Abstract
Although Bordetella pertussis has been observed to survive inside macrophages, its ability to resist or evade degradation in phagolysosomes has not been defined. We here investigated the trafficking of B. pertussis upon entry into human macrophages. During the first hours following phagocytosis, a high percentage of bacteria were destroyed within acidic compartments positive for the lysosome-associated membrane proteins (LAMP). However, roughly one-fourth of the bacteria taken up evade this initial killing event, remaining in nonacidic compartments. Forty-eight hours after infection, the number of intracellular bacteria per cell increased, suggesting that B. pertussis is capable of replicating in this type of compartment. Viable bacteria accumulated within phagosomal compartments positive for the early endosomal marker Rab5 but not the late endosomal marker LAMP. Moreover, B. pertussis-containing phagosomes acquired exogenously added transferrin, indicating that intracellular bacteria have access to extracellular components and essential nutrients via the host cell recycling pathway. Overall, these results suggest that B. pertussis survives and eventually replicates in compartments with characteristics of early endosomes, potentially contributing to its extraordinary ability to persist within hosts and populations.
Bordetella pertussis colonizes the human respiratory tract, causing a disease known as whooping cough or pertussis, which affects around 4 million people worldwide and causes more than 300,000 deaths each year. Despite high vaccination rates, whooping cough remains a serious threat to human health and its incidence has been increasing in recent years in vaccinated populations. Although some potential contributors to initial colonization have been described, the mechanisms that allow this pathogen to evade immune clearance and to cause the extraordinarily prolonged disease known in China as Bai Ri Ke (100-day cough) are not known.
B. pertussis expresses a number of potent virulence factors, adhesins, and toxins (23) with known or predicted roles during infection. Although B. pertussis is described as an extracellular pathogen, several studies indicate that the immunomodulatory properties of several of these virulence factors enable the bacterium to persist within epithelial cells and leukocytes (1, 3, 22, 24), leading to speculation that the infection might also comprise an intracellular stage. The dual extra- and intracellular locations of B. pertussis are also consistent with the reported need for both cellular and humoral immune responses for bacterial elimination from the respiratory tract (12, 17, 33, 38).
It is presumed that macrophages play an important role in the clearance of B. pertussis (21). However, in vitro studies indicated that B. pertussis is capable of surviving intracellularly in human macrophages for several days in the absence of opsonins (11). Moreover, B. pertussis was found viable in alveolar macrophage cells of mice for more than 21 days after infection (16). These observations have led to speculation that alveolar macrophages might represent an intracellular niche for B. pertussis (16, 41). The recovery of viable B. pertussis from human hosts several weeks after infection (19, 30) and the observation of B. pertussis within pulmonary alveolar macrophages of HIV-infected children (7) and in infants with confirmed B. pertussis pneumonia (28) provide support for this theory.
Efforts to characterize the interaction between B. pertussis and human macrophages have been mainly focused on B. pertussis adherence. Several B. pertussis virulence factors facilitate interaction with phagocytes. B. pertussis fimbriae mediate the binding to the very late antigen 5 receptor on monocytes and macrophages, inducing the upregulation of complement receptor 3 (CR3: CD11b/CD18) (14). CR3 expression is further upregulated by pertussis toxin and filamentous hemagglutinin (FHA) (18, 42). It has been demonstrated that CR3 serves as a docking molecule for B. pertussis binding by FHA (18, 31), which eventually leads to B. pertussis uptake in a nonbactericidal way (15). However, little is known about the fate of B. pertussis inside macrophages. Recent studies by our group showed that neutrophil uptake of B. pertussis in the absence of specific antibodies leads to the failure of lysosomal maturation and bacterial clearance (20). These observations are intriguing evidence that B. pertussis has mechanisms that can allow for evasion of phagolysosome biogenesis. However, short-lived neutrophils are unlikely to provide a prolonged reservoir of bacteria, and evasion of phagolysosome biogenesis in macrophages appears to be an important aspect of the persistence of many other pathogens (9, 10, 29, 40).
The aim of this study was to determine the fate of B. pertussis following phagocytosis by macrophages. Although many ingested bacteria were rapidly killed by macrophages, a significant fraction of internalized B. pertussis was capable of evading phagosome-lysosome fusion, surviving for days and eventually replicating in nonacidic compartments with characteristics of early endosomes. These results reveal a pathway that may contribute to both the extraordinarily long persistence of the coughing illness caused by B. pertussis and its ability to persist within largely immune populations.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. pertussis strain B213, a streptomycin-resistant derivate of Tohama I, was transformed with plasmid pCW505 (43) (kindly supplied by A. A. Weiss, Cincinnati, OH), which induces cytoplasmic expression of green fluorescent protein (GFP) without affecting growth or antigen expression (43). Bacteria were stored at −70°C and recovered by growth on Bordet-Gengou (BG) agar plates supplemented with 15% defibrinated sheep blood (bBGA) at 35°C for 3 days. Virulent bacteria were subsequently plated on bBGA, cultured for 20 h at 35°C, and used in phagocytosis experiments.
Antibodies.
The following antibodies were used: monoclonal antibody (MAb) against human lysosome-associated membrane protein 1 (LAMP-1) (Pharmingen, San Diego, CA), MAb against human Rab5 (Pharmingen, San Diego, CA). CY3-conjugated goat F(ab)2 fragments of anti-rabbit immunoglobulin (Jackson InmunoResearch, West Grove, PA), CY3-conjugated goat F(ab)2 fragments of anti-mouse immunoglobulin (Molecular Probes, Eugene, OR). Immunoglobulin G (IgG) fractions from pooled sera of pertussis patients with high anti-B. pertussis antibody titers, as measured by enzyme-linked immunosorbent assay (27), were isolated as previously described (32). Polyclonal rabbit anti-B. pertussis antiserum was obtained as described elsewhere (15).
Flow cytometry.
Phycoerythrin (PE)-conjugated MAbs to CD14, to CD206, and to CD11b; allophycocyanin-conjugated MAbs to CD11c; PE Cy5-conjugated MAbs to CD1a; and the respective isotype controls were obtained from BD Biosciences. GFP-expressing B. pertussis was used in experiments designed to identify the phenotype of infected cells.
After staining, cells were washed and fixed in 1% paraformaldehyde before analysis on a FACScalibur flow cytometer. Data were processed using the CellQuest software (BD Biosciences). Histograms were draw from and mean fluorescence intensity values were determined on the gated populations.
Macrophage isolation.
Blood mononuclear cells from healthy volunteers were isolated by Ficoll-Paque (GE Healthcare, Uppsala, Sweden) gradient centrifugation as previously described (5). The mononuclear cell layer was washed and suspended in Dulbecco's modified Eagle medium (DMEM) containing 10% inactivated autologous normal human serum (NS), added to six-well tissue culture plates (107 cells/well), and incubated for 2 h at 37°C in 5% CO2. Nonadherent cells were then removed by gentle washing (three times with DMEM-10% NS), and adherent cells were cultured for an additional 6 days in DMEM plus 10% NS prior to the addition of B. pertussis. Compared with recently isolated monocytes (day 0), an increase in CD14, CD11c, CD11b, and CD206 was observed upon 6 days of differentiation, as expected for macrophage differentiation. Forty-eight hours after B. pertussis infection, cells still exhibited morphology typical of macrophages and a surface phenotype of CD14+, CD1a−, CD11b+, Cd11c+, and CD206+, as assessed by flow cytometry. It is noteworthy that this was also the phenotype of the cells bearing B. pertussis growth, as confirmed by gating on B. pertussis-positive cells and collecting 10,000 gated events from the sample.
Macrophage infection assays.
GFP-expressing B. pertussis was suspended in DMEM plus 0.2% bovine serum albumin (BSA; Sigma), and the cells were infected at a multiplicity of infection (MOI) of either 20 or 50 bacteria per cell. Bacterial inocula were quantified by plating appropriate dilutions on bBGA. To facilitate bacterial interaction with macrophages, plates were centrifuged for 5 min at 640 × g. After 20 min of incubation at 37°C with 5% CO2, nonadherent bacteria were removed by three washing steps. Unless indicated otherwise, 100 μg/ml polymyxin B sulfate (Sigma) was added for 1 h to kill extracellular bacteria (6). Thereafter, the antibiotic concentration was decreased to 5 μg/ml. At appropriate times of incubation (2, 24, and 48 h after infection), monolayers were washed and B. pertussis intracellular survival was determined as follows. Infected monolayers were scraped, and the number of viable eukaryotic cells was determined by Trypan blue dye exclusion. Next, macrophages were lysed with 0.1% saponin in sterile water and serial dilutions of lysates were rapidly plated onto bBGA plates to enumerate CFU.
Control experiments to assess the efficacy of antibiotic bactericidal activity were performed in parallel. Briefly, samples of 5 × 108 bacteria were incubated with antibiotics for 1 h at 37°C and plated on BG agar. This resulted in a 99.999% decrease in CFU. Additionally, the number of CFU in cell culture supernatants was examined. No viable bacteria were detected at any time postinfection.
In selected experiments, GFP-expressing B. pertussis was opsonized with human IgG (200 μg/ml) for 30 min at 37°C prior to incubation with macrophages at an MOI of 20.
Quantification of phagocytosis.
Opsonized or nonopsonized GFP-expressing B. pertussis was suspended in DMEM plus 0.2% BSA. Macrophages were infected at an MOI of 20 bacteria per cell as described before. After 20 min of incubation at 37°C with 5% CO2, nonadherent bacteria were removed by three washing steps prior to fixation with paraformaldehyde. The number of adherent and phagocytosed bacteria per cell was estimated by fluorescence microscopy. For this purpose, surface-bound bacteria were detected by incubation with rabbit anti-B. pertussis IgG (30 min at 4°C), followed by incubation with CY3-conjugated goat F(ab)2 fragments of anti-rabbit immunoglobulin for another 30 min at 4°C. In experiments performed with opsonized bacteria, the remaining cell surface-bound bacteria were detected by incubation (30 min at 4°C) with CY3-conjugated goat F(ab)2 of anti-human IgG. To avoid eventual cytophilic binding of antibodies to FcγR, all incubations were done in the presence of 25% heat-inactivated human serum. After washing, samples were analyzed by fluorescence microscopy using a DMLB microscope coupled to a DC100 camera (Leica Microscopy Systems Ltd., Heerbrugg, Switzerland). The numbers of extracellular (red and green) and intracellular (green) bacteria were evaluated by examination of at least 100 macrophages. All experiments were carried out at least three times in triplicate.
Confocal microscopy analysis.
Colocalization studies were performed as described before (32), with minor modifications. Briefly, macrophages incubated with GFP-expressing bacteria at 37°C for 20 min were washed to remove nonattached bacteria and further incubated either with 200 nM Lysotracker DND-99 (Molecular Probes) (5 min at 37°C), followed by fixation with paraformaldehyde, or with 100 μg/ml polymyxin B (1 h at 37°C) for colocalization studies at later time points. At 2, 24, and 48 h postinfection, macrophage samples were incubated with or without Lysotracker stain prior to fixation with paraformaldehyde. Those samples that were not incubated with Lysotracker stain were washed twice with phosphate-buffered saline (PBS) and incubated for 10 min at room temperature with PBS containing 50 mM NH4Cl. After two washing steps, cells were incubated for 30 min with PBS containing 0.1% saponin (Sigma) and 0.5% BSA. Next, cells were incubated for 1 h at 4°C with either mouse anti-human LAMP-1 monoclonal antibodies or mouse anti-human Rab5 antibodies in the presence of 0.1% saponin and 0.5% BSA. After three washing steps, macrophages were incubated with the CY3-conjugated F(ab)2 fragment of goat anti-mouse for 30 min. To avoid cytophilic binding of antibodies to FcγR, all incubations were done in the presence of 25% heat-inactivated human serum. Additionally, isotype controls were run in parallel.
Microscopic analyses were performed using a confocal laser scanning microscope (FV300; Olympus, Tokyo, Japan). The percentage of phagosomes containing bacteria that colocalized with a given marker was calculated by analyzing at least 50 phagosomes per donor.
Transferrin uptake.
Transferrin uptake by macrophages was assayed as described before (39), with minor modifications. Briefly, infected macrophages were depleted of transferrin by incubation in DMEM containing 1% BSA for 1 h at 37°C and further incubated for 10 min at 4°C with 10 μg/ml Alexa transferrin-594 (Molecular Probes) in an excess of BSA (1%) to saturate nonspecific endocytosis. Next, cells were incubated for 5 min at 37°C to allow internalization of the ligand, washed with DMEM containing 1% BSA, and further incubated for another 45 min at 37°C. Finally, the cells were fixed and microscopic analyses were performed using a confocal laser scanning microscope (FV300; Olympus, Tokyo, Japan). At least 50 bacteria per donor were analyzed for colocalization with transferrin in each experiment.
Statistical analysis.
Student's t test (confidence level, 95%) or analysis of variance (ANOVA) was used for statistical data evaluation. The significance of the differences between the mean values of the data evaluated by ANOVA was determined with the least significant difference test at a confidence level of 95%. Results are shown as means and standard deviations (SD).
RESULTS
Time course of B. pertussis survival inside human macrophages.
Previous studies have shown that in the absence of opsonic antibodies, the uptake of B. pertussis by human neutrophils leads to the failure of cellular bactericidal activity (20). However, neutrophils do not survive long enough to be an effective bacterial cellular reservoir. We therefore analyzed the outcome of B. pertussis interaction with human macrophages, a type of immune cell that lives longer in the body and is a well-known cellular reservoir of several other bacterial pathogens.
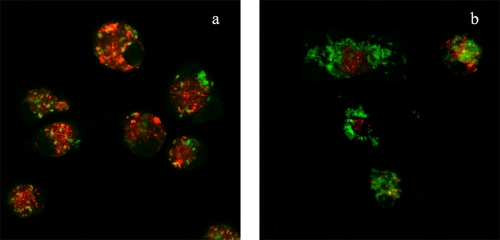
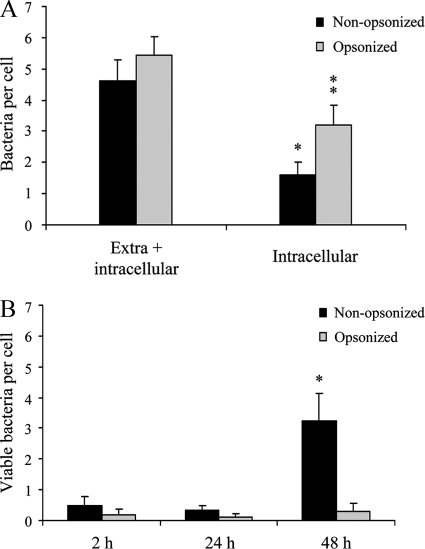
We first examined the time course of the association between B. pertussis and human macrophages. To this end, macrophages were incubated with bacteria for 20 min (MOI, 50), extensively washed, and further incubated for 48 h. A considerable increase in the bacterial loads of macrophages was observed over time, as determined by confocal microscopy (Fig. 1). In control experiments with formalin-inactivated bacteria, no increase in the number of intracellular bacteria was observed under these experimental conditions (data not shown). Interestingly, as time progressed, the number of intracellular bacteria colocalizing with the acidotrophic dye Lysotracker decreased significantly (Fig. 1). Although control experiments showed that B. pertussis was unable to replicate in DMEM plus NS (data not shown), this assay alone could not distinguish bacterial division within macrophages from uptake of bacteria still present in the surrounding of the cell despite the extensive washing. We therefore examined the time course of intracellular bacterial survival following a lower-MOI inoculation that enabled the examination of phagosomes containing individual bacteria. Synchronization of bacterial uptake was achieved by low-speed centrifugation, followed by rapid warming to 37°C for 20 min to allow phagocytosis to be completed. After extensive washing, macrophages were incubated with polymyxin B to kill the remaining extracellular bacteria, and the number of remnant viable (intracellular) bacteria was determined at different times postinfection. The numbers of intracellular and extracellular bacteria before antibiotic treatment were determined by double immunofluorescence staining. About 35% of the macrophage-associated bacteria were found inside cells 20 min after inoculation (Fig. 2A). Figure 2B shows viable intracellular bacteria as assessed by CFU counts after the killing of extracellular bacteria. Relating these to the number of intracellular bacteria, as assessed by double-staining microscopy (Fig. 2A), demonstrated that 70% ± 15% of the ingested bacteria were killed by the macrophages 2 h postinfection. However, the surviving bacteria were able to replicate sevenfold between 24 and 48 h postinfection (Fig. 2B). Importantly, cells supporting bacterial growth 48 h after infection showed macrophage characteristics, as assessed by flow cytometry (data not shown).
FIG. 1.
Increase in the number of B. pertussis cells inside macrophages over time. GFP-expressing B. pertussis cells were incubated with human macrophages (MOI, 50) for 20 min at 37°C. After being washed, the macrophages were incubated for 2 h (panel a) or 48 h (panel b) in DMEM-10% NS and further stained with the acidotropic dye Lysotracker. Results representative of one out of three independent experiments are shown.
FIG. 2.
Phagocytosis and survival of B. pertussis in human macrophages. (A) GFP-expressing B. pertussis cells were incubated with human macrophages (MOI, 20) for 20 min at 37°C. After being washed, the cells were fixed and extracellular and intracellular bacteria were quantified by double immunofluorescence staining. The data represent the mean ± SD of three independent experiments. The number of intracellular IgG-opsonized B. pertussis bacteria was significantly different from the number of intracellular nonopsonized B. pertussis bacteria (*, P < 0.05). (B) GFP-expressing B. pertussis was incubated with human macrophages (MOI, 20) for 20 min at 37°C. After three washing steps, the cells were incubated with polymyxin B to kill extracellular bacteria and the number of CFU of B. pertussis per cell was determined at different time points postinfection. The data represent the mean ± SD of three independent experiments. The number of viable intracellular nonopsonized bacteria per cell at 48 h postinfection was significantly different from the number of viable intracellular bacteria per cell at both 2 and 24 h postinfection. (*, P < 0.05).
Previous studies have shown that antibody opsonization of B. pertussis significantly improves neutrophil uptake of B. pertussis and drastically changes the trafficking inside polymorphonuclear leukocytes (PMNs), leading to efficient bacterial killing (32). A similar effect was observed in this study using macrophages. Figure 2 shows that the presence of specific antibodies enhanced macrophage bacterial uptake and intracellular killing of B. pertussis at initial time points (around 94% ± 5% of the bacteria were killed). Importantly, no increase in intracellular CFU was observed over the 48-h period, suggesting that opsonization prevented the increase in the number of viable intracellular bacteria.
Intracellular trafficking of B. pertussis.
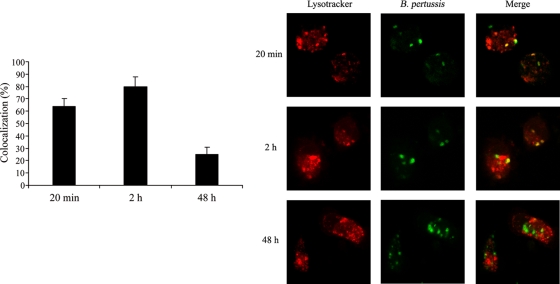
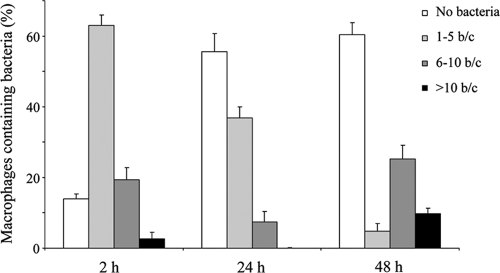
To better define the time course of events involved in intracellular survival and eventual replication of nonopsonized B. pertussis, we followed the maturation of bacterium-containing phagosomes. We first evaluated B. pertussis colocalization with the acidotropic dye Lysotracker at different time points. We found 64% ± 6% of the bacteria in Lysotracker-positive phagosomes as early as 20 min after infection. Two hours after infection, 80% ± 8% the B. pertussis bacteria colocalized with acidic organelles, demonstrating the fusion of bacterial phagosomes with lysosomes at early stages of infection (Fig. 3). These results are consistent with the drop in the number of viable intracellular bacteria observed at these time points, suggesting that those bacteria that were in Lysotracker-positive compartments at early times postinfection were killed. However, at 48 h after infection, coincident with the sevenfold increase in the number of live bacteria inside the macrophages (Fig. 2), most of the B. pertussis-containing vacuoles were nonacidic, as attested by the lack of accumulation of the acidotropic dye in 75% ± 6% of the Bordetella-containing phagosomes (Fig. 3). The increase in the number of intracellular bacteria per cell at 48 h postinfection suggests that some of the surviving bacteria at early time points might have been able to replicate inside the macrophages in nonacidic compartments. To assess this possibility, we monitored the overall levels of macrophage infection by scoring the number of intracellular bacteria per macrophage over time by fluorescence microscopy. One hundred macrophages were examined in each sample. The percentage of macrophages loaded with a certain number of bacteria at each time point was calculated from these data, and the results are depicted in Fig. 4, which shows that at 2 h after infection, most of the macrophages contained one to five bacteria. Twenty-two hours later, an increase in the number of uninfected macrophages was observed, suggesting that some of the macrophages cleared the bacteria upon infection. However, after 48 h, the percentage of macrophages containing more than 10 bacteria significantly increased whereas the percentage of macrophages with one to five bacteria decreased. Together, these results support the hypothesis that although many bacteria were efficiently killed by macrophages, a number of the internalized bacteria were able to avoid lysosome fusion and replicate in nonacidic compartments.
FIG. 3.
Time course of B. pertussis colocalization with the acidotropic dye Lysotracker. GFP-expressing B. pertussis bacteria were incubated with human macrophages (MOI, 20) for 20 min at 37°C. After being washed, B. pertussis-infected macrophages were incubated with Lysotracker (20 min postinfection) or treated with polymyxin B before Lysotracker staining at 2 or 48 h after infection. The bars indicate percentages of Lysotracker-positive phagosomes. The data represent the mean ± SD of three independent experiments. Confocal microscopy images representative of one out of three independent experiments are shown.
FIG. 4.
Quantification of bacterial loads in macrophages over time. Macrophages incubated with GFP-expressing bacteria at 37°C for 20 min were washed and further incubated with polymyxin B to kill the extracellular bacteria. At 2, 24, or 48 h after infection, cells were fixed and the number of bacteria per macrophage (b/c) was analyzed by fluorescence microscopy. The number of intracellular bacteria was determined by analyzing 100 macrophages in each sample. The data represent the mean ± SD of three independent experiments.
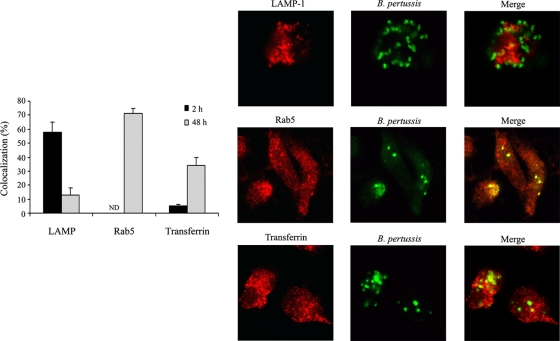
We then investigated the characteristics of the B. pertussis-containing compartments over time. As shown in Fig. 5, B. pertussis-containing phagosomes were mainly positive for the late endosomal/lysosomal marker LAMP-1 at 2 h postinfection, which is in line with the percentage of acidic phagosomes found at this time point (Fig. 3). Forty-eight hours postinfection, the percentage of LAMP-positive B. pertussis phagosomes decreased to 13% ± 5%, suggesting that those bacteria that successfully evaded lysosomal fusion eventually replicated in compartments lacking lysosomal or late endosomal characteristics. Accordingly, further characterization of these bacterium-containing compartments demonstrated that they were mainly positive for Rab5 (Fig. 5), an early endosomal marker.
FIG. 5.
Characterization of B. pertussis-containing phagosome. Macrophages incubated with GFP-expressing B. pertussis at 37°C for 20 min were washed and further incubated with polymyxin B. At 2 or 48 h after infection, macrophages were analyzed by confocal microscopy for Alexa transferrin-594 uptake or LAMP-1 and Rab5 staining. The bars indicate percentages of LAMP-, Rab5-, and transferrin-positive phagosomes. The data represent the mean ± SD of three independent experiments. Representative confocal microscopy images of the colocalization of B. pertussis with LAMP, Rab5, or transferrin in macrophages at 48 h postinfection are shown.
B. pertussis access to exogenous material in macrophages.
Since B. pertussis was found located in early endosomal compartments, we next evaluated whether it has access to nutrients via the recycling pathway. At 2 or 48 h postinfection, macrophages were pulsed with Alexa transferrin-594 as previously described (39) to assess the access of bacterial compartments to extracellular material. As shown in Fig. 5, at 2 h postinfection, only 5% ± 1% of the B. pertussis-containing phagosomes were positive for transferrin. However, after 48 h, the percentage of transferrin-positive phagosomes significantly increased, suggesting that bacteria have access to nutrients via recycling endosomes, as expected for early endosomes and replicative compartments.
DISCUSSION
Many persistent pathogens have evolved strategies to disrupt normal endosomal maturation and fusion with lysosomes. Although B. pertussis has not been traditionally considered an intracellular pathogen, a substantial mass of evidence from cell culture studies argues that this microorganism can invade and persist in many cell types in laboratory cell culture models. Intracellular survival would provide this pathogen with a niche in which it could persist. The present study shows that B. pertussis can not only persists for days inside human macrophages but also increases in numbers in compartments with early endosomal characteristics.
The ability of professional phagocytes to ingest and kill microorganisms is central to innate immunity and host defense. Microbial pathogens that can survive phagocytosis by neutrophils and macrophages have evolved diverse mechanisms to enhance their survival within eukaryotic host cells (26). Some of them, such as Listeria, Shigella, and Rickettsia bacteria, escape into the cytoplasm to avoid lysosomal digestion. Others, like Coxiella burnetii, have adapted to survive within the harsh environment of the lysosome. A greater number of intracellular bacteria inhabit vesicles that do not fuse with lysosomes. Although several in vitro and in vivo studies suggest that B. pertussis is capable of surviving within macrophages (11, 16, 34), the intracellular trafficking of these bacteria has not been determined. CR3 is probably the main receptor involved in nonopsonized B. pertussis binding to phagocytes (25, 31). Binding to CR3 may be advantageous for bacteria, since ligation of this receptor does not activate professional phagocytes (2). Accordingly, nonopsonized B. pertussis failed to trigger a PMN oxidative burst (32), which is consistent with the reported failure of activation of NADPH oxidase by independent ligation of CR3. Recent in vitro studies by our group further showed that after neutrophil uptake, nonopsonized B. pertussis can survive within subcellular structures that do not undergo lysosomal maturation (20). Neutrophils, however, are unlikely cells for the establishment of an intracellular reservoir since they are short-lived. Macrophages, which are longer-lived, are better suited to provide an intracellular niche to extend infection, thereby increasing the opportunity for spread to new hosts. Here we investigated the binding, phagocytosis, and intracellular fate of B. pertussis when it encounters human macrophages. Our results showed that soon after bacterial phagocytosis by macrophages, a considerable number of phagosomes containing B. pertussis fused with lysosomes, as indicated by bacteria colocalizing with LAMP-1 and with the acidotropic dye Lysotracker. However, a large minority of bacteria were found colocalizing with early endosomal markers 2 h after infection and appeared to persist for days thereafter. B. pertussis seems to be unable to survive in acidic and LAMP-positive compartments, as suggested by the concomitant drop in the number of CFU recovered at this time point. These results are in agreement with previous reports indicating that B. pertussis is unable to survive at low pH (35). However, our results further suggest that those bacteria that escape this initial killing event are able to replicate in nonacidic compartments within 48 h after infection. Confocal analysis indicated that, at this time point, the percentage of Lysotracker-positive phagosomes drastically decreased, suggesting that those bacteria that were Lysotracker positive at earlier times postinfection were killed and subsequently degraded. In clear contrast, opsonization of B. pertussis with IgG antibodies not only enhanced macrophage uptake and intracellular killing of B. pertussis but also prevented bacterial replication inside the cell. This is consistent with previous in vivo and in vitro studies showing that FcγR-mediated uptake facilitates B. pertussis clearance, in contrast to uptake via CR3 (15, 32).
The survival of B. pertussis within vacuoles that remain integral to the endosomal network carries the risk of continued sampling of bacterium-derived peptides and their presentation to T cells. However, B. pertussis seems to have evolved strategies to compromise the activation of T cells by the induction of immunosuppressive cytokines such as interleukin-10 and downregulation of HLA-DR and costimulatory molecules in human monocytes (36). Boldrick et al. have profiled gene expression in total peripheral blood mononuclear cells infected with B. pertussis in vitro and have found downregulation of genes that encode major histocompatibility complex class II molecules, the antigen presentation cofactor HLA-DM, the lysosomal protease cathepsin B, and the lysosomal thiol reductase IP-30 (4), all of which are involved in antigen processing and presentation.
Bacterial survival in a particular niche requires the development of an adaptive response generally mediated by the up- and/or downregulation of those genes required for physiological adaptation to the environmental conditions. Although little is known about B. pertussis adaptation to the intracellular environment, both the BvgAS system and the RisAS system have been found to be involved in such a response (22, 37). Although this study was not focused on the bacterial factors involved in survival inside cells, the fact that replication appears to proceed only after 24 h postinfection (Fig. 2B) may reflect a delay before adaptation to environmental conditions encountered within the macrophage.
To our knowledge, this is the first report that provides evidence of an increase in the number of viable intracellular B. pertussis bacteria, suggesting bacterial replication inside human macrophages. Friedman and colleagues (11) did not find intracellular replication. However, several publications since their work have shed light on potential confounding factors in that work, including mononuclear cell treatment prior to infection, extracellular bacterial killing by complement present in human serum (13), and CFU counts from monolayers without taking into account the number of viable macrophages at different time points, among others. With the advantage of this knowledge, our assay were designed to avoid those prior problems.
Confocal analysis revealed that B. pertussis can survive in compartments that are positive for the early endosomal marker Rab5. This small GTPase functions as a regulatory factor in the early endocytic pathway (8). Rab5 retention on bacterium-containing vacuoles might inhibit further maturation of the phagosome, as indicated by the low level of colocalization with LAMP at this time point, limiting the degradative capabilities and enhancing the odds for bacterial survival. Since the ability of bacterial pathogens to survive within a cell depends not only on their ability to avoid degradation in lysosomes but also on their capacity to obtain nutrients, our finding that B. pertussis phagosomes acquire exogenously added transferrin not only confirms our previous observation that B. pertussis retards the maturation of its phagosome but also indicates that there is exchange of material with the recycling compartment, eventually granting the bacteria access to essential nutrients.
Overall, these findings support the hypothesis that B. pertussis can survive and grow within macrophages. This may provide substantial advantages aside from a temporary reprieve from immune effectors. If local proinflammatory signals are required for efficient recruitment/activation of phagocytes, then an intracellular niche could allow the pathogen to survive until local inflammation has been modulated by the many anti-inflammatory mechanisms, allowing it to emerge into a more permissive environment. Alternatively, macrophages could transport bacteria to new sites to initiate new microcolonies. The identification of mutants lacking the ability to survive within macrophages is critical to determining the role of this ability in B. pertussis infection and persistence.
Acknowledgments
This work was partially supported by ANPCyT PICT559 (M.E.R.). M.E.R. is a member of the Scientific Career of CONICET. Y.L. and J.A.H. are doctoral fellows of CONICET. E.T.H. is supported by NIH (GM083113).
Editor: R. P. Morrison
Footnotes
Published ahead of print on 11 January 2010.
REFERENCES
- 1.Abramson, T., H. Kedem, and D. A. Relman. 2008. Modulation of the NF-KB pathway by Bordetella pertussis filamentous hemagglutinin. PLoS One 3:e3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-623. [DOI] [PubMed] [Google Scholar]
- 3.Bassinet, L., P. Gueirard, B. Maitre, B. Housset, P. Gounon, and N. Guiso. 2000. Role of adhesins and toxins in invasion of human tracheal epithelial cells by Bordetella pertussis. Infect. Immun. 68:1934-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boldrick, J. C., A. A. Alizadeh, M. Diehn, S. Dudoit, C. L. Liu, C. E. Belcher, D. Botstein, L. M. Staudt, P. O. Brown, and D. A. Relman. 2002. Stereotyped and specific gene expression programs in human innate immune responses to bacteria. Proc. Natl. Acad. Sci. U. S. A. 99:972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 97:77-89. [PubMed] [Google Scholar]
- 6.Brockmeier, S. L., and K. B. Register. 2000. Effect of temperature modulation and bvg mutation of Bordetella bronchiseptica on adhesion, intracellular survival and cytotoxicity for swine alveolar macrophages. Vet. Microbiol. 73:1-12. [DOI] [PubMed] [Google Scholar]
- 7.Bromberg, K., G. Tannis, and P. Steiner. 1991. Detection of Bordetella pertussis associated with the alveolar macrophages of children with human immunodeficiency virus infection. Infect. Immun. 59:4715-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucci, C., R. G. Parton, I. H. Mather, H. Stunnenberg, K. Simons, B. Hoflack, and M. Zerial. 1992. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70:715-728. [DOI] [PubMed] [Google Scholar]
- 9.Clemens, D., and M. Horwitz. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desjardins, M., and A. Descoteaux. 1997. Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. J. Exp. Med. 185:2061-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman, R. L., K. Nordensson, L. Wilson, E. T. Akporiaye, and D. E. Yocum. 1992. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect. Immun. 60:4578-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hafler, J. P., and A. Pohl-Koppe. 1998. The cellular immune response to Bordetella pertussis in two children with whooping cough. Eur. J. Med. Res. 3:523-526. [PubMed] [Google Scholar]
- 13.Harvill, E. T., P. A. Cotter, and J. F. Miller. 1999. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect. Immun. 67:6109-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazenbos, W., B. van den Berg, and R. van Furth. 1993. Very late antigen-5 and complement receptor type 3 cooperatively mediate the interaction between Bordetella pertussis and human monocytes. J. Immunol. 151:6274-6282. [PubMed] [Google Scholar]
- 15.Hellwig, S. M., H. F. van Oirschot, W. L. Hazenbos, A. B. van Spriel, F. R. Mooi, and J. G. van De Winkel. 2001. Targeting to Fc gamma receptors, but not CR3 (CD11b/CD18), increases clearance of Bordetella pertussis. J. Infect. Dis. 183:871-879. [DOI] [PubMed] [Google Scholar]
- 16.Hellwig, S. M. M., W. L. W. Hazenbos, J. G. J. van de Winkel, and F. R. Mooi. 1999. Evidence for an intracellular niche for Bordetella pertussis in broncho-alveolar lavage cells of mice. FEMS Immunol. Med. Microbiol. 26:203-207. [DOI] [PubMed] [Google Scholar]
- 17.Hewlett, E. L., and S. A. Halperin. 1998. Serological correlates of immunity to Bordetella pertussis. Vaccine 16:1899-1900. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi, Y., S. Claus, and D. Relman. 1994. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18). J. Exp. Med. 180:1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerr, J. R., and R. C. Matthews. 2000. Bordetella pertussis infection: pathogenesis, diagnosis, management, and the role of protective immunity. Eur. J. Clin. Microbiol. Infect. Dis. 19:77-88. [DOI] [PubMed] [Google Scholar]
- 20.Lamberti, Y., M. L. Perez Vidakovics, L. W. van der Pol, and M. E. Rodriguez. 2008. Cholesterol-rich domains are involved in Bordetella pertussis phagocytosis and intracellular survival in neutrophils. Microb. Pathog. 44:501-511. [DOI] [PubMed] [Google Scholar]
- 21.Mahon, B. P., M. S. Ryan, F. Griffin, and K. H. Mills. 1996. Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells. Infect. Immun. 64:5295-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masure, H. R. 1992. Modulation of adenylate cyclase toxin production as Bordetella pertussis enters human macrophages. Proc. Natl. Acad. Sci. U. S. A. 89:6521-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattoo, S., and J. D. Cherry. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18:326-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuirk, P., and K. Mills. 2000. Direct anti-inflammatory effect of a bacterial virulence factor: IL-10-dependent suppression of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. Eur. J. Immunol. 30:415-422. [DOI] [PubMed] [Google Scholar]
- 25.Mobberley-Schuman, P. S., and A. A. Weiss. 2005. Influence of CR3 (CD11b/CD18) expression on phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 73:7317-7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moulder, J. W. 1985. Comparative biology of intracellular parasitism. Microbiol. Rev. 49:298-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagel, J., S. de Graaf, and D. Schijf-Evers. 1985. Improved serodiagnosis of whooping cough caused by Bordetella pertussis by determination of IgG anti-LPF antibody levels. Dev. Biol. Stand. 61:325-330. [PubMed] [Google Scholar]
- 28.Paddock, C. D., G. N. Sanden, J. D. Cherry, A. A. Gal, C. Langston, K. M. Tatti, K. H. Wu, C. S. Goldsmith, P. W. Greer, J. L. Montague, M. T. Eliason, R. C. Holman, J. Guarner, W. J. Shieh, and S. R. Zaki. 2008. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin. Infect. Dis. 47:328-338. [DOI] [PubMed] [Google Scholar]
- 29.Pizarro-Cerdá, J., S. Meresse, R. G. Parton, G. van der Goot, A. Sola-Landa, I. Lopez-Goni, E. Moreno, and J.-P. Gorvel. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66:5711-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preston, N. W. 1986. Recognising whooping cough. Br. Med. J. (Clin. Res. Ed.). 292:901-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Relman, D., E. Tuomanen, S. Falkow, D. T. Golenbock, K. Saukkonen, and S. D. Wright. 1990. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell 61:1375-1382. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez, M. E., S. M. M. Hellwig, D. F. Hozbor, J. Leusen, W.-L. van der Pol, and J. G. J. van de Winkel. 2001. Fc receptor-mediated immunity against Bordetella pertussis. J. Immunol. 167:6545-6551. [DOI] [PubMed] [Google Scholar]
- 33.Ryan, M., G. Murphy, L. Gothefors, L. Nilsson, J. Storsaeter, and K. H. Mills. 1997. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J. Infect. Dis. 175:1246-1250. [DOI] [PubMed] [Google Scholar]
- 34.Saukkonen, K., C. Cabellos, M. Burroughs, S. Prasad, and E. Tuomanen. 1991. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J. Exp. Med. 173:1143-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider, B., R. Gross, and A. Haas. 2000. Phagosome acidification has opposite effects on intracellular survival of Bordetella pertussis and B. bronchiseptica. Infect. Immun. 68:7039-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shumilla, J. A., V. Lacaille, T. M. Hornell, J. Huang, S. Narasimhan, D. A. Relman, and E. D. Mellins. 2004. Bordetella pertussis infection of primary human monocytes alters HLA-DR expression. Infect. Immun. 72:1450-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stenson, T. H., A. G. Allen, J. A. Al-Meer, D. Maskell, and M. S. Peppler. 2005. Bordetella pertussis risA, but not risS, is required for maximal expression of Bvg-repressed genes. Infect. Immun. 73:5995-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storsaeter, J., H. O. Hallander, L. Gustafsson, and P. Olin. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907-1916. [DOI] [PubMed] [Google Scholar]
- 39.Sturgill-Koszycki, S., U. E. Schaible, and D. G. Russell. 1996. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 15:6960-6968. [PMC free article] [PubMed] [Google Scholar]
- 40.Swanson, M., and R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandebriel, R. J., S. M. M. Hellwig, J. P. Vermeulen, J. H. G. Hoekman, J. A. M. A. Dormans, P. J. M. Roholl, and F. R. Mooi. 2003. Association of Bordetella pertussis with host immune cells in the mouse lung. Microb. Pathog. 35:19-29. [DOI] [PubMed] [Google Scholar]
- 42.van't Wout, J., W. N. Burnette, V. L. Mar, E. Rozdzinski, S. D. Wright, and E. I. Tuomanen. 1992. Role of carbohydrate recognition domains of pertussis toxin in adherence of Bordetella pertussis to human macrophages. Infect. Immun. 60:3303-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weingart, C. L., G. Broitman-Maduro, G. Dean, S. Newman, M. Peppler, and A. A. Weiss. 1999. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 67:4264-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]