clearly show the requirement of the cytolethal distending toxin and/or bacterial invasion for cytokine responses (21, 25), most of the observed responses seem independent of these traits.
Chickens are considered to be the main source of human Campylobacter infection. Campylobacter colonization of chickens is rapid and widespread, and results in long-term persistence and shedding (5, 33). Although large numbers of bacteria are in close contact with the epithelial cell layer of particularly the ceca, no intestinal inflammation is seen in these birds (13), in clear contrast to humans. Despite the lack of pathology, several reports have shown induction of immune-associated gene and protein expression after Campylobacter colonization of chicken. Analysis of isolated chicken tissue displayed an increase in cytokine expression (48) and circulating monocytes/macrophages (38), and several different types of chicken cells produce or upregulate cytokines during in vitro infection (32, 34, 49).
Innate immune responses against infectious microbes are for a large part mediated by the activation of Toll-like receptors (TLRs) (31). TLRs are a group of membrane receptors that safeguard the host-microbe boundaries by sensing conserved microbial patterns (41). Bacteria are mainly sensed by TLR2/1/6, TLR4/MD-2, TLR5, and TLR9 that detect lipoproteins, lipopolysaccharide (LPS), flagellin, and DNA, respectively. After TLR activation, a signaling cascade involving the adapter proteins MyD88 (all TLRs but TLR3) or TRIF (TLR3 and TLR4 only) results in the activation and the translocation of nuclear transcription factors such as NF-κB and IRF3, which in turn induce the transcription of cytokines and other immune genes (1).
C. jejuni initiates both MyD88 and TRIF-dependent immune responses through the activation of human TLR2 and TLR4 (17, 43), whereas stimulation of human TLR9 only resulted in low levels of IL-8 secretion (12). Furthermore, C. jejuni is unable to activate human TLR5 (3, 58). The potential of Campylobacter to activate TLRs of the chicken has thus far not been explored. Identification and characterization of the chicken TLRs revealed major species-specific characteristics. For instance, the chicken TLR2 complex does not seem to distinguish between different lipoproteins in contrast to its human counterpart (22, 28), whereas chickens lack TLR9 but express the unique DNA-sensing TLR21 (9; A. M. Keestra et al., unpublished data). Reports on differences in Campylobacter-induced (innate) immune responses between humans and chickens are scarce and somewhat contradictory. Larson et al. showed differences in the amount of IL-8 induction and secretion between C. jejuni-infected human INT407 cells and chicken LMH cells (32), whereas Borrmann et al. reported similar levels of IL-8 upregulation when comparing INT407 and chicken PIC cells (8). In the present study, we for the first time directly compared the potential of Campylobacter to activate individual human and chicken TLRs. Although the majority of TLRs responded similarly to four different Campylobacter strains, chicken TLR21, but not human TLR9, was activated by Campylobacter chromosomal DNA. In addition, Campylobacter was unable to activate MyD88-independent IFN-β transcription via TLR4 signaling in the chicken. Interestingly, live Campylobacter showed no or weak activation of TLRs of both species, suggesting that in vivo differences in bacterial integrity may also influence the innate immune response.
MATERIALS AND METHODS
Bacteria, cell lines, and reagents.
C. jejuni strains 81116 (42), GB18 (14), and RM1221 (40) and the Campylobacter coli strain H1 (a fresh chicken isolate) were routinely cultured on plates with 5% saponin-lysed horse blood (Biotrading) at 37°C under a microaerophilic atmosphere of 10% CO2, 5% O2, and 85% N2. Liquid cultures were grown under the same conditions in heart infusion (HI) broth (Biotrading). Human Mono Mac 6 (MM6) monocytic cells (61) and chicken HD11 macrophages (6) were propagated in RPMI (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (FCS) under 5% CO2 at 37°C. HeLa 57A cells containing a stably transfected NF-κB luciferase reporter plasmid (45), and HEK293 cells were cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen) supplemented with 5% heat-inactivated FCS under 10% CO2 at 37°C. The TLR ligands FSL-1, Pam3CSK4, poly(I:C), and ODN 2006 were purchased from Invivogen. Highly purified LPS from Salmonella enterica serovar Enteritidis 90-13-706 and highly purified lipooligosaccharide (LOS) from C. jejuni 81116 were isolated as described previously (30). Flagellin from serovar Enteritidis was purified as described previously (24).
Preparation of live and lysed Campylobacter.
Campylobacter starter cultures were inoculated in 5 ml of HI broth from saponin plates grown for 48 h at 37°C. After 6 to 8 h of incubation (37°C, shaking at 160 rpm), starter cultures were used to inoculate fresh cultures at a starting optical density at 550 nm (OD550) of 0.00125 in 10 ml of HI broth to virtually exclude the presence of dead bacteria from the plates. After 17 h of growth, bacteria reached an OD550 of ∼1.0, corresponding to 2 × 109 CFU ml−1. Then, 1 ml of this suspension was pelleted by centrifugation (5,000 × g, 10 min, 22°C), washed twice with Dulbecco phosphate-buffered saline (DPBS), and resuspended in DPBS to a final OD550 of 1.0. Live bacteria were analyzed by using the Live/Dead BacLight kit (Invitrogen) and were only used if the percentage dead bacteria was <0.5%. Then, 1 ml of the suspended bacteria was heat killed at 65°C for 30 min and subsequently sonicated on ice (a 15-s pulse followed by a 30-s pause, repeated six times) to further release TLR ligands.
RT-PCR.
MM6 and HD11 cells were cultured in 12-well plates overnight to a confluence of ∼80%. Live or dead bacteria were added at a multiplicity of infection (MOI) of 100. After 2 h of incubation (37°C, 5% CO2), the cells were washed twice with DPBS to remove the cell culture medium and bacteria. RNA from MM6 cells was isolated by using an RNeasy minikit (Qiagen), and RNA from HD11 cells was isolated by using RNA-Bee (Bio-Connect), both according to the manufacturer's protocol. Prior to reverse transcription-PCR (RT-PCR), the RNA was treated with 1 μg of DNase (Fermentas) per μg of RNA for 30 min at 37°C, after which the DNase was inactivated by heating at 65°C for 10 min in the presence of EDTA (2.5 mM, final concentration). Primers (Invitrogen) and probes (Table 1) were designed by using Primer Express software (Applied Biosystems). Probes, labeled with the reporter dye carboxyfluorescein (FAM) and the quencher tetramethyl-6-carboxyrhodamine (TAMRA), were either purchased from Isogen Life Science (chicken GAPDH [glyceraldehyde-3-phosphate dehydrogenase], IL-1β, IL-8 [also known as CXCLi2], iNOS, and beta interferon [IFN-β]) or Eurogentec (human β-actin, IL-1β, IL-8 [CXCL8], and IFN-β). RNA transcript levels were determined by quantitative RT-PCR with an ABI Prism 7000 sequence detection system (Applied Biosystems) using a One Step RT-PCR MasterMix kit for probe assays (Eurogentec). Per reaction, 50 ng of DNase-treated RNA was used. Real-time cycler conditions and normalization against housekeeping genes β-actin (HD11) and GAPDH (MM6) were as described previously (30).
TABLE 1.
RT-PCR primers and probes used in this study
| Gene (species) | Primer (orientation) or probe | Sequence (5′-3′) |
|---|---|---|
| β-actin (human) | Forward | ACCGAGCGCGGCTACAG |
| Reverse | CTTAATGTCACGCACGATTTCC | |
| Probe | (FAM)-TTCACCACCACGGCCGAGC-(TAMRA) | |
| IL-1β (human) | Forward | CGAATCTCCGACCACCACTAC |
| Reverse | TCCATGGCCACAACAACTGA | |
| Probe | (FAM)-AGGGCTTCAGGCAGGCCGC-(TAMRA) | |
| IL-8 (human) | Forward | CTGGCCGTGGCTCTCTTG |
| Reverse | CCTTGGCAAAACTGCACCTT | |
| Probe | (FAM)-CAGCCTTCCTGATTTCTGCAGCTCTGTGT-(TAMRA) | |
| IFN-β (human) | Forward | TTCTCCACGACAGCTCTTTCC |
| Reverse | CACTGACAATTGCTGCTTCTTTG | |
| Probe | (FAM)-TGAGCTACAACTTGCTTGGATTCC-(TAMRA) | |
| GAPDH (chicken) | Forward | GCCGTCCTCTCTGGCAAAG |
| Reverse | TGTAAACCATGTAGTTCAGATCGATGA | |
| Probe | (FAM)-AGTGGTGGCCATCAATGATCCC-(TAMRA) | |
| IL-1β (chicken) | Forward | GCTCTACATGTCGTGTGTGATGAG |
| Reverse | TGTCGATGTCCCGCATGA | |
| Probe | (FAM)-CCACACTGCAGCTGGAGGAAGCC-(TAMRA) | |
| IL-8 (chicken) | Forward | GCCCTCCTCCTGGTTTCAG |
| Reverse | CGCAGCTCATTCCCCATCT | |
| Probe | (FAM)-TGCTCTGTCGCAAGGTAGGACGCTG-(TAMRA) | |
| IFN-β (chicken) | Forward | ACAACTTCCTACAGCACAACAACTA |
| Reverse | GCCTGGAGGCGGACATG | |
| Probe | (FAM)-TCCCAGGTACAAGCACTG-(TAMRA) |
Chromosomal DNA isolation.
Chromosomal DNA was isolated from Campylobacter grown in 100 ml of HI broth at 37°C overnight using CTAB (cetyltrimethylammonium bromide). Briefly, bacteria were pelleted by centrifugation and resuspended in 9.5 ml of TE buffer (10 mM Tris-HCl [pH 7.4], 1 mM EDTA [pH 8.0]). Bacteria were lysed by adding 1 μg of proteinase K and 0.5 ml of 10% sodium dodecyl sulfate, followed by incubation at 37°C for 1.5 h. Subsequently, 1.8 ml of NaCl (5 M) and 1.5 ml of 10% CTAB in 0.7 M NaCl were added, and the mixture was incubated at 65°C for 20 min. DNA was extracted into the aqueous phase after the addition of an equal volume of chloroform-isoamyl alcohol (24:1). After centrifugation (6,000 × g, 10 min, 22°C), the aqueous phase was mixed with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1) and again centrifuged. DNA was precipitated from the aqueous phase with 0.6 volumes of isopropanol and collected by centrifugation (5,000 × g, 5 min, 22°C). The DNA pellet was washed with 5 ml of 70% ethanol, air dried, and resuspended in H2O. DNA concentration was determined by measuring the absorption at 260 nm.
Transfection.
HeLa 57A cells and HEK293 cells were grown overnight to a confluence of ∼60%. Individual wells were transfected with a total amount of 250 ng of plasmid DNA mixture using FuGENE-6 (Roche) at a lipid/DNA ratio of 3:1. Plasmids encoding the human and chicken TLRs and adapter proteins were obtained as described previously (28-30). Human TLR1, TLR2, TLR4, and TLR9 and chicken TLR4 and TLR21 were expressed with their natural leader peptide, and chicken TLR2t2, TLR5, and TLR16 and human TLR5 were expressed with the pFLAG-CMV1 preprotrypsin leader peptide for secreted proteins. Combinations of the following plasmids were transfected: human TLR2, human TLR1, human CD14, and LacZ (62.5 ng per plasmid per well); chicken TLR2t2, chicken TLR16, human CD14, and LacZ (62.5 ng per plasmid per well); human TLR4, human MD-2, human CD14, and LacZ (62.5 ng per plasmid per well); chicken TLR4, chicken MD-2, human CD14, and LacZ (62.5 ng per plasmid per well); human TLR5 and LacZ (125 ng per plasmid per well); chicken TLR5 and LacZ (125 ng per plasmid per well); human TLR9 and LacZ (125 ng per plasmid per well); and chicken TLR21 and LacZ (125 ng per plasmid per well). Cells were incubated with a DNA-FuGENE-6 mixture for 48 h. Prior to stimulation, medium was replaced with fresh DMEM-5% FCS.
Stimulation and luciferase measurement.
Individual wells were stimulated with 2.5 × 107 live or lysed bacteria, 30 μg of chromosomal DNA ml−1, 20 ng of LPS ml−1, 100 ng of Pam3CSK4 ml−1, 100 ng of FSL-1 ml−1, 1 μg of flagellin ml−1, or 0.5 μM ODN 2006. After 5 h of stimulation, the cells were washed twice with 0.5 ml of DPBS and lysed in 100 μl of RLB buffer (Promega) at −80°C. The luciferase activity in thawed lysates was measured in a luminometer (TD-20/20; Turner Designs) using luciferase reagent (Promega). To normalize transfection efficiency, the luciferase results were corrected with LacZ values determined with a β-galactosidase assay (Promega). Statistical analysis was performed by using a paired t test with GraphPad Prism 5 software, where a two-tailed P of <0.05 was considered significant. All experiments were performed three times independently. Chromosomal DNA from all four Campylobacter strains was prepared twice.
RESULTS
Human cytokine responses toward live and disrupted C. jejuni.
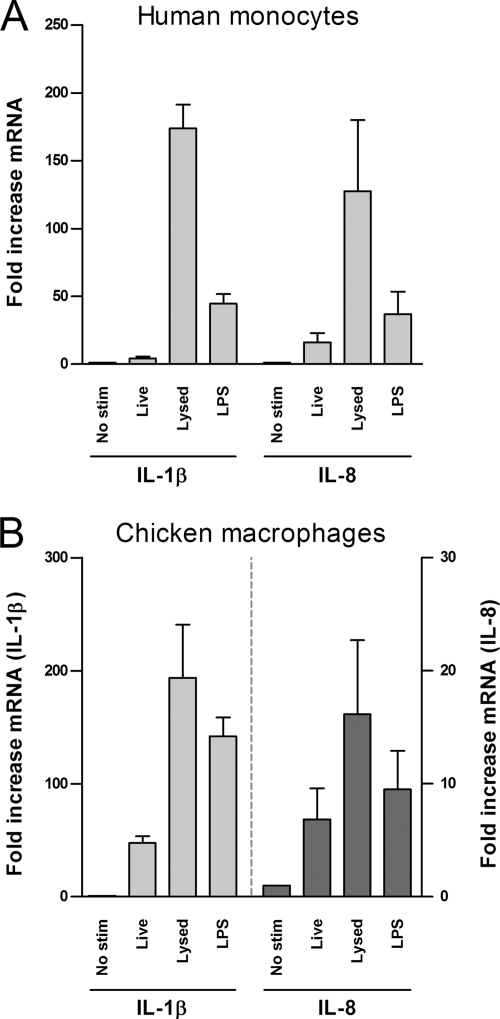
Prior to testing the activation of TLRs by Campylobacter, we assessed the effect of C. jejuni strain 81116 on human MM6 monocytic cells. As host cells likely encounter both live and disrupted C. jejuni during the course of infection, we determined the ability of both viable and lysed C. jejuni to induce innate immune responses. Highly sensitive RT-PCR on isolated RNA was performed to be able to measure early changes (2 h) in IL-1β and IL-8 gene transcription. MM6 cells were infected with live or lysed C. jejuni strain 81116 at an MOI of 100 (5 × 107 CFU ml−1). No significant reduction in viable bacteria was noticed during the period of infection (data not shown). As a positive control, cells were activated by the addition of 1 μg of purified serovar Enteritidis LPS ml−1. This approach demonstrated that live C. jejuni induced minimal upregulation of human IL-1β transcript (∼4-fold increase). In contrast, lysed C. jejuni increased the level of IL-1β transcript by factor ∼175 (Fig. 1A). Similarly, live C. jejuni increased human IL-8 transcript by ∼16-fold, whereas disrupted bacteria induced an ∼125-fold increase in IL-8 mRNA (Fig. 1A). These results indicate that C. jejuni strain 81116 is capable of inducing a powerful innate immune response in human monocytes, particularly once the bacteria disintegrate.
FIG. 1.
Induction of IL-1β and IL-8 by live and disrupted C. jejuni. MM6 cells (A) and chicken HD11 cells (B) were stimulated for 2 h with 5 × 107 CFU of live or lysed C. jejuni strain 81116 ml−1. As a positive control, 1 μg of LPS from serovar Enteritidis was used. IL-1β and IL-8 transcripts were analyzed by RT-PCR and are presented as the fold increase mRNA levels after stimulation compared to nonstimulated cells. Live Campylobacter induced statistically significant levels of IL-1β and IL-8 mRNA in MM6 cells (P < 0.05), whereas in HD11 cells only the induction of IL-1β was statistically significant (P < 0.05). Stimulation of MM6 and HD11 cells with lysed Campylobacter resulted in significantly higher levels of IL-1β and IL-8 mRNA compared to mock stimulation or stimulation with live bacteria (P < 0.05). Values are means ± the standard errors of the mean (SEM) of three independent experiments.
Chicken cytokine responses toward live and lysed C. jejuni.
Since chickens can be colonized heavily by Campylobacter without obvious signs of pathology, we next investigated chicken HD11 macrophages for cytokine expression after infection with C. jejuni. We used similar conditions as described for the MM6 cells, except that primer pairs were optimally adapted to the chicken cytokine gene sequences. In these assays both viable and lysed C. jejuni increased IL-1β and IL-8 mRNA levels (Fig. 1B). Again, the cellular response to disrupted C. jejuni was significantly stronger than observed for live microorganisms (Fig. 1B), although the difference was markedly smaller than observed for the MM6 cells. Overall, the results indicate that C. jejuni strain 81116 activates both human and chicken immune innate genes and that bacterial cell wall integrity is an important determinant of the magnitude of the response in both species.
Disrupted but not live Campylobacter activate both human and chicken TLR2.
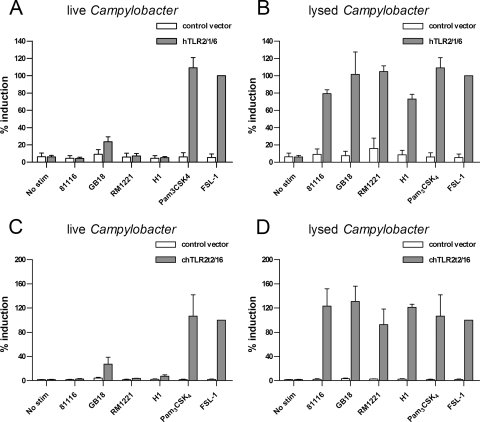
To investigate the contribution of individual TLRs to the Campylobacter innate immune response in humans and chickens, we first tested the ability of live and lysed bacteria to activate the TLR2 complex of both species. Humans recognize bacterial di- and triacylated lipoproteins by the combinations TLR2-TLR6 and TLR2-TLR1, respectively. HeLa 57A cells, which endogenously express TLR6, were transfected with human TLR2 together with TLR1 and stimulated with 5 × 107 CFU ml−1 (MOI of 100) live or lysed Campylobacter. We tested four different Campylobacter strains—C. jejuni 81116, C. jejuni GB18, C. jejuni RM1221, and C. coli H1—to reduce possible strain-specific effects. Cellular activation was measured using the NF-κB luciferase reporter that is stably expressed in HeLa 57A cells. Viable bacteria of none of the strains significantly activated NF-κB compared to control transfected cells after 5 h of infection (Fig. 2A), although strain GB18 tended to show slightly elevated NF-κB activation. Stimulation of live C. jejuni together with the TLR2 ligand Pam3CSK4 yielded a response similar to that obtained with the TLR2 ligand alone, excluding potential inhibition of the TLR/NF-κB response by C. jejuni (data not shown). In contrast to live Campylobacter, disrupted bacteria induced strong levels of NF-κB translocation irrespective of the Campylobacter strain or species tested (Fig. 2B).
FIG. 2.
Activation of human and chicken TLR2 by Campylobacter. HeLa 57A cells expressing human TLR2, TLR1, TLR6, and CD14 (A and B) or chicken TLR2t2, TLR16, and human CD14 (C and D) were stimulated with live or disrupted C. jejuni strain 81116, GB18, or RM1221 or C. coli strain H1 for 5 h. Cells transfected with control vector were stimulated simultaneously to ensure TLR-specific NF-κB activation. Pam3CSK4 (100 ng/ml) and FSL-1 (100 ng/ml) were used as positive controls. Values are the percent induction of NF-κB activation after stimulation with the positive control and are means ± the SEM of three independent experiments. The P values for human and chicken TLR2 responses were as follows: live Campylobacter versus control, not significant; lysed Campylobacter versus control, P < 0.05; lysed versus live Campylobacter, P < 0.05.
To assess the chicken TLR2 response against Campylobacter, HeLa 57A cells were transfected with chTLR2t2 and chTLR16. This receptor complex recognizes both di- and triacylated lipopeptides (28). The addition of live Campylobacter to these cells did not result in significant translocation of NF-κB (Fig. 2C). Again, GB18 showed an increased but not statistically significant NF-κB activation, as was observed for human TLR2/1/6. With lysed bacteria, all four strains were able to potently activate chicken TLR2t2/16-transfected cells (Fig. 2D). These results show that disrupted Campylobacter strains, unlike viable strains, are able to effectively activate both human TLR2 and chicken TLR2.
C. jejuni induces a TLR4-mediated IFN-β response in human but not chicken cells.
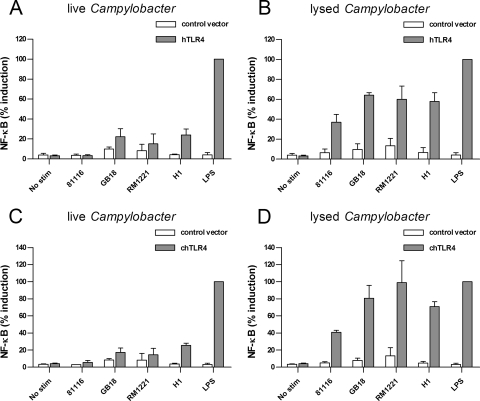
TLR4 is one of the central TLRs in innate immunity. Both lipid A structures with different biological activity toward TLR4 and differences in TLR4 specificity among host species have been described (15, 30, 50). To assess the effect of Campylobacter on the human and chicken TLR4 complex, we expressed TLR4 together with the homologous MD-2 in HeLa 57A cells. Incubation of the cells expressing human TLR4/MD-2 with lysed Campylobacter resulted in high levels of NF-κB translocation for all four strains tested (Fig. 3B). With viable bacteria, moderate human TLR4 activation was detected for the C. coli, while C. jejuni strains yielded no response (Fig. 3A). For cells transfected with chicken TLR4/MD-2, similar results were obtained (Fig. 3C and D), suggesting no major differences in the recognition of Campylobacter by the human and chicken TLR4 complex.
FIG. 3.
Activation of human and chicken TLR4 by Campylobacter. HeLa 57A cells expressing human TLR4, MD-2, and CD14 (A and B) or chicken TLR4, MD-2 and human CD14 (C and D) were stimulated with live or lysed C. jejuni strain 81116, GB18, or RM1221 or C. coli strain H1 for 5 h. Cells transfected with control vector were stimulated simultaneously to ensure TLR-specific NF-κB activation. LPS from serovar Enteritidis (20 ng/ml) was used as a positive control. Values are the percent induction of NF-κB activation after stimulation with the positive control and are means ± the SEM of three independent experiments. The P values for human and chicken TLR4 responses were as follows: live Campylobacter versus control, not significant, except for C. coli H1 (P < 0.05); lysed Campylobacter versus control, P < 0.05; lysed versus live Campylobacter, P < 0.05 for chicken TLR4 and P < 0.001 for human TLR4.
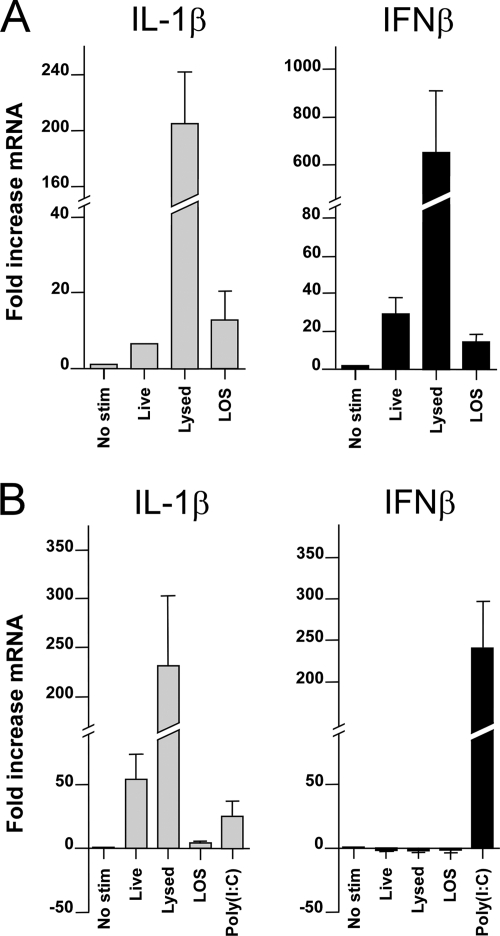
As the mammalian TLR4/MD-2 complex not only signals via the MyD88-dependent pathway but also through the MyD88-independent pathway, we next compared the upregulation of IFN-β mRNA mediated via the MyD88-independent pathway in MM6 and HD11 cells. Cells were stimulated with viable and lysed Campylobacter strains, as well as with purified C. jejuni 81116 LOS. Both viable and killed C. jejuni, as well as C. jejuni LOS, induced elevated levels of IFN-β transcript in the human cells (Fig. 4A). In contrast, stimulation of chicken cells with neither live nor dead C. jejuni, nor stimulation with purified LOS, induced IFN-β transcription, although a strong increase was observed after stimulation of the cells with the TLR3 agonist with poly(I:C) (Fig. 4B). These results indicate a major difference in response to Campylobacter between human and chicken cells.
FIG. 4.
Induction of IFN-β by live, lysed, and purified LOS of C. jejuni. MM6 cells (A) and chicken HD11 cells (B) were stimulated for 2 h with 5 × 107 CFU of live or lysed C. jejuni strain 81116 ml−1 or 1 μg of LOS ml−1. As a positive control, 500 ng of poly(I:C) ml−1 with FuGENE-6 was used. IL-1β and IFN-β transcripts were analyzed by RT-PCR and are presented as the fold increase in mRNA levels after stimulation compared to nonstimulated cells. Stimulation of MM6 cells with live Campylobacter, lysed Campylobacter, or LOS induced statistically significant levels of IL-1β and IFN-β mRNA (P < 0.05). In HD11 cells, stimulation with live Campylobacter, lysed Campylobacter, or LOS but not with poly(I:C) induced statistically significant levels of IL-1β, whereas IFN-β was only induced (P < 0.05) after stimulation with poly(I:C). Values are means ± the SEM of three independent experiments.
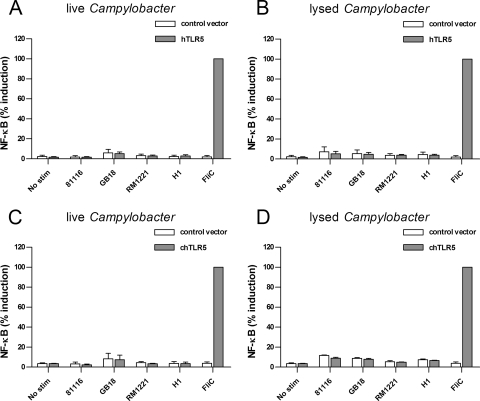
Campylobacter is unable to activate chicken TLR5.
TLR5 is considered to be important for maintenance of intestinal barrier (55). Stimulation of human TLR5 transfected HeLa 57A cells with either live or lysed Campylobacter showed a general lack of activation for all strains tested (Fig. 5A and B), confirming previous reports (3, 58). The identification and cloning of chicken TLR5 in our lab presented the opportunity to investigate its sensitivity toward Campylobacter flagellin. Whereas purified serovar Enteritidis flagellin induced a robust NF-κB response in transfected HeLa 57A cells, stimulation with both live and disrupted Campylobacter strains did not result in activation of chTLR5 (Fig. 5C and D). Also, purified native flagellin of C. jejuni strain 81116 did not activate chTLR5, even at concentrations of >10 μg ml−1 (data not shown). These results indicate that the difference in Campylobacter intestinal pathology between humans and chicken is not due to differential recognition of flagellin by the respective TLR5 receptors.
FIG. 5.
Activation of human and chicken TLR5 by Campylobacter. HeLa 57A cells expressing human TLR5 (A and B) or chicken TLR5 (C and D) were stimulated with live or disrupted C. jejuni strains 81116, GB18, or RM1221 or C. coli strain H1 for 5 h. Cells transfected with control vector were stimulated simultaneously to ensure TLR-specific NF-κB activation. Flagellin from serovar Enteritidis (1 μg ml−1) was used as a positive control. None of the Campylobacter strains, either live or lysed, induced statistically significant activation of NF-κB in HeLa 57A cells transfected with human or chicken TLR5. Values are the percent induction of NF-κB activation after stimulation with the positive control and are means ± the SEM of three independent experiments.
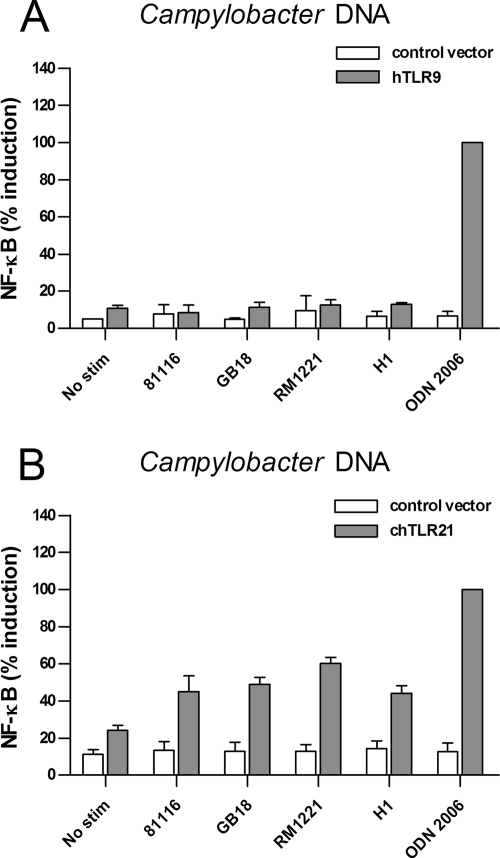
Campylobacter chromosomal DNA is sensed by chicken TLR21 but not human TLR9.
In the mammalian species, bacterial DNA is sensed by TLR9. This receptor is absent in chicken. Instead, chicken express TLR21 which responds to DNA and acts as a functional homologue of mammalian TLR9 (9; Keestra et al., unpublished). To test these receptors for the ability to respond to Campylobacter DNA, we expressed human TLR9 and chicken TLR21 in HEK293 cells. HEK293 cells were used in these experiments since HeLa 57A lack the ability to sense DNA and activate NF-κB when transfected with human TLR9. After 5 h of stimulation of HEK293 cells with chromosomal DNA (30 μg ml−1) from any of the four Campylobacter strains, no significant NF-κB activation was detected for the cells expressing human TLR9. Increasing the internalization of Campylobacter DNA using the transfection reagent FuGENE-6 did not result in NF-κB activation either (data not shown). Stimulation with the synthetic TLR9 ligand ODN 2006 caused a 10-fold increase in TLR9-mediated NF-κB activation, indicating the functionality of the TLR9 receptor under the conditions used (Fig. 6A). The stimulatory effect of ODN 2006 was maintained in the presence of 30 μg of chromosomal Campylobacter DNA/ml (data not shown), excluding potential inhibitory factors in the DNA preparation as a cause for the lack of TLR9 activation. Similar sets of experiments with cells expressing chicken TLR21 demonstrated strong activation of NF-κB by ODN 2006, as well the chromosomal DNA of all four Campylobacter strains (Fig. 6B). These results suggest that, in addition to the response to Campylobacter LOS, humans and chickens may also differ in their ability to detect and respond to Campylobacter DNA.
FIG. 6.
Activation of human TLR9 and chicken TLR21 by Campylobacter. HEK293 cells expressing human TLR9 (A) or HeLa 57A cells expressing chicken TLR21 (B) were stimulated with 30 μg of purified chromosomal DNA ml−1 from C. jejuni strain 81116, GB18, or RM1221 or C. coli strain H1 for 5 h. Cells transfected with control vector were stimulated simultaneously to ensure TLR-specific NF-κB activation. ODN 2006 (0.5 μM) was used as positive control. None of the chromosomal Campylobacter DNA significantly activated TLR9. In contrast, DNA from all Campylobacter strains induced significant NF-κB activation in TLR21-transfected HEK 293 cells (P < 0.05). Values are the percent induction of NF-κB activation after stimulation with the positive control and are means ± the SEM of three independent experiments.
DISCUSSION
Although Campylobacter is the most common cause of human bacterial enteritis, the mechanism that induces inflammation remains obscure. Similarly, the characteristics that determine its lifestyle as a harmless commensal in many animals, including chickens, are still to be elucidated (54). In many bacterial infections, the level of recognition by the innate immune system of the host plays a critical role in determining whether or not pathology is induced. For instance, Yersinia pestis normally expresses an LPS that is not recognized by mammalian TLR4. In strains that artificially express TLR4-stimulating LPS, virulence is completely overcome by an activated host immune system (39). Likewise, studies with knockout mice clearly show the need for TLR activation to control bacterial virulence: TLR5 knockout mice exhibit increased bacterial load and inflammation in the intestine (56), TLR9 deficiency leads to uncontrolled Neisseria meningitidis infection (47), and MyD88-deficient mice are more sensitive to a wide range of bacterial infections (16, 36, 51). The extent to which TLR activation influences Campylobacter infection in humans and animal species is currently unknown. We present here the first systematic comparison of the human and chicken TLR response to Campylobacter. We provide evidence of Campylobacter activation of human and chicken TLR2 and TLR4 and the lack of activation of human and chicken TLR5. Major differences in the human and chicken TLR response to Campylobacter are the failure of chicken cells to produce IFN-β after LOS stimulation in contrast to human cells and the activation of chicken TLR21 but not human TLR9 by Campylobacter chromosomal DNA. Another striking result is the poor activation of TLRs by viable Campylobacter compared to lysed bacteria. This may indicate bacterial integrity in the intestine as a potential determinant of inflammatory pathology.
The activation of the human and chicken TLR2 complexes by Campylobacter indicates that Campylobacter lipoproteins can act as TLR2 ligand and are recognized by both species. Based on genome sequence analysis C. jejuni is predicted to express up to 40 different lipoproteins and to produce both di- and triacylated lipoproteins (M. R. de Zoete, unpublished results). Humans can differentiate between di- and triacylated lipoproteins by utilizing either TLR2-TLR1 or TLR2-TLR6 combinations. Chickens have two versions of TLR2 (TLR2t1 and TLR2t2) that differ mainly in a 192-amino-acid region in the central region of the ligand-binding domain), one TLR16 protein (also known as TLR1t1 and TLR1LA), and a truncated TLR1t2 protein (also known as TLR1LB) (11, 52). The combinations of chicken TLR2t2/TLR16 and chicken TLR2t1/TLR1LB sense both di- and triacylated lipopeptides, whereas the TLR2t1/TLR16 complex only seems to respond to high concentrations of triacylated peptide (22, 28). The fact that both di- and/or triacylated peptides appear to be recognized by the different TLR2 complexes likely explains the TLR2 response to Campylobacter in humans and chicken.
For TLR4, differences in LPS specificity between humans and chickens are reported (30). We found that Campylobacter induced potent NF-κB activation via human TLR4/MD-2 and chicken TLR4/MD-2 (Fig. 3). One striking difference between humans and chickens, however, was the absence of C. jejuni-induced IFN-β upregulation through TLR4/MD-2 in chicken cells. Stimulation of these cells with the TLR3 ligand poly(I:C) yielded a potent IFN-β response, indicating that the cells are capable of producing this cytokine after TLR activation. In mammals, IFN-β is induced through TLR4 via the MyD88-independent TRIF pathway (53). At present, the adapter protein TRAM, which links the TLR4 signaling domain to the TRIF protein, has not been identified in the chicken genome (30), which may explain the inability to induce a TLR4/MD-2-dependent IFN-β response in this species. IFN-β is a key inducer of systemic inflammation, illustrated by the complete resistance to endotoxic shock in IFN-β knockout mice (27). Also, TLR4-induced IFN-β signaling is critical to control the growth of the intracellular bacterium Chlamydia pneumoniae by enhancing the production of IFN-γ, the major macrophage-activating cytokine (46). The impact of the absence of this pathway during Campylobacter colonization of chickens is unclear. Although additional research is required to determine the precise role of the type I and II IFNs in bacterial infection in chickens, it can be speculated that a relative insensitivity for LOS-induced systemic inflammation and macrophage activation may help to retain the commensal nature of Campylobacter in the chicken gut.
TLR5 signaling plays an important role in preventing intestinal pathology, as demonstrated by TLR5-deficient mice, which develop spontaneous intestinal colitis (56). The successful evasion of human TLR5 by Campylobacter (Fig. 5) (3, 58) may contribute to intestinal inflammation. Likewise, the lack of intestinal pathology in chickens could be the result of recognition of Campylobacter flagellin by the chicken version of TLR5. However, our data indicate equal unresponsiveness of TLR5 from both species to Campylobacter flagellin. Therefore, TLR5 evasion seems unlikely to be a major cause of the difference in inflammation between infected humans and chickens.
While the ability of CpG DNA to activate chicken cells has been reported for years (18, 59), the receptor mediating this activation remained elusive due to the absence of a TLR9 homologue. The recent identification of chicken TLR21 as the receptor that senses DNA presented for the first time the opportunity to test the stimulatory potential of Campylobacter chromosomal DNA. TLR21 was activated by chromosomal DNA of all tested Campylobacter strains (Fig. 6). In contrast, HEK293 cells transfected with human TLR9 did not show an increase in NF-κB activation, although the cells did respond to the TLR9 ligand ODN 2006. These results substantiate that C. jejuni DNA is a very weak TLR9 ligand (12). The recognition of both synthetic ODN 2006 and bacterial chromosomal DNA suggest broad ligand specificity for chTLR21. In contrast, mammalian TLR9 (human and murine TLR9) display species-specific ligand recognition and show distinctive responses to ODN 2006. Although highly speculative, these results may indicate that during evolution the presence of a stronger selective advantage for broad recognition of DNA ligands resulted in a divergence between mammals and birds, which may have contributed to the loss of the ligand-specific TLR9 and the gain of the less-specific TLR21 receptor in chickens (44, 52). The role of TLR21 activation by Campylobacter during the colonization of chickens remains speculative. It can be imagined that in the nutrient-rich environment of the chicken cecum, bacterial lysis and thus the availability of TLR21 ligand is limited. Alternatively, it can be argued that activation of this receptor may induce an effective but mild local immune response that helps to retain the host-microbe balance. Lacking a response to Campylobacter DNA may result in spread of the bacteria to the deeper tissue, resulting in an uncontrolled infection and enteritis. In addition, as DNA signaling is known to provide strong adjuvant activity in both humans and chickens (10, 19, 35, 37), it can be speculated that the differential TLR activation by Campylobacter DNA may influence the host antibody response during infection. However, much more knowledge of the in vivo expression and function of TLR9 and TLR21 in the guts of humans and chickens is needed to determine the exact role of the bacterial DNA as a determinant of infection.
Another major conclusion of our work is that live bacteria induce relatively weak TLR responses compared to disrupted bacteria. This observation was noted for both defined TLR2 and TLR4 responses and for MM6 cells and, to a lesser extent, HD11 cells that express multiple TLR receptors. The weak immune response of intact bacteria could not be contributed to a lack of physical interaction between the bacteria and the TLRs on the cell surface, since centrifugation of Campylobacter onto TLR-expressing HeLa 57A cells did not result in increased levels of TLR activation (data not shown). When only heat-killed bacteria were used, the overall responses were significantly lower (de Zoete, unpublished) than with disrupted bacteria. This suggests that bacterial lysis may be needed to expose their full TLR-stimulative potential. In a hostile environment, Campylobacter will likely be disrupted, e.g., after exposure to antimicrobial peptides or after phagocytosis by macrophages and dendritic cells. It is currently unknown whether Campylobacter viability and lysis is different in the human gut compared to the nutrient-rich environment of the chicken cecum and thus may contribute to the difference in intestinal inflammatory response.
Taken together, our results indicate that Campylobacter is sensed by chicken TLRs in a fashion largely comparable to that of human TLRs. Major differences in the TLR responses are the lack of MyD88-independent IFN-β production in chickens after TLR4 activation and the potent activation of chTLR21 by chromosomal Campylobacter DNA. Together with the apparent requirement for bacterial cell lysis for strong innate immune activation, these data may provide a valuable basis for further elucidation of the basis for the different outcomes of Campylobacter infection of human and chicken intestines.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 28 December 2009.
REFERENCES
- 1.Akira, S. 2006. TLR signaling. Curr. Top. Microbiol. Immunol. 311:1-16. [DOI] [PubMed] [Google Scholar]
- 2.Allos, B. M. 2001. Campylobacter jejuni Infections: update on emerging issues and trends. Clin. Infect. Dis. 32:1201-1206. [DOI] [PubMed] [Google Scholar]
- 3.Andersen-Nissen, E., K. D. Smith, K. L. Strobe, S. L. Barrett, B. T. Cookson, S. M. Logan, and A. Aderem. 2005. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl. Acad. Sci. U. S. A. 102:9247-9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakhiet, M., F. S. Al-Salloom, A. Qareiballa, K. Bindayna, I. Farid, and G. A. Botta. 2004. Induction of alpha and beta chemokines by intestinal epithelial cells stimulated with Campylobacter jejuni. J. Infect. 48:236-244. [DOI] [PubMed] [Google Scholar]
- 5.Beery, J. T., M. B. Hugdahl, and M. P. Doyle. 1988. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 54:2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beug, H., A. von Kirchbach, G. Doderlein, J. F. Conscience, and T. Graf. 1979. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell 18:375-390. [DOI] [PubMed] [Google Scholar]
- 7.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 8.Borrmann, E., A. Berndt, I. Hanel, and H. Kohler. 2007. Campylobacter-induced interleukin-8 responses in human intestinal epithelial cells and primary intestinal chick cells. Vet. Microbiol. 124:115-124. [DOI] [PubMed] [Google Scholar]
- 9.Brownlie, R., J. Zhu, B. Allan, G. K. Mutwiri, L. A. Babiuk, A. Potter, and P. Griebel. 2009. Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligodeoxynucleotides. Mol. Immunol. [DOI] [PubMed]
- 10.Chu, R. S., O. S. Targoni, A. M. Krieg, P. V. Lehmann, and C. V. Harding. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cormican, P., A. T. Lloyd, T. Downing, S. J. Connell, D. Bradley, and C. O'Farrelly. 2009. The avian Toll-Like receptor pathway-subtle differences amidst general conformity. Dev. Comp. Immunol. 33:967-973. [DOI] [PubMed] [Google Scholar]
- 12.Dalpke, A., J. Frank, M. Peter, and K. Heeg. 2006. Activation of Toll-like receptor 9 by DNA from different bacterial species. Infect. Immun. 74:940-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhillon, A. S., H. L. Shivaprasad, D. Schaberg, F. Wier, S. Weber, and D. Bandli. 2006. Campylobacter jejuni infection in broiler chickens. Avian Dis. 50:55-58. [DOI] [PubMed] [Google Scholar]
- 14.Endtz, H. P., C. W. Ang, N. van Den Braak, B. Duim, A. Rigter, L. J. Price, D. L. Woodward, F. G. Rodgers, W. M. Johnson, J. A. Wagenaar, B. C. Jacobs, H. A. Verbrugh, and A. van Belkum. 2000. Molecular characterization of Campylobacter jejuni from patients with Guillain-Barre and Miller Fisher syndromes. J. Clin. Microbiol. 38:2297-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erridge, C., E. Bennett-Guerrero, and I. R. Poxton. 2002. Structure and function of lipopolysaccharides. Microbes Infect. 4:837-851. [DOI] [PubMed] [Google Scholar]
- 16.Feng, C. G., C. A. Scanga, C. M. Collazo-Custodio, A. W. Cheever, S. Hieny, P. Caspar, and A. Sher. 2003. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J. Immunol. 171:4758-4764. [DOI] [PubMed] [Google Scholar]
- 17.Friis, L. M., M. Keelan, and D. E. Taylor. 2009. Campylobacter jejuni drives MyD88-independent interleukin-6 secretion via Toll-like receptor 2. Infect. Immun. 77:1553-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He, H., T. L. Crippen, M. B. Farnell, and M. H. Kogut. 2003. Identification of CpG oligodeoxynucleotide motifs that stimulate nitric oxide and cytokine production in avian macrophage and peripheral blood mononuclear cells. Dev. Comp. Immunol. 27:621-627. [DOI] [PubMed] [Google Scholar]
- 19.He, H., V. K. Lowry, C. L. Swaggerty, P. J. Ferro, and M. H. Kogut. 2005. In vitro activation of chicken leukocytes and in vivo protection against Salmonella enteritidis organ invasion and peritoneal S. enteritidis infection-induced mortality in neonatal chickens by immunostimulatory CpG oligodeoxynucleotide. FEMS Immunol. Med. Microbiol. 43:81-89. [DOI] [PubMed] [Google Scholar]
- 20.Hickey, T. E., S. Baqar, A. L. Bourgeois, C. P. Ewing, and P. Guerry. 1999. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infect. Immun. 67:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hickey, T. E., A. L. McVeigh, D. A. Scott, R. E. Michielutti, A. Bixby, S. A. Carroll, A. L. Bourgeois, and P. Guerry. 2000. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect. Immun. 68:6535-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higuchi, M., A. Matsuo, M. Shingai, K. Shida, A. Ishii, K. Funami, Y. Suzuki, H. Oshiumi, M. Matsumoto, and T. Seya. 2008. Combinational recognition of bacterial lipoproteins and peptidoglycan by chicken Toll-like receptor 2 subfamily. Dev. Comp. Immunol. 32:147-155. [DOI] [PubMed] [Google Scholar]
- 23.Hu, L., M. D. Bray, M. Osorio, and D. J. Kopecko. 2006. Campylobacter jejuni induces maturation and cytokine production in human dendritic cells. Infect. Immun. 74:2697-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim, G. F., G. H. Fleet, M. J. Lyons, and R. A. Walker. 1985. Method for the isolation of highly purified Salmonella flagellins. J. Clin. Microbiol. 22:1040-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johanesen, P. A., and M. B. Dwinell. 2006. Flagellin-independent regulation of chemokine host defense in Campylobacter jejuni-infected intestinal epithelium. Infect. Immun. 74:3437-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, M. A., S. Totemeyer, D. J. Maskell, C. E. Bryant, and P. A. Barrow. 2003. Induction of proinflammatory responses in the human monocytic cell line THP-1 by Campylobacter jejuni. Infect. Immun. 71:2626-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karaghiosoff, M., R. Steinborn, P. Kovarik, G. Kriegshauser, M. Baccarini, B. Donabauer, U. Reichart, T. Kolbe, C. Bogdan, T. Leanderson, D. Levy, T. Decker, and M. Muller. 2003. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat. Immunol. 4:471-477. [DOI] [PubMed] [Google Scholar]
- 28.Keestra, A. M., M. R. de Zoete, R. A. van Aubel, and J. P. M. van Putten. 2007. The central leucine-rich repeat region of chicken TLR16 dictates unique ligand specificity and species-specific interaction with TLR2. J. Immunol. 178:7110-7119. [DOI] [PubMed] [Google Scholar]
- 29.Keestra, A. M., M. R. de Zoete, R. A. van Aubel, and J. P. M. van Putten. 2008. Functional characterization of chicken TLR5 reveals species-specific recognition of flagellin. Mol. Immunol. 45:1298-1307. [DOI] [PubMed] [Google Scholar]
- 30.Keestra, A. M., and J. P. M. van Putten. 2008. Unique properties of the chicken TLR4/MD-2 complex: selective lipopolysaccharide activation of the MyD88-dependent pathway. J. Immunol. 181:4354-4362. [DOI] [PubMed] [Google Scholar]
- 31.Kopp, E., and R. Medzhitov. 2003. Recognition of microbial infection by Toll-like receptors. Curr. Opin. Immunol. 15:396-401. [DOI] [PubMed] [Google Scholar]
- 32.Larson, C. L., D. H. Shah, A. S. Dhillon, D. R. Call, S. Ahn, G. J. Haldorson, C. Davitt, and M. E. Konkel. 2008. Campylobacter jejuni invade chicken LMH cells inefficiently and stimulate differential expression of the chicken CXCLi1 and CXCLi2 cytokines. Microbiology 154:3835-3847. [DOI] [PubMed] [Google Scholar]
- 33.Lee, M. D., and D. G. Newell. 2006. Campylobacter in poultry: filling an ecological niche. Avian Dis. 50:1-9. [DOI] [PubMed] [Google Scholar]
- 34.Li, Y. P., H. Ingmer, M. Madsen, and D. D. Bang. 2008. Cytokine responses in primary chicken embryo intestinal cells infected with Campylobacter jejuni strains of human and chicken origin and the expression of bacterial virulence-associated genes. BMC Microbiol. 8:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahmood, M. S., M. Siddique, I. Hussain, A. Khan, and M. K. Mansoor. 2006. Protection capability of recombinant plasmid DNA vaccine containing VP2 gene of very virulent infectious bursal disease virus in chickens adjuvanted with CpG oligodeoxynucleotide. Vaccine 24:4838-4846. [DOI] [PubMed] [Google Scholar]
- 36.Mancuso, G., A. Midiri, C. Beninati, C. Biondo, R. Galbo, S. Akira, P. Henneke, D. Golenbock, and G. Teti. 2004. Dual role of TLR2 and myeloid differentiation factor 88 in a mouse model of invasive group B streptococcal disease. J. Immunol. 172:6324-6329. [DOI] [PubMed] [Google Scholar]
- 37.McCluskie, M. J., and A. M. Krieg. 2006. Enhancement of infectious disease vaccines through TLR9-dependent recognition of CpG DNA. Curr. Top. Microbiol. Immunol. 311:155-178. [DOI] [PubMed] [Google Scholar]
- 38.Meade, K. G., F. Narciandi, S. Cahalane, C. Reiman, B. Allan, and C. O'Farrelly. 2009. Comparative in vivo infection models yield insights on early host immune response to Campylobacter in chickens. Immunogenetics 61:101-110. [DOI] [PubMed] [Google Scholar]
- 39.Montminy, S. W., N. Khan, S. McGrath, M. J. Walkowicz, F. Sharp, J. E. Conlon, K. Fukase, S. Kusumoto, C. Sweet, K. Miyake, S. Akira, R. J. Cotter, J. D. Goguen, and E. Lien. 2006. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 7:1066-1073. [DOI] [PubMed] [Google Scholar]
- 40.Parker, C. T., B. Quinones, W. G. Miller, S. T. Horn, and R. E. Mandrell. 2006. Comparative genomic analysis of Campylobacter jejuni strains reveals diversity due to genomic elements similar to those present in C. jejuni strain RM1221. J. Clin. Microbiol. 44:4125-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasare, C., and R. Medzhitov. 2003. Toll-like receptors: balancing host resistance with immune tolerance. Curr. Opin. Immunol. 15:677-682. [DOI] [PubMed] [Google Scholar]
- 42.Pearson, B. M., D. J. Gaskin, R. P. Segers, J. M. Wells, P. J. Nuijten, and A. H. van Vliet. 2007. The complete genome sequence of Campylobacter jejuni strain 81116 (NCTC11828). J. Bacteriol. 189:8402-8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rathinam, V. A., D. M. Appledorn, K. A. Hoag, A. Amalfitano, and L. S. Mansfield. 2009. Campylobacter jejuni-induced activation of dendritic cells involves cooperative signaling through Toll-like receptor 4 (TLR4)-MyD88 and TLR4-TRIF axes. Infect. Immun. 77:2499-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roach, J. C., G. Glusman, L. Rowen, A. Kaur, M. K. Purcell, K. D. Smith, L. E. Hood, and A. Aderem. 2005. The evolution of vertebrate Toll-like receptors. Proc. Natl. Acad. Sci. U. S. A. 102:9577-9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez, M. S., J. Thompson, R. T. Hay, and C. Dargemont. 1999. Nuclear retention of IκBα protects it from signal-induced degradation and inhibits nuclear factor κB transcriptional activation. J. Biol. Chem. 274:9108-9115. [DOI] [PubMed] [Google Scholar]
- 46.Rothfuchs, A. G., D. Gigliotti, K. Palmblad, U. Andersson, H. Wigzell, and M. E. Rottenberg. 2001. IFN-α/β-dependent, IFN-γ secretion by bone marrow-derived macrophages controls an intracellular bacterial infection. J. Immunol. 167:6453-6461. [DOI] [PubMed] [Google Scholar]
- 47.Sjolinder, H., T. H. Mogensen, M. Kilian, A. B. Jonsson, and S. R. Paludan. 2008. Important role for Toll-like receptor 9 in host defense against meningococcal sepsis. Infect. Immun. 76:5421-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith, C. K., M. Abuoun, S. A. Cawthraw, T. J. Humphrey, L. Rothwell, P. Kaiser, P. A. Barrow, and M. A. Jones. 2008. Campylobacter colonization of the chicken induces a proinflammatory response in mucosal tissues. FEMS Immunol. Med. Microbiol. 54:114-121. [DOI] [PubMed] [Google Scholar]
- 49.Smith, C. K., P. Kaiser, L. Rothwell, T. Humphrey, P. A. Barrow, and M. A. Jones. 2005. Campylobacter jejuni-induced cytokine responses in avian cells. Infect. Immun. 73:2094-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steeghs, L., A. M. Keestra, A. van Mourik, H. Uronen-Hansson, P. van der Ley, R. Callard, N. Klein, and J. P. M. van Putten. 2008. Differential activation of human and mouse Toll-like receptor 4 by the adjuvant candidate LpxL1 of Neisseria meningitidis. Infect. Immun. 76:3801-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 52.Temperley, N. D., S. Berlin, I. R. Paton, D. K. Griffin, and D. W. Burt. 2008. Evolution of the chicken Toll-like receptor gene family: a story of gene gain and gene loss. BMC Genomics 9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uematsu, S., and S. Akira. 2007. Toll-like receptors and type I interferons. J. Biol. Chem. 282:15319-15323. [DOI] [PubMed] [Google Scholar]
- 54.van Putten, J. P. M., L. B. van Alphen, M. M. S. M. Wösten, and M. R. de Zoete. 2009. Molecular mechanisms of Campylobacter infection. Curr. Top. Microbiol. Immunol. 337:197-229. [DOI] [PubMed] [Google Scholar]
- 55.Vijay-Kumar, M., J. D. Aitken, and A. T. Gewirtz. 2008. Toll-like receptor-5: protecting the gut from enteric microbes. Semin. Immunopathol. 30:11-21. [DOI] [PubMed] [Google Scholar]
- 56.Vijay-Kumar, M., C. J. Sanders, R. T. Taylor, A. Kumar, J. D. Aitken, S. V. Sitaraman, A. S. Neish, S. Uematsu, S. Akira, I. R. Williams, and A. T. Gewirtz. 2007. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Invest. 117:3909-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker, R. I., M. B. Caldwell, E. C. Lee, P. Guerry, T. J. Trust, and G. M. Ruiz-Palacios. 1986. Pathophysiology of Campylobacter enteritis. Microbiol. Rev. 50:81-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watson, R. O., and J. E. Galan. 2005. Signal transduction in Campylobacter jejuni-induced cytokine production. Cell Microbiol. 7:655-665. [DOI] [PubMed] [Google Scholar]
- 59.Xie, H., R. B. Raybourne, U. S. Babu, H. S. Lillehoj, and R. A. Heckert. 2003. CpG-induced immunomodulation and intracellular bacterial killing in a chicken macrophage cell line. Dev. Comp. Immunol. 27:823-834. [DOI] [PubMed] [Google Scholar]
- 60.Zheng, J., J. Meng, S. Zhao, R. Singh, and W. Song. 2008. Campylobacter-induced interleukin-8 secretion in polarized human intestinal epithelial cells requires Campylobacter-secreted cytolethal distending toxin- and Toll-like receptor-mediated activation of NF-κB. Infect. Immun. 76:4498-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ziegler-Heitbrock, H. W., E. Thiel, A. Futterer, V. Herzog, A. Wirtz, and G. Riethmuller. 1988. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int. J. Cancer 41:456-461. [DOI] [PubMed] [Google Scholar]