Abstract
Pneumococcal polysaccharide-based vaccines are effective in preventing pneumococcus infection; however, some drawbacks preclude their widespread use in developing and undeveloped countries. Here, we evaluated the protective effects of ATP-dependent caseinolytic protease (ClpP), pneumolysin mutant (ΔA146 Ply), putative lipoate-protein ligase (Lpl), or combinations thereof against pneumococcal infections in mice. Vaccinated mice were intraperitoneally and/or intranasally challenged with different pneumococcal strains. In intraperitoneal challenge models with pneumococcal strain D39 (serotype 2), the most striking protection was obtained with the combination of the three antigens. Similarly, with the intranasal challenge models, (i) additive clearance of bacteria in lungs was observed for the combination of the three antigens and (ii) a combination vaccine conferred complete protection against intranasal infections of three of the four most common pneumococcal strains (serotypes 14, 19F, and 23F) and 80% protection for pneumococcal strain 6B. Even so, immunity to this combination could confer protection against pneumococcal infection with a mixture of four serotypes. Our results showed that the combination vaccine was as effective as the currently used vaccines (PCV7 and PPV23). These results indicate that system immunization with the combination of pneumococcal antigens could provide an additive and broad protection against Streptococcus pneumoniae in pneumonia and sepsis infection models.
Streptococcus pneumoniae (pneumococcus) commonly colonizes the upper respiratory tract asymptomatically and was estimated, in 2005, to kill 1.6 million people every year, most of whom were children aged <5 years in developing and undeveloped countries (36). As far as we know, 91 capsular polysaccharide serotypes have been identified in S. pneumoniae (33); among these, serotypes 23F, 19F, 14, and 6B are the four most epidemic strains worldwide (2, 5, 15, 17, 25, 26, 29). Moreover, and of recent concern, the widespread use of antibiotics, leading to the development of antibiotic resistance or multidrug resistance against S. pneumoniae, is increasing (9, 12, 26).
Heptavalent protein-polysaccharide conjugate vaccine (PCV7) and 23-valent pneumococcal polysaccharide vaccine (PPV23) are the two vaccines currently being used against S. pneumoniae. Both of these vaccines are polysaccharide-based formulations and effective in preventing invasive pneumococcal infections; however, some drawbacks, such as high cost, the limited polysaccharides covered, poor immunogenicity in the very young and the very old, and serotype replacement (22, 24, 26, 36), limit their wider use.
Alternatively, in an attempt to overcome the disadvantages of polysaccharide-based vaccines, a number of studies have been focusing on the screening and evaluation of protein-based vaccine candidates. Pneumococcal protein vaccine candidates, such as nontoxic pneumolysin derivates, pneumococcal surface proteins (PspA and PspC), pneumococcal surface adhesin (PsaA), and ATP-dependent caseinolytic proteases (ClpP), have been studied and shown to provide protection against S. pneumoniae. In addition, another surface protein, putative lipoate-protein ligase (Lpl), has been suggested to be a vaccine candidate, which could effectively elicit a high IgG titer and reduce the blood bacterial load (30). These vaccine candidates are shared by all S. pneumoniae. Of note, it is generally recognized that pneumolysin localized in the cytoplast in a soluble monomer, and its release was dependent on or independent of autolysin (3, 4, 18, 19). A recent study, which showed that pneumolysin was also partially localized on the cell wall (34), strengthened its utility as a vaccine candidate. Additive protections were obtained with combinations of these protein candidates. However, previous evaluations have been based only on intraperitoneal challenge models of pneumococcal disease and were not, to our knowledge, been performed in pneumonia models (7, 10, 13, 20, 23, 30, 37).
In the present study, putative lipoate-protein ligase (Lpl), ClpP, and Ply toxoid were expressed, purified, and confirmed to express on all of the pneumococcal strains used here. A focal pneumococcal pneumonia model, mimicking the natural pneumococcal infection, was used to evaluate pneumococci on lung colonization. We also set up models of invasive diseases, which were used to evaluate their systemic protective effects against pneumococcal infections. System vaccination with the combination of three antigens was sufficient to provide complete protection against pneumococcal serotypes 14, 19F, and 23F. In addition, this vaccination regimen conferred protection against the intranasal infection of a mixture of serotypes 14, 6B, 19F, and 23F. We now report the details of the protective effects elicited by ΔA146 Ply, ClpP, Lpl, and combinations thereof against pneumococcal infections.
MATERIALS AND METHODS
Mice.
The BALB/c mice used in these studies were female and 6 to 8 weeks old and were obtained from and raised at the Chongqing Medical University at Chongqing. All animal experiments were done in accordance with the Institutional Animal Care and Use Committee's guidelines at the Chongqing Medical University at Chongqing.
Bacterial strains and growth conditions.
Escherichia coli DH5α (Invitrogen) and E. coli BL21(DE3) (Novagen, Inc., Madison, WI) were used as the hosts for plasmid cloning and expression of recombinant proteins and were cultured in Luria broth supplemented with ampicillin or kanamycin antibiotics. S. pneumoniae CPM8 chromosomal DNA was a gift from D. A. Morrison. S. pneumoniae strain D39 (NCTC 7466, serotype 2) and R6 were purchased from the National Collection of Type Cultures (London, United Kingdom). Pneumococcal strains CMCC 31436 (serotype 3), CMCC 31207 (serotype 6B), CMCC 31614 (serotype 14), CMCC 31693 (serotype 19F), and CMCC 31759 (serotype 23F) were obtained from the China Medical Culture Collection (CMCC, Beijing, China). S. pneumoniae was grown on Trypticase soy agar plates supplemented with 5% sheep blood (blood agar) or in C+Y medium. Cultures in the late exponential phase were frozen and stored at −80°C in C+Y medium. The viability of bacterial stocks was analyzed prior to challenge.
Pneumococcal antigens, immunizations, and enzyme-linked immunosorbent assays (ELISAs).
ClpP and Lpl were recombinant ClpP/pET32α (34) and Lpl/pET32 that contain a plasmid-encoded S-tag, a Trx protein, and a polyhistidine tag. Mutation in Ply (ΔA146 Ply) (20) was constructed by using site-directed mutagenesis by overlap extension (16). The wild-type pneumolysin (wt-Ply) and ΔA146 Ply were recombinant wt-Ply/pW28 and ΔA146 Ply/pW28, having only a His6 tag at the N terminus. The negative control protein was the purified plasmid-encoded S-tag plus Trx protein. The 23-valent pneumococcal polysaccharide vaccine PPV23 was purchased from Chengdu Institute of Biological Products (Chengdu, China), whereas pneumococcal 7-valent conjugate vaccine PCV7 (Prevnar) was purchased from Wyeth Corp.
Female BALB/c mice, weighing 16 to 18 g, were immunized three times at 14-day intervals with 10 μg of protein in alum adjuvant (3:1 [vol/vol]) (Inject Alum no. 77161; Pierce, Rockford, IL). In brief, mice were primed subcutaneously with either ΔA146 Ply, Lpl, ClpP, ΔA146 Ply plus Lpl, ΔA146 Ply plus ClpP, Lpl plus ClpP, ΔA146 Ply plus Lpl plus ClpP, or negative control protein. Mice were boosted intraperitoneally with the same doses on days 14 and 28. Blood samples were collected 7 or 14 days after the final immunization accordingly, and sera were stored at −20°C for further assays and uses. PPV23 and PCV7 were used as positive controls, and 0.1 ml of PPV23 or PCV7 was used to immunize mice on day 0.
IgG titers were determined by ELISA analysis. For measurement of protein antigen specific IgG titers, antibody levels were determined as described elsewhere (10) by using plates coated with purified ClpP, Lpl, ΔA146 Ply, or negative control protein. For measurement of polysaccharide (PS)-specific IgG titers, ELISA was performed as described previously (21) with modifications. Briefly, 96-well plates were coated with the pneumococcal serotypes 14 and 19F. Serum samples were diluted 1/100 in phosphate-buffered saline (PBS)-T buffer and were added to plates, followed by incubation for 2 h at room temperature. After washing, horseradish peroxidase-labeled goat anti-mouse IgG (Novagen) and TMB substrate (Tiangen) were used to detect bound serotype-specific IgG. The resulting change in optical density was determined on an ELISA plate reader at 450 nm.
ΔA146 Ply hemolytic activity and neutralization effect.
Hemolytic activities of wild-type Ply and ΔA146 Ply were determined as previously established (20). For the neutralization assay, anti-ΔA146 Ply polyclonal serum and control serum were serially diluted in a total volume of 50 μl of PBS in 96-well plates. An equal volume of 2% (vol/vol) human red blood cell suspension (The First Affiliated Hospital of Chongqing Medical University, Chongqing, People's Republic of China) was added to each dilution, following by adding 50 μl of wt-Ply at a final concentration of 250 ng/ml and then incubation at 37°C for 45 min. Plates were then centrifuged for 1 min at 1,000 × g, the supernatant was carefully transferred to 96-well flat-bottom plates, and the absorbance was read at 540 nm.
Challenge of animals.
For the intraperitoneal challenge models, at 2 weeks after the last immunization BALB/c mice were administrated the virulent pneumococcal strain D39 at 1,500 times the 50% lethal dose (LD50; 1.5 × 105 CFU) or 150 times the LD50 (2.0 × 104 CFU).
In the focal pneumonia models, BALB/c mice were intranasally inoculated with pneumococcal strain 31614 (serotype 14, 1.5 × 106 CFU) or 31693 (serotype 19F, 107 CFU). The lungs were removed at day 5 after challenge and homogenized in PBS. Samples were serially diluted and plated onto blood agar plates, and viable counts were determined after overnight incubation.
For intranasal challenge with pneumococcal strain D39, mice inspired the virulent pneumococcal strain D39 at 104 (7.5 × 107 CFU) or 103 (6.0 × 106 CFU) times the LD50 in a volume of 40 μl under injection anesthesia (10% chloral hydrate, 3 ml/kg [body weight]). To further test the protective efficacy of the combination vaccine, vaccinated mice were intranasally administered either pneumococcal serotype 3 (strain 31436, 7.5 × 106 CFU), 6B (strain 31207, 2.0 × 108 CFU), 14 (strain 31614, 1.0 × 108 CFU), 19F (strain 31693, 1.5 × 108 CFU), or 23F (strain 31759, 2.0 × 108 CFU) in 30 μl of PBS or a mixture of four strains (serotypes 6B [2.0 × 108 CFU], 14 [1.0 × 108 CFU], 19F [1.5 × 108 CFU], and 23F [2.0 × 108 CFU]) in 40 μl of PBS. Survival was monitored for 21 days. Blood and lung samples of dying mice were collected and suspended in PBS, followed by centrifugation at 750 × g for 10 min. Then, 15 μl of the supernatants was plated on blood agar plates, followed by incubation overnight. Several clones with different characteristic were picked and suspended in sterile deionized water for PCR. Primers specific to each serotype were synthesized as described by Brito et al. (8).
Passive immunization.
Antisera from control protein- or combination-immunized animals were prepared from vaccinated mice and subjected to incubation at 56°C for 30 min to inactivate complement. Then, 200 μl of antisera (undiluted or 1/15 diluted antisera) was intraperitoneally transferred to animals immediately before infection with pneumococcal strain D39 (600 CFU).
To determine further whether the protective serum component was antigen-specific antibodies, diluted antisera (1/10) were adsorbed either with wild-type strain R6 or with ΔLpl R6, ΔClpP R6, and ΔPly R6 strains, respectively. Briefly, to deprive the serum samples of Lpl-, ClpP-, and Ply-specific antibodies, the sera were adsorbed with wild-type strain R6, whereas to prepare antigen-specific antibodies, the sera were first adsorbed with ΔLpl R6, ΔClpP R6, and ΔPly R6 strains, respectively. The supernatant was obtained after centrifugation, and then was subjected to filter through a 0.2-μm-pore-size filter. The resultant sera were equally mixed together, which are referred to here as antigen-specific antibodies. Before passively transfer to mice, ELISA was used to measure antigen-specific IgG titer in sera. Then, 200-μl portions of adsorbed antisera were intraperitoneally delivered to BALB/c mice, followed by intraperitoneal infection with pneumococcal strain D39 (600 CFU) immediately. All animals were carefully observed for 21 days.
Construction of ΔLpl R6, ΔClpP R6, and ΔPly R6 mutants.
The construction of insertion-deletion mutations of clpP, lpl, and ply (Δgene::ermB) in S. pneumoniae has been well established by using the long flanking homology polymerase reaction (28). The 760-bp ermB cassette was PCR amplified with DAM212 and DAM213 from S. pneumoniae CPM8 DNA (11). Upstream and downstream fragments were PCR amplified with their specific primers (P1 and P3 for upstream fragment; P2 and P4 for downstream fragment; see Table 4). Overlap extension was performed to generate the Up-Erm-Dw fusion fragment. The resulting fragment was used to transform S. pneumoniae R6 as described elsewhere (28). Briefly, pneumococcal strain R6 was exposed to the DNA for 90 min at 37°C after treatment with competence-stimulating peptide. The ensuing culture was plated on blood agar plates containing 0.25 μg of erythromycin/ml. Erm insertion at the loci of Lpl, ClpP, and Ply was confirmed by colony PCR.
TABLE 4.
Primers used for the construction of ΔLpl R6, ΔClpP R6, and ΔPly R6 mutants
| Primer | Sequence (5′-3′)a |
|---|---|
| DAM212 | CCGGGCCCAAAATTTGTTTGAT |
| DAM213 | AGTCGGCAGCGACTCATAGAAT |
| P1-Ply | TTGATTAAGAGAGGAGATGTTGTAG |
| P3-Ply | Cassette 1-TCTTCTACCTCCTAATAAGTTCCTG |
| P2-Ply | GGAGAATGCTTGCGACAA |
| P4-Ply | Cassette 2-TAGGGCTGACATGGTTTG |
| P1-Lpl | ACCAACTTCTTTTTTTGATATTTAT |
| P3-Lpl | Cassette 1-AAAATGATGGTTATTGATGTTACTC |
| P2-Lpl | AGTTGCCAAATTGAATTTGACTCCT |
| P4-Lpl | Cassette 2-AACCTTCCAGTCTAATCTATCTTCG |
| P1-ClpP | ACCAACGTCAAATTTTTGTAGTAGA |
| P3-ClpP | Cassette 1-TTCATTTCTCCTTTTGAGTTTTAAT |
| P2-ClpP | TTTTTTCTTCATCTTTTTTGTCTCC |
| P4-ClpP | Cassette 2-ATGATGATAGAAGGCAAACTCGACT |
Cassette 1 refers to the reverse complementary sequence of DAM212, and cassette 2 refers to the reverse complementary sequence of DAM213.
Statistical analysis.
IgG titers and the numbers of CFU (log10) were compared by using the Mann-Whitney U test. For survival studies, data were analyzed by using the log-rank test and the Fisher exact test, and the Mann-Whitney U was used to analyze the difference in median survival time. Values were considered statistically significant at a P value of <0.05.
RESULTS
Identification of antigen-specific IgG titers.
The IgG titers in sera of each protein and PSs were tested at day 35. ELISA analysis of sera from groups of mice immunized with these purified antigens, either singly or in combination, showed antigen-specific antibody responses that were significantly higher than that achieved with control protein (P < 0.05; Table 1 . Furthermore, comparable IgG titers of each antigen in combinations were detectable. Serum IgG antibodies to PS types 14 and 19F were produced in vaccinated mice with PPV23 and PCV7 (data not shown).
TABLE 1.
Antibody titers (total IgG) response after three immunization with antigens in alum
| Immunization group | Mean antibody titera (log10) ± SD in response to protein antigen |
|||
|---|---|---|---|---|
| Lpl | ClpP | ΔA146 Ply | Control proteinb | |
| Control | 3.706 ± 0.2 | |||
| Alum | (<2) | (<2) | (<2) | (<2) |
| Lpl | 5.445 ± 0.252* | |||
| ClpP | 4.803 ± 0.155* | |||
| ΔA146 Ply | 4.264 ± 0.392* | (<2) | ||
| Lpl+ClpP | 5.565 ± 0.131 | 4.963 ± 0.135 | ||
| Lpl+ΔA146 Ply | 5.505 ± 0.190 | 4.445 ± 0.33 | ||
| ClpP+ΔA146 Ply | 4.803 ± 0.155 | 4.304 ± 0.311 | ||
| Lpl+ClpP+ ΔA146 Ply | 5.605 ± 0.155 | 4.953 ± 0.123 | 4.154 ± 0.227 | |
The antibody titer is defined as the reciprocal of the dilution of serum giving 2.1-fold of the highest absorbance value versus the background level at 450 nm, as determined by ELISA. (<2), No reactivity. *, Significantly higher versus the control group (P < 0.05). The antibody titer was measured 1 week after the third immunization.
The control protein consisted of the purified plasmid-encoded S-tag amino acid residues plus the Trx protein.
Effect of ΔA146 Ply on hemolysis and hemolysis neutralization is mediated by anti-ΔA146 Ply polyclonal serum.
Purified ΔA146 Ply and wt-Ply were tested for hemolytic activity. Hemolytic activity of wt-Ply was exhibited at concentrations from 5 to 25 ng/ml, whereas no hemolytic activity for human erythrocytes was observed for ΔA146 Ply, even at concentrations of 500,000 ng/ml. This observation confirmed the previous result that ΔA146 Ply could not lyse erythrocytes (20), indicating that ΔA146 Ply is a safe candidate for further use as a vaccine.
We next tested whether anti-ΔA146 Ply polyclonal serum could prevent wt-Ply from lysing human erythrocytes. wt-Ply exhibited its hemolytic activity in serum diluted 480-fold, and 50% lysis of human red blood cells could be blocked when the pooled serum was diluted 2,840-fold.
Prevention of lung colonization in vaccinated mice.
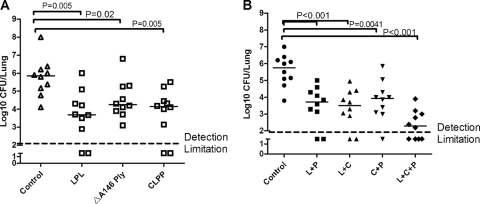
Two focal pneumonia models were set up with two different serotype strains. In the strain 31614 (serotype 14) intranasal infection model, all three proteins were capable of reducing the bacterial load in the lung by >10-fold compared to control protein-immunized mice, especially Lpl (P < 0.05; Fig. 1A ). A similar result was observed in the strain 31693 (serotype 19F) infection model. Compared to the control protein, ClpP and Lpl reduced the median bacterial load nearly 100-fold (P = 0.005 and P = 0.01, respectively [data not shown]), and immunity to ΔA146 Ply was also effective against pneumococcal colonization in lungs, which resulted in a >10-fold-reduced median bacterial load (P = 0.05 [data not shown]). In a second experiment, the most dramatic protection against lung colonization of strain 31693 was achieved by immunization with the three antigens combined, which reduced the number of lung CFU ∼10-fold more than that achieved with Lpl plus ClpP and >20-fold more than those with ClpP plus ΔA146 Ply and Lpl plus ΔA146 Ply (P < 0.05; Fig. 1B).
FIG. 1.
Protection elicited by Lpl, ClpP, and ΔA146 Ply against pneumococcal lung colonization. BALB/c mice were immunized with the indicated antigens and intranasally challenged with S. pneumoniae as follows: 1.5 × 106 CFU of the 31614 strain (A) and 107 CFU of the 31693 strain (B). The lung colonization of individual mice is shown at day 5 after challenge, indicating the median CFU per lung (horizontal lines). The broken line on the y axis indicates no lung infection. Detection limitation, 100 CFU. In panel B, the L, P, and C represent Lpl, ΔA146 Ply, and ClpP, respectively.
Prevention of pneumonia and sepsis in vaccinated mice.
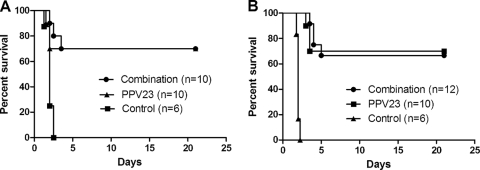
In an initial experiment (Table 2), vaccinated mice were challenged with pneumococcal strain D39 at 1,500 times the LD50 (1.5 × 105 CFU). Sepsis in control mice invariably precedes death. Vaccinated mice survived significantly longer than control mice, except for Lpl group. We observed that 41.7% protection against the 1,500-LD50 challenge dose was achieved with the three antigens combined (combination vaccine) and with no signs of morbidity in the surviving mice (Table 2). Under this high-dose challenge, mice that received the three-antigen combination vaccine (combination vaccine) survived significantly longer than those immunized with a single antigen or those immunized with combinations of two antigens (Mann-Whitney U test, P < 0.05), except for ΔA146 Ply plus ClpP. In addition, 70% of combination-vaccinated mice were protected from the challenge with pneumococcal strain D39 at the 150-LD50 (2.0 × 104 CFU) challenge dose, which was as protective as the positive control of 70% protection (Fig. 2A). Subsequent study using the less-virulent pneumococcal strain TIGR4 (8 × 107 CFU) as the challenge strain showed that Lpl could significantly prolong median survival time to infection; moreover, complete protection was also achieved when mice were vaccinated with the combination vaccine. The median survival time was significantly longer than that achieved by vaccination with ClpP plus ΔA146 Ply (P < 0.05; data not shown).
TABLE 2.
Survival of mice intraperitoneally challenged with S. pneumoniae D39a
| Group | No. of mice | Median survival time (days)b | % Survivalc |
|---|---|---|---|
| Control | 12 | 1.8 | 0 |
| Lpl | 12 | 2.0 | 8.3 |
| ClpP | 12 | 2.5* | 25 |
| ΔA146 Ply | 12 | 2.5* | 25 |
| Lpl+ClpP | 12 | 3.0* | 16.7 |
| Lpl+ΔA146 Ply | 12 | 3.0* | 25 |
| ClpP+ΔA146 Ply | 12 | 5.0* | 16.7 |
| Lpl+ClpP+ΔA146 Ply | 12 | 10.0*† | 41.7d |
The challenge dose was 1.5 × 105 CFU (1,500 LD50).
*, Differences in median survival times between vaccinated groups and control group were analyzed by using the Mann-Whitney U test (P < 0.05); †, this value is significantly different from the value observed from control mice (P = 0.0373 as determined by the Fisher exact test).
Calculated as the percentage of living mice at the end of the observation period.
Significantly longer than those achieved with Lpl plus ΔA146 Ply and Lpl plus ClpP (Mann-Whitney U test; P < 0.05).
FIG. 2.
Protection of vaccinated mice from pneumococcal strain D39 infections. (A) BALB/c mice were challenged intraperitoneally with strain D39 at an LD50 of 150 (2.0 × 104 CFU; combination versus PPV23, P = 0.5206; combination versus control, P < 0.0001) (A) or intranasally with strain D39 at an LD50 of 103 (7.5 × 106 CFU; combination versus PPV23, P = 0.9720; combination versus control, P < 0.0001) (B). The results are represented in survival curves and analyzed by the log-rank test. n refers to the number of animals. For all experiments, control protein-immunized mice served as negative controls.
Mice immunized with ΔA146 Ply regimen were conferred significant protection against pneumococcal strain D39 infection at a 104-LD50 challenge dose (7.5 × 107 CFU), whereas significant protection was not observed in the Lpl and ClpP immunization groups (Table 3). A 58.3% level of protection was achieved when mice were vaccinated with ClpP plus ΔA146 Ply. Similar protection pattern was observed with intranasal challenge with pneumococcal serotype 3 strain 31436 (data not shown). In another set of experiment, 66.7% of the mice immunized with the combination vaccine survived intranasal challenge of pneumococcal strain D39 at 103 times the LD50 (6.0 × 106 CFU), which was as protective as PPV23 (Fig. 2B). Control mice invariably succumbed to infection.
TABLE 3.
Survival of mice intranasally challenged with S. pneumoniae D39a
| Group | No. of mice | Median survival time (days)b | % Survivalc |
|---|---|---|---|
| Control | 12 | 1.25 | 0 |
| Lpl | 12 | 1.25 | 16.7 |
| ClpP | 12 | 1.75 | 16.7 |
| ΔA146 Ply | 12 | 15* | 50* |
| Lpl+ClpP | 12 | 1.25 | 16.7 |
| Lpl+ΔA146 Ply | 12 | 17* | 50* |
| ClpP+ΔA146 Ply | 12 | 21* | 58.3† |
| Lpl+ClpP+ΔA146 Ply | 12 | 13.5* | 50* |
The challenge dose was 7.5 × 107 CFU (104 LD50).
*, Significant protection in median survival times was observed for mice immunized with ΔA146 Ply, ΔA146 Ply plus ClpP, ΔA146 Ply plus Lpl, and ΔA146 plus ClpP plus Lpl (P < 0.01).
Calculated as the percentage of living mice at the end of the observation period. Values significantly different from the value observed with control mice are indicated (*, P = 0.0137; †, P < 0.01 [Fisher exact test]).
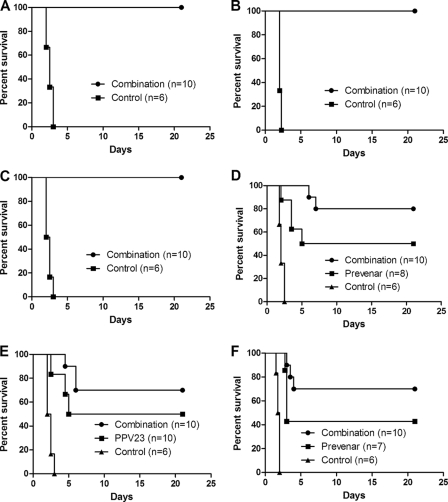
Considering the best protection against pneumococcal infections elicited by the combination vaccine, we next tested whether this vaccine could provide broad protection against different pneumococcal strains (Fig. 3). Vaccinated BALB/c mice were intranasally challenged with pneumococcal serotype 3, 6B, 14, 19F, 23F or with a combination of four serotypes (6B, 14, 19F, and 23F). Complete protection against serotypes 14, 19F, and 23F was achieved in mice that received the combination vaccine, and 80 and 50% protection was achieved against serotype 6B in mice receiving the combination vaccine and PCV7, respectively. A total of 70% of the mice immunized with the combination vaccine were protected from infection from pneumococcal serotype 3 (virulent strain; 7.5 × 106 CFU), whereas only 50% mice survived the challenge in the PPV23-immunized group, but a survival difference was not noted between the two groups. All mice that succumbed to infection were confirmed to have pneumonia and bacteremia due to serotype-specific infection, whereas the surviving mice had no bacteremia and pneumonia at the end of the observation period (Table 5).
FIG. 3.
Protection conferred by combination against multiple pneumococcal strain infections. BALB/c mice were immunized with the combination of Lpl plus ClpP plus ΔA146 Ply and intranasally challenged with 2.0 × 108 CFU of strain 31759 (PS type 23F; P < 0.0001) (A); 1.5 × 108 CFU of strain 31693 (PS type 19F; P < 0.0001) (B); 1.0 × 108 CFU of strain 31614 (PS type 14; P < 0.0001) (C); 2.0 × 108 CFU of strain 31207 (PS type 6B; PCV7 versus combination, P = 0.1189; combination versus control, P < 0.0001; PCV7 versus control, P = 0.0012) (D); 7.5 × 106 CFU of strain 31436 (PS type 3; combination versus PPV23, P = 0.7873; combination versus control, P < 0.0001; PPV23 versus control, P = 0.0005) (E); or with a mixture of serotype 6B (2.0 × 108 CFU), serotype 14 (1.0 × 108 CFU), serotype 23F (2.0 × 108 CFU), and serotype 19F (1.5 × 108 CFU) (combination versus PCV7, P = 0.1823; combination versus control, P < 0.0001; PCV7 versus control, P = 0.0005) (F). Survival was monitored for 21 days after challenge. The results are represented in survival curves and analyzed by the log-rank test. n refers to the number of animals. For all experiments, control protein immunized mice served as negative controls.
TABLE 5.
Bacteremia in vaccinated mice infected with different S. pneumoniae serotypesa
| Serotype | Dose (CFU) | No. of mice |
% of mice with bacteremiab |
Pc | ||
|---|---|---|---|---|---|---|
| Control | Combination | Control | Combination | |||
| 3 | 7.5 × 106 | 6 | 10 | 100 | 30 | 0.0114 |
| 6B | 2.0 × 108 | 6 | 10 | 100 | 20 | 0.007 |
| 14 | 1.0 × 108 | 6 | 10 | 100 | 0 | <0.001 |
| 19F | 1.5 × 108 | 6 | 10 | 100 | 0 | <0.001 |
| 23F | 2.0 × 108 | 6 | 10 | 100 | 0 | <0.001 |
“Combination” refers to a combination of three antigens.
That is, the bacteremia status as determined at the time of death or at the end of the observation period.
P values were determined by using the Fisher exact test.
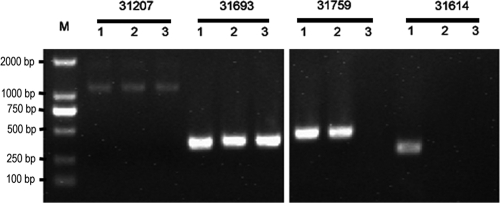
We next tested whether the combination vaccine could provide protection against the challenge with a mixture of the four most common pneumococcal serotypes (serotypes 6B, 14, 19F, and 23F; Fig. 3F). A 70% protection was achieved in the combination vaccine group, and a 42.8% protection was achieved in the PCV7 group. Colony PCR was used to determine the bacterium serotypes responsible for death in the present study. The dying mice in the combination vaccine group were confirmed to have serotype 6B and 19F infections in the lungs and blood, whereas in the PCV7-vaccinated mice, serotypes 6B, 19F, and 23F were responsible for the deaths. Surprisingly, serotype 14 was not detectable in either groups. All serotype strains were detectable in lung and blood samples in control mice (Fig. 4).
FIG. 4.
Serotype-specific pneumococci responsible for the death of mice infected with a mixture of four strains. M, marker. Strains 31207 (serotype 6B), 31693 (serotype 19F), 31759 (serotype 23F), and 31614 (serotype 14) are indicated. Lanes 1, 2, and 3 denote the negative control, PCV7, and the combination vaccine, respectively.
Passive antiserum transfer from immunized animals provides protection against S. pneumoniae sepsis in mice.
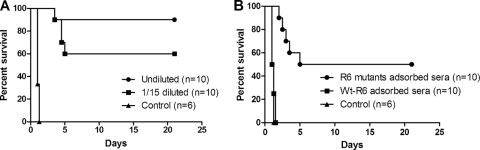
Antisera (complement inactivated) from alum- or combination-immunized mice were intraperitoneally transferred to BALB/c mice at the same time they were infected with strain D39 at a 6-LD50 challenge dose. A 90% protection was achieved for mice that received antisera from combination vaccine-immunized mice. A total of 60% of the mice were protected from death when passively transferred with 1/15-diluted antisera (Fig. 5A). Mice that received control antisera were not protected from S. pneumoniae infection.
FIG. 5.
Pneumococcal sepsis survival studies using passively intraperitoneally transferred antisera in BALB/c mice. (A) Passive transfer of antisera or diluted (1:15) antisera immediately, followed by challenge with D39 strain at a 6-LD50 dose (600 CFU) (log-rank; undiluted versus control, P < 0.0001; 1/15 diluted, P < 0.0001; median survival, diluted and undiluted sera, undefined; control, 1.0 day). (B) Passive transfer of control antisera adsorbed with R6 (control), combination antisera adsorbed with R6, and combination antisera adsorbed with R6 mutants, immediately followed by a D39 challenge at a 6-LD50 dose (600 CFU). Control antisera served as the controls (wt-R6 adsorbed versus R6 mutant adsorbed, P = 0.0023). The results are represented in survival curves and were analyzed by using the log-rank test.
No protection was seen in infected mice receiving combination-specific antiserum adsorbed with strain R6; in contrast, 50% of the mice survived infection when they received antigen-specific antibodies (Fig. 5B). Compared to antigen-specific IgG antibodies in sera adsorbed with R6 mutants, ELISA analysis of the combination antisera adsorbed with R6 strains revealed a drastic reduction in the three antigen-specific IgG reactivities (data not shown), indicating that the protection elicited by the combination vaccine was provided by Lpl-, ClpP-, and Ply-specific antibodies in this model.
DISCUSSION
The most dramatic protection against S. pneumoniae sepsis was obtained with a combination of ΔA146 Ply plus ClpP plus Lpl. Besides. Our observations thus strengthen the conclusion that the combination of pneumococcal antigens could provide additive protection against pneumococcal sepsis (10, 32). Our study also showed that the protection elicited was antibody mediated, since no protection was seen when antigen-specific antibodies were withdrawn (see Fig. 4). Although similar passive protection was not performed here in the intranasal model, we believe that the antibody titer correlates with the protection in either model, because mice boosted twice with ΔA146 Ply with a high antigen-specific IgG titer survived significantly longer than mice boosted once with ΔA146 Ply (P < 0.05 [log-rank analysis]; data not shown).
In the present study, three pneumococcal antigens were used in combinations to test their protective efficacy. Considering the potential antagonism, ELISA was used to reveal whether there were detectable antagonisms between antigens. It has been reported that no immune responses were detected when mice were immunized with ClpP plus Lpl plus ACE097 (30). In the present study, compatible IgG levels of each protein were detected, which suggests that, on the one hand, ΔA146 Ply could be used in combination with ClpP plus Lpl and, on the other hand, the immune response failure of their combination may result from the incorporation of ACE097.
Vaccination with each antigen conferred protection in sepsis models and could reduce the pneumococcal load. However, except for pneumolysin, significant protection against intranasal challenge with pneumococcal strain D39 at 7.5 × 107 CFU was not observed. A possible explanation for this is that the pneumolysin contributes as a toxin to the overall virulence of the pneumococcus D39 in intranasal infection, so protection elicited by pneumolysin would be more predominant than that achieved by Lpl and ClpP. Also, we may attribute their ineffectiveness to the lethal challenge dosage, which masked the differences between groups in this model, and this in turn emphasizes the importance of immunity to ΔA146 Ply in this combination.
We observed, in the control mice, a very similar number of CFU both at inoculation and 5 days later. We have also observed that some control mice exhibited clinical symptoms 3 to 4 days postinoculation, e.g., the animals were moribund, but they ultimately survived. It seems that the number of pneumococci initially increased and then decreased afterward because of the animal's natural immunity, so it is possible that after 5 days the CFU count in the lungs may be lower than or equal to the inoculation dosage. In comparison to the CFU in control mice, although there are still ∼10,000 CFU in single-antigen-vaccinated mice, the CFU counts in vaccinated mice were significantly reduced.
Pneumolysin is a very important virulence factor essential for the survival of pneumococci in airway tracts and required for bacterial spread from the lungs to the blood and have a direct cytotoxic effect on alveolar cells (27). Immunization with PdB, PdA, or even wide-type pneumolysin could confer protection against pneumococcal sepsis (32). We observed here that ΔA146 Ply alone elicited protection against the development of bacteremia (data not shown); surprisingly, ΔA146 Ply alone elicited protection that was as effective as the combination vaccine against intranasal challenge with the D39 strain. Nevertheless, there was evidence that pneumolysin elicited protection against nine pneumococcal serotypes, but protection varied and even failed to protect against one more pneumococcal strain (1). Moreover, in contrast to a previous study (6), our observation showed that ΔA146 Ply could confer protection against carriage or colonization. Taken together, these findings indicate that it would be more reasonable to use a combination formulation other than pneumolysin alone to achieve a broad protection against pneumococcal infections.
We described and revealed here for the first time the differential protection pattern elicited by Lpl, ClpP, ΔA146 Ply, and combinations against intranasal and intraperitoneal pneumococcal infections. Naturally, S. pneumoniae colonizes the upper respiratory tract first and then proliferates and evades the alveolar epithelial capillary endothelium and epithelial basement membrane to cause bacteremia when the host is in a status of hypoimmunity or is immunocompromised. It seems that intranasal challenge more closely imitates natural infection. Accordingly, it would be useful to test whether a vaccine candidate is effective in order to reduce colonization and block the development of bacteremia, especially for pneumococcal vaccine candidates. Apparently, this testing ability was missing in intraperitoneal models. With regard to pneumolysin, for example, if protection was simply based on the intraperitoneal infection model, the protection against bacteremia (data not shown) or against intranasal infection (see Table 3) would be lost, since it is widely accepted that pneumolysin plays an important role in this process of bacterial invasion of the bloodstream. Although we emphasize the importance of protection against intranasal infection, we do not completely discount the protection value against intraperitoneal infection, since this model could be also regarded as very important supplementary evidence against a more severe pneumococcal infection.
This is a very promising preclinical result, showing a complete protection against pneumococcal infections (i.e., with serotypes 14, 19F, and 23F) in mice that have been vaccinated with the combination vaccine, especially because these are three of the four most clinically important S. pneumoniae serotypes. More importantly, protection elicited by the combination vaccine was as effective as that resulting from the PPV23 and PCV7 vaccines.
We also demonstrated for the first time that this combination could provide protective immunity from acute pneumonia caused by challenge with a mixture of four different pneumococcal strains, since S. pneumoniae often colonizes the nasopharynx with multiple serotypes in a single person (14). As can be seen in Table 5, the combination vaccine significantly protected mice from developing bacteremia. Thus, the development of bacteremia in control mice indicated that the observed protection elicited by the combination vaccine was both the result of protection in the lung and protection against the development of bacteremia (Table 5).
Cell wall separation of group B streptococcus (PcsB) is thus another most promising treatment candidate (13), since it is involved in the maintenance of cell morphology and is essential for the growth of S. pneumoniae (31, 35). Immunization with PcsB has shown protective effects in both intranasal and intraperitoneal challenge models. Therefore, it may be promising to combine PcsB with ΔA146 Ply. Moreover, considering the fact that three is better than two, we may recommend a third candidate in combination with PcsB and ΔA146 Ply to have a wider coverage and better protection against pneumococcal infections.
Acknowledgments
This study is supported by Natural Science Foundation grants of Chongqing key projects (NO.CSTC, 2008BA7027) and National Natural Science Foundation grants of China (no. 30671868 and no. 30371275).
Editor: V. J. DiRita
Footnotes
Published ahead of print on 28 December 2009.
REFERENCES
- 1.Alexander, J. E., R. A. Lock, C. C. A. M. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansman, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslan, G., G. Emekdas, M. Bayer, M. S. Serin, N. Kuyucu, and A. Kanik. 2007. Serotype distribution of Streptococcus pneumoniae strains in the nasopharynx of healthy Turkish children. Indian J. Med. Res. 125:582-587. [PubMed] [Google Scholar]
- 3.Balachandran, P., S. K. Hollingshead, J. C. Paton, and D. E. Briles. 2001. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 183:3108-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry, A. M., R. A. Lock, D. Hansman, and J. C. Paton. 1989. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect. Immun. 57:2324-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogaert, D., N. T. Ha, M. Sluijter, N. Lemmens, R. de Groot, and P. W. M. Hermans. 2002. Molecular epidemiology of pneumococcal carriage among children with upper respiratory tract infections in Hanoi, Vietnam. J. Clin. Microbiol. 40:3903-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briles, D. E., S. K. Hollingshead, J. C. Paton, E. W. Ades, F. W. van Ginkel, and W. H. Benjamin, Jr. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339-348. [DOI] [PubMed] [Google Scholar]
- 8.Brito, D. A., M. Ramirez, and H. de Lencastre. 2003. Serotyping Streptococcus pneumoniae by multiplex PCR. J. Clin. Microbiol. 41:2378-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brueggemann, A. B., S. L. Coffman, P. Rhomberg, H. Huynh, L. Almer, A. Nilius, R. Flamm, and G. V. Doern. 2002. Fluoroquinolone resistance in Streptococcus pneumoniae in United States since 1994-1995. Antimicrob. Agents Chemother. 46:680-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao, J., D. P. Chen, W. C. Xu, T. M. Chen, S. X. Xu, J. Y. Luo, Q. Zhao, B. Z. Liu, D. S. Wang, X. M. Zhang, Y. L. Shan, and Y. B. Yin. 2007. Enhanced protection against pneumococcal infection elicited by immunization with the combination of PspA, PspC, and ClpP. Vaccine 25:4996-5005. [DOI] [PubMed] [Google Scholar]
- 11.Claverys, J. P., A. Dintilhac, E. V. Pestova, B. Martin, and D. A. Morrison. 1995. Construction and evaluation of new drug resistance cassettes for gene disruption mutagenesis in Streptococcus pneumoniae, using an Ami test platform. Gene 164:123-128. [DOI] [PubMed] [Google Scholar]
- 12.Doern, G. V., K. P. Heilmann, H. K. Huynh, P. R. Rhomberg, S. Coffman, and A. B. Brueggemann. 2001. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999-2000, including a comparison of resistance rates since 1994-1995. Antimicrob. Agents Chemother. 45:1721-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giefing, C., A. L. Meinke, M. Hanner, T. Henics, D. B. Minh, D. Gelbmann, U. Lundberg, B. M. Senn, M. Schunn, A. Habel, B. Henriques-Normark, A°. Or̈tqvist, M. Kalin, A. von Gabain, and E. Nagy. 2008. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J. Exp. Med. 205:117-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray, B. M., G. M. Converse III, and H. C. Dillon, Jr. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923-933. [DOI] [PubMed] [Google Scholar]
- 15.Ho, P. L., K. F. Lam, F. K. H. Chow, Y. L. Lau, S. S. Wong, S. L. E. Cheng, and S. S. Chiu. 2004. Serotype distribution and antimicrobial resistance patterns of nasopharyngeal and invasive Streptococcus pneumoniae isolates in Hong Kong children. Vaccine 22:3334-3339. [DOI] [PubMed] [Google Scholar]
- 16.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 17.Jefferson, T., E. Ferroni, F. Curtale, P. G. Rossi, and P. Borgia. 2006. Streptococcus pneumoniae in Western Europe: serotype distribution and incidence in children less than 2 years old. Lancet Infect. Dis. 6:405-410. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, M. 1977. Cellular location of pneumolysin. FEMS Microbiol. Lett. 2:243-245. [Google Scholar]
- 19.Kadioglu, A., J. N. Weiser, J. C. Paton, and P. W. Andrew. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nature 6:294-296. [DOI] [PubMed] [Google Scholar]
- 20.Kirkham, L. A. S., A. R. Kerr, G. R. Douce, G. K. Paterson, D. A. Dilts, D. F. Liu, and T. J. Mitchell. 2006. Construction and immunological characterization of a novel nontoxic protective pneumolysin mutant for use in future pneumococcal vaccines. Infect. Immun. 74:586-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobrynski, L. J., A. O. Sousa, A. J. Nahmias, and F. K. Lee. 2005. Cutting edge: antibody production to pneumococcal polysaccharides requires CD1 molecules and CD8-T cells. J. Immunol. 174:1787-1790. [DOI] [PubMed] [Google Scholar]
- 22.Koskela, M., M. Leinonen, V. M. Haiva, M. Timonen, and P. H. Makela. 1986. First and second dose antibody responses to pneumococcal polysaccharide vaccine in infants. Pediatr. Infect. Dis. 5:45-50. [DOI] [PubMed] [Google Scholar]
- 23.Kwon, H. Y., A. D. Ogunniyi, M. H. Choi, S. N. Pyo, D. K. Rhee, and J. C. Paton. 2004. The ClpP protease of Streptococcus pneumoniae modulates virulence gene expression and protects against fatal pneumococcal challenge. Infect. Immun. 72:5646-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyaw, M. K., R. Lynfield, W. Schaffner, A. S. Craig, J. Hadler, A. Reingold, A. R. Facklam, J. H. Jorgensen, J. Besser, E. R. Zell, A. Schuchat, and C. G. Whitney. 2006. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 354:1455-1463. [DOI] [PubMed] [Google Scholar]
- 25.Lauderdale, T. L., M. M. Wagener, H. M. Lin, H. I. Fei, W. Y. Lee, K. S. Hseih, J. F. Lai, and C. C. Chiou. 2006. Serotype and antimicrobial resistance patterns of Streptococcus pneumoniae isolated from Taiwanese children: comparison of nasopharyngeal and clinical isolates. Diagn. Microbiol. Infect. Dis. 56:421-426. [DOI] [PubMed] [Google Scholar]
- 26.Liu, Y. D., H. Wang, M. J. Chen, Z. Sun, R. Zhao, L. Zhang, H. Y. Wang, H. Zhang, L. P. Wang, Y. Z. Chu, Y. Liu, and Y. X. Ni. 2008. Serotype distribution and antimicrobial resistance patterns of Streptococcus pneumoniae isolated from children in China younger than 5 years. Diagn. Microbiol. Infect. Dis. 61:256-263. [DOI] [PubMed] [Google Scholar]
- 27.Maus, U. A., M. Srivastava, J. C. Paton, M. Mack, M. B. Everhart, T. S. Blackwell, J. W. Christman, D. Schlöndorff, W. Seeger, and J. Lohmeyer. 2004. Pneumolysin-induced lung injury is independent of leukocyte trafficking into the alveolar space. J. Immun. 173:1307-1312. [DOI] [PubMed] [Google Scholar]
- 28.Meng, J. P., Y. B. Yin, X. M. Zhang, Y. S. Huang, K. Lan, F. Cui, and S. X. Xu. 2008. Identification of Streptococcus pneumoniae genes specifically induced in mouse lung tissues. Can. J. Microbiol. 54:58-65. [DOI] [PubMed] [Google Scholar]
- 29.Miche, N., M. Watson, F. Baumann, P. Perolat, and B. Garin. 2005. Distribution of Streptococcus pneumoniae serotypes responsible for penicillin resistance and the potential role of new conjugate vaccines in New Caledonia. J. Clin. Microbiol. 43:6060-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morsczeck, C., T. Prokohova, J. Sigh, M. Bille-Nielsen, J. Petersen, A. Boysen, T. Kofoed, N. Frimodt-Møller, P. Nyborg-Nielsen, and P. Schrotz-King. 2008. Streptococcus pneumoniae: proteomics of surface proteins for vaccine development. Clin. Microbiol. Infect. 14:74-81. [DOI] [PubMed] [Google Scholar]
- 31.Ng, W. L., K. M. Kazmierczak, and M. E. Winkler. 2004. Defective cell wall synthesis in Streptococcus pneumoniae R6 depleted for the essential PcsB putative murein hydrolase or the VicR (YycF) response regulator. Mol. Microbiol. 53:1161-1175. [DOI] [PubMed] [Google Scholar]
- 32.Ogunniyi, A. D., M. Grabowicz, D. E. Briles, J. Cook, and J. C. Paton. 2007. Development of a vaccine against invasive pneumococcal disease: based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 75:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, I. H., D. G. Pritchard, R. Cartee, A. Brandao, M. C. C. Brandileone, and M. H. Nahm. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 45:1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price, K. E., and A. Camilli. 2009. Pneumolysin localizes to the cell wall of Streptococcus pneumoniae. J. Bacteriol. 191:2163-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinscheid, D. J., B. Gottschalk, A. Schubert, B. J. Eikmanns, and G. S. Chhatwal. 2001. Identification and molecular analysis of PcsB, a protein required for cell wall separation of group B Streptococcus. J. Bacteriol. 183:1175-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. 2007. Pneumococcal conjugate vaccine for childhood immunization. Wkly. Epidemiol. Rec. 82:93-104. [PubMed] [Google Scholar]
- 37.Wu, H. Y., M. H. Nahm, Y. Guo, M. W. Russell, and D. E. Briles. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 175:839-846. [DOI] [PubMed] [Google Scholar]