Abstract
5C12 HuMAb is a human monoclonal antibody against the A subunit of Shiga toxin 2 (Stx2). We have previously shown that 5C12 HuMAb effectively neutralizes the cytotoxic effects of this toxin by redirecting its transport within the cell and also by neutralizing the toxin's ability to inhibit protein synthesis. The 5C12 HuMAb and its recombinant IgG1 version protect mice at a dose of 0.6 μg against a lethal challenge of Stx2. The contribution of the Fc region to this observed neutralization activity of the 5C12 antibody against Stx2 was investigated in this study. Using recombinant DNA technology, 5C12 isotype variants (IgG1, IgG2, IgG3, and IgG4) and antibody fragments [Fab, F(ab′)2] were expressed in Chinese hamster ovary cells and evaluated in vitro and in vivo. All four 5C12 isotype variants showed protection in vitro, with the IgG3 and IgG4 variants showing the highest protection in vivo. The Fab and F(ab′)2 fragments also showed protection in vitro but no protection in the mouse toxicity model. Similar results were obtained for a second HuMAb (5H8) against the B subunit of Stx2. The data suggest the importance of the Fc region for neutralization activity, but it is not clear if this is related to the stability of the full-length antibody or if the Fc region is required for effective elimination of the toxin from the body.
Approximately 20,000 cases of Shiga toxin (Stx)-producing Escherichia coli (STEC) infections, in which the O157:H7 serotype is the most prevalent serotype, are reported annually in the United States (for recent reviews, see references 6, 9, 10, 23, and 31). Transmission of E. coli O157:H7 is most frequently associated with the consumption of contaminated food (e.g., ground beef or spinach) or drinking unpasteurized dairy products. Infections can also be acquired through person-to-person contact. Infected individuals typically develop abdominal pain and bloody diarrhea 2 to 5 days following exposure. STEC infections are self-limiting and usually resolve in 7 to 10 days. However, in 10 to 15% of children under the age of 5 or in the elderly, E. coli O157:H7 infections can develop into diarrhea-associated hemolytic uremic syndrome (HUS), a serious, life-threatening complication (22, 26, 28, 31). HUS is associated with hemolytic anemia and thrombocytopenia as a result of the destruction of red blood cells and platelets, followed by acute renal failure. There are no effective therapies against HUS, and supportive therapies include dialysis and kidney transplantation. Thus, the best treatment for HUS is prevention or amelioration of the E. coli O157:H7 infection, as no protective therapies are presently available. Antibiotic therapy for treatment of E. coli O157:H7 infections does not shorten the infection period and, in fact, may increase the risk of developing HUS (34).
The primary virulence factor for HUS is Shiga toxin 2 (Stx2), which is one of two antigenically distinct toxins produced by STEC. Stx2, like Stx1, consists of a single A subunit (32 kDa) linked to a ring of five B subunits (7 kDa) (18). The A subunit possesses RNA N-glycosidase activity, which cleaves a specific adenine residue in the 28S rRNA, resulting in the inhibition of protein synthesis. The B subunits are responsible for binding to the host cell receptor, globotriaosyl ceramide (Gb3; Galα[1-4]-Galβ[1-4]-Glcβ1-ceramide). The A subunit is cleaved as it is internalized through clathrin-dependent or independent endocytosis and translocated via the retrograde pathway to the cytosol where it inhibits protein synthesis (3, 4, 19).
Therapies that inhibit cell binding, interfere with the intracellular transport of Stx2, or inhibit enzymatic activity are under development by several research groups. One of the most promising therapies is the use of Stx2-neutralizing human monoclonal antibodies, particularly those directed against the A subunit (13, 14). One of these antibodies, 5C12, was shown to protect mice and gnotobiotic piglets from the fatal complications of Stx2 (13, 24, 25). A recombinant 5C12 antibody also demonstrated similar protective activity in these two animal models (1). We have recently shown that 5C12 neutralizes the toxicity of Stx2 for HeLa cells by blocking the retrograde transport of the toxin to the cytosol where the A subunit inhibits protein synthesis (11). In the present study, we investigated the contribution of the Fc portion of 5C12 to its in vitro and in vivo toxin-neutralizing activity by evaluating the efficacies of the Fabs and F(ab′)2 fragments of 5C12 in the HeLa cell and mouse toxicity assays. Smaller antibody fragments are advantageous for clinical use because of their lower immunogenicity and production costs. A comparison of a human monoclonal antibody against the B subunit of Stx2 (5H8) and its Fab fragment was performed to determine if similar results are obtained. We also investigated the contribution of the Fc functions by comparing the in vitro and in vivo neutralizing activities of the recombinant 5C12 isotype variants (e.g., IgG1, IgG2, IgG3, and IgG4).
MATERIALS AND METHODS
Construction of vectors expressing recombinant 5C12 isotype variants and Fab and F(ab′)2 fragments.
The expression vectors for the recombinant 5C12 isotype variants and Fab and F(ab′)2 fragments are based on the vector designed for the expression of 5C12 IgG1 (1). This vector was designed to be modular in nature such that different cassettes containing various Fc regions could be exchanged for the IgG1 Fc region as NheI/XbaI fragments. The original 5C12 IgG1 expression vector, p5C12IgG1, does not contain the DHFR expression cassette, which was cloned on a different vector, pdhfrExpress. To simplify the transfection process, the DHFR expression cassette was cloned as an EcoRI fragment into the EcoRI site upstream of the light-chain expression cassette to generate p5C12IgG1dhfr. The Fc regions from human IgG2, IgG3, and IgG4 were obtained from M. Preston (Harvard University) (20), as NheI/BamHI fragments cloned into pCRII. The three Fc regions were cloned into p5C12IgG1dhfr as NheI/BamHI fragments to replace the IgG1 Fc region to generate p5C12IgG2, p5C12IgG3, and p5C12IgG4, which contain the IgG2, IgG3, and IgG4 Fc regions, respectively.
The 5C12 Fab expression vector (p5C12Fab) contained the IgG1 CH1 region through the arginine at amino acid position 222, based on the Kabat numbering system (8). Two 5C12 F(ab′)2 expression vectors, which contained IgG2 Fc regions of different lengths, were constructed. The p5C12F(ab′9)2 expression vector contained the first 8 amino acids of the CH2 region (Kabat amino acid number 251), while the p5C12F(ab′26)2 expression vector contained the first 25 amino acids of the CH2 region (Kabat amino acid number 268). The two 5C12 antibody fragments were generated using PCR methodology, and the expression vectors containing these fragments were created by replacement of the 5C12 IgG1 cassette with either the 5C12 Fab or the 5C12 F(ab′)2 cassette. The sequences of the fragments containing the expression cassettes of the DHFR gene and the r5C12 isotype variants or antibody fragments were confirmed by double-stranded sequencing of these expression cassettes. The resulting isotypes and Fab and F(ab′)2 fragments are referred to as recombinant 5C12 (r5C12) IgG2 (or IgG3 or IgG4), r5C12 Fab, and r5C12 F(ab′)2, respectively.
The 5C12 isotype variants and the Fab and F(ab′)2 expression vectors were transfected into CHO DG44 cells, a double DHFR mutant cell line (kindly provided by L. Chasin, Columbia University, New York, NY) (32, 33), as previously described (1). Selection in the presence of G418 and methotrexate and the screening and production of these antibodies have been previously described (1). Each antibody was scaled up to a CELLineAD1000 Bioreactor (Integra Biosciences, Inc., East Dundee, IL) as previously described (1) for in vitro and in vivo testing of these antibodies. The antibodies produced by these cell lines are referred to as r5C12 IgG2, r5C12 IgG3, r5C12 IgG4, r5C12 Fab, r5C12 F(ab′9)2, and r5C12 F(ab′26)2. The antibodies were quantitated by a human kappa or IgG enzyme-linked immunosorbent assay (ELISA; Bethyl Laboratories, Inc., Montgomery, TX) and were used without further purification. The r5C12 IgG1 was either purified by protein A chromatography (HiTrap protein A HP column; GE Healthcare Corp., Piscataway, NJ) or used unpurified, depending on the experiment performed.
Construction of vectors expressing recombinant 5H8 IgG1 and Fab fragment.
The sequences of genes encoding the heavy- and light-chain variable regions of the 5H8 IgG1 were determined from the 5H8-producing hybridoma cell line as previously described (1). The 5C12 heavy- and light-chain variable region cassettes in p5C12IgG1 and p5C12Fab were replaced with the corresponding 5H8 heavy- and light-chain variable cassettes as AflII/NheI and DraIII/BsiWI fragments. The sequences of the fragments containing the expression cassettes of the DHFR gene and those of the r5H8 IgG1 and Fab fragment were confirmed by double-stranded sequencing of these expression cassettes. The resulting IgG1 and Fab fragment are referred to as r5H8 IgG1 and r5H8 Fab, respectively.
Generation of Fab and F(ab′)2 fragments from the 5C12- and 5H8-producing hybridoma cell lines.
Purified Fab was obtained by the chemical cleavage of 5C12 IgG1 produced by the parent hybridoma cell line (13, 25). The 5C12 IgG1 produced by this hybridoma cell line will be referred to as 5C12 HuMAb. The 5C12 HuMAb was produced as previously described (1, 25) and purified by protein A chromatography (IPA300; Repligen Corp., Waltham, MA). Fab and F(ab′)2 fragments were produced by papain or pepsin digestion, respectively (2). The resulting Fab and F(ab′)2 fragments are referred to as 5C12 Fab and 5C12 F(ab′)2, respectively. The 5H8 Fab was produced using the same methodology.
HeLa cell cytotoxicity assay.
The r5C12 isotype variants and Fab and F(ab′)2 fragments were evaluated for their ability to neutralize the toxic effects of Stx2 (Phoenix Laboratory, Boston, MA) on HeLa cells as previously described (1, 25). For comparison, the 5C12 HuMAb, Fab, and F(ab′)2 were included. Briefly, HeLa cells (CCL-2; American Tissue Culture Collection, Manassas, VA) were seeded at 1.4 × 104 cells/well on 96-well plates in McCoy's 5A medium (Mediatech, Inc., Herndon, VA) containing 10% fetal bovine serum (Invitrogen Corp., Carlsbad, CA) and incubated overnight at 37°C in 5% CO2. The r5C12 isotype variants, 5C12 HuMAb, and antibody fragments were serially diluted 2-fold in medium from a starting concentration of either 250 or 100 ng/ml down to 7.8 or 3.12 ng/ml, respectively. Antibody or antibody fragment (100 μl) was premixed with Stx2 (100 μl, 50 ng/ml; concentration determined to result in an ∼70% kill of HeLa cells) at 37°C for 60 min before being transferred onto the HeLa cells. Each antibody or antibody fragment was assayed in triplicate. The HeLa cells were incubated at 37°C for 48 h in 5% CO2. Control wells contained HeLa cells only or HeLa cells plus Stx2. The cells were washed with phosphate-buffered saline (PBS) and fixed in 2% formalin, and the plates were developed with crystal violet as previously described (1, 25). The absorbance (OD) of each well was read at 590 nm. The percentage of cell survival, which is a measurement of the toxin neutralization, was calculated by the following formula: [(ODtoxin+antibody/fragment − ODtoxin only)/(ODno toxin − ODtoxin only)] × 100. The HeLa cell cytotoxicity data were analyzed using one-way analysis of variance (ANOVA) and Tukey's multiple comparison test (Prism 5 version 5.0; GraphPad Software, Inc., San Diego, CA). Neutralization activity comparisons were considered significant if the P value was <0.05.
Similarly, the neutralizing activities of the r5H8 IgG1 and r5H8 Fab were compared to those of their parents, 5H8 IgG1 and Fab. The r5H8 and 5H8 IgG1 and their respective Fab fragments were serially diluted 2-fold in medium from a starting concentration of 1,000 ng/ml down to 7.8 or 31.5 ng/ml.
Mouse toxicity model.
The murine Stx toxicity model was used to evaluate the ability of the r5C12 isotype variants and antibody fragments, as well as the 5C12 Fab and F(ab′)2 fragments, to protect the mice against a lethal dose of Stx2. The r5C12 IgG1 and 5C12 HuMAb were included as controls. Dose response studies (15 to 25 g) were performed using groups of 10 mice aged 3 to 4 weeks (female Swiss-Webster; Taconic, Germantown, NY). The r5C12 isotype variants, 5C12 HuMAb, and antibody fragments were administered intraperitoneally (i.p.) at doses ranging from 0.075 to 5 μg/mouse in 200 μl of PBS. The doses of the r5H8 and 5H8 IgG1s and Fabs ranged from 0.625 to 10 μg/mouse in 200 μl of PBS. After 4 h, Stx2 (25 ng/mouse; dose equal to the 100% lethal dose [LD100]) was administered i.p. A control group of 10 mice received PBS instead of antibody. Mice were observed and scored a minimum of four times daily until the experiment was terminated. The survival data were analyzed using a parametric (log rank test) method (Prism 4 version 4.0; GraphPad Software, Inc.). All mouse procedures were approved by the Tufts University Institutional Animal Care and Use Committee.
RESULTS
Construction of CHO cell lines expressing r5C12 isotype variants or Fab and F(ab′)2 fragments.
The expression vector, p5C12IgG1dhfr, was modularly designed such that isotype variants and Fab and F(ab′)2 fragments of this antibody were expressed in CHO DG44 cells. Transfectants were selected in medium containing G418 (200 μg/ml) but lacking ribonucleosides and deoxyribonucleosides. Methotrexate was added at a concentration of 5 nM and was increased to 500 nM over a period of 4 to 6 months. Secretion of the isotype variants or antibody fragments was monitored by using a human IgG or kappa ELISA, respectively. Specificity of these antibodies or fragments against Stx2 was demonstrated by an Stx2-specific ELISA (data not shown).
Neutralization of Stx2 in the HeLa cytotoxicity assay.
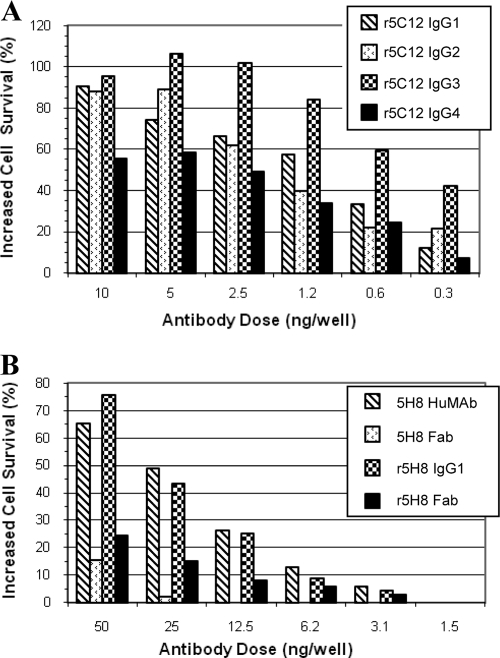
The ability of the r5C12 isotype variants, r5C12 Fab, and r5C12 F(ab′)2 to neutralize the cytotoxic effects of Stx2 on HeLa cells was evaluated. For comparison, 5C12 HuMAb, 5C12 Fab, and 5C12 F(ab′)2 were included in the study. The percentage of increased cell survival in the presence of various amounts of antibody or antibody fragment and 5 ng Stx2 is summarized in Fig. 1 and 2. The data represent the mean percentage of increased survival of cells from Stx2-induced toxicity and death in the presence of various isotype variants or their Fab and F(ab′)2 fragments compared to that for Stx2-only cells. The neutralization activities of the r5C12 isotypes were compared at doses ranging from 0.3 to 10 ng (Fig. 1A). Overall, the four isotypes showed a ≥50% increased survival at doses of ≥2.5 ng. At the three highest doses, the activities of the four isotypes were not significantly different (P < 0.05) from each other within a dose group, except for the IgG3 and IgG4 activities at a dose of 2.5 ng. Within the 1.25-ng-dose group, only the activities of two pairs, IgG2/IgG3 and IgG3/IgG4, were significantly different. The activities of the four isotypes were similar in the 0.6-ng-dose group, and only the activities of two pairs, IgG1/IgG3 and IgG3/IgG4, were significantly different in the lowest-dose group (0.3 ng).
FIG. 1.
(A) Neutralization of Stx2 by r5C12 isotype variants in a HeLa cell cytotoxicity assay. The percent survival of HeLa cells in the presence of 5 ng of Stx2 and various quantities of r5C12 IgG1, IgG2, IgG3 or IgG4 (0.3 to 10 ng) was measured. The percent survival values are from at least two independent assays (triplicate wells) and represent the mean of these values. (B) Neutralization of Stx2 by r5H8 antibody or Fab. The neutralization activities of the 5H8 Fab fragments were compared to those of their parent IgG1 antibodies. Although neither Fab fragment showed good neutralization activity, the parent IgG1 antibodies showed good protection (>40%) only at doses of 25 and 50 ng.
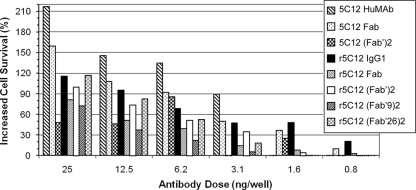
FIG. 2.
Comparison of the neutralization activities of the 5C12 antibody fragments to those of their parent IgG1 antibodies in a HeLa cell cytotoxicity assay. The percentage of increased survival of HeLa cells in the presence of 5 ng of Stx2 and various amounts of antibody or antibody fragments (0.8 to 25 ng) was determined. The percent survival values are from at least two independent assays (triplicate wells) and represent the mean of these values. Overall, the majority of the observed differences in neutralization activities were not statistically significant when analyzed by one-way ANOVA.
In a separate experiment, r5C12 IgG1, r5C12 Fab, and r5C12 F(ab′)2 were compared to their hybridoma-derived equivalent antibody or antibody fragment (Fig. 2). Five of the antibodies or antibody fragments showed 100% increased survival at a dose of 25 ng. The r5C12 IgG1 showed neutralization activities ranging from 20% to 116% increased survival at doses ranging from 0.8 to 25 ng, whereas the 5C12 HuMAb showed increased survival from 90% to 217% at doses ranging from 3.1 to 25 ng, but no activity at the two lower doses (0.8 and 1.6 ng). The Fab and F(ab′)2 fragments showed various levels of increased survival at the different doses, with only the 5C12 Fab and r5C12 F(ab′)2 showing increased survival at the two lower doses. The r5C12 F(ab′9)2 and r5C12 F(ab′26)2 fragments, which are IgG2 isotype fragments, showed a >20% increased survival only at doses of ≥6.25 ng. However, one-way ANOVA and Tukey's comparisons showed that the majority of the neutralization activities of the full-length antibodies and antibody fragments were not significantly different within a dose group. One exception was the 5C12 HuMAb at a dose of 25 ng, which had significantly higher activity than the others. The activities of the 5C12 Fab and the 5C12 F(ab′)2 at the 20 ng dose were also significantly different. The activities of the 5C12 HuMAb and r5C12 IgG1 at doses of 1.6, 3.1, 6.2 and 12.5 ng were similar but were significantly different at the lowest dose of 0.8 ng. Among the Fab and F(ab′)2 groups, significant differences in neutralization activity were noted between 5C12 HuMAb and r5C12 F(ab′9)2 at 12.5 ng, 5C12 HuMAb and r5C12 F(ab′26)2 at 6.2 ng, and 5C12 HuMAb and r5C12 F(ab′9)2 at 3.1 ng.
The r5H8 IgG1 and 5H8 HuMAb and their respective Fab fragments showed a pattern of neutralization whereby the full-length IgG1 antibodies were more effective than the Fab fragments (Fig. 1B). The 5H8 HuMAb is specific against the B subunit of Stx2 and has been previously shown to have lower neutralizing activity in the HeLa cell cytotoxicity assay than does the 5C12 HuMAb (13, 25). Therefore, higher doses of antibody were used in this study. The r5H8 IgG1 and 5H8 HuMAb showed between 20 and 75% increased cell survival at doses of ≥12.5 ng. At the six doses, there were no significant differences between the activities of the r5H8 IgG1 and 5H8 HuMAb. The same was true for their respective Fab fragments. At the three highest doses (12.5, 25, and 50 ng), the neutralization activities of the full-length IgG1s were significantly greater than the activities of the Fab fragments. At the three lower doses (1.5, 3.1, and 6.2 ng), the activities were similar, with the exceptions of 5H8 IgG1 and 5H8 Fab at doses of 3.1 and 6.2 ng.
Stx2 mouse toxicity model.
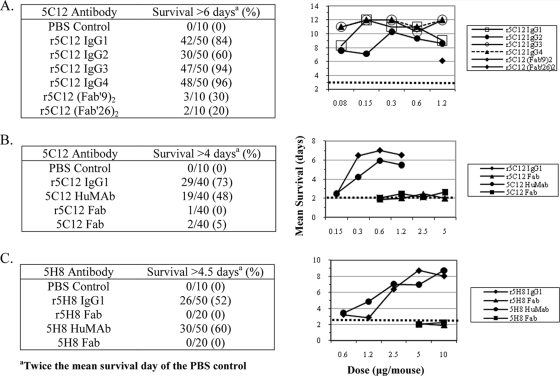
The r5C12 isotypes and r5C12 Fab and r5C12 F(ab′)2 fragments were evaluated in the mouse toxicity model to determine if they significantly prolonged survival (Fig. 3 and Table 1). The 5C12 HuMAb and 5C12 Fab were also included in this study. In the first experiment, mice were given either the r5C12 antibodies (0.08 to 1.20 μg/mouse) or F(ab′)2 fragments (1.20 μg/mouse) i.p. 4 h prior to Stx2 (25 ng/mouse i.p., which killed all the mice) (Fig. 3A). The control group, which received PBS, had a mean survival of 3.0 days, with none surviving >6 days. The r5C12 IgG3 and IgG4 groups showed the highest percentages of survival (94 and 96%, respectively) and the r5C12 IgG2 group the lowest (60%). All four r5C12 isotype groups showed significant prolonged survival at all five doses tested compared to that for the PBS control group (Table 1). The r5C12 IgG3 and r5C12 IgG4 isotype groups showed a ≥90% survival at the lowest dose of 0.08 ng/mouse. At a dose of 1.2 μg/mouse, the r5C12 F(ab′9)2 and r5C12 F(ab′26)2 groups showed significant prolonged survival, with a mean survival of 6.1 and 5.8 days, respectively. However, these survival rates were lower than those of all of the r5C12 isotype groups (Table 1).
FIG. 3.
Survival rates of antibody-treated groups against the lethal effects of Stx2 in the mouse toxicity model. Mice (10 per group) were treated with either four (5C12) or five (5H8) doses of IgG antibodies or one or two doses of Fab antibodies to evaluate their neutralization activities against Stx2. For the multiple-dose groups, the concentration of antibody was doubled. To simplify the survival data, all dose groups were combined for each antibody (left panels). The mean survival data for all groups are shown in the right panels. Detailed survival data are included in Tables 1 to 3. (A) r5C12 isotype variant groups and two r5C12 Fab groups. For the isotype variant groups, doubling the concentration from 0.08 to 1.2 μg/mouse (five doses) was tested. The Fab antibodies were tested at a single dose only (1.2 μg/mouse). PBS control group survival is indicated by a dotted line (3 days). (B) 5C12 IgG1 and 5C12 Fab groups (r5C12 and HuMAb). The IgG1 and Fab antibodies were tested at four doses, 0.15 to 1.2 μg/mouse and 0.6 to 5 μg/mouse, respectively. PBS control group survival is indicated by a dotted line (2 days). (C) 5H8 IgG1 and 5H8 Fab groups (r5H8 and HuMAb). The two IgGl antibodies were tested at five doses (0.6 to 10 μg/mouse) and the two Fab antibodies at two doses (5 and 10 μg/mouse). PBS control group survival is indicated by a dotted line (2.25 days).
TABLE 1.
Neutralization activities of r5C12 isotype variants and r5C12 F(ab′)2 fragments against Stx2 in the mouse toxicity model
| Antibody and dose (μg/mouse) | Survival rate (%)a | No. of days (mean ± SD)b | P valuec |
|---|---|---|---|
| PBS control | 0 | 3.0 ± 0.3 | |
| r5C12 IgG1 | |||
| 1.20 | 70 | 9.0 ± 4.6 | 0.0074 |
| 0.60 | 90 | 11.0 ± 2.7 | <0.0001 |
| 0.30 | 90 | 11.0 ± 2.9 | <0.0001 |
| 0.15 | 100 | 12.0 ± 0.0 | <0.0001 |
| 0.08 | 60 | 8.3 ± 4.6 | 0.0052 |
| r5C12 IgG2 | |||
| 1.20 | 60 | 8.6 ± 4.2 | <0.0001 |
| 0.60 | 70 | 9.3 ± 4.1 | 0.0001 |
| 0.30 | 80 | 10.3 ± 3.2 | <0.0001 |
| 0.15 | 40 | 7.1 ± 4.2 | 0.0006 |
| 0.08 | 50 | 7.6 ± 4.5 | 0.0009 |
| r5C12 IgG3 | |||
| 1.20 | 100 | 12.0 ± 0.0 | <0.0001 |
| 0.60 | 80 | 10.3 ± 3.3 | <0.0001 |
| 0.30 | 100 | 12.0 ± 0.0 | <0.0001 |
| 0.15 | 100 | 12.0 ± 0.0 | <0.0001 |
| 0.08 | 90 | 11.0 ± 2.7 | <0.0001 |
| r5C12 IgG4 | |||
| 1.20 | 100 | 12.0 ± 0.0 | <0.0001 |
| 0.60 | 90 | 11.0 ± 2.8 | <0.0001 |
| 0.30 | 100 | 12.0 ± 0.0 | <0.0001 |
| 0.15 | 100 | 12.0 ± 0.0 | <0.0001 |
| 0.08 | 90 | 11.0 ± 2.5 | <0.0001 |
| r5C12 (Fab′9)2 | |||
| 1.20 | 30 | 6.1 ± 4.0 | 0.0046 |
| r5C12 (Fab′26)2 | |||
| 1.20 | 20 | 5.8 ± 3.2 | <0.0001 |
Percentage of mice (10 per group) that survived through day 12. Mice euthanized on day 12 were given a survival score of 12 days.
Mean survival (days) for each group.
P values calculated for the comparison of the mean survival of the PBS control group and each treated group by parametric (log rank test) analysis.
The design of the second experiment was similar to that of the first experiment. The neutralization activities of the r5C12 IgGl and r5C12 Fab were compared to those of their hybridoma-derived equivalent at four different doses (Fig. 3B and Table 2). The combined survival data of all doses for each antibody group show that the two IgG1 antibodies have a >48% survival and their two Fab fragments have a ≤5% survival (Fig. 3B). The combined r5C12 IgG1 data suggest that the r5C12 IgG1 showed greater protection than does the 5C12 HuMAb (Fig. 3B), but this is in part due to the difference in percentage of survival of mice in the 0.3-μg-dose groups (Table 2). However, the r5C12 IgG1 groups did show higher survival rates and mean survival days than did their 5C12 HuMAb groups at the same doses, but the P value (P = 0.0092) was significant for only one dose (0.3 μg/mouse) (Table 2). The two IgG1 groups showed significant prolonged survival at the three highest doses (1.2, 0.6, and 0.3 μg/mouse), but neither of their Fab groups showed significant prolonged survival compared to that for the PBS control group (mean survival of 1.96 days). All the mice in the two Fab groups died at all four doses, with a mean survival of ≤2.45 days (Fig. 3B and Table 2).
TABLE 2.
Neutralization activities of 5C12 IgG1 antibodies and 5C12 Fab fragments against Stx2 in the mouse toxicity model
| Antibody and dose (μg/mouse) | Survival rate (%)a | No. of days (mean ± SD)b | P valuec |
|---|---|---|---|
| PBS control | 0 | 1.96 ± 0.17 | |
| r5C12 IgG1 | |||
| 1.2 | 90 | 6.50 ± 1.59 | <0.0001 |
| 0.6 | 100 | 7.00 ± 0.00 | <0.0001 |
| 0.3 | 90 | 6.47 ± 1.67 | <0.0001 |
| 0.15 | 10 | 2.37 ± 1.63 | 0.5488 |
| r5C12 Fab | |||
| 5.0 | 0 | 1.99 ± 0.33 | 0.0210 |
| 2.5 | 0 | 2.45 ± 1.62 | 0.4082 |
| 1.2 | 0 | 2.01 ± 0.33 | 0.3676 |
| 0.6 | 0 | 1.88 ± 0.21 | 0.0789 |
| 5C12 HuMAb | |||
| 1.2 | 70 | 5.48 ± 2.45 | 0.0007 |
| 0.6 | 80 | 5.95 ± 2.22 | 0.0007 |
| 0.3 | 30 | 4.23 ± 2.23 | 0.0042 |
| 0.15 | 10 | 2.46 ± 1.63 | 0.3544 |
| 5C12 Fab | |||
| 5.0 | 0 | 2.63 ± 1.57 | 0.0210 |
| 2.5 | 0 | 2.11 ± 0.68 | 0.4082 |
| 1.2 | 0 | 2.45 ± 1.62 | 0.3676 |
| 0.6 | 0 | 2.03 ± 0.30 | 0.0789 |
Percentage of mice (10 per group) that survived through day 7. Mice euthanized on day 7 were given a survival score of 7 days.
Mean survival (days) for each group.
P values were calculated for the comparison of the mean survival of the PBS control group and each treated group by parametric (log rank test) analysis.
The neutralization activities of the r5H8 IgG1 and 5H8 HuMAb and their Fab fragments were also evaluated in the mouse toxicity model (Fig. 3C and Table 3). The combined survival data of all doses for each antibody show that the two 5H8 IgG1 groups have similar survival rates (52 to 60%) and the two Fab groups had no mice surviving. The two IgG1 antibodies showed significant prolonged survival at the three highest doses (2.5, 5, and 10 μg/mouse) compared to that for the PBS control group (mean survival of 2.25 days) (Table 3). Neither Fab fragment prolonged survival at the two doses tested. The 5H8 HuMab data from this study are consistent with our previous report, which shows that the 5H8 HuMAb has lower neutralization activity against the toxic effects of Stx2 (13, 25).
TABLE 3.
Neutralization activities of 5H8 IgG1 antibodies and Fab fragments against Stx2 in the mouse toxicity model
| Antibody and dose (μg/mouse) | Survival rate (no. of surviving mice/total no. of mice [%])a | No. of days (mean ± SD)b | P value for control group vs treated groupc |
|---|---|---|---|
| Control | 0/10 (0) | 2.25 ± 0.42 | |
| r5H8 IgG1 | |||
| 10.0 | 9/10 (90) | 8.05 ± 2.14 | <0.0001 |
| 5.0 | 10/10 (100) | 8.73 ± 0.00 | <0.0001 |
| 2.5 | 6/10 (60) | 6.40 ± 3.01 | <0.0001 |
| 1.2 | 0/10 (0) | 2.87 ± 0.83 | 0.0370 |
| 0.6 | 1/10 (10) | 3.23 ± 2.02 | 0.0673 |
| r5H8 Fab | |||
| 10.0 | 0/10 (0) | 1.91 ± 0.15 | 0.0247 |
| 5.0 | 0/10 (0) | 2.10 ± 0.28 | 0.3493 |
| 5H8 HuMAb | |||
| 10.0 | 10/10 (100) | 8.73 ± 0.0 | <0.0001 |
| 5.0 | 7/10 (70) | 6.96 ± 2.95 | 0.0001 |
| 2.5 | 7/10 (70) | 7.03 ± 2.75 | <0.0001 |
| 1.2 | 4/10 (40) | 4.86 ± 3.38 | 0.0571 |
| 0.6 | 1/10 (10) | 3.43 ± 2.12 | 0.0525 |
| 5H8 Fab | |||
| 10.0 | 0/10 (0) | 2.24 ± 0.39 | 0.8088 |
| 5.0 | 0/10 (0) | 2.01 ± 0.29 | 0.1469 |
Percentage of mice (10 per group) that survived through day 8. Mice euthanized on day 8 were given a survival score of 8 days.
Mean survival (days) for each group.
P values were calculated for the comparison of the mean survival of the control group and each treated group by parametric (log rank test) analysis.
DISCUSSION
A major focus of our research group is the development of efficacious therapies against Stx1 and Stx2. We have developed a panel of human monoclonal antibodies against Stx1 and Stx2 that show efficacy in vitro and in vivo (13, 14). Of these, the 5C12 HuMAb, an IgG1 antibody against the Stx2A subunit, is highly efficacious in the mouse toxicity model (13, 24), as well as in the gnotobiotic piglet model (25). In the piglet model, the 5C12 HuMAb is fully protective at a dose of 0.4 mg/kg of body weight against E. coli O157:H7 challenge after the onset of diarrhea. The 5C12 HuMAb was produced as a recombinant IgG1 monoclonal antibody (r5C12 IgG1) in CHO cells and shown to be as efficacious in vitro and in vivo in the mouse toxicity model as the parent 5C12 HuMAb (1). The present study was undertaken to determine if isotype variants or Fab or F(ab′)2 fragments of r5C12 IgG1 and 5C12 HuMAb show efficacy in vitro and in vivo similar to that of their respective parent antibody. Via recombinant DNA methodologies, isotype variants and Fab and F(ab′)2 antibody fragments were produced in CHO cells by using a modularly designed expression vector. These human antibodies and antibody fragments were evaluated both in vitro and in vivo for neutralization activity against Stx2. The r5C12 isotype variants showed good effectiveness both in vitro and in vivo. Comparison of the activities of the 5C12 full-length antibodies and their antibody fragments showed a general trend whereby the full-length antibodies had greater activities. Although, the Fab and F(ab′)2 fragments also showed good in vitro activity at the higher doses, the Fab fragments showed no protection in the mouse toxicity model, and the two F(ab′)2 fragments showed only marginal activity. Parallel studies of a second HuMAb against the B subunit of Stx2 were also included for comparison. Overall, the 5H8 full-length antibodies showed greater neutralization activities in vitro and in vivo than did the Fab fragments at the higher doses.
The differences between the in vitro and in vivo activities of the full-length IgG antibodies compared to those of the antibody fragments are further amplified if the differences in molecular size are considered. The predicted sizes of the Fab and (Fab′) fragments are ∼50 kDa and ∼105 kDa, which are approximately one-third and two-third the size of the full-length IgG molecules, respectively. In the mouse toxicity model, the Fab fragments may be quickly cleared by the kidneys due to their smaller size and, thus, provide no protection to the Stx2-exposed mice. Protection was not observed if the toxin was administered 15 min, instead of 4 h, after injecting the Fab fragments (data not shown). The F(ab′)2 fragments are larger than the Fab fragments and should not be cleared as quickly by the kidneys, thereby affording some protection to the mice.
It is likely that no single factor is responsible for the observed differences in the neutralization activities. Differences in the molecular sizes of the full-length antibodies and antibody fragments, differences in the binding affinities and/or biological half-lives, and the effector functions mediated by the Fc regions may be important contributing factors. However, understanding the relationship of each of these factors to the in vitro and in vivo neutralization activities of each antibody and antibody fragment is not a simple task. For example, size alone may not account for the differences in neutralization activity of the full-length IgG antibodies and antibody fragments, but the absence of the effector functions mediated by the Fc region may. Two important effector functions of the Fc regions that could contribute to the removal of antibody/toxin complexes from the body are activation of complement and binding with Fcγ receptors. These properties do not play a role in neutralizing the toxin in vitro but play a major role in the clearance of the toxin in vivo. Complement components and Fc parts bind to their receptors on macrophages in liver and spleen, which phagocytose complement and antibody bound antigen and degrade the antigen within phagolysosomes to clear from the body. All four isotypes except IgG4 activate complement, and all of them except IgG2 bind Fc receptors. Fc receptor binding but not complement binding may play a role in the clearance of antibody/toxin complexes, since the in vivo data show that all IgG isotype variants except IgG2 were effective in neutralizing the toxin. Nonetheless, IgG2 protected 50% of mice at the lowest dose. This suggests that the binding affinity and/or the biological half-life of an antibody may also influence its in vivo neutralization activity.
The relationship of the binding affinity of an antibody or antibody fragment to its neutralization activity is unclear. For example, all four 5C12 isotype variants showed good activity in vitro and in vivo, suggesting the potential importance of a full-length Fc fragment for protection against Stx2. However, the effector functions may not contribute directly to the observed neutralization activities because, overall, the activities of the four isotype variants were similar in vitro. Yet, the full-length antibodies appear to have greater neutralization activities than do the antibody fragments in vitro, suggesting that the presence of the Fc region is associated with increased activity and therefore may affect the binding affinity or the biological half-life. A second example illustrating the complexity of the relationship of binding affinity to activity is the comparison of the neutralization activities of three HuMAbs against the A subunit of Stx2. The order of binding affinities (based on the dissociation constant value) of these HuMAbs is 2F10 > 3E9 > 5C12, yet the 5C12 HuMAb showed greater neutralization activity in vitro and in vivo than did both the 2F10 and 3E9 HuMAbs (25). Clearly, understanding the mechanistic relationship of the Fc region with the ability of an antibody to neutralize the lethal effects of Stx2 is beyond the scope of this study.
Several groups have developed effective monoclonal anti-Stx1 and anti-Stx2 antibodies that protect mice against the toxic effects of Stx1 or Stx2, respectively (1, 5, 12-14, 24, 27, 35). The human 5C12 monoclonal antibody also protects gnotobiotic piglets against the fatal systemic complications of STEC producing Stx2 (25). Two groups report the development of a recombinant Fab fragment against Stx1 (7) or an scFv against Stx2A (12). The in vitro neutralizing activities of these two recombinant antibody fragments are similar, requiring 10 μg/ml to obtain 50% cell viability. The scFv against Stx2A was evaluated in a BALB/c mouse enterohemorrhagic E. coli (EHEC) infection model, and at best, 60% survival was observed when 2 mg scFv/mouse was administered (12). While immunotherapeutics for treatment against Stx2 and HUS are attractive, there are several disadvantages of this type of therapy, including instability, high production costs, short shelf life, and additional testing costs because the production system is animal-derived. If the Fab fragments had shown good neutralization activities, they could have been produced in much higher yields in E. coli or yeast expression systems, thereby reducing production costs. With the exception of the monoclonal antibodies described by Mukherjee et al. (13, 14), the other monoclonal antibodies are not of human origin, and thus, immunogenicity may be an additional problem. To complement immunotherapeutics against Stx, other therapies are under development by several laboratories and include small molecule inhibitors (21; D. E. Akiyoshi and S. Tzipori, unpublished data), vaccines (29, 30), and Gb3 mimics (15-17). However, it remains to be shown if any of these alternative therapeutics are as efficacious as the immunotherapeutics.
Acknowledgments
This work was supported by Public Health Service grants RO1-AI041326 and RO1-DK58993 from the National Institutes of Health.
We thank Julia Dilo for technical assistance and Anne Kane (Phoenix Laboratories, Boston, MA) for the purified Stx2.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 19 January 2010.
REFERENCES
- 1.Akiyoshi, D. E., C. M. Rich, S. O'Sullivan-Murphy, L. Richard, J. Dilo, A. Donohue-Rolfe, A. S. Sheoran, S. Chapman-Bonofiglio, and S. Tzipori. 2005. Characterization of a human monoclonal antibody against Shiga toxin 2 expressed in Chinese hamster ovary cells. Infect. Immun. 73:4054-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrew, S. M., and J. A. Titus. 2003. Fragmentation of immunoglobulin G. Curr. Protoc. Cell Biol. 16:16.4. [DOI] [PubMed] [Google Scholar]
- 3.Bonifacino, J. S., and R. Rojas. 2006. Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell Biol. 7:568-579. [DOI] [PubMed] [Google Scholar]
- 4.Cherla, R. P., S. Y. Lee, and V. L. Tesh. 2003. Shiga toxins and apoptosis. FEMS Microbiol. Lett. 228:159-166. [DOI] [PubMed] [Google Scholar]
- 5.Edwards, A. C., A. R. Melton-Celsa, K. Arbuthnott, J. R. Stinson, C. K. Schmitt, H. C. Wong, and A. O'Brien. 1998. Vero cell neutralization and mouse protective efficacy of humanized monoclonal antibodies against Escherichia coli toxins Stx1 and Stx2, p. 388-392. In J. B. Kasper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, DC.
- 6.Gyles, C. L. 2007. Shiga toxin-producing Escherichia coli: an overview. J. Anim. Sci. 85:E45-E62. [DOI] [PubMed] [Google Scholar]
- 7.Inoue, K., K. Itoh, H. Nakao, T. Takeda, and T. Suzuki. 2004. Characterization of a Shiga toxin 1-neutralizing recombinant Fab fragment isolated by phage display system. Tohoku J. Exp. Med. 203:295-303. [DOI] [PubMed] [Google Scholar]
- 8.Kabat, E. A., T. T. Wu, H. M. Perry, K. S. Gottesman, and C. Foeller. 1992. Sequences of proteins of immunological interest, 5th ed. NIH publication no. 91-3242. U.S. Department of Health and Human Services, Public Health Service, NIH, Bethesda, MD.
- 9.Karch, H., P. I. Tarr, and M. Bielaszewska. 2005. Enterohaemorrhagic Escherichia coli in human medicine. Int. J. Med. Microbiol. 295:405-418. [DOI] [PubMed] [Google Scholar]
- 10.Karmali, M. A. 2004. Infection by Shiga toxin-producing Escherichia coli: an overview. Mol. Biotechnol. 26:117-122. [DOI] [PubMed] [Google Scholar]
- 11.Krautz-Peterson, G., S. Chapman-Bonofiglio, K. Boisvert, H. Feng, I. M. Herman, S. Tzipori, and A. S. Sheoran. 2008. Intracellular neutralization of Shiga toxin 2 by an A subunit-specific human monoclonal antibody. Infect. Immun. 76:1931-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma, Y., X. Mao, J. Li, H. Li, Y. Feng, H. Chen, P. Luo, J. Gu, S. Yu, H. Zeng, G. Guo, K. Yang, and Q. Zou. 2008. Engineering an anti-Stx2 antibody to control severe infections of EHEC O157:H7. Immunol. Lett. 121:110-115. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee, J., K. Chios, D. Fishwild, D. Hudson, S. O'Donnell, S. M. Rich, A. Donohue-Rolfe, and S. Tzipori. 2002. Human Stx2-specific monoclonal antibodies prevent systemic complications of Escherichia coli O157:H7 infection. Infect. Immun. 70:612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherjee, J., K. Chios, D. Fishwild, D. Hudson, S. O'Donnell, S. M. Rich, A. Donohue-Rolfe, and S. Tzipori. 2002. Production and characterization of protective human antibodies against Shiga toxin 1. Infect. Immun. 70:5896-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulvey, G., P. I. Kitov, P. Marcato, D. R. Bundle, and G. D. Armstrong. 2001. Glycan mimicry as a basis for novel anti-infective drugs. Biochimie 83:841-847. [DOI] [PubMed] [Google Scholar]
- 16.Neri, P., S. I. Nagano, S. Yokoyama, H. Dohi, K. Kobayashi, T. Miura, T. Inazu, T. Sugiyama, Y. Nishida, and H. Mori. 2007. Neutralizing activity of polyvalent Gb3, Gb2 and galacto-trehalose models against Shiga toxins. Microbiol. Immunol. 51:581-592. [DOI] [PubMed] [Google Scholar]
- 17.Nishikawa, K., K. Matsuoka, M. Watanabe, K. Igai, K. Hino, K. Hatano, A. Yamada, N. Abe, D. Terunuma, H. Kuzuhara, and Y. Natori. 2005. Identification of the optimal structure required for a Shiga toxin neutralizer with oriented carbohydrates to function in the circulation. J. Infect. Dis. 191:2097-2105. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien, A. D., and R. K. Holmes. 1987. Shiga and Shiga-like toxins. Microbiol. Rev. 51:206-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paton, J. C., and A. W. Paton. 2006. Shiga toxin “goes retro” in human primary kidney cells. Kidney Int. 70:2049-2051. [DOI] [PubMed] [Google Scholar]
- 20.Preston, M. J., A. A. Gerceker, M. E. Reff, and G. B. Pier. 1998. Production and characterization of a set of mouse-human chimeric immunoglobulin G (IgG) subclass and IgA monoclonal antibodies with identical variable regions specific for Pseudomonas aeruginosa serogroup O6 lipopolysaccharide. Infect. Immun. 66:4137-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saenz, J. B., T. A. Doggett, and D. B. Haslam. 2007. Identification and characterization of small molecules that inhibit intracellular toxin transport. Infect. Immun. 75:4552-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheiring, J., S. P. Andreoli, and L. B. Zimmerhackl. 2008. Treatment and outcome of Shiga-toxin-associated hemolytic uremic syndrome (HUS). Pediatr. Nephrol. 23:1749-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serna, A., IV, and E. C. Boedeker. 2008. Pathogenesis and treatment of Shiga toxin-producing Escherichia coli infections. Curr. Opin. Gastroenterol. 24:38-47. [DOI] [PubMed] [Google Scholar]
- 24.Sheoran, A. S., S. Chapman, P. Singh, A. Donohue-Rolfe, and S. Tzipori. 2003. Stx2-specific human monoclonal antibodies protect mice against lethal infection with Escherichia coli expressing Stx2 variants. Infect. Immun. 71:3125-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheoran, A. S., S. Chapman-Bonofiglio, B. R. Harvey, J. Mukherjee, G. Georgiou, A. Donohue-Rolfe, and S. Tzipori. 2005. Human antibody against Shiga toxin 2 administered to piglets after the onset of diarrhea due to Escherichia coli O157:H7 prevents fatal systemic complications. Infect. Immun. 73:4607-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegler, R., and R. Oakes. 2005. Hemolytic uremic syndrome; pathogenesis, treatment, and outcome. Curr. Opin. Pediatr. 17:200-204. [DOI] [PubMed] [Google Scholar]
- 27.Smith, M. J., A. R. Melton-Celsa, J. F. Sinclair, H. M. Carvalho, C. M. Robinson, and A. D. O'Brien. 2009. Monoclonal antibody 11E10 which neutralizes Shiga toxin type 2 (Stx2), recognizes three regions on the Stx2 A subunit, blocks the enzymatic action of the toxin in vitro, and alters the overall cellular distribution of the toxin. Infect. Immun. 77:2730-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarr, P. I., C. A. Gordon, and W. L. Chandler. 2005. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073-1086. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji, T., T. Shimizu, K. Sasaki, Y. Shimizu, K. Tsukamoto, H. Arimitsu, S. Ochi, S. Sugiyama, K. Taniguchi, P. Neri, and H. Mori. 2008. Protection of mice from Shiga toxin-2 toxemia by mucosal vaccine of Shiga toxin 2B-His with Escherichia coli enterotoxin. Vaccine 26:469-476. [DOI] [PubMed] [Google Scholar]
- 30.Tsuji, T., T. Shimizu, K. Sasaki, K. Tsukamoto, H. Arimitsu, S. Ochi, K. Taniguchi, M. Noda, P. Neri, and H. Mori. 2008. A nasal vaccine comprising B-subunit derivative of Shiga toxin 2 for cross-protection against Shiga toxin types 1 and 2. Vaccine 26:2092-2099. [DOI] [PubMed] [Google Scholar]
- 31.Tzipori, S., A. Sheoran, D. Akiyoshi, A. Donohue-Rolfe, and H. Trachtman. 2004. Antibody therapy in the management of Shiga toxin-induced hemolytic uremic syndrome. Clin. Microbiol. Rev. 17:926-941, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urlaub, G., and L. A. Chasin. 1980. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc. Natl. Acad. Sci. U. S. A. 77:4216-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urlaub, G., P. J. Mitchell, E. Kas, L. A. Chasin, V. L. Funanage, T. T. Myoda, and J. Hamlin. 1986. Effect of gamma rays at the dihydrofolate reductase locus: deletions and inversions. Somat. Cell Mol. Genet. 12:555-566. [DOI] [PubMed] [Google Scholar]
- 34.Wong, C. S., S. Jelacic, R. L. Habeeb, S. L. Watkins, and P. I. Tarr. 2000. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J. Med. 342:1930-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamagami, S., M. Motoki, T. Kimura, H. Izumi, T. Takeda, Y. Katsuura, and Y. Matsumoto. 2001. Efficacy of postinfection treatment with anti-Shiga toxin (Stx) 2 humanized monoclonal antibody TMA-15 in mice lethally challenged with Stx-producing Escherichia coli. J. Infect. Dis. 184:738-742. [DOI] [PubMed] [Google Scholar]