Abstract
The complement system is important for host resistance to hematogenously disseminated candidiasis. However, modulation of complement activation by cell wall components of Candida albicans has not been characterized. Although intact yeast display mannan on the surface, glucan, typically located in the interior, becomes exposed during C. albicans infection. We show here the distinct effects of mannan and glucan on complement activation and opsonophagocytosis. Previous studies showed that intact cells are resistant to initiation of complement activation through the alternative pathway, and antimannan antibody reverses this resistance via an Fc-independent mechanism. The present study shows that this mannan-dependent resistance can be overcome by periodate-borohydride conversion of mannose polysaccharides to polyalcohols; cells treated with periodate-borohydride initiate the alternative pathway without the need for antibody. These observations identify an inhibitory role for intact mannan in complement activation. Next, removal of the surface-displayed mannan by acid treatment of periodate-borohydride cells exposes glucan. Glucan-displaying cells or purified β-glucan initiate the alternative pathway when incubated with the purified proteins of the alternative pathway alone, suggesting that C. albicans glucan is a natural activator of the alternative pathway. Finally, ingestion of mannan-displaying cells by human neutrophils requires anti-mannan antibody, whereas ingestion of glucan-displaying cells requires complement. These results demonstrate a contrasting requirement of natural antibody and complement for opsonophagocytosis of C. albicans cells displaying mannan or glucan. Thus, differential surface expression of mannan and glucan may influence recognition of C. albicans by the complement system.
Mannan is predominant (39) on the surface of intact Candida albicans cells and masks β-glucan and chitin in the interior (7). However, recent studies found that glucan may become exposed during C. albicans infection (45) or by treatment with caspofungin (44, 45). The phenomenon of glucan unmasking during infection was initially suggested by studies from the Cassone group. They found that the fraction of murine immune serum reactive with C. albicans β-glucan was protective in a mouse model of hematogenously disseminated candidiasis (6). This anti-glucan antibody-mediated protection was confirmed with both antiserum produced by a β-1,3 glucan conjugate vaccine and a monoclonal antibody (MAb) specific for β-glucan (40). Subsequently, Wheeler et al. (45) demonstrated expression of glucan on the surface of C. albicans cells retrieved from the kidneys of infected mice with anti-glucan antibody. They also reported exposure of glucan on C. albicans following treatment with caspofungin at subinhibitory doses both in vivo and in vitro (44, 45). These studies illustrate dynamics in the display of mannan and glucan on the cell surface. They also raise the possibility that variability in surface expression of mannan and glucan might have other biological consequences, e.g., activation of the complement system.
The complement system has an essential role in host innate clearance of initial infections and influences the effector functions of induced immunity. Activation of the complement cascade leads to production of chemotactic agents for recruitment of phagocytes and to deposition of opsonic C3 fragments on the surface of microbes targeted for clearance by phagocytes. Complement activation may occur through the classical pathway, the alternative pathway, or the lectin pathway. Although initiation of the classical pathway begins with C1q recognition of the Fc region of antibody-microbe complex, initiation of the alternative pathway begins with binding of metastable fluid-phase C3b or C3(H2O) to the microbial surface in an antibody independent manner (35). Thus, alternative pathway activation of complement represents an innate defense, independent of the induced immunity; strategies for evasion of alternative pathway-mediated initiation of complement activation are common in microbes (52). An important role for the complement system in host resistance to systemic candidiasis has been well established with experimental animals deficient in C3 (13, 42), mannan binding lectin A/C (20), or factors B and C2 (20). Furthermore, protection by a murine anti-mannan IgM antibody or its IgG3 variant requires an intact complement system in a mouse model of hematogenously disseminated candidiasis (17).
Our previous studies found that intact yeast cells of serotypes A and B of C. albicans are resistant to complement activation and that anti-mannan antibody is required for initiation of both the classical and alternative pathways (3, 26, 50, 51). The intrinsic resistance of intact C. albicans yeast cells to alternative pathway activation was demonstrated in a serum-free assay that consisted of the six alternative pathway proteins (3, 50). Further studies revealed that anti-mannan antibody facilitates alternative pathway activation in an Fc-independent manner (3).
The role of Candida glucan in complement activation has not been studied. Glucan of nonencapsulated Cryptococcus neoformans (46) or Blastomyces dermatitidis (48, 49) is cell surface displayed and contributes to initiation of complement activation. Therefore, C. albicans glucan that is exposed during infection may influence the outcome of the interaction of Candida with the human complement system.
The objective of the present study was to evaluate the relative contributions of mannan and glucan to complement activation and C3 binding by C. albicans. The results show that mannan contributes to the resistance of C. albicans to complement activation and that glucan is a natural activator of the complement system. Thus, masking glucan by mannan may represent a strategy for evasion of complement recognition.
MATERIALS AND METHODS
Yeast and chemical treatment of yeast.
C. albicans 3153A (serotype A) and CA1 (serotype B, provided by Jim E. Cutler, Louisiana State University Health Sciences Center and Research Institute for Children, New Orleans, LA) were used for all experiments. Yeast cells were grown at 37°C in a medium containing glucose, peptone, and yeast extract; inactivated with formaldehyde; and stored in phosphate-buffered saline (PBS; 1.9 mM NaH2PO4, 8.1 mM Na2HPO4, 154 mM NaCl [pH 7.2]) at −80°C as described previously (3, 51).
Some yeast cells were treated with periodate, periodate-borohydride, or periodate-borohydride acid (Smith degradation) for a controlled degradation of carbohydrate components of the cell wall as described previously (14). Briefly, 0.3 ml of packed yeast cells was incubated in the dark on rotation at 4°C in 30 ml of 0.04 M NaIO4 for 96 h and then for another 30 min in the presence of equal moles of ethylene glycol for consumption of excess periodate and washed with water; this procedure produced periodate cells. Some periodate cells were subsequently incubated with 60 mg of NaBH4 in 40 ml of water for 10 h at room temperature; the pH was neutralized with HCl, and the cells were washed with water and named periodate-borohydride cells. Some periodate-borohydride cells were then incubated in 40 ml of 0.5 M HCl for 8 h at room temperature and washed with water; these cells were named Smith cells. Chemically treated cells were stored in PBS at −80°C.
Characterization of the surface structures of chemically treated cells.
The effect of chemical treatment on the integrity of the cell-surface displayed mannan was evaluated with three anti-mannan MAbs: M1g1, a human monoclonal recombinant anti-mannan IgG antibody (47); B6, a murine MAb reactive with an acid-stable mannan fraction (16); and B6.1, a murine MAb reactive with an acid-labile mannan fraction (16). Both B6 and B6.1 were a generous gift of J. Cutler. Untreated or treated yeast cells were incubated with either M1g1, B6, or B6.1 for 30 min in PBS-1% bovine serum albumin (BSA) at room temperature and washed; the amount of cell-bound anti-mannan antibody was quantified by using goat anti-human IgG-fluorescein isothiocyanate (FITC) or anti-mouse immunoglobulin-FITC (Southern Biotech, Birmingham, AL) and flow cytometry with a Quanta SC MPL at 488-nm excitation (Beckman Coulter, Miami, FL). The integrity of the surface-displayed mannan was also assessed for its reactivity with natural antibody in normal human serum (NHS). Untreated or chemically treated cells were incubated in NHS; cell-bound antibody was quantified by flow cytometry using FITC-conjugated goat antibody specific either for human IgG or for all major isotypes of human immunoglobulins (Southern Biotech).
The presence of glucan components on the surface of Smith cells was determined with natural antibody in NHS that had been differentially adsorbed either with intact yeast cells (3) to remove anti-mannan antibody or with Baker's yeast glucan (G5011; Sigma, St. Louis, MO) at 10 mg/ml of serum for 1 h on ice (49) to remove anti-glucan antibody. Natural antibody bound to Smith cells was quantified by flow cytometry using FITC-conjugated goat antibody specific either for human IgG or for all major isotypes of human immunoglobulins. The identity of natural antibody in NHS reactive with Smith cells was determined by competition for binding of anti-glucan antibody with laminarin (CarboMer, San Diego, CA), a soluble β-1,3-glucan with some β-1,6-linked branches (37). Smith cells were incubated in NHS in the presence of 0 to 10,000 μg of laminarin/ml, and cell-bound antibody was quantified with flow cytometry using FITC-conjugated anti-human IgG antibody.
Quantitative analysis of C3 deposition onto yeast cells.
Kinetics of C3 deposition onto yeast cells was determined as described previously (3). Briefly, kinetics of C3 activation and binding was analyzed at 37°C in GVB (0.1% gelatin, 5 mM sodium Veronal, 142 mM NaCl [pH 7.3]) that contained 107 yeast cells/ml, 40% pooled normal human serum (untreated or yeast adsorbed), and either 5 mM EGTA and 5 mM MgCl2 to limit complement activation to the alternative pathway or 1.5 mM CaCl2 and 1 mM MgCl2 to permit the activity of both the classical and the alternative pathways (12). Binding of C3 fragments to 5,000 single yeast cells was quantified with FITC-conjugated goat anti-human C3 antibody (Kent Laboratories, Bellingham, WA) and flow cytometry. Some experiments utilized NHS that had been adsorbed with Baker's yeast glucan, as described above, to determine the contribution of naturally occurring anti-glucan antibody to complement activation by Smith cells.
Human serum depleted of C1q, C2, or factor B (Quidel, Santa Clara, CA; or CompTech, Tyler, TX) was used to determine the requirement of the classical or alternative pathway for initiation of C3 deposition onto periodate-borohydride cells. Each serum was adsorbed with intact yeast (3) to remove natural antibodies and used for kinetic analysis of C3 deposition. In some experiments, purified factor B (Quidel) at 40% of the physiological concentration (50) was added to the reaction mixture containing factor B-depleted serum to restore the alternative pathway activity.
The requirement for antibody in alternative pathway initiation was assessed with a serum-free alternative pathway assay as described previously (50). The assay contained only the six alternative pathway proteins: C3, factors B, D, H, I and properdin (38) (Quidel or CompTech). Smith cells or periodate-borohydride cells were incubated with the proteins in GVB containing 1 mM MgCl2, and the kinetics of C3 deposition were determined as described above. Some assays included untreated cells for comparison in the absence or presence of M1g1 at 10 μg/ml (3).
Quantitative analysis of alternative pathway activation by curdlan.
Curdlan (CarboMer, San Diego, CA) is a chitin-free, water-insoluble β-1,3-glucan (18). It was dissolved in 10% NaOH at 70°C and then precipitated by acid neutralization, washed, and resuspended in water. Curdlan particles at 0.5 mg/ml were incubated in microfuge tubes with the six alternative pathway proteins in GVB containing 1 mM MgCl2 for various times (50), washed, and treated with goat anti-human C3 antibody (Kent Laboratories, Bellingham, WA), followed by rabbit anti-goat IgG-horseradish peroxidase (Southern Biotech). After a washing step, the particles were serially diluted in a microtiter plate with PBS and incubated for 30 min with SureBlue, a peroxidase substrate (KPL, Gaithersburg, MD). The reaction was stopped with 1 M H3PO4, and the amount of particle-bound C3 was reported as titers producing an optical density of 0.5 at 450 nm. Curdlan particles incubated in the serum-free mixture without factor B were used as a negative control (2).
Immunofluorescence microscopic analysis of C3 binding.
Yeast cells from some experiments were incubated with FITC-labeled anti-C3 antibody, washed, mounted in Vectashield (Vector Laboratories, Burlingame, CA), and examined with an Olympus BX51 equipped with an F-view imaging sensor (Olympus, Center Valley, PA) under the control of MicroSuite software (Olympus) as described previously (3).
Elution of cell-bound C3 with hydroxylamine.
Untreated or chemically treated yeast cells were incubated for 6 min in a complement reaction mixture containing 40% NHS prepared as described above and washed. The yeast cells were then incubated for 1 h at 37°C in 500 μl of 0.2 M NaHCO3 at pH 10 alone as a control or in the presence of 1 M hydroxylamine as described previously (25) and washed. Cell-bound C3 was detected with FITC-conjugated anti-C3 antibody and quantified with flow cytometry as described above.
Phagocytosis of untreated or chemically treated C. albicans cells by human neutrophils.
Peripheral blood was collected from normal adult donors with informed consent, and neutrophils were isolated with Histopaque 1119 and Histopaque 1077 according to the manufacturer's instructions (Sigma) and resuspended in RPMI 1640. Neutrophil viability was assessed by trypan blue exclusion, and the cell density was determined by flow cytometry with phycoerythrin-conjugated anti-human CD13 (BioLegend, San Diego, CA). A phagocytosis assay mixture was prepared in microfuge tubes that contained 106 neutrophils/ml in RPMI 1640 alone as a no-serum control or in the presence of 20% NHS that was either untreated, heat treated for 30 min at 56°C to inactivate C3, yeast adsorbed (3), yeast adsorbed and heat inactivated, glucan adsorbed as described above, or glucan adsorbed and heat inactivated. The mixture was warmed to 37°C, and phagocytosis was initiated by the addition of yeast cells at 2 × 106/ml. The reaction was stopped after a 30-min incubation at 37°C by the addition of ice-cold PBS. Cells were immediately collected by centrifugation at 100 × g for 15 min at 4°C, resuspended in 0.5 ml of PBS-1% BSA, and mounted in duplicate by cytocentrifugation on uncoated glass slides. Neutrophils with ingested or attached yeast cells were visualized microscopically with Wright-Giemsa stain, and at least 250 neutrophils from 10 or more microscopic fields were counted. The percentage of neutrophils that were associated with yeast was compared between the treatments and the no-serum control by analysis of variance, followed by post hoc analysis using the Bonferroni t test by use of SigmaPlot 11 (Systat Software, Chicago, IL).
RESULTS
Periodate-based chemical treatments alter the structure of cell surface-displayed mannan.
A periodate-based chemical treatment (14) was utilized to alter the surface-displayed mannan or to remove the mannan layer to expose the glucan component in a stepwise fashion. Periodate oxidation cleaves the covalent bond between two adjacent hydroxylated carbons and generates two aldehydes. Thus, mannose units contained in mannan are all expected to be susceptible to periodate oxidation except α-1,3-linked units that are less abundant in C. albicans mannan (39). In contrast, C. albicans glucan is expected to be resistant to periodate oxidation as it is composed of β-1,3-linked glucose units connected via β-1,3 linkages to chains of β-1,6-glucan (22). Consequently, periodate oxidation of mannan opens the ring structure of mannose units, yielding polyaldehydes, some of which may exist as cyclic acetals (14). These polyaldehydes are reduced by borohydride to polyalcohols that can be subsequently removed by acid hydrolysis, known as Smith degradation, to expose periodate oxidation-resistant glucan (14). With this stepwise treatment procedure, three types of C. albicans 3153A yeast cells were generated: periodate cells, periodate-borohydride cells, and periodate-borohydride-acid cells (Smith cells). In an initial analysis, periodate cells showed no activity in complement activation and therefore were not included in detailed characterization of the cell surface structures.
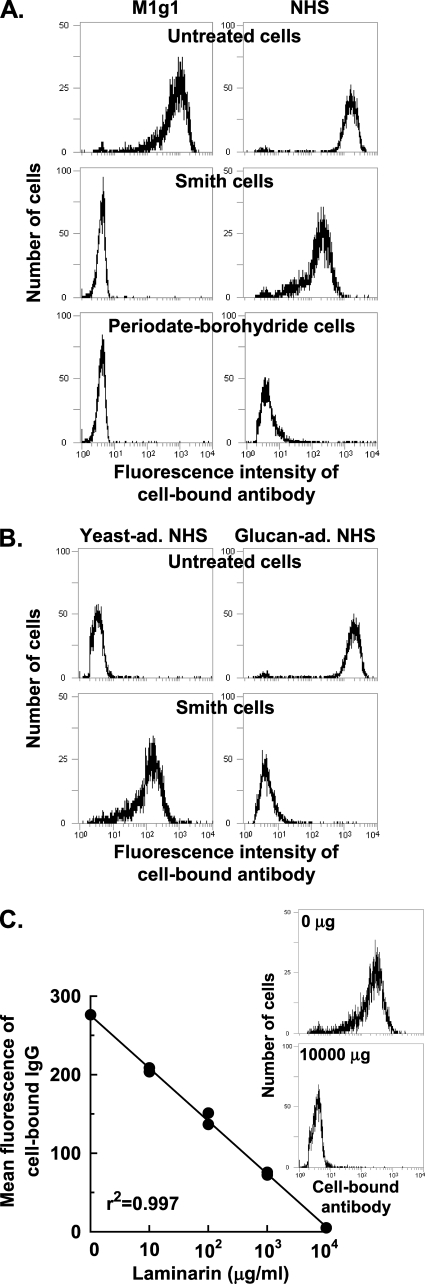
We first assessed the integrity of mannan displayed on the cell surface of chemically treated cells using MAbs specific for mannan epitopes with flow cytometry. Compared to untreated cells, periodate-borohydride cells and Smith cells showed only background binding with either M1g1 (Fig. 1A), a human recombinant anti-mannan antibody (47), or two murine antibodies (data not shown) with one (B6.1) specific for an acid-labile mannan fraction and the other (B6) for an acid-stable mannan fraction (16).
FIG. 1.
Surface characteristics of chemically treated cells. (A) Binding activity of untreated and treated cells for M1g1 (a mannan-specific human MAb) or natural antibody in NHS. (B) Binding activity of Smith cells and untreated cells for natural antibody in NHS differentially adsorbed with intact yeast (yeast-ad.) or with glucan (glucan-ad.). (C) Binding activity of Smith cells for natural antibody in NHS in the presence of increasing amounts of laminarin, a soluble β-glucan. Cell-bound antibody was quantified with FITC-conjugated anti-human IgG antibody and flow cytometry. The flow cytometry histograms shown in panel A and panel B are representative of three or more experiments. The data shown in panel C were pooled from two independent experiments with the insert showing representative flow cytometry histograms.
The binding activity of the chemically treated cells for natural antibody was next determined as appreciable amounts of natural anti-mannan antibody are present in pooled NHS (26, 31, 51). Incubation of periodate-borohydride cells in NHS did not yield detectable amounts of cell-bound antibody as determined with two different FITC-labeled antibodies, one specific for human IgG (Fig. 1A) and the other specific for all major isotypes of human immunoglobulins (data not shown). In contrast, Smith cells exhibited a novel ability to bind a natural antibody in NHS (Fig. 1A).
The specificity of the antibody in NHS that was reactive with Smith cells was determined in two ways. In the first, differentially adsorbed sera were used. Binding of this natural antibody to Smith cells could be abolished by adsorption of serum with isolated yeast glucan but not with intact, untreated yeast cells (Fig. 1B). To avoid bias in detection, the lack of binding of natural antibody to Smith cells in glucan-adsorbed serum was assessed with two different detection antibodies, one specific for human IgG (Fig. 1B) and one specific for all major isotypes of human immunoglobulins (data not shown). In the second approach, the soluble β-glucan laminarin (37) was used as a competitor for binding of anti-glucan antibody in NHS to Smith cells. In the presence of increasing amounts of laminarin, binding of natural antibody to Smith cells was effectively reduced to a background level (r2 = 0.99; Fig. 1C), thus confirming the exposure of β-glucan on Smith cells. This observation is consistent with a recent report that Candida glucan is resistant to periodate oxidation as demonstrated with a MAb specific for β-1,3-linked glucose residues (41).
Taken together, these results indicate that periodate-borohydride cells display an altered mannan layer that is reactive neither with anti-mannan MAbs nor with natural antibody in NHS. In contrast, Smith cells, with the loss of the mannan layer, display a glucan layer that is reactive with natural anti-glucan antibody found in NHS.
Surface-displayed mannan influences interaction of C. albicans yeast cells with the complement system.
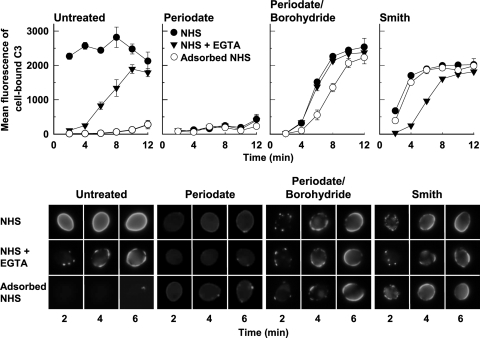
The effects of structural alterations in mannan on the kinetics and the distribution of initiation sites for C3 deposition on the cell surface were compared between untreated and treated yeast cells under three serum conditions: (i) NHS that contains natural anti-mannan antibody (26, 51), (ii) yeast-adsorbed NHS that is deficient in natural anti-mannan antibody (26, 51), or (iii) EGTA-treated NHS where natural antibody is present but the serum complement activity is limited to the alternative pathway (12). Untreated yeast cells showed three distinct patterns of complement activation (Fig. 2, upper left and lower left). In NHS, the initiation of C3 deposition on yeast cells occurred without a noticeable delay in a uniform, synchronous pattern over the entire cell surface, a characteristic of anti-mannan antibody-mediated classical pathway activation (3, 51). Yeast adsorption of NHS essentially abolished the ability of the serum to deposit C3 onto the cell surface, suggesting an intrinsic resistance of the cell surface to complement activation in the absence of anti-mannan antibody (3, 50, 51). Finally, EGTA-treatment of NHS produced a delay in the initiation of C3 binding but did not diminish the ultimate amount of C3 deposition, seen as an expansion of foci of C3 to eventually cover the entire cell surface. Given that alternative pathway-mediated complement activation is typically independent of antibody (35), the emergence of C3 binding in EGTA-treated NHS but not in yeast-adsorbed NHS indicates a requirement of anti-mannan antibody for alternative pathway activation by intact C. albicans yeast cells. Antibody-dependent initiation of C3 binding to C. albicans through the alternative pathway has been previously established (3, 50).
FIG. 2.
Effect of alterations in the mannan structure on the kinetics of C3 activation (upper panel) and on the distribution of sites for initial C3 deposition (lower panel). Untreated yeast cells, periodate cells, periodate-borohydride cells, or Smith cells were incubated in NHS, NHS-EGTA, or yeast-adsorbed NHS for 2 to 12 min. Cell-bound C3 was detected with FITC-goat anti-human C3 antibody and either quantified with flow cytometry (upper panel, mean fluorescence of cell-bound C3 ± the standard error of the means from two independent experiments) or visualized with immunofluorescence microscopy (lower panel).
The patterns of C3 binding to the chemically treated cells were markedly different under the three serum conditions described above. Periodate treatment reduced C3 binding to a background level under these serum conditions (Fig. 2, upper middle left and lower middle left). In contrast, periodate-borohydride treatment produced a rapid C3 deposition following a short delay with all three sera (Fig. 2, upper middle right and lower middle right); this rapid C3 deposition occurred as expanding foci of initial C3 over the entire cell surface. The similarity in the C3 binding patterns for periodate-borohydride cells in the presence or absence of either natural anti-mannan antibody or EGTA suggests an antibody-independent complement activation. This interpretation is supported by absence of binding of natural antibody from NHS to periodate-borohydride cells (Fig. 1A). On the other hand, acid treatment of periodate-borohydride cells to generate Smith cells produced a surface that allowed a rapid initiation of C3 deposition over the entire cell surface in both untreated NHS and NHS that had been adsorbed with untreated yeast cells, in contrast to a delayed and patchy deposition of initial C3 in EGTA-treated NHS (Fig. 2, upper right and lower right). These observations indicate that complement activation by Smith cells involves both the classical pathway, likely mediated by natural anti-glucan antibody that is not removed by adsorption with intact yeast cells, and the alternative pathway. Taken together, results shown in Fig. 2 identify a regulatory role for the surface-displayed mannan in complement activation by intact C. albicans yeast.
Periodate-borohydride treated mannan initiates the alternative pathway of complement activation in the absence of anti-mannan antibody.
Previous studies established a requirement of anti-mannan antibody for initiation of C3 deposition onto untreated C. albicans yeast cells (3, 50, 51), shown here as a suppressed initiation of complement activation in yeast-adsorbed NHS (Fig. 2, left panel). However, periodate-borohydride treatment converted the mannan layer that is normally inert to complement activation into a surface that initiated C3 deposition in a manner indistinguishable between NHS, NHS-EGTA, and yeast-adsorbed NHS (Fig. 2, middle right panel), suggesting an antibody-independent alternative pathway mechanism for complement activation.
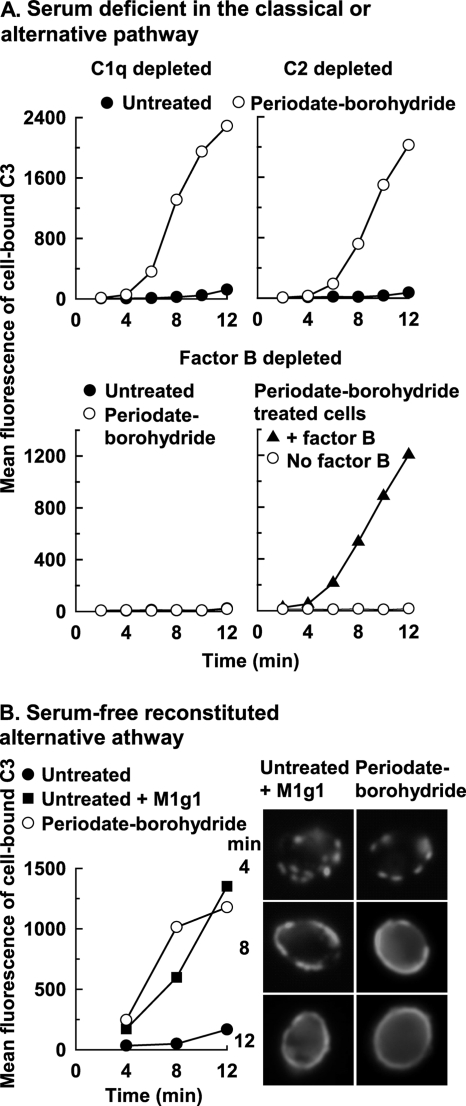
We further determined whether complement activation by periodate-borohydride cells was limited to the alternative pathway, using human serum that was depleted of classical pathway component C1q or C2 or alternative pathway component factor B. A kinetic analysis revealed a rapid deposition of C3 onto periodate-borohydride cells, after a short delay, only in C1q- or C2-depleted serum that contains an intact alternative pathway (Fig. 3A, upper panel) but not in factor B-depleted serum that contains a defective alternative pathway (Fig. 3A, lower left). As a control, addition of purified factor B restored C3 opsonization of periodate-borohydride cells to factor B-depleted serum (Fig. 3A, lower right). As expected, no appreciable amounts of C3 deposition were detected on untreated cells in these sera in the absence of anti-mannan antibody (Fig. 3A, upper panel and lower left).
FIG. 3.
Antibody-independent initiation of the alternative complement pathway by periodate-borohydride-treated C. albicans cells. (A) Kinetics for C3 deposition onto untreated cells or periodate-borohydride cells was analyzed in yeast-adsorbed normal human serum depleted of C1q or C2 (upper panel) or factor B (lower panel, left). Restoration of alternative pathway-mediated C3 binding to periodate-borohydride cells was determined in factor B-depleted serum supplemented with exogenous factor B (lower panel, right). Cell-bound C3 was quantified with FITC-goat anti-human C3 antibody and flow cytometry. A representative experiment from two is shown. (B) Patterns of C3 activation and binding by periodate-borohydride cells in the presence of the six alternative pathway proteins were determined by flow cytometry (left) or immunofluorescence microscopy (right). Untreated cells with or without M1g1 were included for comparison. A representative experiment from two is shown.
The lack of a requirement for antibody in activation of the alternative pathway by periodate-borohydride cells was then assessed using a serum-free assay that contained only the six alternative pathway proteins (3, 50). C3 deposition occurred on periodate-borohydride cells in a pattern that was similar to those observed in NHS, NHS-EGTA, or yeast-adsorbed NHS (Fig. 2) as determined by kinetics analysis (Fig. 3B, left) and immunofluorescence microcopy (Fig. 3B, right). As a comparison, accumulation of appreciable amounts of C3 on untreated cells occurred only when the anti-mannan MAb M1g1 was added to the six-protein mixture (Fig. 3B). These observations support the hypothesis that the intact mannan surface of C. albicans yeast cells is resistant to C3 deposition in the absence of anti-mannan antibody and indicate that such resistance is reversed by the structural alterations of the mannan introduced by periodate-borohydride treatment.
Cell surface-displayed mannan provides acceptors for C3 binding.
C3 binds to an acceptor through either an amide linkage to an amino group or an ester linkage to a hydroxyl group. Unlike the amide bond, an ester linkage is susceptible to hydrolysis by hydroxylamine (30). The observation (Fig. 2) that suppression of C3 deposition by periodate oxidation of mannose polysaccharides to polyaldehydes, as observed with periodate cells, could be reversed by borohydride reduction of polyaldehydes to polyalcohols (14), as seen with periodate-borohydride cells, suggested a role for hydroxyl-containing structures in C3 binding. To test this possibility, untreated cells or periodate-borohydride cells were first incubated in NHS for 6 min to allow initiation of C3 activation and binding through both the classical and the alternative pathways. Opsonized cells were then incubated either in a carbonate buffer alone as a control or in the buffer containing hydroxylamine, and the amount of cell-bound C3 was quantified. Hydroxylamine treatment released 91% ± 2% of cell-bound C3 from untreated cells and 83% ± 5% of cell-bound C3 from periodate-borohydride cells. This observation indicates that the hydroxyl groups contained in mannan are the main site for C3 binding.
Glucan-displaying cells are antibody-independent activators of complement.
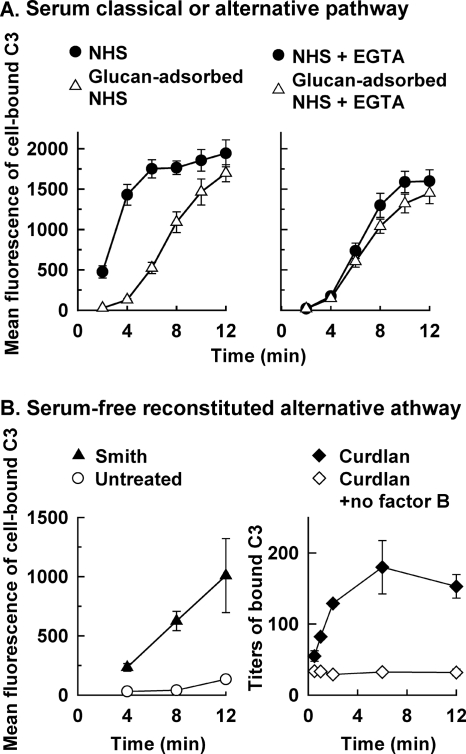
In addition to naturally occurring anti-mannan antibody, natural anti-Candida glucan antibody is also present in serum from normal adults (8), but the role of antiglucan antibody in complement activation has not been determined. The observation that Smith cells displayed glucan on the surface (Fig. 1) and activated complement in yeast-adsorbed NHS (Fig. 2, right panel) led to an analysis of the role of natural anti-glucan antibody in complement activation by C. albicans yeast cells. The kinetics of C3 deposition on Smith cells incubated in NHS or glucan-adsorbed NHS was compared either in the absence of EGTA to assess the activity of both the classical and the alternative pathways or in the presence of EGTA to assess alternative pathway activity alone. Glucan adsorption of serum inhibited the characteristic early kinetics for classical pathway-mediated C3 deposition onto Smith cells (Fig. 4A, left), but it had no discernible effect on the kinetics of alternative pathway-mediated C3 deposition (Fig. 4A, right). In contrast, kinetics of C3 accumulation on untreated cells was similar between NHS and glucan-adsorbed NHS (data not shown).
FIG. 4.
Kinetics of C3 activation and binding by glucan-displaying Smith cells or the β-glucan curdlan. (A) Antibody-dependent and antibody-independent initiation of C3 deposition on Smith cells incubated in NHS or glucan-adsorbed NHS was assessed either in the absence of EGTA (left) to allow the activity of both the classical and the alternative pathways or in the presence of EGTA (right) to limit complement activation to the alternative pathway. Cell-bound C3 was quantified with FITC-antibody for human C3 and flow cytometry. The results are means from two independent experiments ± the standard error of the means. (B) Contribution of β-glucan to antibody-independent activation of a reconstituted alternative pathway by Smith cells (left) or by the pure β-glucan curdlan (right) was determined. C3 bound to Smith cells, or untreated cells as a comparison, was quantified as described above, and the data are reported as means from two independent experiments ± the standard error of the means. C3 bound to curdlan particles incubated with the complete pathway, or with a partial pathway without factor B as a negative control, was quantified by enzyme-linked immunosorbent assay, and the data are mean titers from three independent experiments ± the standard error of the means.
The results shown in Fig. 4A suggest that glucan-displaying Smith cells, unlike mannan-displaying intact C. albicans, may be able to initiate complement activation independent of antibody. This possibility was evaluated with two different approaches. First, the ability of Smith cells to activate the alternative pathway in an antibody-independent manner was determined in a serum free assay containing the six alternative pathway proteins alone (Fig. 4B, left). The kinetics of C3 accumulation on Smith cells via the reconstituted alternative pathway was similar to those observed in glucan adsorbed NHS or EGTA-treated glucan adsorbed NHS (Fig. 4A). Second, the ability of β-glucan alone to activate C3 binding was analyzed because removal of the surface-displayed mannan layer may have also exposed chitin. Curdlan, a water-insoluble, chitin-free β-1,3-glucan (18), was used for this analysis. Incubation of curdlan with the six alternative pathway proteins led to accumulation of C3 on the β-1,3-glucan particles (Fig. 4B, right), suggesting a potential for the glucan component of C. albicans in complement activation.
We then determined the usage of hydroxyl groups in C3 binding to Smith cells. Approximately 70% ± 5% of C3 bound to Smith cells could be released by hydroxylamine treatment, indicating an important role for polysaccharides as a C3 acceptor.
These observations together suggest that glucan-displaying C. albicans cells have an intrinsic ability to activate C3 through the alternative pathway in an antibody-independent fashion but require anti-glucan antibody for initiation of the classical pathway.
Complement is required for opsonophagocytosis of glucan-displaying cells.
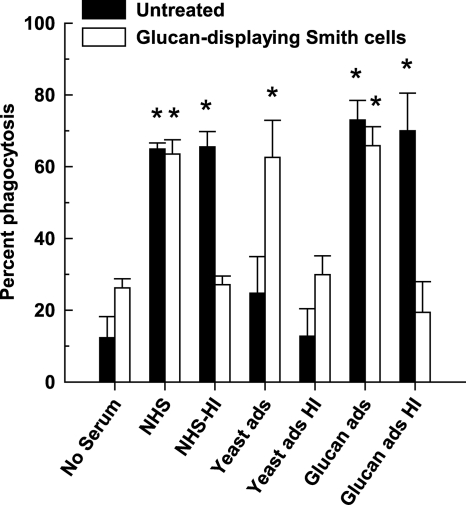
Previous studies found that natural anti-mannan antibody alone facilitates phagocytic killing of C. albicans yeast cells in the absence of complement (26). We evaluated the relative contributions of complement and natural antibody to phagocytosis of glucan-displaying Smith cells or intact untreated cells by human neutrophils in NHS that had been adsorbed either with intact yeast cells to remove anti-mannan antibody or with isolated yeast glucan to remove anti-glucan antibody and/or treated with heat for inactivation of C3. Phagocytosis of untreated intact cells was quantitatively similar in untreated NHS, heat-inactivated NHS or glucan-adsorbed and heat-inactivated NHS (P < 0.001, compared to the no-serum control), but it was reduced to a background level in yeast-adsorbed NHS (Fig. 5). These results demonstrate a requirement of natural anti-mannan antibody for ingestion of intact cells by human neutrophils. In contrast, phagocytosis of glucan-displaying Smith cells was quantitatively similar in NHS, yeast-adsorbed NHS, or glucan-adsorbed NHS (P < 0.001, compared to the no-serum control), but it was reduced to a background level in heat-inactivated NHS that still contains natural anti-glucan antibody (Fig. 5). Thus, complement opsonins are required for ingestion of glucan-displaying cells by human neutrophils.
FIG. 5.
Phagocytosis of untreated cells or glucan-displaying Smith cells of C. albicans by human neutrophils. Yeast cells were incubated with human neutrophils from peripheral blood in the absence of serum as a control or in the presence of 20% serum that was untreated (NHS), heat inactivated (NHS-HI), yeast adsorbed (Yeast ads), yeast adsorbed and heat inactivated (Yeast ads HI), glucan-adsorbed (Glucan ads), or glucan adsorbed and heat inactivated (Glucan ads HI). Neutrophils and yeast cells were visualized by using Wright-Giemsa stain and microscopy, and the percentage of neutrophils that were associated with yeast cells was determined. The data are shown as means ± the standard error of the means from at least two independent experiments using neutrophils from different donors. *, P < 0.001 between treatment and no-serum control for each yeast type.
Influence of mannan and glucan on complement activation and C3 binding is similar between serotypes A and B yeast cells of C. albicans.
There are two serotypes of C. albicans (19), and both are frequently recovered from asymptomatic individuals or candidiasis patients (1, 5). To determine whether the role of mannan and glucan in complement activation is similar between these two serotypes, we extended the studies described above to CA1 cells, a serotype B of C. albicans that has been used for studies of murine anti-mannan antibody-mediated protection (9) and for characterization of complement activation (28, 50, 51). Similar to serotype A 3153A cells, CA1 cells treated with any one of the three chemical methods lost the ability to bind to anti-mannan antibodies, but CA1 cells subject to Smith degradation displayed glucan (data not shown). Furthermore, these chemically treated CA1 cells activated and bound C3 in a manner similar to 3153A cells, as shown in Fig. 2 and 4A (data not shown). Finally, hydroxylamine treatment released most of C3 bound to CA1 cells that were untreated or had been treated with periodate-borohydride or Smith degradation (data not shown). Thus, mannan and glucan modulate complement opsonization in a similar manner for prototype strains of serotype A and serotype B.
DISCUSSION
The complement system plays an important role in host resistance to hematogenously disseminated candidiasis (13, 17, 20, 42). Initiation of complement activation requires recognition of the microbial surface structures by the complement system (36). On the cell surface of C. albicans, mannan is predominant (39), and it masks glucan in the interior (7). However, C. albicans glucan may become exposed during infection (45) or by treatment with caspofungin (44, 45). Glucan of nonencapsulated C. neoformans (46) or B. dermatitidis (48, 49) is cell surface displayed and contributes to complement activation. The activity of C. albicans glucan and the interplay of mannan and glucan in modulation of complement activation have not been studied.
The major objective of the present study was to determine the relative contributions of mannan and glucan to complement activation and C3 binding by C. albicans. Comparison of the patterns of C3 deposition onto intact cells and cells with altered mannan structures revealed three characteristics of mannan in modulation of complement activation by C. albicans. First, anti-mannan antibody is required for complement opsonization of C. albicans yeast cells. Disruption of the ring structure of mannose units by periodate-borohydride treatment abolished both the binding of anti-mannan antibodies (Fig. 1A) and the rapid classical pathway-mediated initiation of C3 deposition observed with intact cells in NHS (Fig. 2). This correlation between binding of anti-mannan antibody and initiation of complement activation supports the previous findings that either polyclonal or monoclonal anti-mannan antibodies restore C3 opsonization of C. albicans yeast to pooled NHS that has been adsorbed with intact yeast cells or chemically purified mannan (3, 51).
Second, mannan appears to serve as an acceptor for covalent binding of C3 to C. albicans yeast cells. Binding of C3 to an acceptor occurs through an ester linkage to a hydroxyl group or through an amide bond to an amino group; the ester linkage is susceptible to hydroxylamine hydrolysis (30). The data from the present study show that (i) C3 deposition occurred on intact cells, and ca. 90% of the cell-bound C3 could be eluted with hydroxylamine; (ii) C3 deposition was abolished on periodate-treated cells that display modified mannose units with aldehyde groups (and possibly acetals); and (iii) reduction of polyaldehydes to polyalcohols with borohydride restored C3 deposition, and ca. 80% of the cell-bound C3 could be eluted with hydroxylamine. These observations indicate a correlation between binding of C3 fragments and the presence of the hydroxyl groups in mannose polysaccharides displayed on intact cells or in polyalcohols displayed on periodate-borohydride-treated cells. Polysaccharides as a major C3 acceptor have also been reported for C. neoformans (27), B. dermatitidis (49), and Aspergillus fumigatus (29).
Third, mannan contributes to the intrinsic resistance of C. albicans to complement activation through the alternative pathway in the absence of anti-mannan antibody. The results show that periodate-borohydride-treated cells lost the ability to bind anti-mannan antibodies or natural antibody in NHS (Fig. 1A) but gained a novel ability to activate complement in an antibody-independent manner (Fig. 2, middle right panel and Fig. 3) via the alternative pathway (Fig. 3). This observation supports and extends previous studies that the intrinsic resistance of intact C. albicans yeast cells to complement activation could be overcome by anti-mannan antibody (3, 50) in an Fc-independent fashion (3). Thus, conversion of an inert surface for complement activation to an activating surface by perturbation of mannan with periodate-borohydride treatment (Fig. 1, 2, and 3) suggests that intact mannan is an inhibitor to complement activation.
How intact mannan inhibits complement activation is currently unknown. There is a striking similarity between the pattern of antibody-independent alternative pathway-mediated C3 deposition onto periodate-borohydride treated cells and the pattern of antibody-dependent alternative pathway-mediated C3 deposition onto intact cells. In both cases, a rapid kinetics of C3 accumulation following a short delay was seen as expanding unconnected discrete foci of C3 that covered the entire cell surface over time (Fig. 2 and 3). This similarity suggests that the effect of anti-mannan antibody or perturbation in the mannan structure on alternative pathway initiation appears similar, one that promotes seeding of initial C3 leading to alternative pathway activation. Indeed, an earlier study demonstrated the influence of anti-mannan antibody on the formation of the alternative pathway C3 convertase on the cell surface at disconnected, discrete sites (3). C. albicans, being a human commensal, may have acquired structural features in the intact mannan that either inhibit the binding of initial C3 and/or complement factors required for alternative pathway activation or favor the binding of negative regulators. The latter possibility has been implied by the observation that C. albicans naturally binds to factor H (34), an inhibitor to alternative pathway initiation. Thus, periodate-borohydride-treated cells may offer a new experimental approach to understanding why the intact cell surface of C. albicans is resistant to the alternative pathway of complement activation.
In contrast to mannan-displaying intact C. albicans yeast cells that are resistant to complement activation (Fig. 2 and 3B) (3, 50), glucan-displaying Smith cells, after the removal of the mannan layer (Fig. 1), showed robust complement activation (Fig. 2, right). Adsorption of NHS by β-glucan (Fig. 4A, left), but not by intact mannan-displaying yeast cells (Fig. 2, right), inhibited the rapid initiation of C3 deposition onto Smith cells. However, glucan adsorption did not abolish the delayed initiation of C3 deposition (Fig. 4A, right), and Smith cells showed an ability to initiate activation of the reconstituted alternative pathway (Fig. 4B, left). These observations suggest two distinct mechanisms of complement activation by glucan-displaying cells: an anti-glucan antibody-dependent classical pathway and an anti-glucan antibody-independent alternative pathway. To our knowledge, this is the first experimental evidence for the involvement of Candida β-glucan in complement activation.
The findings of the present study did not rule out the possibility for activation of the alternative pathway by other cell wall components, such as chitin, that may have been unmasked by the removal of mannan. However, the implication of C. albicans β-glucan as an intrinsic activator of the complement system is supported by several lines of evidence. Our results show that curdlan (18), a chitin-free β-glucan, activates the reconstituted alternative pathway under a serum-free condition (Fig. 4B, right). This observation is consistent with a previous report that curdlan activates the alternative pathway of guinea pigs (33). Purified glucans from different sources have been demonstrated to activate the alternative pathway (10, 15, 21). The role of β-glucan in complement activation has also been studied with zymosan (11), B. dermatitidis (48, 49), and nonencapsulated C. neoformans (46). Common to these systems are the display of β-glucan on the surface and activation of the alternative pathway in the absence of anti-glucan antibody.
C. albicans glucan as an activator of the complement system is masked by cell surface-displayed mannan. This masking is analogous to the interplay between BAD1 and β-glucan in B. dermatitidis. BAD1 is a cell surface-displayed protein antigen and adhesin (4, 24) and is inert to complement activation (48). Genetic disruption of BAD1 expression or chemical addition of exogenous β-glucan to the cell surface enhanced C3 deposition onto B. dermatitidis yeast cells (48). Thus, masking of glucan from complement recognition may represent an immune evasion strategy.
Mannan and glucan also differ in their role in opsonophagocytosis of C. albicans by human neutrophils. The observation of efficient opsonophagocytosis of intact yeast cells in either NHS or heat-inactivated NHS but not in yeast-adsorbed NHS (Fig. 5) indicates that anti-mannan antibody alone is sufficient as an opsonin. These results are supported by an earlier report that natural anti-mannan antibody is sufficient in mediating opsonophagocytic killing of C. albicans yeast cells by human neutrophils (26). In contrast, efficient opsonophagocytosis of glucan-displaying cells requires complement. Heat inactivation of NHS abolished serum opsonic activity, whereas glucan or yeast adsorption did not inhibit opsonophagocytosis (Fig. 5). This observation is consistent with other reports that complement opsonins are required for phagocytosis of glucan-displaying nonencapsulated C. neoformans by human macrophages (23, 32). The inability of natural anti-glucan antibody alone to promote phagocytosis as seen in heat-inactivated and yeast-adsorbed NHS may be attributed to the quantity and the isotype specificity. Natural anti-β-glucan antibody is present in low amounts in normal individuals, 1 log lower than that of anti-mannan antibody (8), and the prevailing subclass is IgG2 (8, 23). Among the Fc receptors expressed by human neutrophils, FcγRII is the only receptor for IgG2 (43). However, it is a low-affinity receptor (43). Therefore, natural anti-β-glucan antibody may not be an effective opsonin for phagocytosis.
Acknowledgments
We thank Kensaku Nakayama for discussion of the chemical treatment procedures. We also thank Mindy Engevik and Sindy Chaves for their assistance.
This study was supported by National Institutes of Health grants AI014209, AI037194, and AI044786 (T.R.K.) and AI052139 and GM063119 (M.X.Z.).
Editor: G. S. Deepe, Jr.
Footnotes
Published ahead of print on 22 December 2009.
REFERENCES
- 1.Auger, P., C. Dumas, and J. Joly. 1979. A study of 666 strains of Candida albicans: correlation between serotype and susceptibility to 5-fluorocytosine. J. Infect. Dis. 139:590-594. [DOI] [PubMed] [Google Scholar]
- 2.Bjornson, A. B., P. I. Magnafichi, R. D. Schreiber, and H. S. Bjornson. 1987. Opsonization of bacteroides by the alternative complement pathway reconstructed from isolated plasma proteins. J. Exp. Med. 165:777-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boxx, G. M., C. T. Nishiya, T. R. Kozel, and M. X. Zhang. 2009. Characteristics of Fc-independent human antimannan antibody-mediated alternative pathway initiation of C3 deposition to Candida albicans. Mol. Immunol. 46:473-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandhorst, T. T., M. Wuthrich, T. Warner, and B. Klein. 1999. Targeted gene disruption reveals an adhesin indispensable for pathogenicity of Blastomyces dermatitidis. J. Exp. Med. 189:1207-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brawner, D. L., and J. E. Cutler. 1989. Oral Candida albicans isolates from nonhospitalized normal carriers, immunocompetent hospitalized patients, and immunocompromised patients with or without acquired immunodeficiency syndrome. J. Clin. Microbiol. 27:1335-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bromuro, C., A. Torosantucci, P. Chiani, S. Conti, L. Polonelli, and A. Cassone. 2002. Interplay between protective and inhibitory antibodies dictates the outcome of experimentally disseminated candidiasis in recipients of a Candida albicans vaccine. Infect. Immun. 70:5462-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calderone, R. A., and P. C. Braun. 1991. Adherence and receptor relationships of Candida albicans. Microbiol. Rev. 55:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiani, P., C. Bromuro, A. Cassone, and A. Torosantucci. 2009. Anti-beta-glucan antibodies in healthy human subjects. Vaccine 27:513-519. [DOI] [PubMed] [Google Scholar]
- 9.Cutler, J. E. 2005. Defining criteria for anti-mannan antibodies to protect against candidiasis. Curr. Mol. Med. 5:383-392. [DOI] [PubMed] [Google Scholar]
- 10.Czop, J. K., and K. F. Austen. 1985. Properties of glycans that activate the human alternative complement pathway and interact with the human monocyte beta-glucan receptor. J. Immunol. 135:3388-3393. [PubMed] [Google Scholar]
- 11.Fearon, D. T., and K. F. Austen. 1977. Activation of the alternative complement pathway due to resistance of zymosan-bound amplification convertase to endogenous regulatory mechanisms. Proc. Natl. Acad. Sci. U. S. A. 74:1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine, D. P., S. R. Marney, Jr., D. G. Colley, J. S. Sergent, and R. M. Des Prez. 1972. C3 shunt activation in human serum chelated with EGTA. J. Immunol. 109:807-809. [PubMed] [Google Scholar]
- 13.Gelfand, J. A., D. L. Hurley, A. S. Fauci, and M. M. Frank. 1978. Role of complement in host defense against experimental disseminated candidiasis. J. Infect. Dis. 138:9-16. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein, I. J., G. W. Hay, B. A. Lewis, and F. Smith. 1965. Controlled degradation of polysaccharides by periodate oxidation, reduction, and hydrolysis, p. 361-370. In General polysaccharides, vol. V. Academic Press, Inc., New York, NY. [Google Scholar]
- 15.Hamuro, J., U. Hadding, and D. Bitter-Suermann. 1978. Solid phase activation of alternative pathway of complement by beta-1,3-glucans and its possible role for tumour regressing activity. Immunology 34:695-705. [PMC free article] [PubMed] [Google Scholar]
- 16.Han, Y., T. Kanbe, R. Cherniak, and J. E. Cutler. 1997. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect. Immun. 65:4100-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han, Y., T. R. Kozel, M. X. Zhang, R. S. MacGill, M. C. Carroll, and J. E. Cutler. 2001. Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental, hematogenously disseminated candidiasis. J. Immunol. 167:1550-1557. [DOI] [PubMed] [Google Scholar]
- 18.Harada, T., A. Misaki, and H. Saito. 1968. Curdlan: a bacterial gel-forming β-1,3-glucan. Arch. Biochem. Biophys. 124:292-298. [DOI] [PubMed] [Google Scholar]
- 19.Hasenclever, H. F., and W. O. Mitchell. 1961. Antigenic studies of Candida. I. Observation of two antigenic groups in Candida albicans. J. Bacteriol. 82:570-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Held, K., S. Thiel, M. Loos, and F. Petry. 2008. Increased susceptibility of complement factor B/C2 double knockout mice and mannan-binding lectin knockout mice to systemic infection with Candida albicans. Mol. Immunol. 45:3934-3941. [DOI] [PubMed] [Google Scholar]
- 21.Inal, S., K. Nagaki, S. Ebisu, K. Kato, and S. Kotani. 1976. Activation of the alternative complement pathway by water-insoluble glucans of streptococcus mutans: the relation between their chemical structures and activating potencies. J. Immunol. 117:1256-1260. [PubMed] [Google Scholar]
- 22.Iorio, E., A. Torosantucci, C. Bromuro, P. Chiani, A. Ferretti, M. Giannini, A. Cassone, and F. Podo. 2008. Candida albicans cell wall comprises a branched β-d-(1→6)-glucan with β-d-(1→3)-side chains. Carbohydr. Res. 343:1050-1061. [DOI] [PubMed] [Google Scholar]
- 23.Keller, R. G., G. S. Pfrommer, and T. R. Kozel. 1994. Occurrences, specificities, and functions of ubiquitous antibodies in human serum that are reactive with the Cryptococcus neoformans cell wall. Infect. Immun. 62:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein, B. S., P. M. Sondel, and J. M. Jones. 1992. WI-1, a novel 120-kilodalton surface protein on Blastomyces dermatitidis yeast cells, is a target antigen of cell-mediated immunity in human blastomycosis. Infect. Immun. 60:4291-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozel, T. R., R. R. Brown, and G. S. Pfrommer. 1987. Activation and binding of C3 by Candida albicans. Infect. Immun. 55:1890-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozel, T. R., R. S. MacGill, A. Percival, and Q. Zhou. 2004. Biological activities of naturally occurring antibodies reactive with Candida albicans mannan. Infect. Immun. 72:209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozel, T. R., and G. S. Pfrommer. 1986. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect. Immun. 52:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozel, T. R., L. C. Weinhold, and D. M. Lupan. 1996. Distinct characteristics of initiation of the classical and alternative complement pathways by Candida albicans. Infect. Immun. 64:3360-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozel, T. R., M. A. Wilson, T. P. Farrell, and S. M. Levitz. 1989. Activation of C3 and binding to Aspergillus fumigatus conidia and hyphae. Infect. Immun. 57:3412-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Law, S. K., N. A. Lichtenberg, and R. P. Levine. 1979. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J. Immunol. 123:1388-1394. [PubMed] [Google Scholar]
- 31.Lehmann, P. F., and E. Reiss. 1980. Comparison by ELISA of serum anti-Candida albicans mannan IgG levels of a normal population and in diseased patients. Mycopathologia 70:89-93. [DOI] [PubMed] [Google Scholar]
- 32.Levitz, S. M., and A. Tabuni. 1991. Binding of Cryptococcus neoformans by human cultured macrophages: requirements for multiple complement receptors and actin. J. Clin. Invest. 87:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsushita, M. 1990. Curdlan, a (1-3)-β-d-glucan from Alcaligenes faecalis var. myxogenes IFO13140, activates the alternative complement pathway by heat treatment. Immunol. Lett. 26:95-97. [DOI] [PubMed] [Google Scholar]
- 34.Meri, T., A. Hartmann, D. Lenk, R. Eck, R. Wurzner, J. Hellwage, S. Meri, and P. F. Zipfel. 2002. The yeast Candida albicans binds complement regulators factor H and FHL-1. Infect. Immun. 70:5185-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pangburn, M. K., and H. J. Muller-Eberhard. 1984. The alternative pathway of complement. Springer Semin. Immunopathol. 7:163-192. [DOI] [PubMed] [Google Scholar]
- 36.Pangburn, M. K., K. L. Pangburn, V. Koistinen, S. Meri, and A. K. Sharma. 2000. Molecular mechanisms of target recognition in an innate immune system: interactions among factor H, C3b, and target in the alternative pathway of human complement. J. Immunol. 164:4742-4751. [DOI] [PubMed] [Google Scholar]
- 37.Read, S. M., G. Currie, and A. Bacic. 1996. Analysis of the structural heterogeneity of laminarin by electrospray-ionization-mass spectrometry. Carbohydr. Res. 281:187-201. [DOI] [PubMed] [Google Scholar]
- 38.Schreiber, R. D., M. K. Pangburn, P. H. Lesavre, and H. J. Muller-Eberhard. 1978. Initiation of the alternative pathway of complement: recognition of activators by bound C3b and assembly of the entire pathway from six isolated proteins. Proc. Natl. Acad. Sci. U. S. A. 75:3948-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki, S. 1997. Immunochemical study on mannans of genus Candida. I. Structural Invest. of antigenic factors 1, 4, 5, 6, 8, 9, 11, 13, 13b, and 34. Curr. Top. Med. Mycol. 8:57-70. [PubMed] [Google Scholar]
- 40.Torosantucci, A., C. Bromuro, P. Chiani, F. De Bernardis, F. Berti, C. Galli, F. Norelli, C. Bellucci, L. Polonelli, P. Costantino, R. Rappuoli, and A. Cassone. 2005. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 202:597-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torosantucci, A., P. Chiani, C. Bromuro, F. De Bernardis, A. S. Palma, Y. Liu, G. Mignogna, B. Maras, M. Colone, A. Stringaro, S. Zamboni, T. Feizi, and A. Cassone. 2009. Protection by anti-beta-glucan antibodies is associated with restricted β-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS. One 4:e5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsoni, S. V., A. M. Kerrigan, M. J. Marakalala, N. Srinivasan, M. Duffield, P. R. Taylor, M. Botto, C. Steele, and G. D. Brown. 2009. Complement C3 plays an essential role in the control of opportunistic fungal infections. Infect. Immun. 77:3679-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unkeless, J. C. 1989. Function and heterogeneity of human Fc receptors for immunoglobulin G. J. Clin. Invest. 83:355-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wheeler, R. T., and G. R. Fink. 2006. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wheeler, R. T., D. Kombe, S. D. Agarwala, and G. R. Fink. 2008. Dynamic, morphotype-specific Candida albicans beta-glucan exposure during infection and drug treatment. PLoS Pathog. 4:e1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson, M. A., and T. R. Kozel. 1992. Contribution of antibody in normal human serum to early deposition of C3 onto encapsulated and nonencapsulated Cryptococcus neoformans. Infect. Immun. 60:754-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, M. X., M. C. Bohlman, C. Itatani, D. R. Burton, P. W. Parren, S. C. St Jeor, and T. R. Kozel. 2006. Human recombinant antimannan immunoglobulin G1 antibody confers resistance to hematogenously disseminated candidiasis in mice. Infect. Immun. 74:362-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, M. X., T. T. Brandhorst, T. R. Kozel, and B. S. Klein. 2001. Role of glucan and surface protein BAD1 in complement activation by Blastomyces dermatitidis yeast. Infect. Immun. 69:7559-7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, M. X., and B. Klein. 1997. Activation, binding, and processing of complement component 3 (C3) by Blastomyces dermatitidis. Infect. Immun. 65:1849-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, M. X., and T. R. Kozel. 1998. Mannan-specific immunoglobulin G antibodies in normal human serum accelerate binding of C3 to Candida albicans via the alternative complement pathway. Infect. Immun. 66:4845-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, M. X., D. M. Lupan, and T. R. Kozel. 1997. Mannan-specific immunoglobulin G antibodies in normal human serum mediate classical pathway initiation of C3 binding to Candida albicans. Infect. Immun. 65:3822-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zipfel, P. F., R. Wurzner, and C. Skerka. 2007. Complement evasion of pathogens: common strategies are shared by diverse organisms. Mol. Immunol. 44:3850-3857. [DOI] [PubMed] [Google Scholar]