Abstract
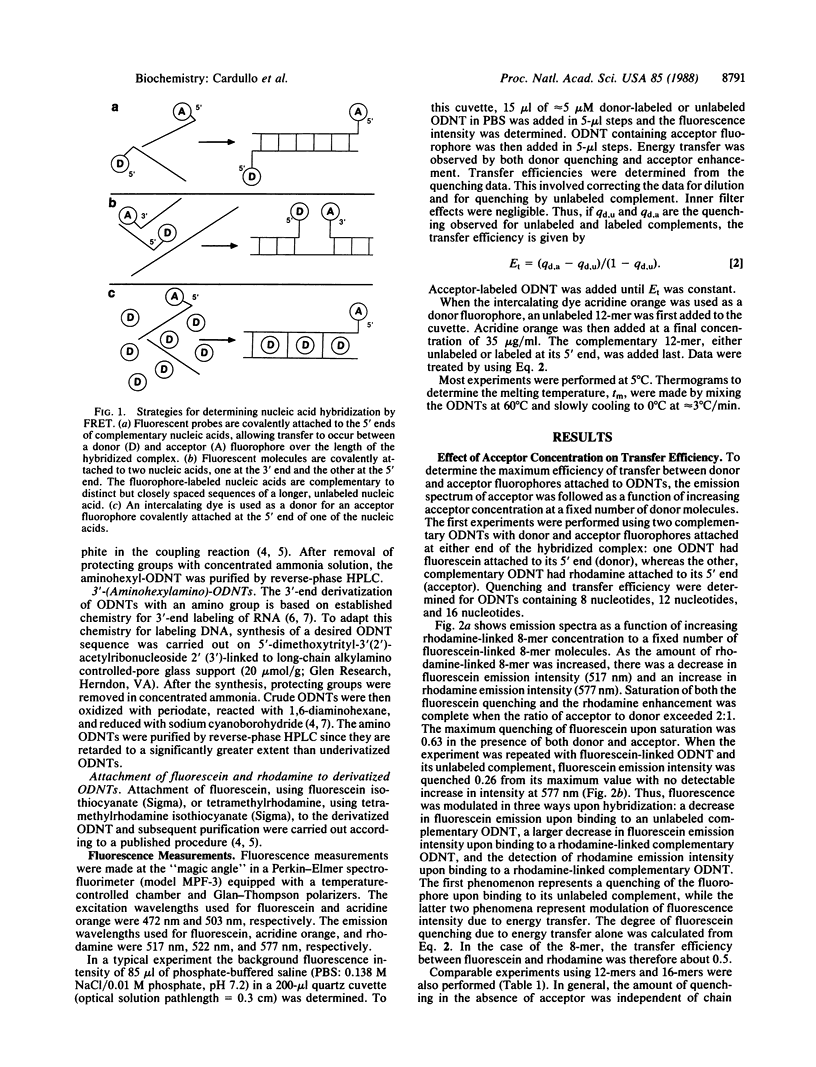
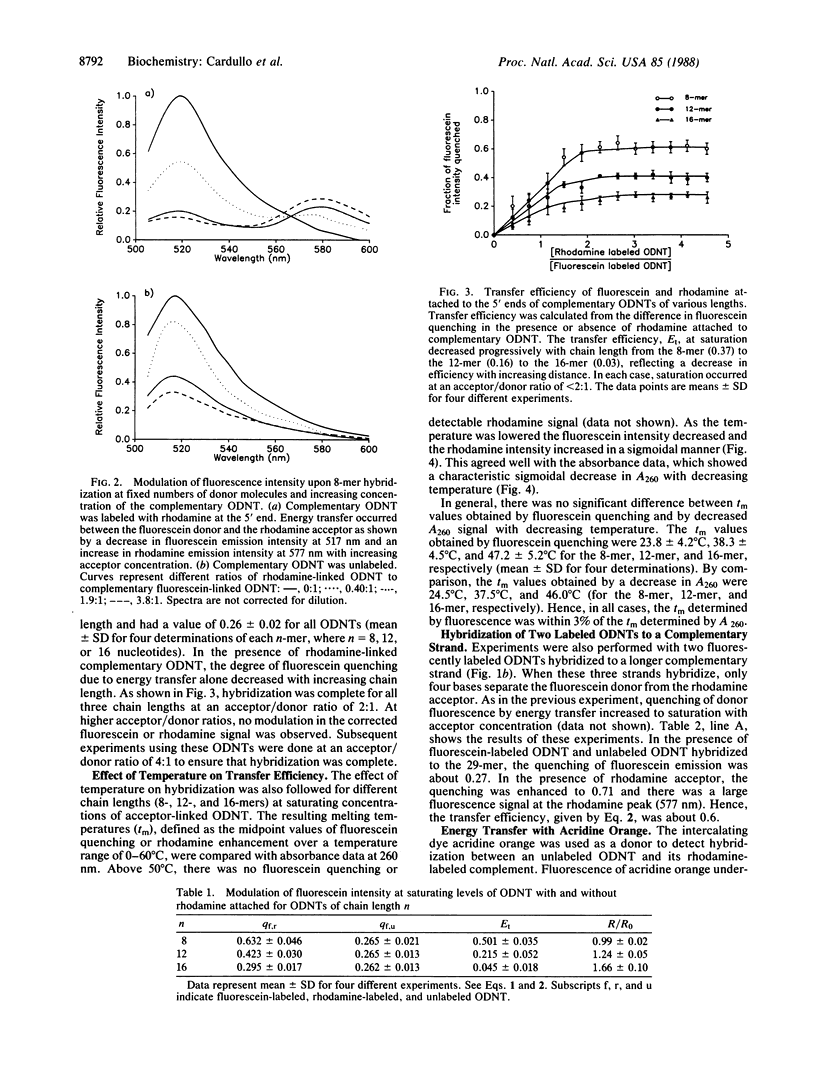
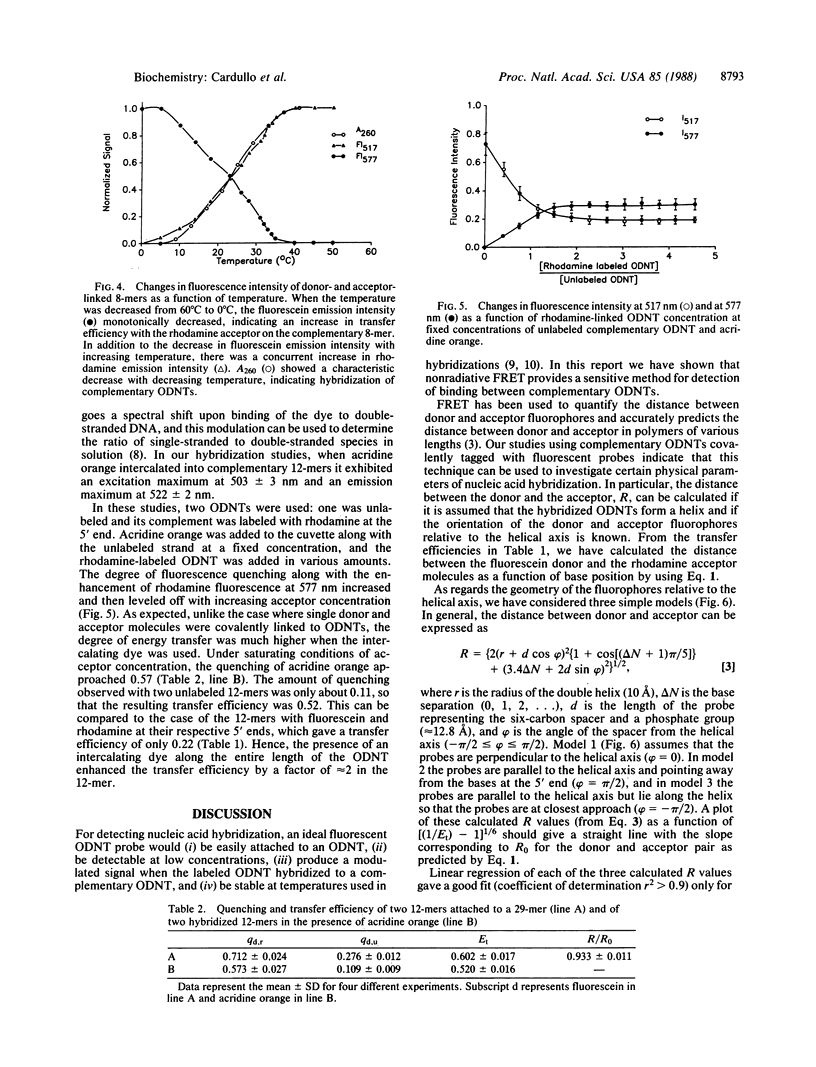
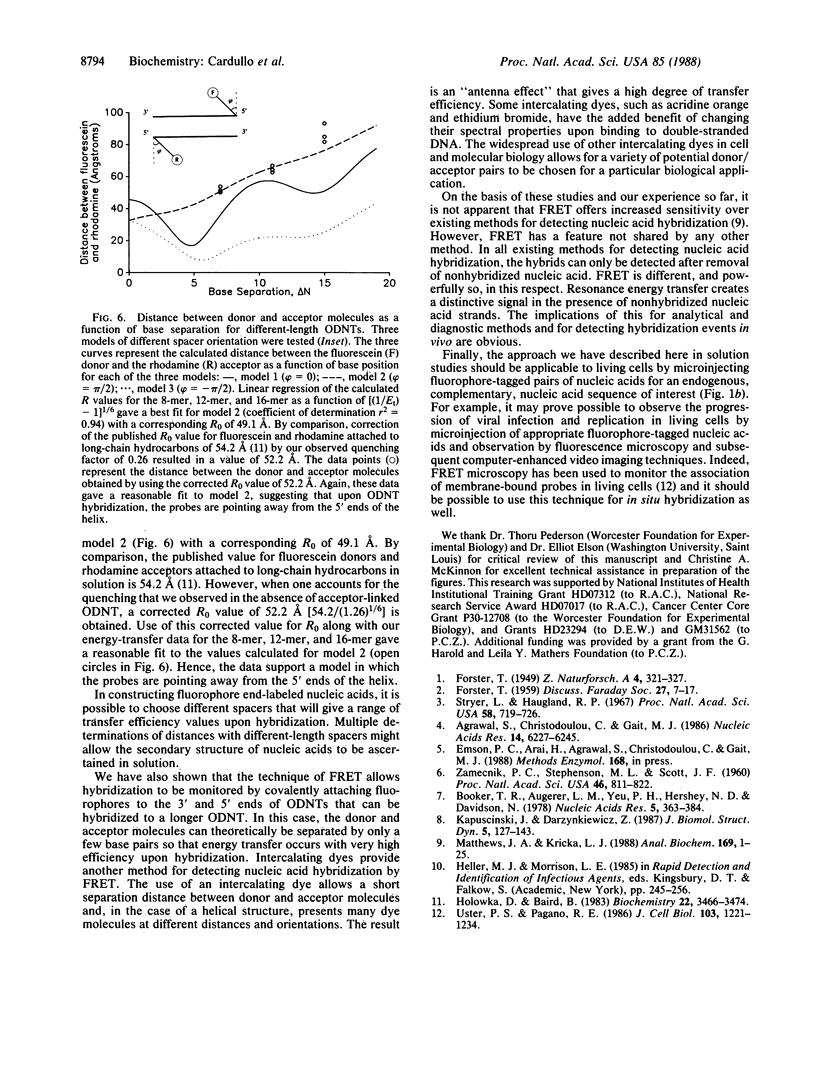
Three approaches were used to study hybridization of complementary oligodeoxynucleotides by nonradiative fluorescence resonance energy transfer. (i) Fluorescein (donor) and rhodamine (acceptor) were covalently attached to the 5' ends of complementary oligodeoxynucleotides of various lengths. Upon hybridization of the complementary oligodeoxynucleotides, energy transfer was detected by both a decrease in fluorescein emission intensity and an enhancement in rhodamine emission intensity. In all cases, fluorescein emission intensity was quenched by about 26% in the presence of unlabeled complement. Transfer efficiency at 5 degrees C decreased from 0.50 to 0.22 to 0.04 as the distance between donor and acceptor fluorophores in the hybrid increased from 8 to 12 to 16 nucleotides. Modeling of these hybrids as double helices showed that transfer efficiency decreased as the reciprocal of the sixth power of the donor-acceptor separation R, as predicted by theory with a corresponding R0 of 49 A. (ii) Fluorescence resonance energy transfer was used to study hybridization of two fluorophore-labeled oligonucleotides to a longer, unlabeled oligodeoxynucleotide. Two 12-mers were prepared that were complementary to two adjacent sequences separated by four bases on a 29-mer. The adjacent 5' and 3' ends of the two 12-mers labeled with fluorescein and rhodamine exhibited a transfer efficiency of approximately 0.60 at 5 degrees C when they both hybridized to the unlabeled 29-mer. (iii) An intercalating dye, acridine orange, was used as the donor fluorophore to a single rhodamine covalently attached to the 5' end of one oligodeoxynucleotide in a 12-base-pair hybrid. Under these conditions, the transfer efficiency was approximately 0.47 at 5 degrees C. These results establish that fluorescence modulation and nonradiative fluorescence resonance energy transfer can detect nucleic acid hybridization in solution. These techniques, with further development, may also prove useful for detecting and quantifying nucleic acid hybridization in living cells.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Christodoulou C., Gait M. J. Efficient methods for attaching non-radioactive labels to the 5' ends of synthetic oligodeoxyribonucleotides. Nucleic Acids Res. 1986 Aug 11;14(15):6227–6245. doi: 10.1093/nar/14.15.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowka D., Baird B. Structural studies on the membrane-bound immunoglobulin E-receptor complex. 1. Characterization of large plasma membrane vesicles from rat basophilic leukemia cells and insertion of amphipathic fluorescent probes. Biochemistry. 1983 Jul 5;22(14):3466–3474. doi: 10.1021/bi00283a025. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Interactions of acridine orange with double stranded nucleic acids. Spectral and affinity studies. J Biomol Struct Dyn. 1987 Aug;5(1):127–143. doi: 10.1080/07391102.1987.10506381. [DOI] [PubMed] [Google Scholar]
- Matthews J. A., Kricka L. J. Analytical strategies for the use of DNA probes. Anal Biochem. 1988 Feb 15;169(1):1–25. doi: 10.1016/0003-2697(88)90251-5. [DOI] [PubMed] [Google Scholar]
- Stryer L., Haugland R. P. Energy transfer: a spectroscopic ruler. Proc Natl Acad Sci U S A. 1967 Aug;58(2):719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uster P. S., Pagano R. E. Resonance energy transfer microscopy: observations of membrane-bound fluorescent probes in model membranes and in living cells. J Cell Biol. 1986 Oct;103(4):1221–1234. doi: 10.1083/jcb.103.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L., Scott J. F. PARTIAL PURIFICATION OF SOLUBLE RNA. Proc Natl Acad Sci U S A. 1960 Jun;46(6):811–822. doi: 10.1073/pnas.46.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]



