Abstract
Aspergillus fumigatus is the most frequent cause of invasive mold infections worldwide. Platelets contribute to inflammation and promote thrombosis, characteristically seen in aspergillosis, and might be involved both in antifungal defense and in the histopathological process. In the experiments reported here, in vitro activation of platelets by conidia, swollen conidia, and hyphae from A. fumigatus was assessed by flow cytometry and enzyme immunoassays. THP-1 monocytes and human monocytes with and without platelets were cultured with hyphae from A. fumigatus, and the release of interleukin-8 (IL-8) was measured by enzyme immunoassays. A. fumigatus potently induced the expression of CD62-p and CD63 and the release of CD40 ligand, RANTES, and Dickkopf homolog 1 in platelets, with particularly enhancing effects of hyphae compared with conidia. The hypha-mediated activation of platelets further enhanced the release of IL-8 both in THP-1 monocytes and in human adherent monocytes. In conclusion, we have found that A. fumigatus is a potent inducer of platelet-mediated inflammation, potentially promoting protective as well as harmful responses during aspergillosis.
Aspergillosis is the most common mold infection worldwide, and Aspergillus fumigatus accounts for more than 90% of the cases (6). In contrast to most human pathogens, which are encountered infrequently, A. fumigatus spores are inhaled on a daily basis, and occasionally, exposure to large numbers of conidia can occur. The first-line host defense against Aspergillus infection is based on innate immunity mediated by monocytes/macrophages and neutrophils (11, 12, 17, 18). The adaptive immune system responds to a pathogen only after it has been recognized by the innate immune system (11). While inadequate immune responses may predispose to invasive disease, overly robust responses can result in immune-mediated inflammatory tissue damage. Thus, imbalanced immune responses to A. fumigatus may result in a spectrum of human disease states ranging from allergic bronchopulmonary aspergillosis to invasive aspergillosis in the immunocompromised host (9). Despite better diagnostic tools and therapeutic advances, the infection is difficult to diagnose and treat, and the outcome of invasive aspergillosis is often fatal.
Several lines of evidence support a role for platelets in inflammation (10). Platelet-mediated inflammation has been demonstrated during various acute and chronic infections, and it has been suggested that platelets contribute to antimicrobial defenses (5, 8). Very little is known about the role of platelets in defense against Aspergillus infection. In this connection, it is interesting that important risk groups for invasive aspergillosis, e.g., patients with chemotherapy-induced neutropenia and recipients of hematopoietic stem cell transplants (6, 13), very often have concurrent thrombocytopenia in addition to neutropenia. Furthermore, it has been reported that liver transplant recipients with thrombocytopenia have a considerably higher incidence of fungal infection than nonthrombocytopenic patients (3). There are some reports on the interaction between A. fumigatus and platelets, showing inhibition of fungal growth potentially involving the release of known platelet-derived microbial peptides, as well as direct physical interaction between platelets and conidia or hyphae (4, 16).
Aspergillus fumigatus is angioinvasive, leading to intravascular thrombosis and dissemination of the fungus through the bloodstream (1, 2, 21). In view of the well-known role of thrombocytes in vascular thrombosis in general, it is also possible that thrombocytes contribute to the vascular damage and thrombosis which are a hallmark of invasive aspergillosis.
To further study the possible role of platelets in the immune response and pathogenesis of Aspergillus fumigatus, we have examined the effect of conidia and hyphae on relevant platelet-related inflammatory mediators. We have also examined the ability of Aspergillus-exposed platelets to modulate inflammatory responses in monocytes.
MATERIALS AND METHODS
Preparation of Aspergillus conidia and hyphae.
A. fumigatus (ATCC MYA 1163) was grown on Sabouraud agar at 28°C for 7 days. The agar contained penicillin (12 mg/liter) and streptomycin (40 mg/liter) to prevent bacterial contamination. Conidia were harvested by gently scraping the medium and solubilizing in phosphate-buffered saline (PBS) with 0.05% Tween. The suspension was filtered through a 25-mm-diameter Easy Pressure syringe filter holder (Gelman laboratory, New York, NY) with polypropylene separators (10-μm pore size). The conidial concentration was determined by counting in a Bürker chamber. Live conidia were stored at 4°C with a weekly turnover to ensure the best possible viability and were diluted to correct concentrations in RPMI 1640 (PAA Laboratories, Pasching, Austria) prior to use. In some experiments, the conidia were incubated at 37°C under 5% CO2 for 6 or 18 h to generate swollen conidia and hyphae, respectively.
Preparation and stimulation of citrated PRP.
Preparation of citrated platelet-rich plasma (PRP), obtained from healthy volunteers, was performed as previously described (15). PRP (6.0 × 108 platelets/ml) was incubated at 22°C with 10 μM SFLLRN (synthesized at The Biotechnology Centre of Oslo, Oslo, Norway) in Tris-buffered saline (20 mM Tris and 150 mM NaCl [pH 7.4]). Conidia, swollen conidia at a concentration of 6.0 × 107/ml, hyphae grown from the same conidial concentration, or a combination of SFLLRN and the conidia or hyphae were dissolved in medium as described in the preceding paragraph. Some experiments were done with hyphae separated from platelets with 0.4-μm-pore-sized cell culture inserts (BD Falcon, Franklin Lakes, NJ). At different time points, aliquots were removed and centrifuged at 13,000 × g for 5 min to obtain platelet-free (and Aspergillus-free) plasma (PFP), which was stored at −80°C until cytokine measurement or was used for further in vitro experiments with THP-1 monocytes and adherent monocytes (see below).
Preparation and stimulation of THP-1 and adherent monocytes.
The human monocytic cell line THP-1 (American Type Culture Collection, Manassas, VA) was cultured in RPMI 1640 (PAA Laboratories, Pasching, Austria) with or without PRP, hyphae from A. fumigatus in PRP as described above, and hyphae from A. fumigatus at the same concentration at 37°C under 5% CO2. In a separate set of experiments, THP-1 monocytes were also incubated with PFP from unstimulated and A. fumigatus (hypha)-activated (120 min) PRP (see above).
In a separate set of experiments, peripheral blood mononuclear cells (PBMC) (5 × 106/ml), isolated from heparinized blood from six healthy volunteers by Isopaque-Ficoll (Lymphoprep; Nycomed Pharma, Oslo, Norway) gradient centrifugation, were incubated in RPMI medium with 10% fetal calf serum (FCS; PAA Laboratories) in 12-well cell culture clusters (Costar, Corning, NY) at 37°C under 5% CO2 for 45 min and washed carefully three times in PBS at 37°C to obtain adherent monocytes, which were verified by microscopy. Stimulation and culturing of adherent monocytes with hyphae from A. fumigatus were done as described for THP-1 monocytes with adjusted platelet and hyphal concentrations to yield the same ratios.
After 4 h, cell- and hypha-free supernatants were harvested and stored at −80°C until further analysis.
Flow cytometry.
The labeled platelets were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San Diego, CA) as described previously (7). Briefly, light scatter and fluorescence channels were set at logarithmic gain, and platelets were gated based on forward and side scatter properties as well as fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies against CD41 or CD61 (BD Pharmingen, San Jose, CA). Platelet activation was checked by the well-documented surface exposure of the integral α-granule membrane protein P-selectin (CD62P) and the lysosomal integral membrane protein CD63 using FITC-conjugated monoclonal antibodies against CD62P and CD63 (both from BD Pharmingen), respectively. Isotype control antibodies were used as appropriate, and positive cells were defined by a fluorescence intensity gate containing only 1% of the events observed with the nonspecific antibody. Altogether, 10,000 positive events were analyzed each time, and the CXP software (Beckman Coulter, Miami, FL) was used for data processing.
EIAs.
Concentrations of regulated-on-activation, normal T-cell expressed and secreted (RANTES/CCL5), the tumor necrosis factor (TNF) superfamily member LIGHT (TNFSF14), interleukin-8 (IL-8/CXCL8), monocyte chemotactic protein 1 (MCP-1/CCL2), and Dickkopf homolog 1 (DKK-1) were measured by enzyme immunoassays (EIAs) from R&D Systems. Soluble CD40 ligand (sCD40L) was measured by an EIA from Bender Medsystems (Vienna, Austria). The intra- and interassay coefficients of variation were <10% for all assays.
Ethics.
Informed consent for blood collection was obtained from all individuals. The study was approved by the local ethics committee.
Statistical analyses.
Data are presented as means ± standard errors of the means (SEM) unless otherwise stated. The Wilcoxon matched-pair test was used in the paired situation. Coefficients of correlation were calculated by the Spearman rank test. P values (2-sided) were considered significant at <0.05.
RESULTS
A. fumigatus induces platelet-activation.
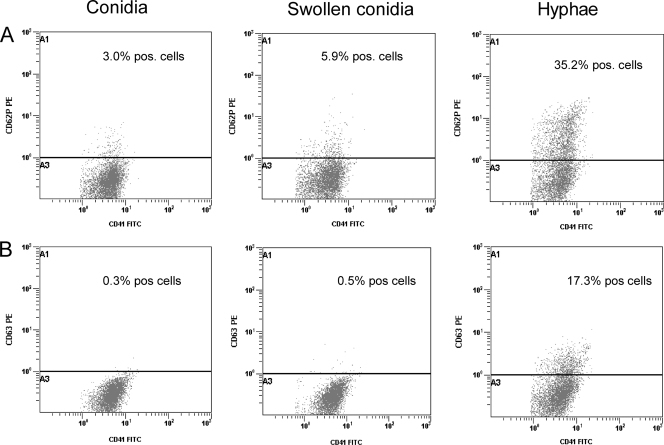
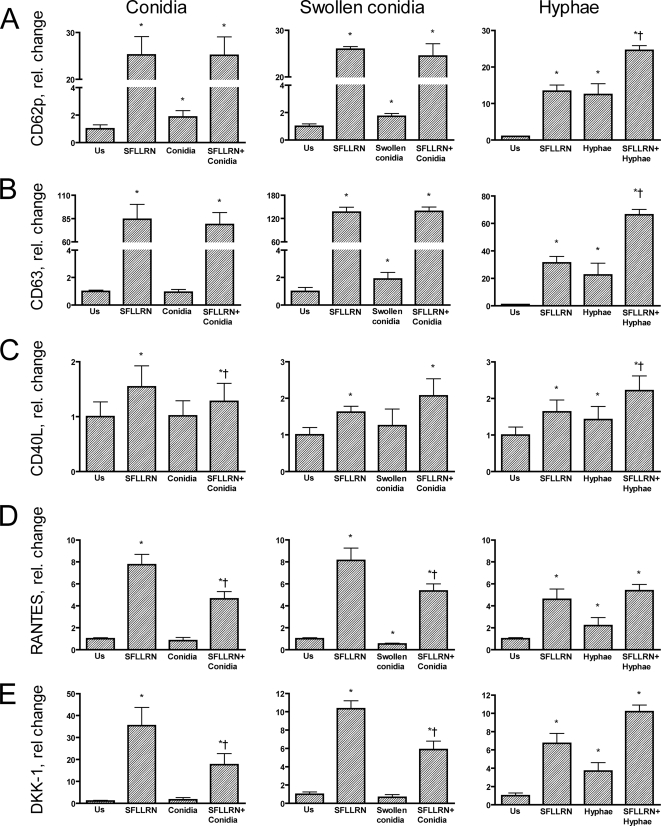
When the ability of A. fumigatus to induce platelet activation was investigated, several significant findings were made (Fig. 1 to 3). First, resting conidia, swollen conidia, and in particular hyphae markedly induced an increase in the surface expression of CD62P as assessed by flow cytometry. In fact, the hypha-induced expression of CD62P was similar to the effect of the thrombin receptor agonist SFLLRN, and the combination of these two stimuli showed an additive effect on CD62P expression. Second, a similar pattern was seen for the expression of the lysosomal integral membrane protein CD63, although no effect of resting conidia was observed. Third, in contrast to the effects of conidia and hyphae on the membrane expression of CD62P and CD63, only hyphae induced a significant increase in the release of RANTES and CD40L, with a particularly pronounced effect on CD40L. Fourth, we have recently shown that upon activation, platelets may release significant amounts of DKK-1, an important regulator of the wingless (Wnt) signaling pathways, which are involved in inflammation and vascular development (22), and notably, hyphae, but not conidia, induced a marked release of DKK-1 upon platelet activation. Finally, while combined stimulation with SFLLRN and swollen conidia or hyphae showed an enhancing effect on CD40L, the combination of SFLLRN and resting or swollen conidia attenuated the SFLLRN-induced release of RANTES and DKK-1. The release of TNFSF14 (LIGHT) was very low in all experiments, with no significant effects of conidia or hyphae either alone or in combination with SFLLRN (data not shown).
FIG. 1.
Expression of CD62P (A) and CD63 (B) in platelets upon stimulation with conidia (left), swollen conidia (center), and hyphae (right) from Aspergillus fumigatus as assessed by flow cytometry after incubation for 120 min.
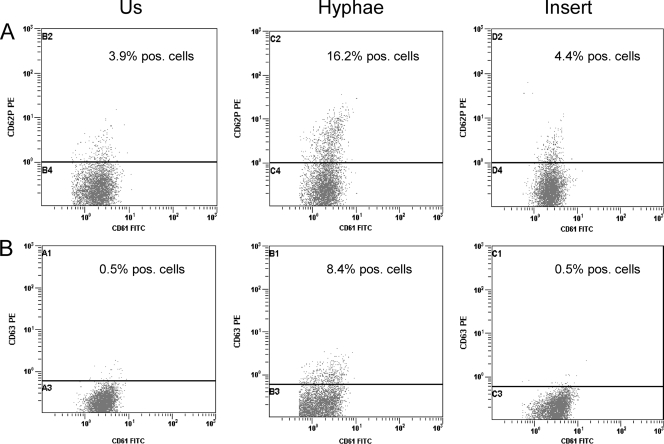
FIG. 3.
Expression of CD62P (A) and CD63 (B) in unstimulated (Us) platelets (left) compared with that in platelets stimulated with hyphae from Aspergillus fumigatus (center) and with that in hyphae separated from platelets with cell culture inserts (right) as assessed by flow cytometry after incubation for 120 min.
Aspergillus is known to produce secondary metabolites that potentially could induce platelet activation. However, experiments (n = 3) with cell culture inserts showed that direct contact between platelets and Aspergillus hyphae is necessary to achieve increased expression of CD62P and CD63 (Fig. 3).
Effect of hypha-activated platelets on chemokine release in THP-1 monocytes.
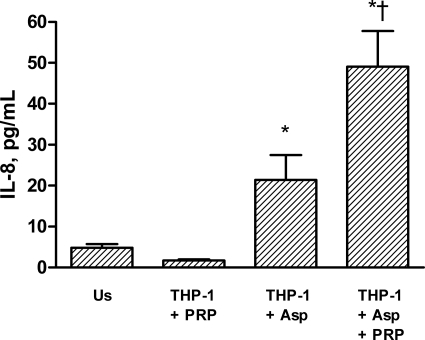
Platelets are known to induce inflammatory responses in adjacent cells such as monocytes/macrophages (10). To further elucidate the inflammatory interaction between A. fumigatus and platelets, we therefore examined the ability of hypha-activated platelets to induce the release of the inflammatory chemokines IL-8 and MCP-1 in THP-1 monocytes. While there was no release of IL-8 in unstimulated THP-1 cells or in cells cocultured with unstimulated PRP, hyphae induced a modest and significant release of IL-8 in THP-1 monocytes after culture for 4 h (Fig. 4). More importantly, this hypha-mediated increase in IL-8 release was markedly enhanced when THP-1 cells were incubated with hyphae and PRP, suggesting that the interaction between hyphae and platelets promotes inflammatory responses in THP-1 monocytes (Fig. 4). The increase in IL-8 levels in the coculture experiments could potentially reflect enhanced release from Aspergillus-activated platelets. However, neither conidia nor hyphae from A. fumigatus induced any detectable amounts of IL-8 (the detection limit of the assay was 2 pg/ml) when they were cultured with PRP for 120 min (data not shown). In contrast to the enhancing effect of coculture with hyphae and PRP on IL-8 release in THP-1 cells, PFP from PRP that had been stimulated with hyphae for 4 h did not induce a significant increase in IL-8 release after incubation for an additional 4 h with THP-1 monocytes (1.8 ± 0.7 pg/ml versus 3.6 ± 1.7 pg/ml for unstimulated and PFP-exposed THP-1 cells, respectively; n = 2). This finding suggests that the inflammatory response in THP-1 monocytes may be dependent on direct contact between monocytes, platelets, and hyphae. Finally, in contrast to the effect on IL-8, hyphae and PRP did not induce the release of MCP-1 when cocultured with THP-1 monocytes (20 ± 5.9 pg/ml versus 25 ± 7.6 pg/ml for THP-1 plus PRP and THP-1 plus PRP plus hyphae, respectively; n = 6; P = 0.3), suggesting that the Aspergillus/platelet-induced inflammatory response in THP-1 monocytes has some degree of selectivity.
FIG. 4.
Release of IL-8 from THP-1 monocytes after culture for 4 h with or without unstimulated PRP, hyphae from Aspergillus fumigatus, or both. Data are given as means ± SEM (n = 11). *, P < 0.05 versus unstimulated THP-1 monocytes (Us). †, P < 0.05 versus THP-1 monocytes plus hyphae and THP-1 monocytes plus PRP.
Effect of hypha-activated platelets on chemokine release in human adherent monocytes.
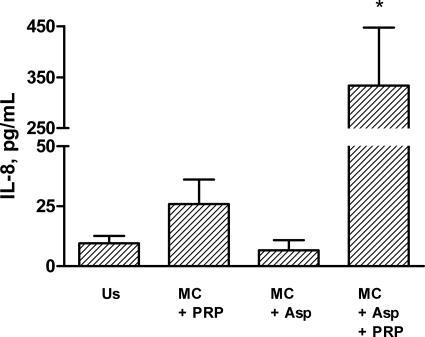
As with THP-1 cells, when human adherent monocytes (n = 6) were incubated with hyphae and PRP, there was a significant increase in IL-8 release over that in cells that were incubated with hyphae or PRP alone (Fig. 5). In fact, the IL-8 response was even more pronounced in adherent human monocytes than in THP-1 cells, further supporting the in vivo relevance of the inflammatory interaction between hyphae, platelets, and monocytes. Moreover, as with THP-1 cells, hyphae and PRP had no effect on MCP-1 release in adherent human monocytes (22 ± 6.3 pg/ml versus 20 ± 5.5 pg/ml for monocytes plus PRP and monocytes plus PRP plus hyphae, respectively; n = 6; P = 1.0).
FIG. 5.
Release of IL-8 from human monocytes after culture for 4 h with or without unstimulated PRP, hyphae from Aspergillus fumigatus, or both. Data are given as means ± SEM (n = 6). *, P < 0.05 for comparisons with unstimulated monocytes (Us), monocytes plus hyphae (MC + Asp), and monocytes plus PRP.
DISCUSSION
Previous in vitro studies have suggested that platelets may be involved in the immune response against Aspergillus fumigatus, but their role in aspergillosis is still unclear. In the present study, we report several new findings concerning the interaction between platelets and Aspergillus with potential relevance to the role of platelets in aspergillosis.
Our findings indicate that A. fumigatus induces the activation of α-granules and lysosomal granules, resulting in enhanced expression of membrane-bound (i.e., CD63 and CD62P) as well as soluble (i.e., RANTES, CD40L, and DKK-1) mediators. Although RANTES, CD40L, and DKK-1 are released from α-granules, the release mechanisms of CD40L differ from those of the two other molecules (15). Thus, the ability of A. fumigatus to enhance the release of all these mediators underscores its capability to promote platelet activation in a broad way. Moreover, hyphae from A. fumigatus induced platelet responses to the same extent as thrombin receptor activation, and for some of the responses (e.g., CD63 and CD62P), coactivation with hyphae markedly enhanced the SFLLRN-mediated responses. These findings indicate that A. fumigatus is a potent activator of platelets, which may act synergistically with thrombin activation.
Molds such as A. fumigatus differ from most other pathogens in their ability to switch between different morphological phenotypes, inducing different immune response patterns (19). The present study clearly shows that conidia and hyphae differ in the ability to activate platelets. Thus, while hyphae potently induced platelet activation, as assessed by all the parameters measured (except for LIGHT release), there was no significant release of RANTES, CD40L, or DKK-1 when platelets were exposed to conidia. Moreover, resting conidia attenuate the release of RANTES, CD40L, and DKK-1 in SFLLRN-activated platelets, while hyphae had the opposite effect. Although the responses were more modest than those for hyphae, swollen and even resting conidia induced the upregulation of membrane-bound CD62P, and swollen conidia induced the upregulation of CD63, suggesting that the release of soluble mediators and the upregulation of membrane-bound markers may be somewhat differently regulated upon exposure to conidia. Hyphae, which are the fungal morphotype responsible for invasive disease, are likely to come into contact with platelets during infection. However, it is also conceivable that inhaled conidia may encounter platelets in both interstitial tissue and alveolar spaces in the presence of inflammation and hemorrhage, which occur frequently during Aspergillus infection (20).
Monocytic phagocytes play an important role in the innate immune response to A. fumigatus by ingesting and inactivating conidia. It is therefore interesting that we could demonstrate that hypha-activated platelets may enhance the inflammatory response in monocytes as measured by the release of the inflammatory chemokine IL-8. We found no enhancing effect on IL-8 release when platelets were replaced by PFP from hypha-activated PRP in the coculture experiments with THP-1 monocytes, suggesting that direct contact between monocytes, hyphae, and platelets is necessary. This finding may support previous studies by Weyrich et al. showing that platelet-mediated release of IL-8 in monocytes is dependent on direct contact between activated platelets and monocytes (24).
Our findings with human monocytes suggest that the inflammatory interaction between platelets, monocytes, and Aspergillus also occur in vivo, potentially contributing to the inflammatory responses seen in invasive aspergillosis. Hence, RANTES, released from platelets upon exposure to hyphae, may induce monocyte arrest in an inflamed endothelium (23). The colocalization of platelets, monocytes, and hyphae in inflamed vessel walls may promote inflammatory responses, which may be relevant in Aspergillus infection, where the histopathological hallmarks include angioinvasion, thrombosis, and vascular events. It has been reported previously that the histological patterns of tissue injury in invasive pulmonary aspergillosis differ between neutropenic and nonneutropenic patients (20). Thus, inflammatory necrosis is the predominant pattern in nonneutropenic patients, while angioinvasion is most frequent in neutropenic patients. Since most neutropenic patients also have an often profound thrombocytopenia, normal platelet numbers may not be necessary for Aspergillus-associated angioinvasion and thrombosis, but inflammatory active thrombocytes may well contribute to the marked inflammatory necrosis in nonneutropenic patients without thrombocytopenia.
A novel finding in the current study was that hyphae from A. fumigatus promoted a platelet-mediated release of DKK-1. DKK-1 is a major regulator of the Wnt signaling pathway, a complex cascade of mediators that have been shown to be involved in a wide range of physiological and pathophysiological responses, such as inflammation, angiogenesis, and regulation of cell survival (14). Recently, we reported that DKK-1 also may play a major role in platelet-dependent endothelial activation and inflammation (22), and Wnt signaling has been shown to be involved in the pathogenesis of various disorders ranging from cancer to atherosclerosis and septicemia (14, 22). It is tempting to hypothesize that DKK-1 and the Wnt pathway could also be involved in A. fumigatus-induced inflammation and vascular pathology. Our findings should encourage further studies of the role of the Wnt signaling pathway and DKK-1 in responses to Aspergillus infection.
In the current study, we show that A. fumigatus is a potent inducer of platelet-mediated inflammation, potentially promoting further inflammatory responses in monocytes. These findings may reflect a role for platelets in the protective immune response against Aspergillus infection. On the other hand, inflammatory necrosis, thrombosis, and fungal invasion of blood vessels are well-known histopathological hallmarks in patients with invasive infections caused by A. fumigatus, and Aspergillus-induced activation of platelets with release of inflammatory mediators may well contribute to these harmful consequences of invasive aspergillosis. The specific importance of platelets for the pathogenesis of Aspergillus infection may differ between patient groups according to the presence or absence of thrombocytopenia. We believe that further studies are warranted to elucidate the possible importance of platelets in Aspergillus infection.
FIG. 2.
Relative change in the expression of CD62P (A) and CD63 (B) on platelet surfaces and release of CD40L (C), RANTES (D), and DKK-1 (E) in platelets stimulated with SFLLRN (10 μM) alone or in combination with conidia (left), swollen conidia (center), or hyphae (right) from Aspergillus fumigatus. The expression of CD62P and CD63 was assessed by flow cytometry (percentage of positive platelets), and the release of CD40L, RANTES, and DKK-1 was measured by an EIA in platelet-free supernatants after incubation for 120 min. The levels of release of CD40L, RANTES, and DKK-1 in unstimulated cells were 2.5 ± 0.35 ng/ml, 9.1 ± 0.8 pg/ml, and 0.1 ± 0.02 pg/ml, respectively. Data are given as means ± SEM (n = 6). *, P < 0.05 versus unstimulated (Us) cells. †, P < 0.05 versus SFLLRN.
Editor: G. S. Deepe, Jr.
Footnotes
Published ahead of print on 14 December 2009.
REFERENCES
- 1.Berenguer, J., M. C. Allende, J. W. Lee, K. Garrett, C. Lyman, N. M. Ali, J. Bacher, P. A. Pizzo, and T. J. Walsh. 1995. Pathogenesis of pulmonary aspergillosis. Granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am. J. Respir. Crit. Care Med. 152:1079-1086. [DOI] [PubMed] [Google Scholar]
- 2.Bodey, G. P., and S. Vartivarian. 1989. Aspergillosis. Eur. J. Clin. Microbiol. Infect. Dis. 8:413-437. [DOI] [PubMed] [Google Scholar]
- 3.Chang, F. Y., N. Singh, T. Gayowski, M. M. Wagener, S. M. Mietzner, J. E. Stout, and I. R. Marino. 2000. Thrombocytopenia in liver transplant recipients: predictors, impact on fungal infections, and role of endogenous thrombopoietin. Transplantation 69:70-75. [DOI] [PubMed] [Google Scholar]
- 4.Christin, L., D. R. Wysong, T. Meshulam, R. Hastey, E. R. Simons, and R. D. Diamond. 1998. Human platelets damage Aspergillus fumigatus hyphae and may supplement killing by neutrophils. Infect. Immun. 66:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damas, J. K., M. Jensenius, T. Ueland, K. Otterdal, A. Yndestad, S. S. Froland, J. M. Rolain, B. Myrvang, D. Raoult, and P. Aukrust. 2006. Increased levels of soluble CD40L in African tick bite fever: possible involvement of TLRs in the pathogenic interaction between Rickettsia africae, endothelial cells, and platelets. J. Immunol. 177:2699-2706. [DOI] [PubMed] [Google Scholar]
- 6.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 7.Hognestad, A., A. Michelsen, F. Brosstad, J. K. Damas, T. Holm, S. Simonsen, J. K. Kjekshus, P. Aukrust, and A. K. Andreassen. 2004. Platelet activation in heart transplant recipients. Clin. Transplant. 18:142-147. [DOI] [PubMed] [Google Scholar]
- 8.Holme, P. A., F. Muller, N. O. Solum, F. Brosstad, S. S. Froland, and P. Aukrust. 1998. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEB J. 12:79-89. [DOI] [PubMed] [Google Scholar]
- 9.Hope, W. W., and D. W. Denning. 2004. Invasive aspergillosis: current and future challenges in diagnosis and therapy. Clin. Microbiol. Infect. 10:2-4. [DOI] [PubMed] [Google Scholar]
- 10.May, A. E., P. Seizer, and M. Gawaz. 2008. Platelets: inflammatory firebugs of vascular walls. Arterioscler. Thromb. Vasc. Biol. 28:s5-s10. [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov, R., and C. Janeway, Jr. 2000. Innate immunity. N. Engl. J. Med. 343:338-344. [DOI] [PubMed] [Google Scholar]
- 13.Mikulska, M., A. M. Raiola, B. Bruno, E. Furfaro, M. T. Van Lint, S. Bregante, A. Ibatici, V. Del Bono, A. Bacigalupo, and C. Viscoli. 2009. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant. 44:361-370. [DOI] [PubMed] [Google Scholar]
- 14.Niehrs, C. 2006. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25:7469-7481. [DOI] [PubMed] [Google Scholar]
- 15.Otterdal, K., T. M. Pedersen, and N. O. Solum. 2004. Release of soluble CD40 ligand after platelet activation: studies on the solubilization phase. Thromb. Res. 114:167-177. [DOI] [PubMed] [Google Scholar]
- 16.Perkhofer, S., B. E. Kehrel, M. P. Dierich, J. P. Donnelly, W. Nussbaumer, J. Hofmann, C. von Eiff, and C. Lass-Florl. 2008. Human platelets attenuate Aspergillus species via granule-dependent mechanisms. J. Infect. Dis. 198:1243-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romani, L. 2004. Immunity to fungal infections. Nat. Rev. Immunol. 4:1-13. [DOI] [PubMed] [Google Scholar]
- 18.Schneemann, M., and A. Schaffner. 1999. Host defense mechanism in Aspergillus fumigatus infections. Contrib. Microbiol. 2:57-68. [DOI] [PubMed] [Google Scholar]
- 19.Shoham, S., and S. M. Levitz. 2005. The immune response to fungal infections. Br. J. Haematol. 129:569-582. [DOI] [PubMed] [Google Scholar]
- 20.Stergiopoulou, T., J. Meletiadis, E. Roilides, D. E. Kleiner, R. Schaufele, M. Roden, S. Harrington, L. Dad, B. Segal, and T. J. Walsh. 2007. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am. J. Clin. Pathol. 127:349-355. [DOI] [PubMed] [Google Scholar]
- 21.Sugino, K., C. Hasegawa, G. Sano, K. Shibuya, and S. Homma. 2008. Pathophysiological study of chronic necrotizing pulmonary aspergillosis. Jpn. J. Infect. Dis. 61:450-453. [PubMed] [Google Scholar]
- 22.Ueland, T., K. Otterdal, T. Lekva, B. Halvorsen, A. Gabrielsen, W. J. Sandberg, G. Paulsson-Berne, T. M. Pedersen, L. Folkersen, L. Gullestad, E. Oie, G. K. Hansson, and P. Aukrust. 2009. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29:1228-1234. [DOI] [PubMed] [Google Scholar]
- 23.von Hundelshausen, P., K. S. Weber, Y. Huo, A. E. Proudfoot, P. J. Nelson, K. Ley, and C. Weber. 2001. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation 103:1772-1777. [DOI] [PubMed] [Google Scholar]
- 24.Weyrich, A. S., M. R. Elstad, R. P. McEver, T. M. McIntyre, K. L. Moore, J. H. Morrissey, S. M. Prescott, and G. A. Zimmerman. 1996. Activated platelets signal chemokine synthesis by human monocytes. J. Clin. Invest. 97:1525-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]