Abstract
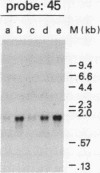
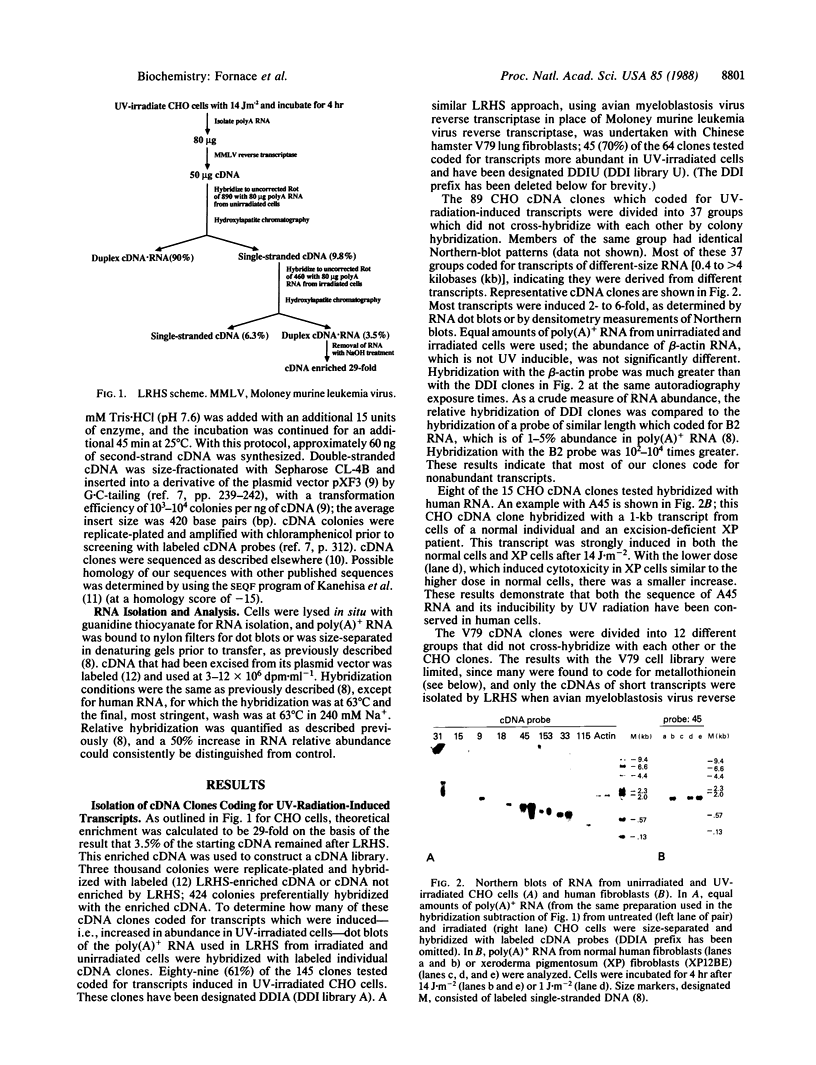
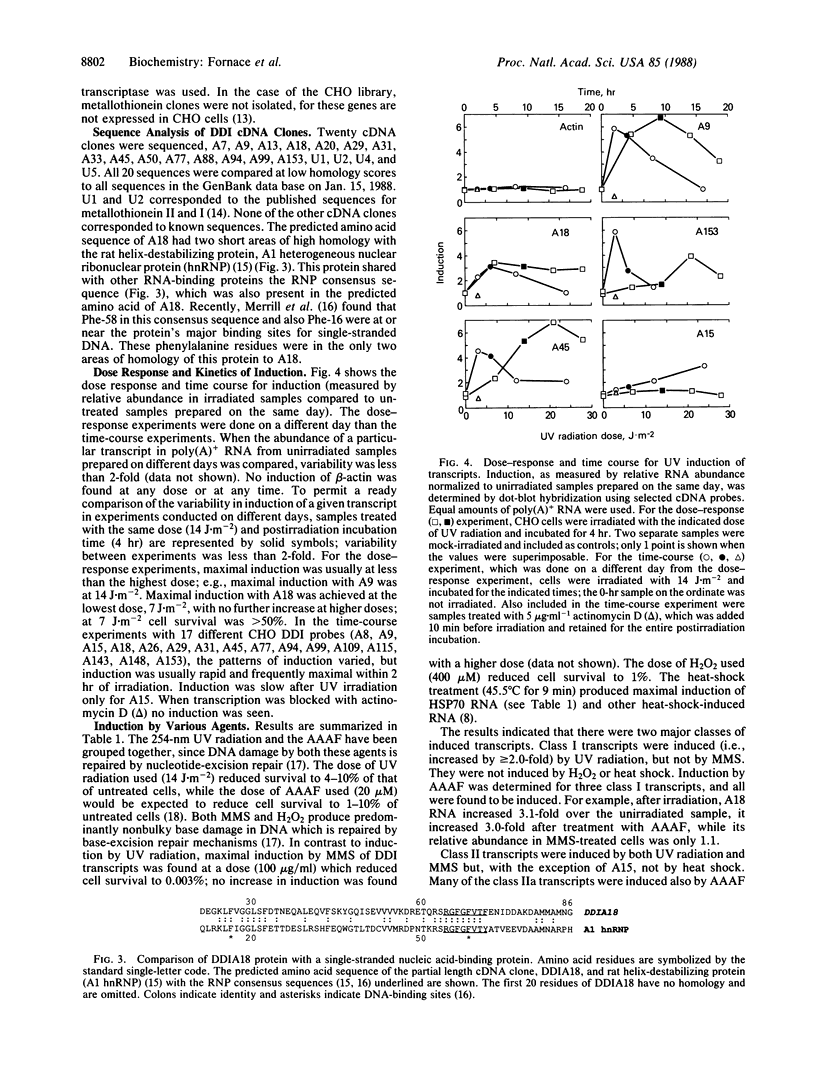
Hybridization subtraction at low ratios of RNA to cDNA was used to enrich for the cDNA of transcripts increased in Chinese hamster cells after UV irradiation. Forty-nine different cDNA clones were isolated. Most coded for nonabundant transcripts rapidly induced 2- to 10-fold after UV irradiation. Only 2 of the 20 cDNA clones sequenced matched known sequences (metallothionein I and II). The predicted amino acid sequence of one cDNA had two localized areas of homology with the rat helix-destabilizing protein. These areas of homology were at the two DNA-binding sites of this nucleic acid single-strand-binding protein. The induced transcripts were separated into two general classes. Class I transcripts were induced by UV radiation and not by the alkylating agent methyl methanesulfonate. Class II transcripts were induced by UV radiation and by methyl methanesulfonate. Many class II transcripts were induced also by H2O2 and various alkylating agents but not by heat shock, phorbol 12-tetradecanoate 13-acetate, or DNA-damaging agents which do not produce high levels of base damage. Since many of the cDNA clones coded for transcripts which were induced rapidly and only by certain types of DNA-damaging agents, their induction is likely a specific response to such damage rather than a general response to cell injury.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amacher D. E., Elliott J. A., Lieberman M. W. Differences in removal of acetylaminofluorene and pyrimidine dimers from the DNA of cultured mammalian cells. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1553–1557. doi: 10.1073/pnas.74.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Pöting A., Mallick U., Rahmsdorf H. J., Schorpp M., Herrlich P. Induction of metallothionein and other mRNA species by carcinogens and tumor promoters in primary human skin fibroblasts. Mol Cell Biol. 1986 May;6(5):1760–1766. doi: 10.1128/mcb.6.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Cobianchi F., SenGupta D. N., Zmudzka B. Z., Wilson S. H. Structure of rodent helix-destabilizing protein revealed by cDNA cloning. J Biol Chem. 1986 Mar 15;261(8):3536–3543. [PubMed] [Google Scholar]
- Coombs A. M., Moss S. H. Effects of peroxide and catalase on near ultraviolet radiation sensitivity in Escherichia coli strains. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Mar;51(3):493–503. doi: 10.1080/09553008714550971. [DOI] [PubMed] [Google Scholar]
- Crawford B. D., Enger M. D., Griffith B. B., Griffith J. K., Hanners J. L., Longmire J. L., Munk A. C., Stallings R. L., Tesmer J. G., Walters R. A. Coordinate amplification of metallothionein I and II genes in cadmium-resistant Chinese hamster cells: implications for mechanisms regulating metallothionein gene expression. Mol Cell Biol. 1985 Feb;5(2):320–329. doi: 10.1128/mcb.5.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr Measurement of M. luteus endonuclease-sensitive lesions by alkaline elution. Mutat Res. 1982 Jun;94(2):263–276. doi: 10.1016/0027-5107(82)90290-1. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Mitchell J. B. Induction of B2 RNA polymerase III transcription by heat shock: enrichment for heat shock induced sequences in rodent cells by hybridization subtraction. Nucleic Acids Res. 1986 Jul 25;14(14):5793–5811. doi: 10.1093/nar/14.14.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Schalch H., Alamo I., Jr Coordinate induction of metallothioneins I and II in rodent cells by UV irradiation. Mol Cell Biol. 1988 Nov;8(11):4716–4720. doi: 10.1128/mcb.8.11.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Herrick G., Alberts B. Nucleic acid helix-coil transitions mediated by helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2133–2141. [PubMed] [Google Scholar]
- Kanehisa M., Klein P., Greif P., DeLisi C. Computer analysis and structure prediction of nucleic acids and proteins. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):417–428. doi: 10.1093/nar/12.1part1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartasova T., Cornelissen B. J., Belt P., van de Putte P. Effects of UV, 4-NQO and TPA on gene expression in cultured human epidermal keratinocytes. Nucleic Acids Res. 1987 Aug 11;15(15):5945–5962. doi: 10.1093/nar/15.15.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill B. M., Stone K. L., Cobianchi F., Wilson S. H., Williams K. R. Phenylalanines that are conserved among several RNA-binding proteins form part of a nucleic acid-binding pocket in the A1 heterogeneous nuclear ribonucleoprotein. J Biol Chem. 1988 Mar 5;263(7):3307–3313. [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Robinson G. W., Nicolet C. M., Kalainov D., Friedberg E. C. A yeast excision-repair gene is inducible by DNA damaging agents. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1842–1846. doi: 10.1073/pnas.83.6.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotem N., Axelrod J. H., Miskin R. Induction of urokinase-type plasminogen activator by UV light in human fetal fibroblasts is mediated through a UV-induced secreted protein. Mol Cell Biol. 1987 Feb;7(2):622–631. doi: 10.1128/mcb.7.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby S. W., Szostak J. W. Specific Saccharomyces cerevisiae genes are expressed in response to DNA-damaging agents. Mol Cell Biol. 1985 Jan;5(1):75–84. doi: 10.1128/mcb.5.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]