Abstract
Cigarette smoke exposure increases the risk of pulmonary and invasive infections caused by Streptococcus pneumoniae, the most commonly isolated organism from patients with community-acquired pneumonia. Despite this association, the mechanisms by which cigarette smoke exposure diminishes host defense against S. pneumoniae infections are poorly understood. In this study, we compared the responses of BALB/c mice following an intratracheal challenge with S. pneumoniae after 5 weeks of exposure to room air or cigarette smoke in a whole-body exposure chamber in vivo and the effects of cigarette smoke on alveolar macrophage phagocytosis of S. pneumoniae in vitro. Bacterial burdens in cigarette smoke-exposed mice were increased at 24 and 48 h postinfection, and this was accompanied by a more pronounced clinical appearance of illness, hypothermia, and increased lung homogenate cytokines interleukin-1β (IL-1β), IL-6, IL-10, and tumor necrosis factor alpha (TNF-α). We also found greater numbers of neutrophils in bronchoalveolar lavage fluid recovered from cigarette smoke-exposed mice following a challenge with heat-killed S. pneumoniae. Interestingly, overnight culture of alveolar macrophages with 1% cigarette smoke extract, a level that did not affect alveolar macrophage viability, reduced complement-mediated phagocytosis of S. pneumoniae, while the ingestion of unopsonized bacteria or IgG-coated microspheres was not affected. This murine model provides robust additional support to the hypothesis that cigarette smoke exposure increases the risk of pneumococcal pneumonia and defines a novel cellular mechanism to help explain this immunosuppressive effect.
Pneumococcal pneumonia, caused by the Gram-positive pathogen Streptococcus pneumoniae, is the most common form of community-acquired pneumonia in the United States and worldwide (24, 26). This organism can disseminate from the respiratory tract and is the leading cause of death from invasive bacterial infections, with antibiotic-resistant strains becoming increasingly more common (18, 26). Cigarette smoke (CS) exposure increases the risk of serious pneumococcal infections in humans (2, 29), although the mechanisms underlying this effect are not known. Consistent with increased risks of many infectious diseases among smokers (3), animal models have been used to demonstrate impairments in host defense against viral (13, 33), fungal (8), and bacterial (11) infections in smoke-exposed animals. To our knowledge, no reports exist which demonstrate the effects of CS exposure on host defense in a murine model of pneumococcal pneumonia, despite the clinical significance of this pathogen.
The alveolar macrophage (AM) is a specifically differentiated resident phagocyte in the pulmonary alveoli that acts to maintain an environment free of pathogens and debris (27). Under normal conditions, AMs constitute the majority of immune cells within the alveolar space and act as a first line of innate host defense in the lung, using an array of receptors to recognize pathogen-associated molecular patterns (PAMPs) and to facilitate phagocytic uptake (36). Normally, AM function is tightly regulated to prevent inappropriate inflammation that could result in lung damage (1), but under conditions which overwhelm their clearance capacity, AMs play additional roles in the generation and subsequent resolution of inflammation and leukocyte recruitment (28, 37). Murine models of pulmonary pneumococcal infection have shown increased mortality (22) and bacterial burden (10) following AM depletion, indicating their importance in the innate host defense against such infections. Phagocytosis of S. pneumoniae is enhanced following opsonization with complement fragments C3b and C3bi, which adhere to the surfaces of bacteria. The critical importance of C3 in this context was recently demonstrated by studies reporting defects in host defense against pneumococcal pneumonia (19, 34).
The increased susceptibility of smokers to pneumococcal pneumonia is incompletely understood, and no reports to date have assessed the effects of CS exposure on AM phagocytosis of pneumococcus, although many studies have demonstrated impairments in phagocytosis of other targets (15, 16, 21, 30, 31). Therefore, we determined the effects of CS exposure on pulmonary host defense against pneumococcal pneumonia in a murine model and assessed the effects of CS on AM phagocytosis of S. pneumoniae in vitro.
MATERIALS AND METHODS
Animals.
Female BALB/c mice aged 8 to 12 weeks were purchased from Charles River Laboratories (Portage, MI) and maintained in the University of Michigan Unit for Laboratory Animal Medicine under specific-pathogen-free conditions. All experiments were conducted in accordance with the Animal Care and Use Committee of the University of Michigan.
CS exposure model.
Smoke from standardized 2R4F research cigarettes (University of Kentucky, Lexington, KY) was generated by a TE-2 cigarette smoking machine (Teague Enterprises, Davis, CA). This device was set up to provide a mixture of both mainstream (smoke pulled through the filter) and side-stream (smoke generated from the lit end of the cigarette) smoke. Animals were exposed for 4 h/day and 5 days/week in a 54-liter glass and Plexiglass whole-body exposure chamber with an electric fan for chamber mixing in standard mouse caging units with wire cage tops, with water available ad libitum. For a gravimetric measure of total suspended particulate matter, high-retention glass-fiber filters (Pall Corporation, East Hills, NY) were weighed prior to exposure and placed in line at the exhaust port for the duration of the exposure. Filters were weighed with correction for room humidity, and the mean concentration of particulates collected during a 4-h exposure was 19.6 mg/m3. Control animals were housed in an identical chamber but were exposed to room air with no smoke.
Preparation of CSE.
A single lot of cigarette smoke extract (CSE), which was used throughout the study, was prepared by drawing (bubbling) the smoke from five 2R4F cigarettes, secured to the inlet port of a glass impinger (Ace Glass, Vineland, NJ), through 50 ml of RPMI 1640 contained in a reservoir. Aliquots of the CSE were stored at −70°C.
S. pneumoniae preparation and pneumonia model.
S. pneumoniae serotype 3 (ATCC 6303; American Type Culture Collection, Manassas, VA) was grown to mid-log phase in Todd-Hewitt broth supplemented with 0.5% yeast extract (Difco, Detroit, MI) at 37°C with 5% CO2. The virulence of this organism was maintained by subculturing bacteria obtained from the spleens of bacteremic mice and storing them at −80°C until further use. Heat-killed S. pneumoniae was prepared by heating bacteria to >90°C for 30 min. Pneumococcal pneumonia was induced in mice by administering 1 × 104 CFU of live S. pneumoniae via the intratracheal route as previously described (17).
Clinical appearance score.
Animals were monitored for health at least once every 6 h for 48 h following infection, and a clinical score for appearance was scored as previously described (11): 0, normal; 1, lack of grooming; 2, piloerection and nasal and ocular discharge; 3, piloerection and hunched posture; and 4, piloerection and hunched posture with partially closed eyes. Core temperatures were determined immediately after euthanasia, by insertion of an electronic thermometer (MicroTherma; BrainTree Scientific) into a surgical incision in the peritoneum.
BAL and AM culture.
Following euthanasia by CO2 asphyxiation, lungs were removed, the trachea was cannulated with a 23-gauge needle, and the lungs were lavaged with 10 ml of ice-cold HEPES-buffered saline solution containing dextrose, sodium EDTA, and penicillin-streptomycin (Invitrogen, Carlsbad, CA) in 0.5-ml increments. In experiments measuring cellular recruitment following pneumococcal challenge, a 2-ml total lavage volume was used. After recovery by bronchoalveolar lavage (BAL), cells were centrifuged at approximately 400 × g, resuspended in RPMI 1640 (Invitrogen), enumerated with a hemocytometer, and plated at a concentration of 1 ×106 cells/ml. After 1 h at 37°C and 5% CO2, the medium was replaced with warm RPMI 1640 supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin, and the cells were cultured overnight. Cell viability was determined using the XTT reagent (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions.
Lung leukocyte enumeration.
After 5 weeks of room air or CS exposure and at 24 and 48 h postinfection, lung leukocytes were obtained from mice by BAL following CO2 asphyxiation, and differential counts were performed on cells following staining with a modified Wright-Giemsa stain (American Scientific Products, McGraw Park, IL).
Determinations of survival, bacterial loads, and cytokines.
Following intratracheal inoculation with S. pneumoniae, survival was assessed every 24 h. For a separate group of mice, lungs and spleens obtained from euthanized mice at 24 and 48 h postinfection were homogenized, serially diluted, and plated on blood agar plates to determine bacterial burdens. Cytokine levels (interleukin-1β [IL-1β], IL-6, IL-10, CXCL2 [MIP-2], CCL2 [MCP-1], transforming growth factor beta [TGF-β], and tumor necrosis factor alpha [TNF-α]) in whole-lung homogenates were determined by the University of Michigan Immunology Core, using commercially available enzyme immunoassay (EIA) kits (R&D Duoset; R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Phagocytosis.
Phagocytosis of IgG-opsonized polystyrene microspheres was performed as previously described (25). Fluorescein isothiocyanate (FITC)-labeled S. pneumoniae, prepared as previously described (5), was either left unopsonized or opsonized with normal or heat-treated rat serum or C3-deficient human serum (Sigma) for 30 min at 37°C and then washed by repeated centrifugation before being added to plate wells at a ratio of 200 bacteria per AM (or 300:1 for unopsonized bacteria). After culture of opsonized FITC-labeled bacteria with AMs, phagocytosis was assessed as previously described (4).
Statistical analysis.
Data were analyzed using Prism 4 (GraphPad Software, La Jolla, CA) software. Survival was evaluated using the log rank test. For parametric data, mean values were compared using Student's t test or one-way analysis of variance (ANOVA), followed by the Bonferroni post hoc test for mean separation. For nonparametric data, the Mann-Whitney and Kruskal-Wallis tests were used for comparisons. In all cases, differences were considered significant if P values were <0.05. Data are presented as mean values ± standard errors of the means (SEM), unless otherwise noted.
RESULTS
Cigarette smoke exposure impairs pulmonary S. pneumoniae clearance and produces a more severe illness.
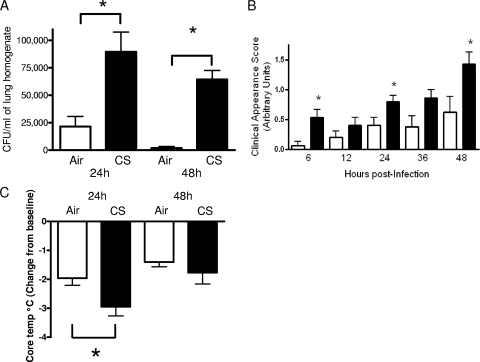
To determine if CS exposure impairs bacterial clearance, animals were exposed to CS or room air (control) for 5 weeks prior to S. pneumoniae challenge. In comparison with mice exposed to room air, bacterial burdens were approximately 4-fold and 35-fold higher in the CS-exposed animals at 24 and 48 h postinfection, respectively (Fig. 1). We did not observe splenic bacterial burdens at either of these time points. To assess morbidity during the course of pneumococcal pneumonia, we evaluated the clinical appearance and core body temperature following S. pneumoniae challenge, as previously described (11). Prior to infection, we did not observe differences in the clinical appearance of these animals. However, the appearance of the CS-exposed animals deteriorated after infection (Fig. 1B). We also observed that this group had significantly reduced core body temperatures at 24 h postinfection, which was consistent with the higher bacterial burdens at this time point (Fig. 1C). While there was a trend toward lower temperatures at 48 h in CS-exposed animals, this difference did not reach statistical significance. However, there was no difference in survival between room air-exposed (53%) and CS-exposed (60%) mice 10 days following S. pneumoniae challenge (data not shown).
FIG. 1.
Cigarette smoke exposure increases pulmonary bacterial burden and worsens clinical signs of pneumococcal pneumonia. Female BALB/c mice were exposed to room air (open bars) or cigarette smoke (CS) (solid bars) for 5 weeks, followed by an intratracheal challenge with live S. pneumoniae (104 CFU). (A) Lung homogenates were assessed for bacterial burdens at 24 and 48 h postinfection. (B) The clinical appearance was evaluated 6 h after infection and every 12 h thereafter, as described in Materials and Methods. (C) Core temperatures were taken at the time of euthanasia, and data are presented as changes from baseline (core temperature of mice that were not infected). Bars represent means ± standard errors of the means (n = 7 or 8 mice per group). *, P < 0.05 compared with air-exposed mice at the same time point, by ANOVA (A and C) and the Kruskal-Wallis test (B).
CS exposure enhances pulmonary cytokine production post-S. pneumoniae challenge.
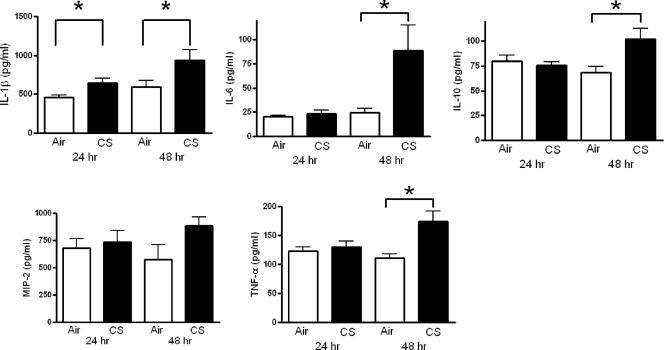
We next measured cytokine levels in lung homogenates in order to determine if the CS-induced impairment in pulmonary clearance of S. pneumoniae was associated with alterations in inflammatory mediator production (Fig. 2). While we observed higher levels of IL-1β in CS-exposed mice 24 h after infection, there were no differences in any other cytokines at this time point. However, at 48 h postinfection, higher levels of IL-1β, IL-6, IL-10, MIP-2 (although not statistically significant), and TNF-α were observed in CS-exposed mice, confirming a more severe infection in these animals. There were no differences in levels of TGF-β at any time point (data not shown).
FIG. 2.
Cytokine levels in lung homogenates obtained from mice following an intratracheal challenge with live S. pneumoniae. Female BALB/c mice were exposed to room air (open bars) or cigarette smoke (CS) (solid bars) for 5 weeks, followed by an intratracheal challenge with live S. pneumoniae (104 CFU). Homogenates were prepared from lungs removed from euthanized mice at 24 and 48 h postinfection and were assessed for IL-1β, IL-6, IL-10, MIP-2, and TNF-α as described in Materials and Methods. *, P < 0.05 versus air-exposed mice, by t test.
Impaired S. pneumoniae clearance in CS-exposed animals is not associated with reduced lung leukocyte number or viability.
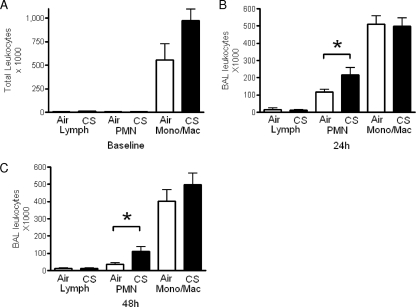
To determine whether the impairments in bacterial clearance were due to CS-mediated deficits in lung leukocytes, we assessed total and differential cell counts and the viability of cells recovered from BAL fluid following 5 weeks of room air or CS exposure. As shown in Fig. 3A, AMs constituted >95% of the recovered leukocytes in both groups of animals, and we observed that the number of resident macrophages in the CS-exposed group was approximately 40% higher (but not statistically significant) than that in the room air-exposed group. In addition, the viability of these cells, as determined by XTT assay, did not differ (data not shown).
FIG. 3.
Elevated neutrophil counts in cigarette smoke-exposed mice following intratracheal challenge with heat-killed S. pneumoniae. Leukocytes were obtained by bronchoalveolar lavage from mice exposed to room air (open bars) or cigarette smoke (CS) (solid bars) for 5 weeks, with no subsequent challenge (A) or 24 h (B) or 48 h (C) following an intratracheal challenge with heat-killed S. pneumoniae (106 CFU-equivalent dose). Differential counts were calculated by multiplying total cell counts by the percentages of lymphocytes (Lymph), neutrophils (PMN), and monocytes/macrophages (Mono/Mac) following differential staining. Bars represent means ± standard errors of the means (n = 4 to 6 mice per group for panel A and 7 or 8 mice per group for panels B and C). *, P < 0.05 by paired t test.
Since leukocyte recruitment to the lungs following pulmonary pneumococcal infection is typically rapid and robust (7), we compared pulmonary leukocyte recruitment in room air- and CS-exposed animals in response to pneumococcal challenge. In the infection model described above, the higher bacterial burdens among the smoke-exposed animals created an unequal challenge at the measured time points with regard to the number of bacteria in the lungs. Therefore, to create conditions of similar challenges, CS- and room air-exposed mice were subsequently given 106 CFU of heat-killed rather than viable S. pneumoniae organisms. While there were no differences in lymphocyte or monocyte/macrophage counts, we observed greater numbers of polymorphonuclear leukocytes (PMNs) in CS-exposed mice 24 and 48 h after intratracheal challenge with heat-killed S. pneumoniae (Fig. 3B and C). These data suggest that CS exposure does not impair bacterial clearance by reducing pulmonary leukocyte recruitment.
Reduced IL-1β, IL-10, and TNF-α production in CS-exposed mice following intratracheal challenge with heat-killed S. pneumoniae.
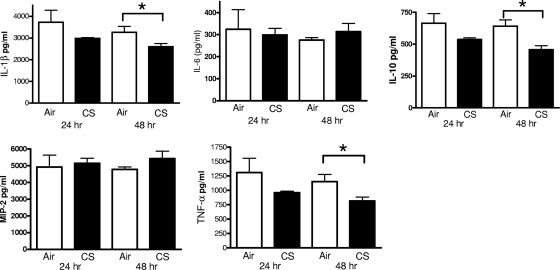
Since the elevated levels of cytokines observed in CS-exposed mice following infection with live S. pneumoniae may reflect higher bacterial burdens rather than differences in the capacity for cytokine production, we also measured cytokines in lung homogenates from room air- and CS-exposed animals following intratracheal challenge with heat-killed bacteria. Although we found no differences in any of the cytokines evaluated 24 h after the instillation of heat-killed S. pneumoniae (Fig. 4), there were modest reductions in IL-1β, IL-10, and TNF-α at 48 h. There were no differences in MCP-1 or TGF-β (data not shown). These data suggest that CS exposure induced a modest suppressive effect on pulmonary cytokine production following the instillation of equal numbers of heat-killed bacteria.
FIG. 4.
Cytokine levels in lung homogenates obtained from mice following intratracheal challenge with heat-killed S. pneumoniae. Female BALB/c mice were exposed to room air (open bars) or cigarette smoke (CS) (solid bars) for 5 weeks, followed by an intratracheal challenge with S. pneumoniae (106 CFU). Lung homogenates were assessed for IL-1β, IL-6, IL-10, MIP-2, and TNF-α as described in Materials and Methods. *, P < 0.05 versus air-exposed mice, by t test.
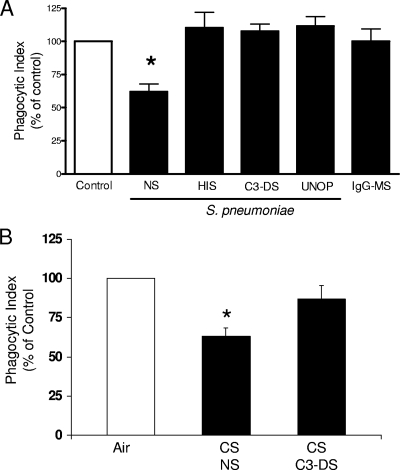
Impaired C3-mediated phagocytosis of S. pneumoniae in AMs treated with CSE.
Since alterations in cytokine production and cellular recruitment did not explain the CS-induced impairment in pulmonary bacterial clearance, we assessed the effects of CSE on the phagocytic capacity of AMs in vitro. As shown in Fig. 5, we observed that CSE reduced AM phagocytosis of serum-opsonized S. pneumoniae, by approximately 40%. To determine the nature of the phagocytic impairment caused by CSE, we assessed phagocytosis of bacteria opsonized with heat-treated serum (to destroy complement), those opsonized with C3-deficient serum, unopsonized bacteria, and IgG-coated microspheres. AM phagocytosis of unopsonized S. pneumoniae or S. pneumoniae opsonized with serum lacking C3 was not impaired. Similar responses were observed using live FITC-labeled S. pneumoniae (data not shown). In addition, CSE did not affect Fc receptor (FcR)-mediated phagocytosis, since the uptake of IgG-labeled microspheres was not different from that of the control. These data suggest that cigarette smoke exposure disables phagocytosis of bacteria opsonized with complement fragments derived from C3 but not other targets.
FIG. 5.
Cigarette smoke extract impairs complement-mediated phagocytosis of S. pneumoniae by AMs in vitro. (A) AMs were cultured overnight with medium alone (control) (open bar) or with medium containing 1% CSE (solid bars), and phagocytosis of S. pneumoniae opsonized with normal rat serum (NS), heat-inactivated rat serum (HIS), C3-deficient serum (C3-DS), or no serum (UNOP) or of IgG-coated microspheres (IgG-MS) was assessed as described in Materials and Methods. (B) Female BALB/c mice were exposed to room air (open bars) or cigarette smoke (CS) (solid bars) for 4 h, and AMs were recovered by lavage and cultured overnight. On the following day, phagocytosis of S. pneumoniae opsonized with normal rat serum (NS) or C3-deficient serum (C3-DS) was assessed. Data were normalized to their respective controls. Bars represent the means ± SEM for three to five experiments. *, P < 0.05 compared with control AMs, by ANOVA.
Impaired C3-mediated phagocytosis in AMs obtained from mice exposed to cigarette smoke for 4 h.
Since smoke exposure impaired phagocytosis after overnight culture with 1% CSE, we next asked if a brief exposure to cigarette smoke would impair complement-mediated phagocytosis of S. pneumoniae in AMs. As shown in Fig. 5B, we observed that phagocytosis of S. pneumoniae opsonized with normal rat serum but not C3-deficient serum was impaired in AMs obtained from mice exposed to cigarette smoke for 4 h. These results confirm our findings using CSE in vitro and suggest that cigarette smoke exposure in vivo reduces complement-mediated phagocytosis of bacteria.
DISCUSSION
Although the link between cigarette smoke exposure and susceptibility to bacterial pneumonia has been recognized for many years, the mechanisms underlying this association are poorly understood. In this report, we demonstrated that CS exposure substantially attenuated pulmonary pneumococcal clearance and produced more severe physiological signs of infection. In addition, the defect in pulmonary bacterial clearance following CS exposure was associated with elevated lung cytokines (IL-1β, IL-6, IL-10, and TNF-α) and a reduction in AM complement-mediated phagocytosis of S. pneumoniae in vitro. These results provide new insight into the mechanisms by which CS exposure compromises pulmonary host defense against pneumococcal infections.
The observed defect in pneumococcal clearance in CS-exposed animals was not due to decreased numbers or viability of resident leukocytes prior to infection. On the contrary, there was a trend toward more macrophages in the lungs of CS-exposed mice. While this trend did not reach statistical significance using our sample size, elevated AM numbers have also been reported for human smokers (23) and for mice following a longer duration of CS exposure (14). Therefore, it is likely that the increased numbers of macrophages observed in our model represent an early stage in the accumulation of AMs. Additionally, under conditions of similar challenge using heat-killed pneumococcus, CS-exposed animals displayed no impairment in leukocyte recruitment postinfection. In contrast, CS exposure enhanced neutrophil recruitment, an observation consistent with other published murine models of pneumococcal pneumonia in which AM function was artificially impaired or the number of AMs was reduced experimentally (9, 22). The higher levels of PMNs in CS-exposed mice challenged with heat-killed S. pneumoniae may indicate a reduction in apoptosis of these cells, since levels of MIP-2, a neutrophil chemoattractant, were only slightly (not significantly) elevated. Despite the CS-associated increase in the number of AMs, which has also been reported to be as much as sixfold higher in smokers, we and others have observed increased susceptibility to pulmonary infections (12).
It is relevant that proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 have been shown to be important in host defense against pneumococcal infection (17, 30, 38). Despite the fact that we observed elevated levels of these proinflammatory cytokines, pulmonary pneumococcal clearance was diminished in CS-exposed mice challenged with live S. pneumoniae. This observation is consistent with the case for smokers with chronic obstructive pulmonary disease (COPD), who exhibit enhanced pulmonary inflammation and increased susceptibility to pulmonary bacterial infections, and a report by Drannik et al., who also observed elevated pulmonary cytokines in CS-exposed mice following the induction of P. aeruginosa pneumonia (11). The increased pulmonary cytokine levels we observed may also have contributed to the higher clinical appearance scores and hypothermia observed in CS-exposed mice infected with live S. pneumoniae. The enhancement of cytokine production in the present study was most likely due to a delay in pulmonary bacterial clearance, since we observed lower levels of these cytokines in CS-exposed animals following intratracheal challenge with heat-killed S. pneumoniae.
Since AMs play an essential role in the orchestration of pulmonary bacterial host defense, we examined the ability of AMs to phagocytose S. pneumoniae following treatment with CSE in vitro. In these experiments, we isolated AMs from mice and treated them overnight with 1% CSE, a level that did not affect cell viability (data not shown). This approach was used in order to observe the direct effects of CSE on AM phagocytic function, since CS exposure modifies extracellular matrix proteins in the lung which are known to suppress AM phagocytic function (21). It is worth mentioning that in experiments run side by side on the same microplate, opsonization with normal rat serum produced roughly twice the level of phagocytosis seen with either form of complement-depleted serum (data not shown), affirming the importance of complement in our system. Our observation that CSE affected complement-mediated phagocytosis but not phagocytosis of IgG-coated beads (FcR-mediated phagocytosis) or bacteria without functional C3 implies that the impairment was not a generalized effect, as might be seen with disruption of cytoskeletal function or membrane trafficking. In addition, when these experiments were conducted using unopsonized pneumococcus, no differences were observed between CSE-pretreated and control AMs. The fact that we observed an impairment of complement-mediated phagocytosis of S. pneumoniae in AMs obtained from mice exposed to cigarette smoke in vivo substantiates these conclusions.
Although AM phagocytosis of unopsonized pneumococcus can occur through receptors such as scavenger receptor A (SR-A) and macrophage receptor with collagenous structure (MARCO) (5, 6), it is inefficient, and multiple lines of evidence support the importance of complement in antipneumococcal innate host defense. For example, genetic defects in C3 are associated with susceptibility to pneumococcal infections in humans (34) and mice (19), and mutant strains of pneumococcus lacking the anticomplement factors pneumococcal surface protein A and pneumolysin display reduced virulence in wild-type mice but not C3 knockout mice (39). Neither of these reports specifically addresses the role of AMs in this effect. However, increased bacterial burdens were seen in C3-deficient mice within 1 h of infection, arguing against a major role for recruited cells such as neutrophils (39). It is worth noting that the study by Kerr et al. (19) employed the use of a large inoculum (106 CFU) given via the intranasal route to demonstrate that C3 plays an essential role in pulmonary bacterial clearance of S. pneumoniae. In our experiments, a relatively low inoculum (104 CFU) of S. pneumoniae was administered to BALB/c mice via the intratracheal route after smoke exposure. We used this low dose of bacteria because BALB/c mice are very susceptible to lethality from S. pneumoniae, and in humans, pneumococcal pneumonia can result from a low inoculum of bacteria that is aspirated from the nasopharynx. The intratracheal route of bacterial challenge was employed in our studies because smoke exposure can induce nasopharyngeal inflammation that might complicate the delivery of S. pneumoniae to mice via the intranasal route. While smoke exposure may have many different immunosuppressive effects, our study suggests that smoking impairs complement-mediated pulmonary clearance of S. pneumoniae.
Our data establish a role for complement in CSE-mediated impairment of AM phagocytosis of pneumococcus, which is a novel finding. In interpreting the relevance of these in vitro data to the living animal, it bears consideration that opsonization of the phagocytic targets was carried out in isolation from either cells or CS constituents, and thus could not be affected by CSE treatment. However, in vivo, opsonization occurs in the same pulmonary milieu, and CS is known to directly cleave C3 (20, 32, 35), suggesting that complement-mediated effects may be more pronounced at the organismal level.
In summary, we have demonstrated that CS exposure impairs pulmonary clearance of S. pneumoniae and that this defect is associated with reduced complement-mediated phagocytosis of this organism by AMs treated with CSE in vitro. Given the important role played by AMs in maintaining a pathogen-free alveolar environment, the functional defects we describe here may well contribute to increased pneumococcal susceptibility in CS-exposed humans.
Acknowledgments
This work was supported by grants HL077417 (P.M.), HL082480 (J.L.C.), and HL078727 (D.M.A.) from the NIH and by the University of Michigan Tobacco Research Network (P.M.). Support for J.C.P. was provided by grant T32ES007062.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 14 December 2009.
REFERENCES
- 1.Ali, F., M. E. Lee, F. Iannelli, G. Pozzi, T. J. Mitchell, R. C. Read, and D. H. Dockrell. 2003. Streptococcus pneumoniae-associated human macrophage apoptosis after bacterial internalization via complement and Fc-gamma receptors correlates with intracellular bacterial load. J. Infect. Dis. 188:1119-1131. [DOI] [PubMed] [Google Scholar]
- 2.Almirall, J., I. Bolibar, M. Serra-Prat, J. Roig, I. Hospital, E. Carandell, M. Agusti, P. Ayuso, A. Estela, A. Torres, et al. 2008. New evidence of risk factors for community-acquired pneumonia: a population-based study. Eur. Respir. J. 31:1274-1284. [DOI] [PubMed] [Google Scholar]
- 3.Arcavi, L., and N. Benowitz. 2004. Cigarette smoking and infection. Arch. Intern. Med. 164:2206-2216. [DOI] [PubMed] [Google Scholar]
- 4.Aronoff, D. M., C. Lewis, C. H. Serezani, K. A. Eaton, D. Goel, J. C. Phipps, M. Peters-Golden, and P. Mancuso. 2009. E-prostanoid 3 receptor deletion improves pulmonary host defense and protects mice from death in severe Streptococcus pneumoniae infection. J. Immunol. 183:2642-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arredouani, M., Z. Yang, A. Imrich, Y. Ning, G. Qin, and L. Kobzik. 2006. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am. J. Respir. Cell Mol. Biol. 35:474-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arredouani, M., Z. Yang, Y. Y. Ning, G. Qin, R. Soininen, K. Tryggvason, and L. R. Kobzik. 2004. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J. Exp. Med. 200:267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergeron, Y., N. Ouellet, A.-M. Deslauriers, M. Simard, M. Olivier, and M. G. Bergeron. 1998. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect. Immun. 66:912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen, P., A. Preston, T. Ling, M. Du, W. Fields, J. Curtis, and J. Beck. 2008. Pneumocystis murina infection and cigarette smoke exposure interact to cause increased organism burden, development of airspace enlargement, and pulmonary inflammation in mice. Infect. Immun. 76:3481-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dockrell, D., M. Lee, D. Lynch, and R. Read. 2001. Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J. Infect. Dis. 184:713-722. [DOI] [PubMed] [Google Scholar]
- 10.Dockrell, D., H. Marriott, L. Prince, V. Ridger, P. Ince, P. Hellewell, and M. Whyte. 2003. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J. Immunol. 171:5380-5388. [DOI] [PubMed] [Google Scholar]
- 11.Drannik, A. G., M. A. Pouladi, C. S. Robbins, S. I. Goncharova, S. Kianpour, and M. R. Stampfli. 2004. Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 170:1164-1171. [DOI] [PubMed] [Google Scholar]
- 12.Fisher, G., K. McNeill, G. Finch, F. Wilson, and D. Golde. 1982. Functional evaluation of lung macrophages from cigarette smokers and nonsmokers. J. Reticuloendothel. Soc. 32:311-321. [PubMed] [Google Scholar]
- 13.Gualano, R., M. Hansen, R. Vlahos, J. Jones, R. Park-Jones, G. Deliyannis, S. Turner, K. Duca, and G. Anderson. 2008. Cigarette smoke worsens lung inflammation and impairs resolution of influenza infection in mice. Respir. Res. 9:53-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hautamaki, R., D. Kobayashi, R. Senior, and S. Shapiro. 1997. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277:2002-2004. [DOI] [PubMed] [Google Scholar]
- 15.Hodge, S., G. Hodge, J. Ahern, H. Jersmann, M. Holmes, and P. Reynolds. 2007. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 37:748-755. [DOI] [PubMed] [Google Scholar]
- 16.Hodge, S., G. Hodge, R. Scicchitano, P. Reynolds, and M. Holmes. 2003. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol. 81:289-296. [DOI] [PubMed] [Google Scholar]
- 17.Hsu, A., D. M. Aronoff, J. Phipps, D. Goel, and P. Mancuso. 2007. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin. Exp. Immunol. 150:332-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadioglu, A., and P. Andrew. 2004. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 25:143-149. [DOI] [PubMed] [Google Scholar]
- 19.Kerr, A., G. Paterson, A. Riboldi-Tunnicliffe, and T. Mitchell. 2005. Innate immune defense against pneumococcal pneumonia requires pulmonary complement component C3. Infect. Immun. 73:4245-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kew, R., B. Ghebrehiwet, and A. Janoff. 1985. Cigarette smoke can activate the alternative pathway of complement in vitro by modifying the third component of complement. J. Clin. Invest. 75:1000-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkham, P., G. Spooner, I. Rahman, and A. Rossi. 2004. Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem. Biophys. Res. Commun. 318:32-37. [DOI] [PubMed] [Google Scholar]
- 22.Knapp, S., J. C. Leemans, S. Florquin, J. Branger, N. A. Maris, J. Pater, N. van Rooijen, and T. van der Poll. 2003. Alveolar macrophages have a protective anti-inflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 167:171-179. [DOI] [PubMed] [Google Scholar]
- 23.Koyama, S., S. Rennard, D. Daughton, S. Shoji, and R. Robbins. 1991. Bronchoalveolar lavage fluid obtained from smokers exhibits increased monocyte chemokinetic activity. J. Appl. Physiol. 70:1208-1214. [DOI] [PubMed] [Google Scholar]
- 24.Levine, O., K. O'Brien, M. Knoll, R. Adegobla, S. Black, S. Cherian, R. Dagan, D. Goldblatt, A. Grange, B. Greenwood, T. Hennessy, K. Klugman, S. Madhi, K. Mulholland, H. Nohynek, M. Santosham, S. Saha, J. Scott, S. Sow, C. Whitney, and F. Cutts. 2006. Pneumococcal vaccination in developing countries. Lancet 367:1880-1882. [DOI] [PubMed] [Google Scholar]
- 25.Mancuso, P., and M. Peters-Golden. 2000. Modulation of alveolar macrophage phagocytosis by leukotrienes is Fc receptor-mediated and protein kinase C-dependent. Am. J. Respir. Cell Mol. Biol. 23:727-733. [DOI] [PubMed] [Google Scholar]
- 26.Mandell, L. A., R. G. Wunderink, A. Anzueto, J. G. Bartlett, G. D. Campbell, N. C. Dean, S. F. Dowell, T. M. File, Jr., D. M. Musher, M. S. Niederman, A. Torres, and C. G. Whitney. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44(Suppl. 2):S27-S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marriott, H., and D. Dockrell. 2007. The role of the macrophage in lung disease mediated by bacteria. Exp. Lung Res. 33:493-505. [DOI] [PubMed] [Google Scholar]
- 28.Mizgerd, J. 2002. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin. Immunol. 14:123-132. [DOI] [PubMed] [Google Scholar]
- 29.Nuorti, J. P., J. C. Butler, M. M. Farley, L. H. Harrison, A. McGeer, M. S. Kolczak, and R. F. Breiman. 2000. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N. Engl. J. Med. 342:681-689. [DOI] [PubMed] [Google Scholar]
- 30.Ortega, E., C. Barriga, and A. Rodriguez. 1994. Decline in the phagocytic function of alveolar macrophages from mice exposed to cigarette smoke. Comp. Immunol. Microbiol. Infect. Dis. 17:77-84. [DOI] [PubMed] [Google Scholar]
- 31.Ortega, E., F. Hueso, M. Collazos, M. Pedrera, C. Barriga, and A. Rodriguez. 1992. Phagocytosis of latex beads by alveolar macrophages from mice exposed to cigarette smoke. Comp. Immunol. Microbiol. Infect. Dis. 15:137-142. [DOI] [PubMed] [Google Scholar]
- 32.Perricone, R., C. de Carolis, G. de Sanctis, and L. Fontana. 1983. Complement activation by cigarette smoke condensate and tobacco infusion. Arch. Environ. Health 38:176-179. [DOI] [PubMed] [Google Scholar]
- 33.Phaybouth, V., S. Wang, J. Hutt, D. McDonald, K. Harrod, and E. Barrett. 2006. Cigarette smoke suppresses Th1 cytokine production and increases RSV expression in a neonatal model. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L222-L231. [DOI] [PubMed] [Google Scholar]
- 34.Picard, C., A. Puel, J. Bustamante, C. Ku, and J. Casanova. 2003. Primary immunodeficiencies associated with pneumococcal disease. Curr. Opin. Allergy Clin. Immunol. 3:451-459. [DOI] [PubMed] [Google Scholar]
- 35.Robbins, R., K. Nelson, G. Gossman, S. Koyama, and S. Rennard. 1991. Complement activation by cigarette smoke. Am. J. Physiol. 260:L254-L259. [DOI] [PubMed] [Google Scholar]
- 36.Staurt, L., and R. Ezekowitz. 2005. Phagocytosis: elegant complexity. Immunity 22:539-550. [DOI] [PubMed] [Google Scholar]
- 37.Thomassen, M., L. Divis, and C. Fisher. 1996. Regulation of human alveolar macrophage inflammatory cytokine production by interleukin-10. Clin. Immunol. Immunopathol. 80:321-324. [DOI] [PubMed] [Google Scholar]
- 38.Wright, J., and A. Churg. 1990. Cigarette smoke causes physiologic and morphologic changes of emphysema in the guinea pig. Am. Rev. Respir. Dis. 142:1422-1428. [DOI] [PubMed] [Google Scholar]
- 39.Yuste, J., M. Botto, J. Paton, D. Holden, and J. Brown. 2005. Additive inhibition of complement deposition by pneumolysin and PspA facilitates Streptococcus pneumoniae septicemia. J. Immunol. 175:1813-1819. [DOI] [PubMed] [Google Scholar]