Abstract
Results of previous studies utilizing bioinformatic approaches in antigen-mining experiments revealed that secreted proteins are among the most frequently recognized antigens from Mycobacterium bovis. Thus, we hypothesized that the analysis of secreted proteins is likely to reveal additional immunogenic antigens that can be used to increase the specificity of diagnostic tests or be suitable vaccination candidates for mycobacterial infections. To test this hypothesis, 382 pools of overlapping peptides spanning 119 M. bovis secreted and potentially secreted proteins were screened for the ability to stimulate a gamma interferon response in vitro using whole blood from tuberculin-positive reactor (TB reactor) cattle. Of the 119 proteins screened, 70 (59%) induced positive responses in the TB reactor animals to various degrees. Strikingly, all but one of the 15 ESAT-6 proteins tested were recognized by at least 30% of the TB reactor animals, with 12 of the 22 most commonly recognized antigens belonging to this protein family. Further analysis of these data demonstrated that there was no significant difference in immunogenicity between the ESAT-6 proteins that were components of potentially intact ESX secretory systems and those corresponding to additional partial esx loci. Importantly for vaccine design, antigenic epitopes in some highly conserved regions shared by numerous ESAT-6 proteins were identified. However, despite this considerable homology, peptide-mapping experiments also revealed that immunodominant peptides were located in regions of amino acid variability.
The incidence of bovine tuberculosis (BTB), a zoonotic infection in cattle caused by Mycobacterium bovis, has been steadily rising in Great Britain over the last 20 years despite the current “test and slaughter” control policy (13). The desire for an effective cattle vaccine to help control the spread of infection has been acknowledged by the British government. To date, the only available vaccine for BTB is M. bovis bacillus Calmette-Guérin (BCG), which has shown various degrees of efficacy in cattle (6, 8, 35). Recent developments show that, using a heterologous prime-boost vaccine strategy, the efficacy of BCG vaccination is significantly improved following boosting with DNA (24), protein (36), or virus (30-32) subunit vaccines. However, vaccination with BCG results in sensitization of animals to bovine tuberculin and compromises the single intradermal comparative tuberculin test (SICCT) currently used for diagnosis of BTB. Thus, the identification of novel immunogenic proteins for use as vaccine candidates and/or as reagents in diagnostic tests able to differentiate M. bovis-infected and uninfected vaccinated animals is of high research priority.
A longstanding concept in tuberculosis research is that active secretion of antigenic proteins by mycobacteria induces strong cellular immune responses in the host. Indeed, in our own antigen-mining experiments with M. bovis-infected cattle, we have previously observed that secreted proteins in general, and members of the ESAT-6 protein family in particular, are among the most frequently recognized antigens from M. bovis. The ESAT-6 protein family comprises 23 small proteins (approximately 100 amino acids) that show amino acid sequence similarity to either ESAT-6 or CFP-10, proteins encoded by the adjacent esxA and esxB genes, respectively, which are located at the ESX-1 locus. The genes encoding the 23 proteins that constitute the ESAT-6 family are dispersed throughout the genome, with the genes for 10 members located within the ESX-1 to ESX-5 loci; the genes encoding the other 13 members are not located within ESX loci 1 to 5. The ESX loci are thought to have originated through multiple duplication events, beginning with the ESX-4 locus (16).
Of all the members of the ESAT-6 protein family, ESAT-6 and CFP-10 have been the most extensively investigated. Although lacking classical signal sequences, ESAT-6 and CFP-10 can be isolated from M. tuberculosis culture filtrate (7, 27) and are thought to be secreted via the ESX-1/type VII secretion system (3). ESAT-6 and CFP-10 form a stable, fully folded structure only when present as a heterodimer (23), the formation of which appears to be crucial for their secretion (17). Given that Rv0287 and Rv0288 (two ESAT-6 proteins that show homology to CFP-10 and ESAT-6, respectively) also form a heterodimer (20), it has been suggested that this may be a general, characteristic feature of all ESAT-6 couplets. Both ESAT-6 and CFP-10 have been demonstrated previously to be strong immunogens in numerous infection models, including humans (18, 22, 29), mice (5), cattle (2, 21), and guinea pigs (1, 15, 26). Furthermore, immune responses directed against other ESAT-6 proteins have been identified in M. bovis-infected cattle (1, 2, 12, 19). Lastly, antigenic epitopes in several ESAT-6 proteins have also been characterized in human studies (4, 25), although these experiments were performed with a limited number of individuals.
The aim of the present study is to investigate the immunogenicity of potential M. bovis secreted antigens by using a cattle model of M. bovis infection. Pools of overlapping peptides representing the ESAT-6 protein family and a complete list of potentially secreted M. bovis antigens identified through in silico analysis of M. tuberculosis H37Rv were assessed for the ability to induce gamma interferon (IFN-γ) production in whole-blood assays. In addition, IFN-γ responses to individual peptides were evaluated to identify the immunodominant epitopes within antigenic proteins.
MATERIALS AND METHODS
Selection of secreted proteins.
One hundred nineteen secreted or potentially secreted proteins were selected for antigen screening (see Table S1 in the supplemental material). Candidate proteins were chosen based on (i) the presence of signal sequences (e.g., Rv0192A and Rv0559c, etc.), (ii) linkage to ESX loci (e.g., Rv3449 and Rv3883c, etc.), (iii) membership in the ESX family (e.g., Rv1197 and Rv1198, etc.), or (iv) prior evidence of secretion given in the literature (e.g., Rv1435c and Rv0867c). Although encoded by regions deleted in M. bovis, the ESAT-6 proteins Rv2347c, Rv3619c, and Rv3620c were included in experiments investigating cross-reactive responses (see Fig. 1, 2, and 3) due to their close sequence similarity to other ESAT-6 proteins expressed in M. bovis. The full list of screened proteins is shown in Table S1 in the supplemental material.
FIG. 1.
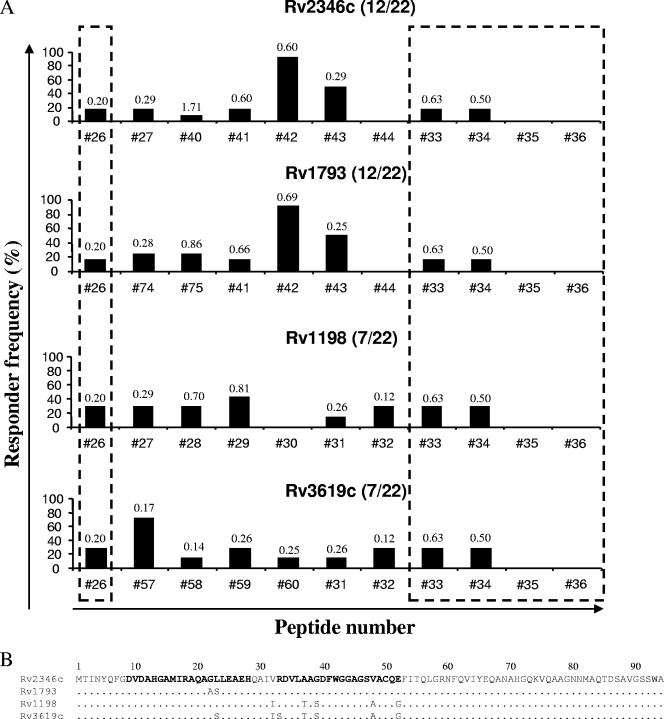
Epitope mapping of the Mtb9.9 subfamily. (A) Overlapping peptides covering proteins Rv2346c, Rv1793, Rv1198, and Rv3619c were screened individually for the ability to stimulate IFN-γ production in whole-blood cultures from TB reactor animals. The data represent the responder frequencies for individual peptides among animals that recognized at least one peptide from each protein (the number of responding animals and the total number of animals are given in parentheses). Regions of the proteins that share identical sequences are indicated by the dashed boxes. The value above each bar denotes the mean ΔOD450 for the animal blood samples responding to the indicated peptide. (B) Amino acid sequences of the specified proteins. Sequences in bold highlight the regions of the protein covered by peptide 57 (amino acids 9 to 28) and peptide 42 (amino acids 33 to 52). Dots represent identical amino acids.
FIG. 2.
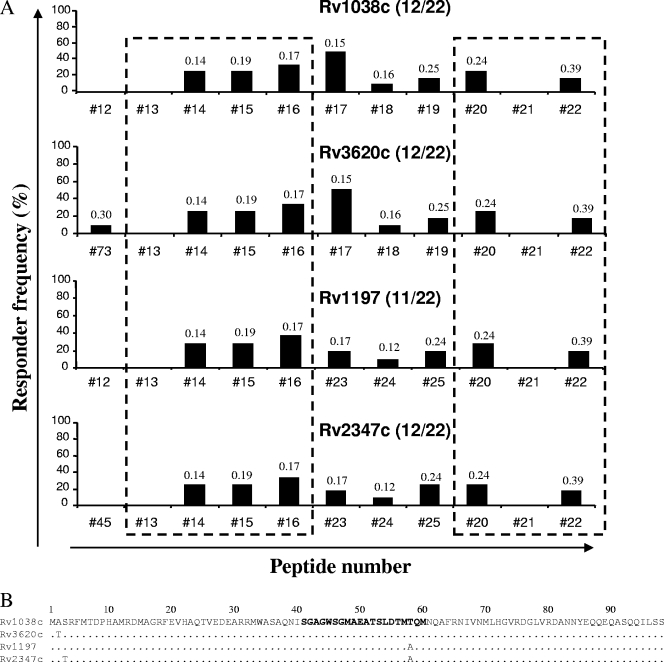
Epitope mapping of the QILSS subfamily. (A) Overlapping peptides covering proteins Rv1038c, Rv3620c, Rv1197, and Rv2347c were screened individually for the ability to stimulate IFN-γ production in whole-blood cultures from TB reactor animals. The data represent the responder frequencies for individual peptides among animals that recognized at least one peptide from each protein (the number of responding animals and the total number of animals are given in parentheses). Regions of the proteins that share identical sequences are indicated by the dashed boxes. The value above each bar denotes the mean ΔOD450 for the animal blood samples responding to the indicated peptide. (B) Amino acid sequences of the specified proteins. The sequence in bold highlights the region of the protein covered by peptide 17.
FIG. 3.
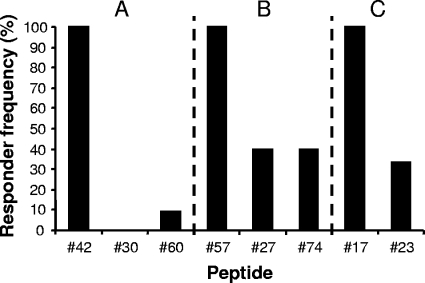
Recognition of protein-specific immunodominant epitopes. Shown are the responder frequencies among peptide 42-responsive animals (A), peptide 57-responsive animals (B), and peptide 17-responsive animals (C) for corresponding peptides from alternative ESAT-6 proteins.
Cattle.
All animals were housed at the Veterinary Laboratories Agency at the time of blood sampling, and procedures were conducted within the limits of a United Kingdom Home Office license, issued under the Animal (Scientific Procedures) Act 1986, which was approved by the local ethical review committee. Heparinized blood samples were obtained from 19 naturally infected, SICTT-positive reactors in herds known to have BTB based on postmortem examination and M. bovis culture from tissues. Heparinized blood samples were also obtained from four animals who were experimentally infected ca. 6 months earlier with an M. bovis field strain from Great Britain (AF 2122/97) by intratracheal instillation of 103 CFU as described previously (11). A detailed postmortem examination of the 23 tuberculin-positive reactor (TB reactor) animals revealed visible tuberculosis lesions in all but two animals, confirming the presence of active disease.
Production and preparation of peptides and antigens.
Peptides representing the selected antigens were commercially produced using the JPT system of membrane-based high-throughput peptide synthesis (JPT Peptide Technologies GmbH, Berlin, Germany). Peptides were synthesized in pools of 20-mers overlapping by 12 amino acids for each of the genes of interest; some genes were represented by more than one pool of peptides. In total, 382 peptide pools containing a total of 4,162 peptides were evaluated. These peptide pools were dissolved in RPMI 1640 (Gibco, United Kingdom) containing 20% dimethyl sulfoxide (DMSO) to obtain a concentration of 1 mg of each peptide/ml, and the peptide pools were used to stimulate whole blood at a final concentration of 5 μg of each peptide/ml. Peptides in the pools corresponding to some antigens were synthesized individually by Mimotopes Pty. Ltd. (Clayton, Australia), dissolved in RPMI 1640 containing 20% DMSO to obtain a concentration of 5 mg/ml, and used individually to stimulate whole blood at a final concentration of 10 μg/ml.
Bovine tuberculin purified protein derivative (PPD-B) was supplied by the Tuberculin Production Unit at the Veterinary Laboratories Agency, Weybridge, Surrey, United Kingdom, and was used at a final concentration of 10 μg/ml. Peptides from ESAT-6 and CFP-10 were formulated to obtain a peptide cocktail as described previously (33) and were used at a final concentration of 5 μg of each peptide/ml. This peptide cocktail was used as a “gold standard” with which to compare the immunogenicities of the other antigens. Staphylococcal enterotoxin B (Sigma-Aldrich, United Kingdom) was included as a positive control at a final concentration of 1 μg/ml, while whole blood was incubated with RPMI 1640 alone as a negative control.
IFN-γ ELISA.
Whole-blood aliquots (250 μl) were added in duplicate to antigen in 96-well plates, and the plates were incubated at 37°C in the presence of 5% CO2 for 24 h, after which plasma supernatants were harvested and stored at −80°C until required. Quantification of IFN-γ in the plasma supernatants was performed using the Bovigam enzyme-linked immunosorbent assay (ELISA) kit (Prionics AG, Switzerland). A result was considered positive if the optical density at 450 nm (OD450) with antigen minus the OD450 without antigen (ΔOD450) was ≥0.1 for both of the duplicate wells (9, 19, 39).
RESULTS AND DISCUSSION
To test the hypothesis that M. bovis secreted proteins are likely to be highly immunogenic, 382 pools of peptides spanning a total of 119 secreted or potentially secreted proteins (see Table S1 in the supplemental material) were screened for the ability to stimulate an IFN-γ response in vitro by using whole-blood samples from TB reactor cattle (19 naturally infected and 4 experimentally infected animals). Samples from all TB reactor animals responded to PPD-B, while samples from 22 of the 23 animals (96%) also responded to the ESAT-6-CFP-10 peptide cocktail, results similar to those reported previously (9). Of the 119 secreted proteins, 49 (41%) failed to induced a positive response in any of the animals studied, while the remaining 70 (59%) were recognized to various degrees, with responder frequencies ranging from 4 to 65%. Strikingly, of the top 22 antigens, i.e., those that induced an IFN-γ response in approximately 40% or more of the TB reactor animals, 12 belonged to the ESAT-6 family of proteins (Table 1). Indeed, of the 15 ESAT-6 proteins tested, all but 1 were recognized by at least 30% of the TB reactor animals.
TABLE 1.
Most frequently recognized M. bovis antigens
| M. tuberculosis designationa | M. bovis designationb | Size (amino acids) | Responder frequency (%) | Antigen details |
|---|---|---|---|---|
| Rv3619c | Deleted | 94 | 70 | ESAT-6-like protein 1 (ESXV) |
| Rv1038c | Mb1067c | 98 | 65 | ESAT-6-like protein 2 (ESXJ) |
| Rv1197 | Mb1229 | 98 | 61 | ESAT-6-like protein 3 (ESXK) |
| Rv1792 | Mb1820 | 98 | 61 | ESAT-6-like protein (ESXM) |
| Rv2347c | Deleted | 98 | 61 | ESAT-6-like protein 7 (ESXP) |
| Rv3020c | Mb3046c | 97 | 61 | ESAT-6-like protein (ESXS) |
| Rv2346c | Mb2375c | 94 | 57 | ESAT-6-like protein 6 (ESXO) |
| Rv3017c | Mb3042c | 120 | 57 | ESAT-6-like protein 8 (ESXQ) |
| Rv3444c | Mb3474c | 100 | 57 | ESAT-6-like protein (ESXT) |
| Rv1860 | Mb1891 | 325 | 57 | Immunogenic protein MPT32 |
| Rv3803c | Mb3833c | 299 | 57 | MPT51/MPB51 antigen protein FbpD |
| Rv1037c | Mb1066c | 94 | 48 | ESAT-6-like protein (ESXI) |
| Rv1198 | Mb1230 | 94 | 48 | ESAT-6-like protein (ESXL) |
| Rv2780 | Mb2802 | 371 | 48 | l-Alanine dehydrogenase (TB43) |
| Rv3445c | Mb3475c | 125 | 43 | ESAT-6-like protein (ESXU) |
| Rv0062 | Mb0063 | 380 | 43 | Possible cellulose CELA1 |
| Rv0129c | Mb0134c | 340 | 43 | Antigen 85 complex C |
| Rv3890c | Mb3919c | 95 | 39 | ESAT-6-like protein (ESXC) |
| Rv3905c | Mb3935c | 103 | 39 | ESAT-6-like protein (ESXF) |
| Rv2376c | Mb2397c | 168 | 39 | Low-molecular-wt antigen CFP-2 |
| Rv3666c | Mb3690c | 541 | 39 | Probable dipeptide-binding lipoprotein DppA |
| Rv1886c | Mb1918c | 325 | 39 | Antigen 85 complex B |
| Rv2518c | Mb2547c | 408 | 39 | Probable conserved lipoprotein LppS |
| Rv1174c | Mb1207c | 110 | 39 | Low-molecular-wt T-cell antigen TB8.4 |
Rv designation for M. tuberculosis H37Rv protein. Boldface denotes ESAT-6 proteins.
Mb designation for M. bovis AF 2122/97 protein. Proteins listed as being deleted are encoded by regions present in M. tuberculosis but not in M. bovis and were included as controls to investigate cross-reactive immune responses.
Five tandem esx genes are located within ESX-1 to ESX-5 loci, which encode novel type VII secretion systems responsible for the export of ESAT-6 family proteins, as well as other proteins (14). The type VII machinery encoded at these loci includes ATP-binding proteins, subtilisin-like membrane-anchored cell wall-associated serine proteases, putative ABC transporters, and other amino-terminally membrane-associated proteins (28). However, the secreted substrates corresponding to each particular ESX locus have yet to be defined. Furthermore, six tandem esx genes are located outside the ESX-1 to ESX-5 loci, raising questions as to how, or if, their encoded proteins are secreted (16). In light of this, we were interested in whether the ESAT-6 family proteins were more frequently recognized if they were expressed as components of potentially intact secretory systems (ESX-1 to ESX-5) than if they were encoded by the partial esx loci. When the results presented herein were combined with immunogenicity data from previous experiments, no significant statistical differences in the levels of immunogenicity of the ESAT-6-like proteins when grouped accordingly were observed (Table 2) (P > 0.6; Mann-Whitney test). Interestingly, Rv2346c induced an IFN-γ response in more than half of the animals studied, despite the fact that its binding partner Rv2347c is deleted in M. bovis. Thus, these data suggest that the immunogenicity of the ESAT-6 family proteins was not inherently dependent on their inclusion as components of distinct intact secretory systems.
TABLE 2.
Responder frequencies for the esx locus proteins
| Protein source | Responder frequency (%) fora: |
|
|---|---|---|
| CFP-10-like protein | ESAT-6-like protein | |
| Complete esx loci | ||
| ESX-1 locus | 70 (Rv3874)b | 80 (Rv3875)b |
| ESX-2 locus | 30 (Rv3891c) | 39 (Rv3890c) |
| ESX-3 locus | 94 (Rv0287)c | 75 (Rv0288)d |
| ESX-4 locus | 43 (Rv3445c) | 57 (Rv3444c) |
| ESX-5 locus | 61 (Rv1792) | 0 (Rv1793) |
| Mean ± SEM | 60 ± 11 | 50 ± 14 |
| Regions of partial esx loci | ||
| Region 1 | 39 (Rv3905c) | 30 (Rv3904c) |
| Region 2 | 61 (Rv3020c) | 16 (3019c)e |
| Region 3 | NAf | 57 (Rv2346c) |
| Region 4 | 48 (Rv1198) | 61 (Rv1197) |
| Region 5 | 65 (Rv1038c) | 48 (Rv1037c) |
| Region 6 | NAf | NAf |
| Mean ± SEM | 53 ± 6 | 42 ± 8 |
Designations of M. tuberculosis H37Rv proteins are given in parentheses.
Information is from reference 33 and unpublished data.
Information is from reference 19.
Information is from reference 12 and H. M. Vordermeier, unpublished data.
Information is from Vordermeier, unpublished.
NA, not applicable. Rv2347c, Rv3620c, and Rv3619c are encoded by regions deleted in M. bovis.
Based on their predicted amino acid sequences, members of the ESAT-6 protein family can be divided into three distinct subgroups: (i) the Mtb9.9 subfamily (consisting of Rv1037c, Rv1198, Rv1793, Rv2346c, and Rv3619c) (4); (ii) the QILSS subfamily (consisting of Rv1038c, Rv1197, Rv1792, Rv2347c, and Rv3620c) (10); and (iii) the TB10.4 homologs (consisting of Rv0288, Rv3019c, and Rv3017c) (25). Given the high degree of amino acid similarity and the fact that immunogenicity was not dependent on the inclusion of the proteins in distinct ESX secretory systems, we speculated that the immune response to these antigens was, in part, driven by the recognition of antigenic epitopes located in conserved regions shared among numerous proteins, effectively increasing the antigenic load of these epitopes. Indeed, when we tested this hypothesis using peptide pools from the ESAT-6 proteins Rv3619c, Rv2347c, and Rv3620c, whose genes are deleted from the M. bovis genome, with blood samples from TB reactor animals, we were able to demonstrate strong immune responses directed against the peptides (Table 1 and data not shown). Therefore, we next investigated whether these immune responses were directed to conserved or nonconserved epitopes located within the ESAT-6 family proteins. To this end, the overlapping peptides contained within the peptide pools for the ESAT-6 family proteins were screened individually for the ability to induce IFN-γ production in TB reactor animals. The ESAT-6 proteins Rv2347c, Rv3619c, and Rv3620c, referred to above, were included as controls in these experiments to identify any cross-reactive epitopes.
Representative results of the epitope-mapping experiments for the Mtb9.9 subfamily antigens Rv2346c, Rv1793, Rv1198, and Rv3619c are shown in Fig. 1. In general, numerous peptides covering almost the entirety of the protein sequences were recognized to various extents by the TB reactor animals. Several of the peptides (peptides 26, 33, and 34) were located in regions of 100% sequence identity among the four individual proteins (Fig. 1A, dashed boxes), suggesting that each protein, when all are expressed simultaneously, may contribute to the available overall epitope concentration, thus accounting in part for the immunodominance of ESAT-6 family proteins. However, other peptides were located in regions of sequence diversity. In animals responsive to antigen Rv2346c, the most frequently recognized peptide was peptide 42. Unsurprisingly, given the high degree of sequence homology between Rv2346c and Rv1793, the central region of Rv1793 also contains the major immunodominant epitope for this peptide (peptide 42). In comparison, both Rv1198 and Rv3619c contain a number of amino acid substitutions within the region spanned by peptide 42 (Fig. 1B, amino acids 32 to 52), resulting in the complete loss of the immunodominance of this portion of the proteins. Figure 1A also demonstrates that the peptide most frequently recognized by animals responsive to Rv3619c was peptide 57. However, despite differing at only one or two amino acid positions, the corresponding regions in Rv2346c and Rv1198 (peptide 27) and Rv1793 (peptide 74) were recognized far less frequently. Given that Rv3619c is deleted in M. bovis, it remains unclear why peptide 57 is recognized to such a degree in TB reactor animals, although we can speculate that the amino acid substitutions seen in this peptide may result in increased affinity of peptide 57 (compared to that of peptide 27 or peptide 74) for bovine major histocompatibility complex class II (MHC-II) molecules.
Similar results were observed for the QILSS subfamily of ESAT-6 family proteins (Fig. 2). With the exception of the N-terminal regions, numerous peptides spanning the majority of the protein sequences were recognized to various extents by the TB reactor animals, with many of these peptides located in regions of 100% sequence identity among the proteins (Fig. 2A, dashed boxes). Peptide 17 was the most frequently recognized peptide for animals responsive to antigen Rv1038c or Rv3620c. However, peptides representing the single amino acid change from threonine to alanine seen at position 58 in both Rv1197 and Rv2347c (Fig. 2B) were less frequently recognized. Although there is a high degree of homology among the ESAT-6 family proteins, several members of the family do not cluster with any of the other proteins. Epitope mapping of one such protein, Rv3020c, revealed the immunodominance of the N-terminal region of this protein, spanned by peptide 55 (data not shown).
In addition to measuring the responder frequencies, we analyzed the magnitudes of the IFN-γ responses to the individual peptides (indicated by the ΔOD450). High IFN-γ levels were detected in response to both frequently recognized peptides (e.g., peptide 42) and those recognized to a lesser extent (e.g., peptide 75) (Fig. 1A), suggesting that the promiscuity of a given peptide epitope and the magnitude of response to the same peptide are not necessarily related.
The results of our peptide-mapping experiments revealed that some of the most frequently recognized peptides were located in regions of sequence diversity. It has been hypothesized that the amino acid substitutions encoded by the duplicated genes for the ESAT-6 protein family may allow for antigenic drift, wherein the regulated expression of functionally similar protein homologs that differ in their immunodominant epitopes results in antigen variation and immune system escape (25). Thus, we investigated the abilities of animals responsive to peptide 42 (contained in Rv2346c and Rv1793) to recognize the corresponding peptides from Rv1198 and Rv3619c (peptides 30 and 60, respectively). Our results show that of the 11 animals that recognized peptide 42, none responded to peptide 30 and only 1 (9%) responded to peptide 60 (Fig. 3A). Similarly, of the five animals that recognized peptide 57, only two (40%) responded to peptide 27 or peptide 74 (Fig. 3B), and of the six animals that recognized peptide 17, only two (33%) responded to peptide 23 (Fig. 3C). Taken together, these results suggest that these protein-specific epitopes within the ESAT-6 protein family could be used in immune kinetic experiments to study whether antigenic drift is a feature of the T-cell recognition of ESAT-6 family proteins.
The data presented herein reveal that the ESAT-6 family proteins contain both major (immunodominant) and minor antigenic epitopes. Furthermore, we have shown that major immunodominant epitopes are located within regions of sequence diversity. Indeed, we have identified key amino acid residues that may be critical for antigenicity, including (i) threonine at position 58 for the QILSS subfamily, (ii) glycine and serine at positions 22 and 23 for the Mtb9.9 subfamily, and (iii) numerous other potential residues between positions 33 and 52 for the Mtb9.9 subfamily. The effect of the amino acid composition at positions 22 and 23 on the antigenicity of the Mtb9.9 proteins has been demonstrated previously in a human study, in which T-cell lines specific for Rv1198 (which has glycine and leucine at positions 22 and 23, respectively) failed to recognize peptides from either Rv1793 (with alanine and serine at positions 22 and 23, respectively) or Rv3619c (with glycine and serine at positions 22 and 23, respectively) (4). Thus, our results generated from a cattle model of M. bovis infection may also have implications for M. tuberculosis antigenicity studies with humans.
We initially hypothesized that sequence homology could lead to the immunodominance of ESAT-6 family members by increasing the antigenic load. However, our results revealed that the regions of homology were not strikingly more frequently recognized than regions of greater variability. It is possible that the homologous ESAT-6 family proteins are expressed in a sequential manner and that epitope concentration for the homologous regions may not occur during natural infection. Alternatively, the epitopes located in the more variable regions of the proteins may demonstrate increased interaction with bovine MHC-II molecules. By employing the virtual-matrix-based prediction program ProPred, which we have shown previously to predict antigenic epitopes in M. bovis (34), we identified predicted binding sequences in peptides from both homologous and variable protein regions (data not shown). However, it still remains possible that epitopes located in the variable regions show greater affinity for bovine MHC-II molecules.
The results of the present study have several implications with regard to vaccine design for mycobacterial infections. Formulations containing proteins/peptides that target the antigen-specific epitopes (e.g., peptides 17, 42, and 55) could be complemented with those that target the homologous regions shared among the different ESAT-6 family proteins (e.g., peptides 16, 20, 26, and 33). Targeting these shared sequences not only will reduce the number of different components within a vaccine but also may exploit a potentially greater antigenic load for these regions. One caveat, however, is that in this study we have measured effector T-cell responses, and future experiments may be required to ensure that appropriate memory T-cell responses are directed to these antigens. Lastly, given that these proteins are also predicted to be secreted in M. tuberculosis, the novel immunogenic antigens indentified herein may be relevant for vaccines to control mycobacterial infections in both cattle and humans.
Supplementary Material
Acknowledgments
This study was funded by the Department for Environment, Food and Rural Affairs (DEFRA), United Kingdom.
We are indebted to Animal Health for identifying naturally M. bovis-infected tuberculin-positive cattle and to the staff of the Animal Service Unit for their dedication to the welfare of the animals housed at VLA.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 19 January 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Aagaard, C., M. Govaerts, V. Meikle, A. J. Vallecillo, J. A. Gutierrez-Pabello, F. Suarez-Guemes, J. McNair, A. Cataldi, C. Espitia, P. Andersen, and J. M. Pollock. 2006. Optimizing antigen cocktails for detection of Mycobacterium bovis in herds with different prevalences of bovine tuberculosis: ESAT6-CFP10 mixture shows optimal sensitivity and specificity. J. Clin. Microbiol. 44:4326-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aagaard, C., M. Govaerts, L. Meng Okkels, P. Andersen, and J. M. Pollock. 2003. Genomic approach to identification of Mycobacterium bovis diagnostic antigens in cattle. J. Clin. Microbiol. 41:3719-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdallah, A. M., N. C. Gey van Pittius, P. A. Champion, J. Cox, J. Luirink, C. M. Vandenbroucke-Grauls, B. J. Appelmelk, and W. Bitter. 2007. Type VII secretion—mycobacteria show the way. Nat. Rev. Microbiol. 5:883-891. [DOI] [PubMed] [Google Scholar]
- 4.Alderson, M. R., T. Bement, C. H. Day, L. Zhu, D. Molesh, Y. A. Skeiky, R. Coler, D. M. Lewinsohn, S. G. Reed, and D. C. Dillon. 2000. Expression cloning of an immunodominant family of Mycobacterium tuberculosis antigens using human CD4+ T cells. J. Exp. Med. 191:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen, P., A. B. Andersen, A. L. Sorensen, and S. Nagai. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359-3372. [PubMed] [Google Scholar]
- 6.Berggren, S. A. 1981. Field experiment with BCG vaccine in Malawi. Br. Vet. J. 137:88-96. [DOI] [PubMed] [Google Scholar]
- 7.Berthet, F. X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 144(Pt. 11):3195-3203. [DOI] [PubMed] [Google Scholar]
- 8.Buddle, B. M., D. Keen, A. Thomson, G. Jowett, A. R. McCarthy, J. Heslop, G. W. De Lisle, J. L. Stanford, and F. E. Aldwell. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10-16. [DOI] [PubMed] [Google Scholar]
- 9.Cockle, P. J., S. V. Gordon, A. Lalvani, B. M. Buddle, R. G. Hewinson, and H. M. Vordermeier. 2002. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect. Immun. 70:6996-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 11.Dean, G. S., S. G. Rhodes, M. Coad, A. O. Whelan, P. J. Cockle, D. J. Clifford, R. G. Hewinson, and H. M. Vordermeier. 2005. Minimum infective dose of Mycobacterium bovis in cattle. Infect. Immun. 73:6467-6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean, G. S., S. G. Rhodes, M. Coad, A. O. Whelan, P. Wheeler, B. Villareal-Ramos, E. Mead, L. Johnson, D. J. Clifford, R. G. Hewinson, and H. M. Vordermeier. 2008. Isoniazid treatment of Mycobacterium bovis in cattle as a model for human tuberculosis. Tuberculosis (Edinb.) 88:586-594. [DOI] [PubMed] [Google Scholar]
- 13.de la Rua-Domenech, R. 2006. Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis (Edinb.) 86:77-109. [DOI] [PubMed] [Google Scholar]
- 14.DiGiuseppe Champion, P. A., and J. S. Cox. 2007. Protein secretion systems in Mycobacteria. Cell. Microbiol. 9:1376-1384. [DOI] [PubMed] [Google Scholar]
- 15.Elhay, M. J., T. Oettinger, and P. Andersen. 1998. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect. Immun. 66:3454-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gey Van Pittius, N. C., J. Gamieldien, W. Hide, G. D. Brown, R. J. Siezen, and A. D. Beyers. 2001. The ESAT-6 gene cluster of Mycobacterium tuberculosis and other high G+C Gram-positive bacteria. Genome Biol. 2:RESEARCH0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guinn, K. M., M. J. Hickey, S. K. Mathur, K. L. Zakel, J. E. Grotzke, D. M. Lewinsohn, S. Smith, and D. R. Sherman. 2004. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 51:359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori, T., M. Sakatani, F. Yamagishi, T. Takashima, Y. Kawabe, K. Nagao, E. Shigeto, N. Harada, S. Mitarai, M. Okada, K. Suzuki, Y. Inoue, K. Tsuyuguchi, Y. Sasaki, G. H. Mazurek, and I. Tsuyuguchi. 2004. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am. J. Respir. Crit. Care Med. 170:59-64. [DOI] [PubMed] [Google Scholar]
- 19.Mustafa, A. S., Y. A. Skeiky, R. Al-Attiyah, M. R. Alderson, R. G. Hewinson, and H. M. Vordermeier. 2006. Immunogenicity of Mycobacterium tuberculosis antigens in Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle. Infect. Immun. 74:4566-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okkels, L. M., and P. Andersen. 2004. Protein-protein interactions of proteins from the ESAT-6 family of Mycobacterium tuberculosis. J. Bacteriol. 186:2487-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollock, J. M., and P. Andersen. 1997. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovis in cattle. Infect. Immun. 65:2587-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravn, P., A. Demissie, T. Eguale, H. Wondwosson, D. Lein, H. A. Amoudy, A. S. Mustafa, A. K. Jensen, A. Holm, I. Rosenkrands, F. Oftung, J. Olobo, F. von Reyn, and P. Andersen. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637-645. [DOI] [PubMed] [Google Scholar]
- 23.Renshaw, P. S., P. Panagiotidou, A. Whelan, S. V. Gordon, R. G. Hewinson, R. A. Williamson, and M. D. Carr. 2002. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6*CFP-10 complex. Implications for pathogenesis and virulence. J. Biol. Chem. 277:21598-21603. [DOI] [PubMed] [Google Scholar]
- 24.Skinner, M. A., D. N. Wedlock, G. W. de Lisle, M. M. Cooke, R. E. Tascon, J. C. Ferraz, D. B. Lowrie, H. M. Vordermeier, R. G. Hewinson, and B. M. Buddle. 2005. The order of prime-boost vaccination of neonatal calves with Mycobacterium bovis BCG and a DNA vaccine encoding mycobacterial proteins Hsp65, Hsp70, and Apa is not critical for enhancing protection against bovine tuberculosis. Infect. Immun. 73:4441-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skjot, R. L. V., I. Brock, S. M. Arend, M. E. Munk, M. Theisen, T. H. Ottenhoff, and P. Andersen. 2002. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect. Immun. 70:5446-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skjot, R. L. V., T. Oettinger, I. Rosenkrands, P. Ravn, I. Brock, S. Jacobsen, and P. Andersen. 2000. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect. Immun. 68:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tekaia, F., S. V. Gordon, T. Garnier, R. Brosch, B. G. Barrell, and S. T. Cole. 1999. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber. Lung Dis. 79:329-342. [DOI] [PubMed] [Google Scholar]
- 29.van Pinxteren, L. A., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vordermeier, H. M., K. Huygen, M. Singh, R. G. Hewinson, and Z. Xing. 2006. Immune responses induced in cattle by vaccination with a recombinant adenovirus expressing mycobacterial antigen 85A and Mycobacterium bovis BCG. Infect. Immun. 74:1416-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vordermeier, H. M., S. G. Rhodes, G. Dean, N. Goonetilleke, K. Huygen, A. V. Hill, R. G. Hewinson, and S. C. Gilbert. 2004. Cellular immune responses induced in cattle by heterologous prime-boost vaccination using recombinant viruses and bacille Calmette-Guerin. Immunology 112:461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vordermeier, H. M., B. Villarreal-Ramos, P. J. Cockle, M. McAulay, S. G. Rhodes, T. Thacker, S. C. Gilbert, H. McShane, A. V. Hill, Z. Xing, and R. G. Hewinson. 2009. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 77:3364-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vordermeier, M., A. O. Whelan, and R. G. Hewinson. 2003. Recognition of mycobacterial epitopes by T cells across mammalian species and use of a program that predicts human HLA-DR binding peptides to predict bovine epitopes. Infect. Immun. 71:1980-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waddington, F. G., and D. C. Ellwood. 1972. An experiment to challenge the resistance to tuberculosis in B.C.G. vaccinated cattle in Malawi. Br. Vet. J. 128:541-552. [DOI] [PubMed] [Google Scholar]
- 36.Wedlock, D. N., M. Denis, M. A. Skinner, J. Koach, G. W. de Lisle, H. M. Vordermeier, R. G. Hewinson, S. van Drunen Littel-van den Hurk, L. A. Babiuk, R. Hecker, and B. M. Buddle. 2005. Vaccination of cattle with a CpG oligodeoxynucleotide-formulated mycobacterial protein vaccine and Mycobacterium bovis BCG induces levels of protection against bovine tuberculosis superior to those induced by vaccination with BCG alone. Infect. Immun. 73:3540-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.