Abstract
We have previously shown that one of the minimal active regions of statherin, a human salivary protein, for binding to Fusobacterium nucleatum is a YQPVPE amino acid sequence. In this study, we identified the FomA protein of F. nucleatum, which is responsible for binding to the statherin-derived YQPVPE peptide. Overlay analysis showed that a 40-kDa protein of the F. nucleatum cell envelope (40-kDa CE) specifically bound to the YQPVPE peptide. The equilibrium association constant between the affinity-purified 40-kDa CE and the YQPVPE peptide was 4.30 × 106. Further, the purity and amino acid sequence analyses of the purified 40-kDa CE revealed approximately 98.7% (wt/wt) purity and a high degree of homology with FomA, a major porin protein of F. nucleatum. Thus, a FomA-deficient mutant failed to bind to the YQPVPE peptide. In addition, increased levels of a FomA-specific mucosal IgA antibody (Ab) and plasma IgG and IgA Abs were seen only in mice immunized nasally with cholera toxin (CT) and the purified 40-kDa FomA protein. Interestingly, saliva from mice that received FomA plus CT as a mucosal adjuvant nasally prevented in vitro binding of F. nucleatum to statherin-coated polyvinyl chloride plates. Taken together, these results suggest that induction of specific immunity to the 40-kDa FomA protein of F. nucleatum, which specifically binds to the statherin-derived peptide, may be an effective tool for preventing the formation of F. nucleatum biofilms in the oral cavity.
Bacterial adherence is often an essential first step in the colonization and establishment of an infection in a susceptible host. Thus, the adherence of bacteria is itself an important virulence factor. Fusobacterium nucleatum is a Gram-negative anaerobe that plays a pivotal role in early colonization during dental plaque formation. Thus, it is known that F. nucleatum bridges not only salivary proteins and other coaggregating oral bacterial strains but also early and late colonizers in the oral cavity (4, 22). In addition, it has been shown that the presence of F. nucleatum is a predisposing factor for some systemic diseases, such as urinary tract infections (32) and intrauterine infections associated with preterm birth (23), as well as for oral diseases, including alveolar abscesses (35) and periodontal disease (18). One may assume that the oral cavity, which is covered with a mucosal membrane, is the most likely portal of entry into the host for this pathogenic organism.
Among human salivary proteins, statherin is known as a unique acidic, carbohydrate-free phosphoprotein (14) that inhibits the primary and secondary precipitation of calcium salts. In addition, statherin is tightly adsorbed to enamel surfaces (13) and facilitates adhesion by F. nucleatum (41), Actinomyces viscosus (28), Actinomyces naeslundii (28), and Porphyromonas gingivalis (1, 2) to its preadsorbed hydroxyapatite surface. Our previous study also showed that of all human salivary proteins, statherin exhibited the strongest ability to bind to F. nucleatum cell surface protein (33).
In order to elicit maximal levels of antigen (Ag)- or pathogen-specific immune responses in both mucosal and systemic lymphoid tissue compartments, it is necessary to use an appropriate mucosal adjuvant (9). Nasal immunization has emerged as perhaps the most effective regimen for inducing both peripheral and mucosal immunity, including salivary secretory IgA (S-IgA) antibody (Ab) responses (25). It is now well accepted that cholera toxin (CT), produced by Vibrio cholerae, is the most potent mucosal adjuvant for the induction of Ag-specific Ab responses when coadministered with a protein Ag (42). Thus, detailed studies have shown that CT induces CD4+ T helper type 2 (Th2) cells for the induction of mucosal S-IgA, systemic subclass IgG1 and IgG2b, and systemic IgE Ab responses (38, 42, 44). Among Th2 cytokines, interleukin-4 (IL-4) notably plays a key role in the mucosal adjuvanticity of CT (29, 39). Further, our recent study showed that nasal application of CT as an adjuvant enhanced mucosal S-IgA Ab responses to a T-cell-independent Ag through cross talk between IL-5 receptor-positive (IL-5R+) B-1a B cells and IL-5-producing CD4+ T cells (15).
We have previously shown that two amino acid alignments (YQPVPE and PYQPQYQ) on the statherin molecule are the most likely segments that bind to F. nucleatum (33). In this study, we show that the 40-kDa FomA protein of F. nucleatum is responsible for interaction with the YQPVPE peptide in the active binding segment of the statherin molecule. In addition, we examined whether induction of FomA-specific Ab responses could prevent the binding of F. nucleatum to the YQPVPE peptide. The outcomes of these studies could lead to the development of effective strategies for the prevention of F. nucleatum adherence and infection as well as for the prevention of its associated diseases.
MATERIALS AND METHODS
Bacterial culture conditions and radiolabeling.
F. nucleatum ATCC 25586 (wild type) was grown in Trypticase soy broth (Becton Dickinson, Sunnyvale, CA) supplemented with yeast extract (1 mg/ml), hemin (5 μg/ml), and menadione (1 μg/ml) (TSB medium) at 37°C under anaerobic conditions (80% N2, 10% CO2, 10% H2) (33). Before the bacteria were harvested, the cells were washed three times with 50 mM phosphate-buffered saline (PBS; pH 7.2) and were suspended in the same buffer. In some experiments, the harvested cells were radiolabeled with the Bolton-Hunter reagent kit (PerkinElmer Japan Co., Ltd., Yokohama, Japan). The specific activity of the iodinated protein was 1.7 mCi (58.1 MBq) per 109 cells.
FomA-deficient mutant of F. nucleatum.
The internal sequence of the fomA gene, which encodes the major porin protein of F. nucleatum, was amplified by PCR. The primer sequences used for PCR were 5′-CGG GAT CCG CTC CAG CTT GGA GAC CAA ATG G-3′ (primer fomABamF1) and 5′-GGA ATT CCC AAC AAC TCC ACT ATT ATG TCC-3′ (primer fomAEcoR2). These primers were inserted at BamHI or EcoRI restriction sites, respectively. The PCR product was digested with BamHI and EcoRI and was then ligated into the suicide vector pSF151 (30). The resultant plasmid, pFOMA151, was electrotransformed into F. nucleatum strain ATCC 25586. Cells were electroporated by a method described previously, with minor modifications (8). Briefly, the harvested F. nucleatum cells were washed with 10% glycerol, and the resultant plasmid, pFOMA151 (5 μg/100 μl of cell suspension), was pulsed with a Bio-Rad gene pulser II (1.8 kV, 25 μF, 400 Ω, and 7 ms) into the competent cells, which were then incubated on ice for 5 min. The cell suspension was then added to 4.0 ml of TSB medium and was incubated at 37°C overnight. The ΔfomA mutant strain SN-3 was subsequently isolated on kanamycin-containing agar plates.
Mice.
Female C57BL/6 mice (6 to 8 weeks old) were purchased from Japan SLC, Inc. (Hamamatsu, Japan). These mice were maintained in the experimental animal facility at Osaka University (Suita, Japan), and all experiments were conducted in accordance with the guidelines provided by the Osaka University Graduate School of Dentistry Animal Care Committee.
Preparation of bacterial envelopes and biotinylation of the synthesized peptide.
F. nucleatum whole cell envelopes (CEs) were prepared by a method described previously, with minor modifications (36). Briefly, the harvested cell suspension was ultrasonicated in an ice bath at 1-min intervals with an ultrasonic disrupter (UR-200P; TOMY Seiko, Tokyo, Japan) emitting 200 W and was allowed to cool for 1 min between ultrasonic treatments. After undisrupted cells were removed by centrifugation (1,000 × g), the supernatants were centrifuged at 100,000 × g for 90 min in order to recover the whole CEs as a sediment. The fraction containing whole CEs was washed three times with 50 mM PBS (pH 7.2) followed by distilled water and was then lyophilized. Analogous peptides (YQPVPE) corresponding to the amino acid sequence of statherin were commercially synthesized and purified at the Sequence and Peptide Synthesis Facility of Aves Labs, Inc. (Tigard, OR). The amino acid sequences and mass values of products were confirmed by mass spectroscopy as well as analytical high-pressure liquid chromatography. The synthesized YQPVPE peptide was biotinylated by using the ECL protein biotinylation module according to the manufacturer's instructions (GE Healthcare UK Ltd., Buckinghamshire, United Kingdom).
Western blot ligand overlay assay.
In order to examine whether F. nucleatum whole CEs possessed the ability to bind the YQPVPE peptide, a ligand overlay assay was performed using the biotinylated YQPVPE peptide (33). F. nucleatum whole CEs were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 15% polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA) and were then transferred to a nitrocellulose membrane. The membrane was then incubated with 1 mg/ml of a PBS solution of the biotinylated YQPVPE peptide at 4°C overnight, followed by incubation with horseradish peroxidase (HRP)-conjugated streptavidin (GE Healthcare UK Ltd.). The F. nucleatum CEs that were bound to the YQPVPE peptide were detected by using an HRP conjugate substrate kit (Bio-Rad Laboratories).
Purification of the F. nucleatum CE protein that binds to the YQPVPE peptide.
The YQPVPE peptide-conjugated affinity column was prepared according to the manufacturer's instructions for CNBr-activated Sepharose 4B (GE Healthcare UK Ltd.) in order to purify CEs binding to the YQPVPE peptide. In brief, lyophilized whole CEs dissolved in 10 mM Tris-HCl buffer (pH 6.7) were applied to the YQPVPE affinity column. The column was washed with 10 volumes of Tris-HCl (pH 6.7), and the components bound to the affinity column were eluted with Tris-HCl containing 0.5 M NaCl. The affinity column purification was repeated in order to obtain highly purified CE. Purified CEs were subjected to the ligand overlay assay in order to confirm their ability to bind to the YQPVPE peptide. The purity (wt/wt) of the YQPVPE peptide-binding CE was calculated by the Experion system (Bio-Rad Laboratories) based on SDS-PAGE.
Biomolecular interaction analysis.
The affinity of binding between the YQPVPE peptide-binding F. nucleatum CE and the synthesized YQPVPE peptide was analyzed by a BIAcore model X system (GE Healthcare UK Ltd.) as described previously (37). One hundred micrograms per milliliter of purified YQPVPE peptide-binding CE or bovine serum albumin (BSA) (Sigma, St. Louis, MO) diluted in 10 nM sodium acetate (pH 4.0) was applied to the surface of a CM5 sensor chip (GE Healthcare UK Ltd.). The synthesized YQPVPE peptide, as an analyte, was diluted with HBSP buffer (10 mM HEPES, 150 mM NaCl, 3.4 mM EDTA, and 0.005% Tween 20 [pH 7.4]) and was then injected at a flow rate of 30 μl/min at 25°C. The concentration of the analyte was adjusted to 1.3, 2.5, 5, 10, and 20 mM. Binding between the purified CE or BSA and the YQPVPE peptide was monitored and presented as resonance units (RU) in a sensorgram. Analysis of these kinetic parameters was conducted with the BIAevaluation software package, version 3.1 (GE Healthcare UK Ltd.), according to the operator's manual.
Amino acid sequence analysis of a 40-kDa CE protein purified by YQPVPE peptide-conjugated column chromatography.
A 40-kDa CE protein purified by YQPVPE peptide-conjugated column chromatography was subjected to amino acid sequence analysis (Nippi Research Institute, Biomatrix, Tokyo, Japan). The amino-terminal sequences of three fragments digested with V8 protease were determined by the Edman degradation method (6) and were deposited in the BLASTp database.
Dot blot assays for binding to the YQPVPE peptide.
To examine the direct binding of wild-type F. nucleatum and the ΔfomA mutant strain SN-3 to the YQPVPE peptide, a dot blot assay was performed as described previously (33). Briefly, cells of both wild-type F. nucleatum and the ΔfomA mutant strain SN-3 were radiolabeled with 125I using a Bolton-Hunter reagent kit (PerkinElmer Japan Co., Ltd.). The specific activity of the iodinated protein was 1.8 mCi (59.2 MBq) per 109 cells. The YQPVPE peptide (1 mM, 100 μl) or lipid-free BSA (1 mM, 100 μl), dissolved in KCl buffer (50 mM KCl solution containing 1 mM KH2PO4, 1 mM CaCl2, and 0.1 mM MgCl2 [pH 6.8]), was immobilized on an Immobilon-P polyvinylidene difluoride (PVDF) membrane with a Bio-Dot apparatus (both from Bio-Rad Laboratories). The membrane was blocked with 5% Block Ace and was incubated overnight at room temperature with 3 ml of 125I-labeled wild-type F. nucleatum or the 125I-labeled ΔfomA mutant strain SN-3. Subsequently, the membrane was washed with KCl buffer containing 100 mM NaCl and was subjected to autoradiography. The binding activity toward the YQPVPE peptide or BSA was determined by the relative densitometric analysis of reaction dots by a PowerPC Mac with the NIH Image program, version 1.6.2 (National Information Service).
Nasal immunization with the F. nucleatum FomA protein.
In order to harvest the lipopolysaccharide (LPS)-free purified FomA protein as an immunogen, lyophilized whole CEs were applied three times to the YQPVPE peptide-conjugated purification column, and the LPS was further removed using a polymyxin B affinity column immobilized on cross-linked 4% agarose beads (Sigma) according to the manufacturer's instructions. The concentration of residual endotoxin was determined by a Limulus ES-II test Wako kit (Wako Pure Chemical Industries, Osaka, Japan). Mice were nasally immunized under anesthesia with 20 μg of this purified LPS-free FomA protein (6 μl per nostril), with or without native CT (1 μg) (List Biological Laboratories Inc., Campbell, CA), three times at weekly intervals.
Sample collection.
Plasma and stimulated saliva were collected on days 0 and 21. Stimulated-saliva samples were collected following intraperitoneal injection of mice with pilocarpine (Sigma) as described previously (16, 42). Mice were sacrificed 7 days after the last immunization (day 21), and nasal wash specimens were obtained by instillation of 1 ml of PBS three times into the posterior opening of the nasopharynx with a 30-gauge hypodermic needle (11).
Mucosal and systemic 40-kDa FomA-specific Ab assays.
Levels of FomA-specific Abs in plasma and external secretions were determined by an enzyme-linked immunosorbent assay (ELISA) (11, 43). Briefly, 96-well Falcon microtest assay plates (BD Biosciences, San Jose, CA) were coated with 1 μg/ml of purified FomA protein in PBS. After wells were blocked with 1% BSA in PBS, 2-fold serial dilutions of samples were added and incubated overnight at 4°C. HRP-labeled goat anti-mouse μ, γ, or α heavy-chain-specific Abs (Southern Biotechnology Associates, Birmingham, AL) were added to individual wells, followed by three washes with PBS-Tween 20 (0.05%). For IgG Ab subclass analysis, biotinylated monoclonal Abs (MAbs) specific for IgG1, IgG2a, IgG2b, or IgG3 (BD Biosciences PharMingen, Franklin Lakes, NJ) and a peroxidase-conjugated goat antibiotin Ab (Vector Laboratories, Burlingame, CA) were used for detection. The color reaction was developed for 15 min at room temperature with 100 μl of 1.1 mM 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid). End point titers were expressed as the reciprocal log2 of the last dilution that gave an optical density at 415 nm 0.1 greater than the background. To enumerate the Ab-forming cells (AFCs), mononuclear cells from the spleen and cervical lymph nodes (CLNs) were isolated aseptically by a mechanical dissociation method using gentle teasing through stainless steel screens as described previously (16, 43). A modified dissociation method based on a previously described protocol (11, 16) was used to isolate nasal passages (NPs). Mononuclear cells from submandibular glands (SMGs) and nasopharynx-associated lymphoreticular tissues (NALT) were isolated by an enzymatic dissociation procedure with collagenase type IV (0.5 mg/ml; Sigma), followed by discontinuous Percoll gradient centrifugation (GE Healthcare UK Ltd.) (11, 16). Mononuclear cells obtained from mucosal and systemic lymphoid tissues were subjected to an enzyme-linked immunospot (ELISPOT) assay in order to determine the numbers of Ag-specific AFCs (11, 16).
F. nucleatum biofilm formation assay on statherin-coated PVC plates.
The assay of inhibition of static F. nucleatum biofilm formation on statherin was performed by using 96-well polyvinyl chloride (PVC) plates according to the method of O'Toole and Kolter (31). Briefly, stationary-phase cultures of F. nucleatum were washed three times and resuspended at a concentration of 5 × 108 cells/ml with KCl buffer (50 mM KCl solution containing 1 mM KH2PO4, 1 mM CaCl2, and 0.1 mM MgCl2 [pH 6.7]). The YQPVPE peptide (25 μg; 100 μl) or stimulated-saliva samples (diluted 1:10 with KCl buffer; 100 μl) from either naïve mice or mice given the 40-kDa FomA protein nasally, with or without CT, were then incubated with F. nucleatum (5 × 107 cells) at 25°C for 3 h. Subsequently, each mixture was added to statherin-coated PVC plates (100 μg/ml; KCl buffer) and was incubated at 25°C overnight. The plates were then washed with KCl buffer, and the wells were stained with 25 μl of 1% crystal violet (CV) and incubated for 15 min. The stained biofilm was extracted in 95% ethanol and diluted (2-fold), and biofilm formation was quantitated by measuring the absorbance at 595 nm.
Statistical analysis.
The results are expressed as the mean ± 1 standard error of the mean (SEM) or 1 standard deviation (SD). All mouse groups were compared with control mice by using an unpaired Mann-Whitney U test with Statview software (Abacus Concepts, Berkeley, CA) designed for Macintosh computers. P values of <0.05 or <0.01 were considered significant.
RESULTS
Isolation and identification of F. nucleatum CE protein binding to the YQPVPE peptide, the minimal active segment.
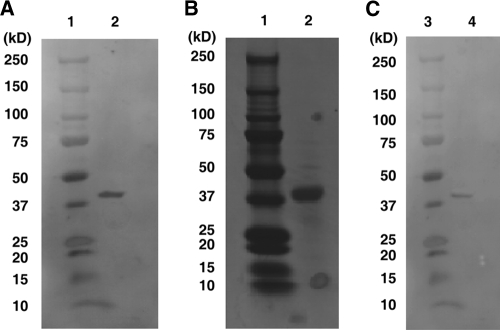
In order to identify F. nucleatum components that specifically bind to the YQPVPE peptide of statherin, whole CEs of F. nucleatum were subjected to a Western blot assay. The YQPVPE peptide specifically bound to a 40-kDa protein of the F. nucleatum CE (40-kDa CE) (Fig. 1A). Further, we purified the 40-kDa CE from the F. nucleatum whole CE fraction by repeated elution of whole CEs through a YQPVPE peptide-coupled, CNBr-activated Sepharose 4B affinity chromatography column. Both the SDS-PAGE gel and Western blot analyses of the purified CE revealed a strong single band with a molecular mass of 40 kDa (Fig. 1B and C). The purity of the 40-kDa CE was approximately 98.7% ± 1.1% (wt/wt).
FIG. 1.
The YQPVPE peptide binds to the 40-kDa cell envelope (CE) protein of F. nucleatum. (A) The F. nucleatum (ATCC 25586) CEs that specifically bind to the YQPVPE peptide were determined by a ligand overlay assay. F. nucleatum whole CEs were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was incubated with the biotinylated YQPVPE peptide followed by horseradish peroxidase (HRP)-conjugated streptavidin for substrate development. Lane 1, molecular mass standard marker; lane 2, F. nucleatum whole CEs. (B and C) SDS-PAGE (B) and ligand overlay analysis (C) of F. nucleatum CEs purified by a YQPVPE peptide-coupled, CNBr-activated Sepharose 4B column. Lanes 1 and 3, molecular mass standard marker; lane 2, YQPVPE affinity column-purified F. nucleatum; lane 4, purified F. nucleatum CE incubated with the biotinylated YQPVPE peptide.
Real-time profiles of the affinity of binding of the YQPVPE peptide to the 40-kDa CE protein.
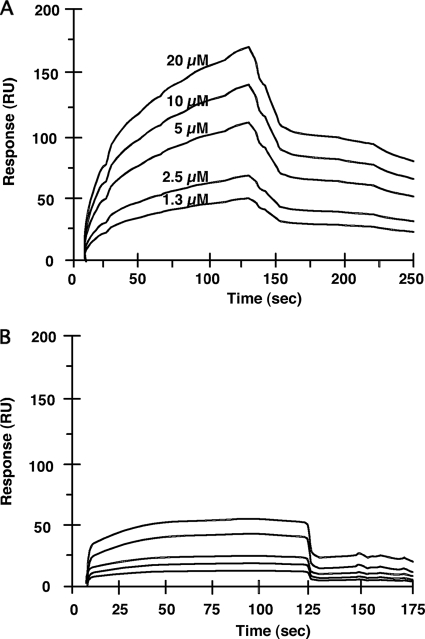
To determine the affinities of interactions between the purified 40-kDa CE and the YQPVPE peptide, direct surface plasmon resonance was assessed using the Biacore X biomolecular interaction monitoring system. The YQPVPE peptide, as an analyte, bound to the immobilized 40-kDa CE with a high rate of association and a low rate of dissociation compared with binding to the control (BSA) (Fig. 2). The values of the association rate constants (kass [1/M·s]), dissociation rate constants (kdiss [1/s]), and equilibrium association constants (Ka, calculated as kass/kdiss [1/M]) were 6.33 × 103, 1.47 × 10−3, and 4.30 × 106, respectively.
FIG. 2.
Biomolecular interactions of affinity column-purified F. nucleatum CEs and the YQPVPE peptide. The affinity of binding of the YQPVPE peptide to immobilized F. nucleatum CEs (A) or BSA (B) was estimated by the BIAcore system. HBSP buffer (10 mM HEPES, 150 mM NaCl, 3.4 mM EDTA, and 0.005% Tween 20 [pH 7.4]) was used as a running buffer, with a flow rate of 30 μl/min. The statherin peptide solution at various concentrations (1.3 μM to 20 μM) was monitored and injected over the 40-kDa CE (100 μg/ml) or BSA (100 μg/ml) on the CM5 sensor chip.
Amino acid sequence of the 40-kDa CE protein.
We next determined the amino acid sequence of the 40-kDa CE by Edman degradation (6). When the 40-kDa CE was specifically digested with V8 protease, the amino-terminal sequences of the three digested fragments were determined to be VAPAWRPNGS, KFXFRLYQXK, and LNXYNYYNVX, respectively. A homology search for the amino acid sequence in the BLASTp database revealed that the 40-kDa CE had a high degree of homology (94%) with FomA, known as a porin protein of F. nucleatum (Fig. 3). Taken together, these observations demonstrate that the YQPVPE peptide binds specifically to FomA, a major protein of the F. nucleatum outer membrane.
FIG. 3.
Homology search for the amino acid alignment of the 40-kDa F. nucleatum component. The 40-kDa CE of F. nucleatum was digested with V8 protease, and the amino-terminal sequences of the three cleaved fragments were determined using the BLASTp database program. The underlined sequences correspond to the identified amino-terminal sequences of F. nucleatum FomA and the 40-kDa CE of the F. nucleatum envelope obtained from the YQPVPE peptide-conjugated affinity column.
Targeted mutagenesis of fomA.
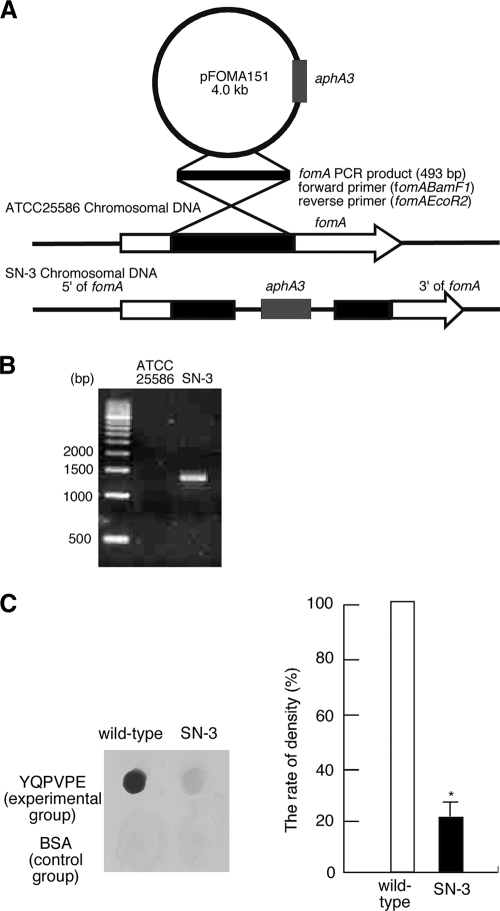
In order to confirm that FomA specifically binds to statherin, we next constructed a FomA-deficient mutant of F. nucleatum. Targeted inactivation of the fomA gene in F. nucleatum strain ATCC 28856 resulted in the generation of the ΔfomA mutant strain SN-3 (Fig. 4A). The mutation was confirmed by PCR analysis of chromosomal DNA with primers fomABamF1 and aphA3F2 (5′-TCC GTA TCT TTT ACG CAG CGG-3′). A fragment spanning the region between fomA and aphA3 was amplified using these primer pairs (Fig. 4B); further examination showed that only a single insert was present in the chromosome, and no transcript was made from the resultant gene (data not shown). Thus, the target gene, fomA, was inactivated in this mutant. We compared the growth rate of the ΔfomA mutant strain SN-3 with that of ATCC 25586 and confirmed that the presence of an antibiotic cassette in the chromosome did not influence the growth rate (data not shown).
FIG. 4.
Construction and functional analysis of the ΔfomA mutant strain SN-3. (A) pFOMA151 contains an internal fragment of fomA and a kanamycin resistance gene (aphA3). SN-3 was produced by single-crossover recombination. (B) The ΔfomA mutant strain SN-3 and the wild-type strain of F. nucleatum (ATCC 25586) were subjected to PCR with forward primer fomABamF1 and reverse primer aphA3F2. (C) Dot blot assay for direct binding activity of ATCC 25586 or mutant strain SN-3 to the YQPVPE peptide. The dot blots show typical results for both the experimental (binding to the YQPVPE peptide) and control (binding to BSA) groups. The level of dot blot density for the control group was subtracted from the level of dot blot density for the experimental group in each experiment. The relative percentage of dot density for each was calculated relative to the dot density for wild-type F. nucleatum bound to the YQPVPE peptide, which was defined as 100%. The graph shows the average percentages of dot density for the binding of wild-type F. nucleatum (100%) or SN-3 (21%) to the YQPVPE peptide. The experiments were performed in triplicate on three separate occasions. Data are expressed as means ± standard deviations. The asterisk indicates a significant difference (P < 0.05) from the result for the wild-type strain.
Wild-type F. nucleatum but not the ΔfomA mutant strain SN-3 binds to the YQPVPE peptide on PVDF membranes.
To examine whether the ΔfomA mutant strain SN-3 bound the YQPVPE peptide, a PVDF membrane adsorbed with the YQPVPE peptide or BSA was incubated with either 125I-labeled wild-type F. nucleatum or the 125I-labeled ΔfomA mutant strain SN-3. The binding activity was visualized by autoradiography, and the relative density of reaction dots was determined by densitometric analysis. The ΔfomA mutant strain SN-3 exhibited reduced levels of binding to the YQPVPE peptide (21%) (Fig. 4C) when the rate of dot density representing wild-type F. nucleatum binding to the YQPVPE peptide had been adjusted to 100%. These results suggest that FomA plays a key role in the binding of F. nucleatum to the YQPVPE amino acid sequences of the statherin molecule.
Induction of FomA-specific immunity.
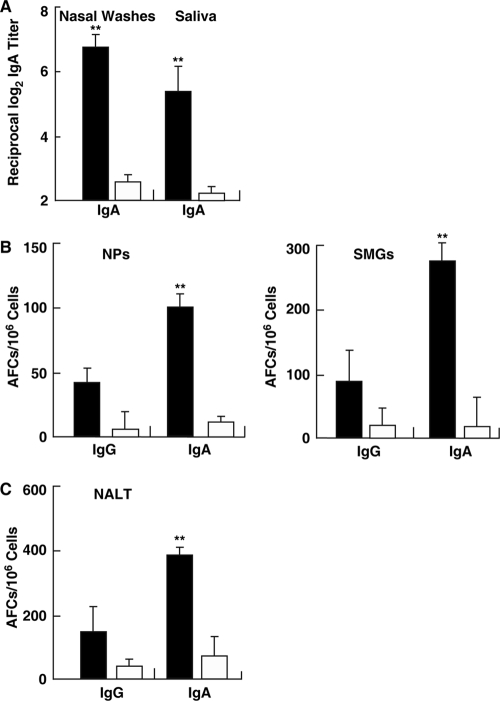
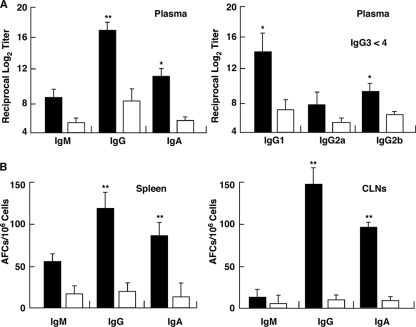
We next examined whether simultaneous nasal administration of LPS-free, purified FomA plus CT as a mucosal adjuvant would enhance FomA-specific immune responses in both mucosal and systemic lymphoid tissues. One microgram of the purified FomA preparation contained <0.1 endotoxin unit (EU). Mice given FomA protein plus CT nasally exhibited significantly higher Ag-specific S-IgA Ab responses in saliva and SMGs than mice given FomA alone nasally (Fig. 5A and B). In addition, FomA-specific S-IgA Ab responses were noted in nasal secretions (Fig. 5A). The laminae propriae of NPs (Fig. 5B) and NALT (Fig. 5C) of mice given FomA plus CT nasally also exhibited elevated AFC responses. Elevated FomA-specific plasma IgA and IgG Ab responses were also seen in mice given nasal FomA plus CT compared with those of mice nasally immunized with FomA alone (Fig. 6A). Thus, significantly increased numbers of FomA-specific IgA and IgG AFCs were seen in the spleens of mice nasally immunized with FomA plus CT (Fig. 6B). IgG subclass Ab analyses revealed that levels of both FomA-specific IgG1 and IgG2b Abs were markedly higher than those for control mouse groups (Fig. 6A). In contrast, relatively low IgG2a Ab responses and no IgG3 Ab responses were seen (Fig. 6A). Taken together, these results show that nasal administration of FomA plus CT as a mucosal adjuvant effectively induced FomA-specific Ab responses in the oral mucosae as well as in other mucosal and systemic immune compartments.
FIG. 5.
FomA-specific immune responses in external secretions and mucosal lymphoid tissues. C57BL/6 mice were nasally immunized weekly for three consecutive weeks either with FomA protein (20 μg) plus cholera toxin (CT; 1 μg) as a mucosal adjuvant (filled bars) or with FomA only (open bars). (A) Seven days after the last immunization, the levels of FomA-specific IgA Abs in nasal washes and saliva were determined by FomA-specific ELISAs. Data are means ± SEMs (n = 15). Double asterisks indicate significant differences (P < 0.01) from results for control mice. (B and C) Seven days after the last immunization, mononuclear cells isolated from NPs, SMGs, and NALT were subjected to Ag-specific ELISPOT assays in order to determine the numbers of IgG and IgA AFCs. Mice immunized nasally with FomA alone were used as controls. Data are means ± SEMs (n = 15). Asterisks indicate significant differences (**, P < 0.01) from results for control mice.
FIG. 6.
Comparison of FomA-specific Ab responses in systemic lymphoid tissues of mice given FomA with (filled bars) or without (open bars) CT. Each mouse group was immunized nasally weekly for three consecutive weeks. (A) Seven days after the last immunization, FomA-specific IgM, IgG, IgA, and IgG subclass Ab responses in plasma were determined by Ag-specific ELISAs. (B) Seven days after the last immunization, mononuclear cells were isolated from spleens and CLNs and were then subjected to Ag-specific ELISPOT assays in order to determine the numbers of IgM, IgG, and IgA AFCs. Data are means ± SEMs (n = 15). Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) from results for control mice.
Inhibition of biofilm formation by saliva from mice given FomA plus CT nasally.
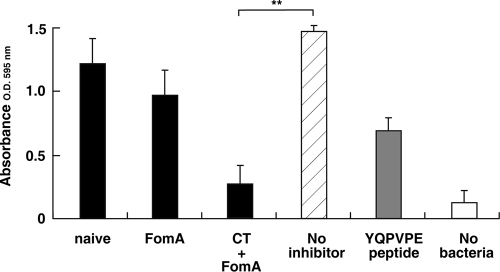
Since F. nucleatum is known to be one of the microorganisms observed microscopically at the axis of dental plaque, and since it readily assembles into the dental plaque biofilm, the inhibitory effects of saliva from mice nasally immunized with FomA plus CT on biofilm formation were examined. Saliva from naïve mice or from mice immunized with FomA alone inhibited biofilm formation minimally (17% and 34%, respectively) (Fig. 7). In contrast, saliva from mice immunized with FomA plus CT showed significant inhibition of biofilm formation (82%) (Fig. 7). The synthetic YQPVPE peptide solution (final concentration, 125 μg/ml) showed moderate inhibition of biofilm formation on statherin-coated PVC plates (53%) (Fig. 7). Taken together, these results showed that F. nucleatum biofilm formation is prevented by saliva containing significant levels of FomA-specific S-IgA Abs.
FIG. 7.
Effect of saliva on the formation of F. nucleatum biofilms on statherin-coated PVC plates. F. nucleatum (5 × 107 bacteria/well) was preincubated with either KCl buffer alone (no inhibitor), saliva from naïve mice, saliva from mice immunized nasally either with FomA alone or with FomA plus CT, or YQPVPE peptide solution at 25°C for 3 h. Subsequently, each mixture, as well as a sample with no bacteria, was added to statherin-coated PVC plates and incubated at 25°C overnight. The resulting biofilm was stained with crystal violet and extracted with 95% ethanol. Biofilm formation was scored by measuring the absorbance at 595 nm. All assays were performed in triplicate, and means and standard deviations are shown. Asterisks indicate significant differences (**, P < 0.01) from results for control mice.
DISCUSSION
We have previously suggested that F. nucleatum and statherin bind through a protein-protein interaction. In this regard, YQPVPE (amino acids [aa] 21 to 26) and PYQPQYQ (aa 33 to 39) were the minimal active regions of the salivary statherin molecule necessary for binding to F. nucleatum (33). This study is the first to show that a 40-kDa protein of the F. nucleatum cell envelope (40-kDa CE) is the major component for binding to salivary statherin. This protein molecule showed high homology with FomA, known as a porin protein in the F. nucleatum outer membrane. In addition, significant levels of Ag-specific S-IgA Ab responses were induced in both mucosal and systemic lymphoid tissues when mice were nasally immunized with FomA plus native CT as a mucosal adjuvant. Interestingly, saliva from mice given FomA plus CT significantly inhibited the formation of F. nucleatum biofilms on statherin-coated PVC plates. Since the attachment of F. nucleatum to the surfaces of teeth and oral mucosae plays a central role in plaque formation and the subsequent development of F. nucleatum-associated periodontitis, as well as playing roles in some systemic diseases, the present study has shed light on the mechanisms of F. nucleatum colonization and the possible prevention of the diseases it mediates.
The present study initially focused on the elucidation of the specific binding site of F. nucleatum to the YQPVPE peptide of statherin, and sequencing analysis showed that the 40-kDa CE specifically bound to the YQPVPE peptide (Fig. 1). When the affinity of binding between the 40-kDa CE and the YQPVPE peptide was examined by surface plasmon resonance technology using a BIAcore system, the values of the association rate constants (kass [1/M·s]), dissociation rate constants (kdiss [1/s]), and equilibrium association constants (Ka, calculated as kass/kdiss [1/M]) were 6.33 × 103, 1.47 × 10−3, and 4.30 × 106, respectively (Fig. 2). The interaction between statherin and P. gingivalis fimbriae resulted in a kass of 2.49 × 103, a kdiss of 1.68 × 10−3, and a Ka of 1.48 × 106 (3). Our results clearly showed that the 40-kDa CE possessed a binding affinity toward statherin higher than that of P. gingivalis fimbriae. Since we found that the 40-kDa CE had a high degree of homology with FomA, known to be a major porin protein of F. nucleatum (21) (Fig. 3), it is possible that the 40-kDa CE plays a role in the interactions between different strains of bacteria, in addition to its binding to salivary protein. Indeed, the 40-kDa major outer membrane protein of F. nucleatum, FomA, has been proposed to be directly involved in binding to Streptococcus sanguinis (17) and P. gingivalis (19, 20). To further confirm the specific binding of FomA to the YQPVPE peptide, we constructed a ΔfomA mutant strain of F. nucleatum, SN-3. When we examined the interactions between F. nucleatum and the statherin-derived YQPVPE peptide, the ΔfomA mutant strain SN-3 clearly showed a level of binding to the statherin peptide significantly lower than that of the wild-type strain (Fig. 4C), although SN-3 maintained a low affinity for the YQPVPE peptide, confirming previous reports that F. nucleatum possesses a variety of lectin-like and non-lectin-like adhesins that are used to attach to host cells (12), extracellular matrix proteins (40), and salivary proteins (33). The YQPVPE peptide-binding activity of SN-3 may be due to these other molecules. Nevertheless, our results clearly showed that the ΔfomA mutant strain SN-3 displayed a significant reduction in binding to the YQPVPE peptide, suggesting that F. nucleatum FomA is most likely an important bacterial anchor or receptor for adherence to teeth and to the oral mucosal surface and thus that it plays a role in subsequent biofilm formation.
It is well known that mucosal immunization results in protective immunity in both mucosal and systemic compartments (24). Among all potential immunization routes, the nasal Ag delivery system is presumably the most efficacious regimen for inducing Ag-specific S-IgA Ab responses in the oral cavity (26). In this regard, nasal administration of purified FomA protein plus native CT induced Ag-specific IgA Ab responses in saliva and SMGs (Fig. 5A and B). Importantly, saliva that contained FomA-specific S-IgA Abs effectively inhibited the binding of F. nucleatum to statherin for the biofilm formation (Fig. 7). However, nasal vaccination with FomA alone did not induce mucosal or systemic immune responses (Fig. 5 and 6). Thus, saliva from mice nasally immunized with FomA alone failed to inhibit biofilm formation (Fig. 7). These results indicate that FomA itself, without CT as a mucosal adjuvant, is not a strong immunogen. Indeed, in support of this view, F. nucleatum is one of many commensal organisms in the oral cavity. Still, since F. nucleatum acts as a bridge between salivary proteins and other coaggregating strains of oral bacteria for plaque formation with early colonizers (i.e., Streptococcus gordonii and Streptococcus oralis) and late colonizers (i.e., P. gingivalis and Actinomyces naeslundii) (4, 22), the induction of FomA-specific S-IgA Abs in saliva is of central importance in the prevention of F. nucleatum colonization. In addition, F. nucleatum is believed to be a pathogen in systemic diseases, such as urinary tract infections (32) and intrauterine infections associated with preterm birth (23), as well as in several oral diseases (20, 35). Thus, induction of FomA-specific S-IgA Abs in saliva appears to be important for the prevention of biofilm formation and of F. nucleatum-associated oral and systemic disease development. To support this view, it has been shown that Ag-specific S-IgA Abs provide effective protective immunity against bacterial (34) and viral (7) pathogens.
Since whole saliva contains Abs from gingival crevicular fluid that originate from serum IgG Abs, it is possible that salivary FomA-specific IgG Abs, as well as S-IgA Abs, are important. Indeed, nasal immunization with FomA plus CT induced significantly increased FomA-specific IgG Ab responses in plasma (Fig. 6A). Further, plasma from mice nasally immunized with FomA plus CT partially inhibited the binding of F. nucleatum to statherin (data not shown). Despite this potential, we did not detect FomA-specific IgG Abs in saliva. Therefore, one could conclude that FomA-specific S-IgA Abs are the major players for the prevention of F. nucleatum-associated biofilm formation in the oral cavity.
Our results clearly showed that the use of CT as a mucosal adjuvant is required for the induction of FomA-specific Ab responses in both mucosal and systemic compartments. However, nasal application of enterotoxins, such as CT and Escherichia coli heat-labile enterotoxin (LT), has been shown to be inappropriate for humans due to central nervous system (CNS) toxicity, resulting in the induction of Bell's palsy (5). In this regard, our group has constructed mutants of CT harboring single-amino-acid substitutions in the ADP-ribosyltransferase active center that render them nontoxic (43) and has developed a novel nontoxic chimeric mucosal adjuvant that combines the nontoxic subunit A of mutant CT (E112K) with the pentameric subunit B of LT from enterotoxigenic E. coli (mCT-A/LT-B) (27, 43). In addition, it has been reported that other nontoxic nasal adjuvants, including a plasmid expressing flt3 ligand cDNA (pFL) and the CpG oligodeoxynucleotide (ODN), successfully induced Ag-specific S-IgA Ab responses in saliva (10, 16). In this regard, we are currently assessing the efficacy of a combined nasal vaccine, i.e., FomA protein with nontoxic mutant CT (mCT-A/LT-B), pFL, and the CpG ODN, for the prevention of oral infection with F. nucleatum.
In summary, the present study identified the 40-kDa CE of F. nucleatum specifically binding to the YQPVPE peptide as FomA, an F. nucleatum outer membrane protein. In this regard, saliva containing FomA-specific S-IgA Abs from mice nasally immunized with FomA protein plus CT markedly inhibited the formation of F. nucleatum biofilms on statherin-coated PVC plates. Understanding of the cellular and molecular mechanisms of initial F. nucleatum colonization in the oral cavity could lead to the development of immunobiological strategies to prevent not only F. nucleatum infection but also its associated oral and systemic diseases.
Acknowledgments
We thank Jerry R. McGhee and Rebekah S. Gilbert for editorial assistance as well as for scientific discussion and critique in the preparation of the manuscript.
This study was supported in part by Grants-in-Aid of Scientific Research (C-19592403 and C-17592179) from the Ministry of Education, Science, Sports and Culture of Japan, by Research for Promoting Technological Seeds from the Japan Science and Technological Agency (13-043), and by U.S. Public Health Service grants DE 012242 and AG 025873.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 14 December 2009.
REFERENCES
- 1.Amano, A., H. T. Sojar, J.-Y. Lee, A. Sharma, M. J. Levine, and R. J. Genco. 1994. Salivary receptors for recombinant fimbrillin of Porphyromonas gingivalis. Infect. Immun. 62:3372-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amano, A., K. Kataoka, P. A. Raj, R. J. Genco, and S. Shizukuishi. 1996. Binding sites of salivary statherin for Porphyromonas gingivalis recombinant fimbrillin. Infect. Immun. 64:4249-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amano, A., T. Nakamura, S. Kimura, I. Morisaki, I. Nakagawa, S. Kawabata, and S. Hamada. 1999. Molecular interaction of Porphyromonas gingivalis fimbriae with host proteins: kinetic analysis based on surface plasmon resonance. Infect. Immun. 67:2399-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradshaw, D. J., P. D. Marsh, G. K. Watson, and C. Allison. 1998. Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect. Immun. 66:4729-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couch, R. B. 2004., Nasal vaccination, Escherichia coli enterotoxin, and Bell's palsy. N. Engl. J. Med. 350:860-861. [DOI] [PubMed] [Google Scholar]
- 6.Edman, P. 1949. A method for the determination of amino acid sequence in peptides. Arch. Biochem. 22:475. [PubMed] [Google Scholar]
- 7.Etchart, N., B. Baaten, S. R. Andersen, L. Hyland, S. Y. C. Wong, and S. Hou. 2006. Intranasal immunization with inactivated RSV and bacterial adjuvants induces mucosal protection and abrogates eosinophilia upon challenge. Eur. J. Immunol. 36:1136-1144. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher, H. M., H. A. Schenkein, R. M. Morgan, K. A. Bailey, C. R. Berry, and F. L. Macrina. 1995. Virulence of Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect. Immun. 63:1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujihashi, K., P. N. Boyaka, and J. R. McGhee. 2008. Host defense at mucosal surfaces, p. 287-304. In R. R. Rich, T. A. Fleisher, W. T. Shearer, et al. (ed.), Clinical immunology: principles and practice, 3rd ed. Mosby/Elsevier, St. Louis, MO.
- 10.Fukuiwa, T., S. Sekine, R. Kobayashi, H. Suzuki, K. Kataoka, R. S. Gilbert, Y. Kurono, P. N. Boyaka, A. M. Kreig, J. R. McGhee, and K. Fujihashi. 2008. A recombination of Flt3 ligand cDNA and CpG ODN as nasal adjuvant elicits NALT dendritic cells for prolonged mucosal immunity. Vaccine 26:4849-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagiwara, Y., J. R. McGhee, K. Fujihashi, R. Kobayashi, N. Yoshino, K. Kataoka, Y. Etani, M.-N. Kweon, S. Tamura, T. Kurata, Y. Takeda, H. Kiyono, and K. Fujihashi. 2003. Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissues. J. Immunol. 170:1754-1762. [DOI] [PubMed] [Google Scholar]
- 12.Han, Y. W., W. Shi, G. T. Huang, S. Kinder Haake, N. H. Park, H. Kuramitsu, and R. J. Genco. 2000. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect. Immun. 68:3140-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hay, D. I. 1973. The isolation from human parotid saliva of a tyrosine-rich acidic peptide which exhibits high affinity for hydroxyapatite surfaces. Arch. Oral Biol. 18:1531-1541. [DOI] [PubMed] [Google Scholar]
- 14.Hay, D. I., and E. C. Moreno. 1989. Statherin and the acidic proline-rich proteins, p. 131-150. In J. Tenovuo (ed.), Human saliva: clinical chemistry and microbiology. CRC Press, Boca Raton, FL.
- 15.Kataoka, K., K. Fujihashi, S. Sekine, T. Fukuiwa, R. Kobayashi, H. Suzuki, H. Nagata, K. Takatsu, S. Shizukuishi, J. R. McGhee, and K. Fujihashi. 2007. Nasal cholera toxin elicits IL-5 and IL-5 receptor α-chain expressing B-1a B cells for innate mucosal IgA antibody responses. J. Immunol. 178:6058-6065. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka, K., J. R. McGhee, R. Kobayashi, K. Fujihashi, S. Shizukuishi, and K. Fujihashi. 2004. Nasal Flt3 ligand cDNA elicits CD11c+ CD8+ dendritic cells for enhanced mucosal immunity. J. Immunol. 172:3612-3619. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman, J., and J. M. DiRienzo. 1989. Isolation of a corncob (coaggregation) receptor polypeptide from Fusobacterium nucleatum. Infect. Immun. 57:331-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kesavalu, L., S. Sathishkumar, V. Bakthavatchalu, C. Matthews, D. Dawson, M. Steffen, and J. L. Ebersole. 2007. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect. Immun. 75:1704-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinder, S. A., and S. C. Holt. 1989. Characterization of coaggregation between Bacteroides gingivalis T22 and Fusobacterium nucleatum T18. Infect. Immun. 57:3425-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinder, S. A., and S. C. Holt. 1993. Localization of the Fusobacterium nucleatum T18 adhesion activity mediating coaggregation with Porphyromonas gingivalis T22. J. Bacteriol. 175:840-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleivdal, H., R. Benz, and H. B. Jensen. 1995. The Fusobacterium nucleatum major outer-membrane protein (FomA) forms trimeric, water-filled channels in lipid bilayer membranes. Eur. J. Biochem. 233:310-316. [DOI] [PubMed] [Google Scholar]
- 22.Kolenbrander, P. E., and J. London. 1993. Adhere today, here tomorrow: oral bacterial adherence. J. Bacteriol. 175:3247-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, H., R. W. Redline, and Y. W. Han. 2007. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J. Immunol. 179:2501-2508. [DOI] [PubMed] [Google Scholar]
- 24.McGhee, J. R., and H. Kiyono. 1999. The mucosal immune system, p. 909-930. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 25.Mestecky, J., R. S. Blumberg, H. Kiyono, and J. R. McGhee. 2003. The mucosal immune system, p. 965-1020. In W. E. Paul (ed.), Fundamental immunology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 26.Moldoveanu, Z., M. L. Clements, S. J. Prince, B. R. Murphy, and J. Mestecky. 1995. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine 11:1006-1012. [DOI] [PubMed] [Google Scholar]
- 27.Momoi, F., T. Hashizume, T. Kurita-Ochiai, Y. Yuki, H. Kiyono, and M. Yamamoto. 2008. Nasal vaccination with the 40-kilodalton outer membrane protein of Porphyromonas gingivalis and a nontoxic chimeric enterotoxin adjuvant induces long-term protective immunity with reduced levels of immunoglobulin E antibodies. Infect. Immun. 76:2777-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niemi, D. L., and I. Johansson. 2004. Salivary statherin peptide binding epitopes of commensal and potentially infectious Actinomyces spp. delineated by a hybrid peptide construct. Infect. Immun. 72:782-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okahashi, N., M. Yamamoto, J. L. Vancott, S. N. Chatfield, M. Bluthmann, T. Hiroi, H. Kiyono, and J. R. McGhee. 1996. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect. Immun. 64:1516-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto, S., S. Kawabata, Y. Terao, H. Fujitaka, Y. Okuno, and S. Hamada. 2004. The Streptococcus pyogenes capsule is required for adhesion of bacteria to virus-infected alveolar epithelial cells and lethal bacterial-viral superinfection. Infect. Immun. 72:6068-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 32.Ribot, S., K. Gal, M. V. Goldblat, and H. H. Eslami. 1981. The role of anaerobe bacteria in the pathogenesis of urinary tract infections. J. Urol. 126:852-853. [DOI] [PubMed] [Google Scholar]
- 33.Sekine, S., K. Kataoka, M. Tanaka, H. Nagata, T. Kawakami, K. Akaji, S. Aimoto, and S. Shizukuishi. 2004. Active domains of salivary statherin on apatitic surfaces for binding to Fusobacterium nucleatum cells. Microbiology 150:2373-2379. [DOI] [PubMed] [Google Scholar]
- 34.Shah, P., D. E. Briles, J. King, Y. Hale, and E. Swiatio. 2009. Mucosal immunization with polyamine transport protein D (PotD) protects mice against nasopharyngeal colonization with Streptococcus pneumoniae. Exp. Biol. Med. 234:403-409. [DOI] [PubMed] [Google Scholar]
- 35.Siqueira, J. F., Jr., and I. N. Rocas. 2009. The microbiota of acute abscesses. J. Dent. Res. 88:61-65. [DOI] [PubMed] [Google Scholar]
- 36.Takada, H., T. Ogawa, F. Yoshimura, K. Otsuka, S. Kokeguchi, K. Kato, T. Umemoto, and S. Kotani. 1988. Immunological activities of a porin fraction isolated from Fusobacterium nucleatum ATCC 10953. Infect. Immun. 56:855-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terao, Y., S. Okamoto, K. Kataoka, S. Hamada, and S. Kawabata. 2005. Protective immunity against Streptococcus pyogenes challenge in mice after immunization with fibronectin-binding protein. J. Infect. Dis. 192:2081-2091. [DOI] [PubMed] [Google Scholar]
- 38.Vadolas, J., J. K. Davis, P. J. Wright, and R. A. Strungnell. 1995. Intranasal immunization with liposomes induces strong mucosal immune responses in mice. Eur. J. Immunol. 25:969-975. [DOI] [PubMed] [Google Scholar]
- 39.Vajdy, M., M. H. Kosco-Vilbois, M. Kopf, G. Kohler, and N. Lycke. 1995. Impaired mucosal immune responses in interleukin 4-targeted mice. J. Exp. Med. 181:41-53. [DOI] [PubMed] [Google Scholar]
- 40.Winkler, J. R., S. R. John, R. H. Kramer, C. I. Hoover, and P. A. Murray. 1987. Attachment of oral bacteria to a basement-membrane-like matrix and to purified matrix proteins. Infect. Immun. 55:2721-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie, H., R. J. Gibbons, and D. I. Hay. 1991. Adhesive properties of strains of Fusobacterium nucleatum of the subspecies nucleatum, vincentii and polymorphum. Oral Microbiol. Immunol. 6:257-263. [DOI] [PubMed] [Google Scholar]
- 42.Xu-Amano, J., R. J. Jackson, K. Fujihashi, H. Kiyono, H. F. Staats, and J. R. McGhee. 1994. Helper Th1 and Th2 cell responses following mucosal or systemic immunization with cholera toxin. Vaccine 12:903-911. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto, S., H. Kiyono, M. Yamamoto, K. Imaoka, K. Fujihashi, F. W. van Ginkel, M. Noda, Y. Takeda, and J. R. McGhee. 1997. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc. Natl. Acad. Sci. U. S. A. 94:5267-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanagita, M., T. Hiroi, N. Kitagaki, S. Hamada, H. Ito, H. Shimauchi, S. Murakami, H. Okada, and H. Kiyono. 1999. Nasopharyngeal-associated lymphoreticular tissue (NALT) immunity: fimbriae-specific Th1 and Th2 cell-regulated IgA responses for the inhibition of bacterial attachment to epithelial cells and subsequent inflammatory cytokine production. J. Immunol. 162:3559-3565. [PubMed] [Google Scholar]