Abstract
The protozoan parasite Trypanosoma cruzi has evolved sophisticated systems to evade the immune response. An important requirement for a productive immune response is recruitment of the appropriate immune cells from the bloodstream to the sites of infection. Here, we show that a mucin expressed and secreted by the metacyclic infective form of T. cruzi, AgC10, is able to interfere with L-selectin-mediated monocyte adhesion. Thus, incubation of U937 monocytic cells stably expressing L-selectin (U937LAM) with AgC10 strongly reduced their adhesion on P-selectin under flow, which is dependent on L-selectin. This treatment also results in a significant inhibition by AgC10 of U937LAM and human primary monocyte adhesion to activated vascular endothelium. This effect was specific for L-selectin, because vascular cell adhesion molecule 1 (VCAM-1)-mediated adhesion was not affected by AgC10 pretreatment. This effect of AgC10 is likely due to its ability to induce L-selectin shedding from the monocyte membrane, since pharmacologic blocking of this shedding prevents AgC10 activity. This is the first description of a mechanism that prevents leukocyte adhesion to the endothelium by a parasite and represents a new potential countermeasure to evade the generation of a correct immune response.
The recruitment of leukocytes from the bloodstream to sites of injury or infection is key to the development of an appropriate immune response (29). This is a multistep process regulated by a cascade of molecular interactions between leukocytes and endothelial cells. The initial contact of leukocytes with the activated endothelium involves rapid and transient interactions that initiate rolling on the surface of endothelial cells under blood flow. These interactions are mediated by proteins of the selectin family, which consists of three members expressed by endothelial cells (P-selectin and E-selectin), leukocytes (L-selectin), and platelets (P-selectin) (16, 24, 35). L-selectin was first identified as a lymphocyte homing receptor (10) and is constitutively expressed in most leukocytes, including naive T cells and monocytes, but is rapidly shed from the surface upon cellular activation (15, 31). Several L-selectin ligands, such as GlyCAM-1, MAdCAM-1, CD34, and Sgp 200, have been identified, as have ligands of other selectins, such as PSGL-1, and P- and E-selectin (27). The requirement of L-selectin for the appropriate homing of the effector immune cells to mount a protective immune response is well documented (28). Interestingly, some pathogens, such as Trypanosoma cruzi (2, 7) or Cryptococcus neoformans (9), express proteins that have been reported to bind to L-selectin.
Trypanosoma cruzi is the protozoan parasite causative of Chagas' disease, a chronic debilitating multisystemic disorder which affects several million people in South and Central America (34). The disease is a complex zoonosis, with mammals as natural reservoir hosts. Transmission is primarily by contact with the contaminated feces of domiciliated blood-sucking triatomine bugs. The parasite is taken in the blood meal of reduviid insects as a trypomastigote, which differentiates into the epimastigotes that multiply extracellularly in the midgut of the insect. In the hindgut, epimastigotes transform into infective nondividing metacyclic trypomastigotes, which are released in the feces. Metacyclic trypomastigotes are able to invade a wide variety of host mammalian cells, phagocytic and nonphagocytic (5, 6). Once inside the cells, the metacyclic forms escape from endocytic vacuoles to the cytoplasm, where they transform into amastigotes, which multiply intracellularly in a variety of mammalian cells. Upon rupture of host cells, the amastigotes differentiate into trypomastigotes that circulate in the blood until they encounter appropriate target cells, and they then go through another intracellular cycle or are taken up by the insect again. From a clinical point of view, the T. cruzi infections proceed in two phases. In the acute phase, circulating blood trypomastigotes are observed and there is a local inflammation at the sites of infection. During the chronic phase, circulating parasites cannot be observed by inspection of blood, but progressive tissue damage occurs, involving the esophagus, colon, and heart (34).
T. cruzi has evolved many sophisticated mechanisms to evade the immune response. Thus, several alterations of the immune response during T. cruzi infection have been described; these include severe immunosuppression in the acute phase, which is thought to facilitate dissemination and establishment of the parasite in the infected host (11). Different mechanisms mediated by various cell types, including T cells (21), adherent cells (26), and immature myeloid cells (14), have been ascribed to immunosuppression. Also, the presence of autoreactive T cells has been shown to be involved in the tissue damage observed in the chronic phase of the disease developed later on after T. cruzi infection (12).
Macrophages, which can be infected by T. cruzi, play a crucial role in the elimination of this parasite, especially in the early acute phase (11). T. cruzi enters initially as metacyclic forms to the body of the infected mammal, and recruitment of monocytes to the site of T. cruzi entry is thus thought to be key to controling the initial phases of the infection. However, there is little understanding of how T. cruzi evades immune cell recognition at sites of infection.
AgC10 is a mucin-like cell surface protein expressed by metacyclic trypomastigotes, the insect-infective form, as well as on amastigotes, the intracellular replicative form in the mammalian host (7). We have previously shown that AgC10 can be released from the parasite surface to the medium by the action of a phospholipase C and has the potential to inhibit macrophage function (1) and T-cell activation (2). More interestingly, AgC10 can bind to L-selectin in T cells (2) and macrophages (7). Therefore, we hypothesized that AgC10 could also interfere with L-selectin-mediated interactions with molecules expressed in the activated endothelium.
For the work described in this paper, we devised a system in which the human monocyte-like U937 cell line accumulation on immobilized P-selectin under physiological flow conditions is dependent on L-selectin expression. Using this system, we show that AgC10 directly purified from T. cruzi inhibits L-selectin-mediated adhesion of monocytes, most likely due to the induction of L-selectin shedding from the monocyte surface. Furthermore, the effect of AgC10 on monocyte L-selectin results in a significant decrease in monocyte adhesion to activated endothelium. This is the first description of a molecule from a parasite having inhibitory properties on cell trafficking and/or migration and represents a new mechanism used by parasites to evade the immune response.
MATERIALS AND METHODS
Cell culture and monocyte isolation.
Pooled human umbilical vein endothelial cells (HUVEC) were isolated and cultured as described previously (30). HUVEC monolayers (subculture 2) were allowed to reach confluence and were used in experiments 48 h later. Monocytes were isolated from whole blood obtained from healthy adult donors. Blood was drawn into citrate buffer anticoagulant and combined with an equal volume of 6% Dextran 70 solution (B. Braun, Bethlehem, PA). This mixture was layered over lymphocyte separation medium (1.078 g/ml; Cellgro, Manassas, VA) and centrifuged for 30 min at 400 × g. Following centrifugation, peripheral blood mononuclear cells (PBMCs) were obtained from the interface which formed between the lymphocyte separation medium and saline solution. Monocytes were negatively selected from the PBMCs using a MACS monocyte isolation kit II (Miltenyi Biotec, Germany). Purified monocytes were maintained in minimal essential medium (MEM) with 0.5% human serum albumin (HSA) at 8°C and used within 2 h following isolation. In this procedure, CD2 (BD Biosciences, San Jose, CA) and CD42b (GeneTex, San Antonio, TX) antibodies were necessary additions to the antibody cocktail to remove platelets and a small number of lymphocytes. The Human Use Committee Board of Brigham and Women's Hospital approved all protocols involving human subjects. U937 cells were obtained from American Type Culture Collection (ATCC), Manassas, VA. U937LAM (22) and U937 cells were cultured in complete RPMI medium as previously described.
Reagents.
Dulbecco's phosphate-buffered saline (DPBS) with or without Ca2+ and Mg2+ (DPBS+ or DPBS−, respectively) and M199 were purchased from BioWhittaker Bioproducts (Walkersville, MD). Recombinant human tumor necrosis factor alpha (TNF-α) was from Genzyme (Cambridge, MA). HSA (albuminate, 25%) was obtained from Baxter Healthcare (Glendale, CA).
Recombinant mouse P-selectin and vascular cell adhesion molecule 1 (VCAM-1) were purchased from R&D Systems. Blocking monoclonal antibody (MAb) for PSGL-1 (clone 5D8.8.12 or PL2) was purchased from Beckman Coulter (Fullerton, CA). Blocking L-selectin MAb LAM 1-4 was kindly provided by T. Tedder. Anti-CD62L (DREG-56) and anti-VLA4 (9C10) were purchased from BD Biosciences (San Jose, CA). Ro 31-9790 was purchased from Roche (Nutley, NJ) and used at 30 μM.
AgC10 purification.
AgC10 was purified as previously described (1, 7). Briefly, T. cruzi metacyclic trypomastigotes were washed and extracted twice with organic mixtures of chloroform/methanol followed by 1-butanol. Aqueous phases were lyophilized and resuspended in ammonium acetate containing propanol and loaded into an octyl Sepharose column. AgC10 was used at 20 μg/ml unless otherwise indicated.
Measurement of monocyte interactions with P-selectin and VCAM-1 under defined flow conditions.
Interactions of monocytes with P-selectin and VCAM-1-coated slides under defined laminar flow conditions in a parallel plate flow chamber were observed as previously described (13, 19). Cells were resuspended in DPBS containing 0.1% (vol/vol) bovine serum albumin (BSA) and 20 mM HEPES, pH 7.4, at 37°C (5 × 105 cells/ml), treated with AgC10 or MAbs where indicated, and perfused over human P-selectin- or VCAM-1-coated coverslips (10 μg/ml). Interactions were recorded with a phase-contrast objective (20×) and a videomicroscope connected to Videolab software (Ed Marcus Laboratories, Boston, MA) to record cell behavior. Accumulation of cells was determined after the initial minute of each flow rate by counting cells in five different fields.
Measurement of monocyte interactions with activated endothelium under defined flow conditions.
The assays were carried out using a parallel plate flow chamber as previously described (20). Briefly, confluent HUVEC monolayers, grown on 25-mm glass coverslips (Carolina Biological Supply, Burlington, NC) coated with 5 μg/ml fibronectin (Sigma, St Louis, MO) were stimulated with TNF-α (25 ng/ml) for 4 h and inserted into the flow chamber. U937 cells, U937LAM cells, or human primary monocytes (0.5 × 106/ml) suspended in flow buffer (PBS/0.1% human serum albumin) were treated with AgC10 or the LAM 1-4 MAb and drawn through the chamber at decreasing flow rates corresponding to an estimated shear stress of 1.5 dynes/cm2, 1.0 dyne/cm2, 0.76 dyne/cm2, and 0.5 dyne/cm2. Cell interactions were determined after the initial minute of each flow rate by counting the number of adherent cells in 4 to 10 different fields recorded using a videomicroscope.
Flow cytometry.
U937 cells, U937LAM cells, or human primary monocytes (1 × 106/ml) were treated with the indicated MAb for 20 min, followed by incubation with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody. Cell surface expression was analyzed using a FACSCalibur (Becton Dickinson).
Statistical analysis.
All results are expressed as the means ± standard deviations (SD) unless otherwise stated. Statistical analyses by ANOVA followed by the Newman Keuls test were performed with GraphPad Prism software and were considered statistically significant at a P value of ≤0.05. Data are the means from at least three different experiments unless otherwise indicated.
RESULTS
L-selectin facilitates monocyte-like U937LAM cell adhesion to P-selectin under flow conditions.
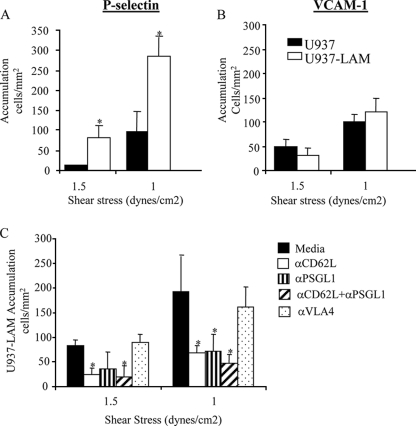
To our knowledge, there are no commercially available L-selectin ligands expressed on high endothelial venules (HEV) that mediate rolling and adhesion in vivo. GlyCAM-1 is the only L-selectin ligand found to support rolling interactions of leukocytes in vitro, but since it is not expressed on the surface of HEV, its physiological role in vivo is not clear (33). Therefore, we have devised a system where we use monocyte-like U937 cells, which constitutively express α4β1 (VLA-4) and PSGL-1 but lack L-selectin, and a second U937 cell stably transfected with human L-selectin (U937LAM) that has been described previously (22). We have tested their ability to bind to physiological adhesion molecules under shear flow conditions. When tested in parallel, more U937LAM cells accumulated on P-selectin in comparison with U937 cells at two different shear stresses tested (Fig. 1A). On the other hand, the levels of accumulation of U937LAM and U937 on VCAM-1 were similar (Fig. 1B). The increased binding to P-selectin observed for U937LAM was dependent on PSGL-1, as expected, but was also L-selectin mediated, since a decrease in binding of U937LAM to P-selectin was observed when these molecules were blocked individually or in combination by the corresponding function-blocking antibodies (Fig. 1C). In contrast, blocking of VLA-4 had no effect, indicating that VLA-4 is not involved in the accumulation of U937LAM on P-selectin. These data confirm the relevance and dependence of L-selectin in our system.
FIG. 1.
U937LAM monocytes use L-selectin to interact with P-selectin under shear flow conditions. Glass coverslips were coated with P-selectin (A and C) or VCAM-1 (B) as described in Materials and Methods, and U937 or U937LAM cells were loaded in the flow chamber at two different shear stresses at 37°C. *, P < 0.01 for U937LAM versus U937. (C) Cells were preincubated with the indicated MAbs (20 μg/ml) for 30 minutes at room temperature before the flow adhesion assay. *, P < 0.01 versus medium.
AgC10 inhibits L-selectin-dependent U937 monocyte adhesion to P-selectin under flow conditions.
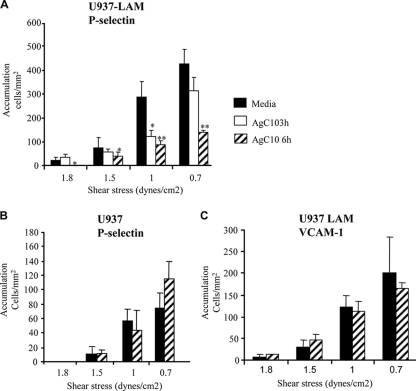
AgC10 binds L-selectin in macrophages; thus, the effect of AgC10 in L-selectin-mediated leukocyte adhesion can now easily be tested using the above system. To evaluate this hypothesis, both U937 and U937LAM monocytes were treated with AgC10, and their binding ability to P-selectin and VCAM-1 was studied under flow conditions. Treatment of U937LAM with AgC10 inhibited adhesion to P-selectin across a broad range of shear stresses tested. This effect was more pronounced after longer times of incubation of cells with AgC10 (Fig. 2A). In contrast, AgC10 did not have any effect on adhesion of U937 cells to P-selectin, suggesting that AgC10 was exerting its inhibitory effect by specifically binding to L-selectin or regulating its expression at the cell surface (Fig. 2B). Moreover, treatment of U937LAM with AgC10 had no effect on adhesion to VCAM-1 (Fig. 2C), further confirming that the observed effect is L-selectin dependent and specific, since as shown in Fig. 1, binding of U937LAM to VCAM-1 is independent of L-selectin expression.
FIG. 2.
AgC10 inhibits L-selectin-dependent monocyte-like U937LAM adhesion to P-selectin under flow conditions. U937LAM (A and C) or U937 (B) cells were incubated with AgC10 (20 μg/ml) for the indicated times at 37°C previous to the flow adhesion study with coverslips coated with P-selectin (A and B) or VCAM-1 (C) at the indicated shear stresses. Data are means ± SD for four different experiments. * indicates a P value of <0.05 and ** indicates a P value of <0.01 for AgC10 versus medium.
AgC10 inhibits L-selectin-dependent U937 monocyte adhesion to P-selectin by inducing L-selectin shedding.
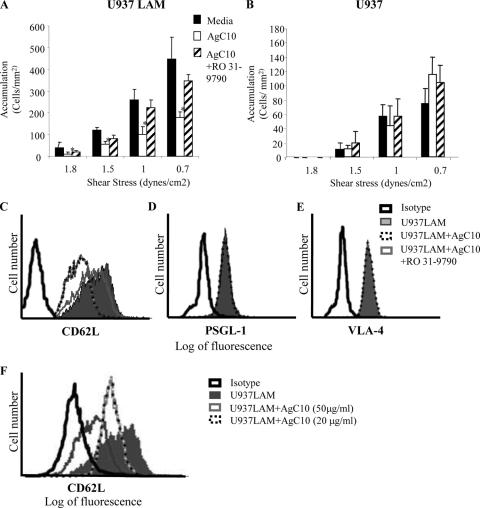
L-selectin is rapidly shed from the cell surface upon cellular activation (15, 31). The fact that the inhibitory effect of AgC10 on adhesion of U937LAM cells was more pronounced after longer times of incubation (Fig. 2) led us to hypothesize that AgC10 was binding to L-selectin, inducing its shedding. To address this question, U937LAM cells were treated with AgC10 in the presence or absence of the L-selectin shedding inhibitor Ro 31-9790 (3) and assayed for adhesion to P-selectin. As shown in Fig. 3, pretreatment of U937LAM with Ro 31-9790 prevented L-selectin shedding in the presence of a high dose (50 μg/ml) of AgC10 (Fig. 3C) and preserved adhesion to P-selectin (Fig. 3A). In contrast, Ro 31-9790 treatment did not have any effect on U937 adhesion (Fig. 3B). AgC10 did not affect PSGL-1 or VLA-4 expression in U937LAM cells (Fig. 3D and E). On the other hand, the effect of AgC10 on L-selectin surface expression was detectable at 20 μg/ml and was dose dependent (Fig. 3F). These results indicate that the L-selectin shedding inhibitor is not affecting monocyte binding through other pathways and supports the conclusion that the effect of AgC10 is due to promotion of L-selectin shedding from the surface of U937LAM cells (Fig. 3B).
FIG. 3.
AgC10 inhibits L-selectin-dependent monocyte-like U937LAM adhesion to P-selectin by inducing L-selectin shedding. U937LAM (A and C to F) or U937 (B) cells were treated with AgC10 (20 μg/ml [A and B] and 50 μg/ml [C to E]) for 6 h at 37°C in the presence or absence of the L-selectin shedding inhibitor Ro 31-9790 (30 μM). (A and B) After the corresponding treatments, cells were inserted in the flow chamber at the indicated shear stresses, and adhesion to P-selectin was quantified. Data are the means from three experiments. *, P < 0.01 for AgC10 versus medium. (C to F) FACS staining of U937LAM cells. After the indicated treatments, cells were stained with specific MAbs to the indicated surface proteins for 20 min, fixed in 1% paraformaldehyde, and analyzed by flow cytometry.
AgC10 inhibits monocyte cell adhesion on TNF-α-activated endothelium via L-selectin.
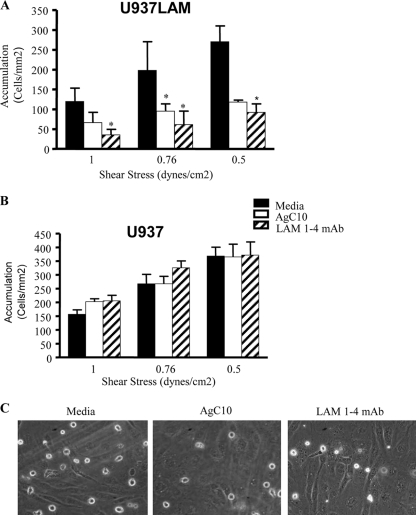
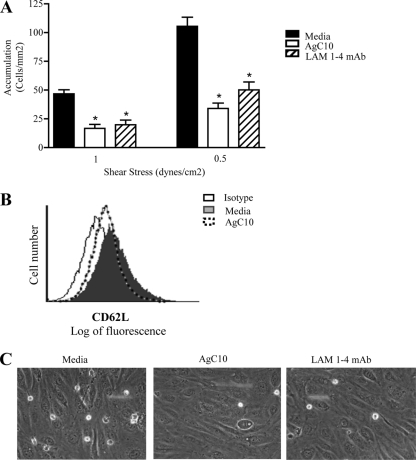
To further investigate the inhibitory effect of AgC10 on L-selectin-mediated adhesion, we examined U937LAM cell adhesion to vascular endothelial cells. TNF-α-activated HUVEC have been reported to express E-selectin, P-selectin, ICAM-1, VCAM-1, and L-selectin ligands (36). Indeed, we found that upon AgC10 treatment, U937LAM binding was significantly inhibited, and the reduction was comparable to that observed when U937LAM cells were treated with the blocking L-selectin antibody LAM 1-4 MAb (Fig. 4A and C). Neither AgC10 nor LAM 1-4 MAb had any effect on adhesion of U937 to TNF-α-treated HUVEC (Fig. 4B), suggesting again that the observed decreased adhesion is related to diminished expression of L-selectin (see Videos S1 [medium], S2 [AgC10], and S3 [LAM 1-4 MAb] in the supplemental data). To corroborate these results, we used human primary monocytes isolated directly from healthy donors and performed similar experiments. We hypothesized that AgC10 is encountering circulating monocytes in the bloodstream, and in a short period of time, this is where AgC10 is binding to L-selectin and may be inducing its shedding, further resulting in decreased adhesion to high endothelial venules (HEV) and recruitment into the infected tissue. Monocytes can rapidly shed L-selectin when activated or incubated at 37°C (22); therefore, we treated the monocytes for 10 min with medium, AgC10, or LAM 1-4 MAb before doing the experiments under flow conditions. The shear stresses tested are adequate for primary monocytes to bind to activated endothelium in our experimental model (22). At both shear stresses tested, AgC10 treatment resulted in a significant inhibition of monocyte binding to activated HUVEC, and this effect was comparable to that seen when monocytes were treated with LAM 1-4 MAb, which also resulted in decreased monocyte adhesion, in agreement with previous results (22) (Fig. 5A). (See Video S4 [medium], S5 [AgC10], and S6 [LAM 1-4 MAb] in the supplemental data). Monocyte-monocyte interactions via L-selectin/PSGL-1 that normally take place under flow conditions at 1.5 dynes/cm2 of shear stress also were not observed when cells were treated with AgC10 (data not shown), corroborating the effect of AgC10 in L-selectin-mediated adhesion. L-selectin loss from the monocyte surface was observed by fluorescence-activated cell sorter (FACS) staining of monocytes treated with AgC10, LAM 1-4, or medium for 10 min at 37°C (Fig. 5B).
FIG. 4.
AgC10 inhibits L-selectin-dependent monocyte-like U937LAM accumulation on activated endothelium. U937LAM (A and C) or U937 (B) cells were treated with AgC10 (20 μg/ml) for 6 h at 37°C or with the L-selectin-blocking MAb LAM 1-4 (15 μg/ml) for 30 min at 37°C. After the corresponding treatments, cells were inserted in the flow chamber at the indicated shear stresses to test monocyte adhesion to activated HUVEC (stimulated 4 h with 25 ng/ml of TNF-α). Data are the means from three experiments. *, P < 0.01 for AgC10 or LAM 1-4 versus medium. (C) Photographs captured from videos imaged at 0.76 dyne/cm2 are representative of three independent experiments with similar results. (See also supplemental video S1 for medium, supplemental video S2 for AgC10, and supplemental video S3 for LAM 1-4 MAb.)
FIG. 5.
AgC10 inhibits L-selectin-dependent accumulation of human primary monocytes on activated endothelium. (A) Monocytes isolated from whole blood (as described in Materials and Methods) were treated with AgC10 (20 μg/ml) or the L-selectin-blocking MAb LAM 1-4 (15 μg/ml) for 10 min at 37°C. After the corresponding treatments, cells were inserted in the flow chamber at the indicated shear stresses to test monocyte adhesion to activated HUVEC (stimulated 4 h with 25 ng/ml of TNF-α). Data are the means from two experiments. *, P < 0.01 for AgC10 or LAM 1-4 versus medium. (B) Monocytes treated as in panel A were stained with a specific MAb to L-selectin for 20 min, fixed in 1% paraformaldehyde, and analyzed by flow cytometry. (C) Photographs captured from videos imaged at 1 dyne/cm2 are representative of results of two independent experiments. (See also supplemental video S4 for medium, supplemental video S5 for AgC10, and supplemental video S6 for LAM 1-4 MAb.)
DISCUSSION
We have previously shown that AgC10, a mucin expressed by infective metacyclic trypomastigotes and amastigotes, is released from the membrane to the medium during infection and binds to L-selectin in human macrophages and mouse and human T cells (2, 7). This results in an inhibition of T-cell and macrophage activation. L-selectin is involved in the initial steps of leukocyte recruitment on the vascular endothelium (25). However, whether AgC10 could interfere with L-selectin-dependent leukocyte adhesion to activated endothelium remained unclear. To address this question, we used an in vitro system to compare the adhesion to activated endothelium and to the inducible adhesion molecules that are expressed by activated endothelium, including P-selectin and VCAM-1, of two monocyte cell lines that differ only in their expression of L-selectin: U937 cells, which do not express L-selectin, and U937LAM cells, which were stably transduced with human L-selectin. GlyCAM-1 is the only described L-selectin ligand that supports rolling interactions of leukocytes in vitro, but since it is not expressed on the surface of HEV, its physiological role in vivo is still unclear (33). Interestingly, in our system, monocytic U937LAM cell accumulation under flow was highly dependent on L-selectin, since U937LAM cells showed that their adhesion to P-selectin, but not to VCAM-1, was abrogated by an L-selectin-blocking MAb. Prior studies have demonstrated that leukocyte-expressing L-selectin recognizes P-selectin and that this pathway supports adhesion under flow conditions (4, 32).
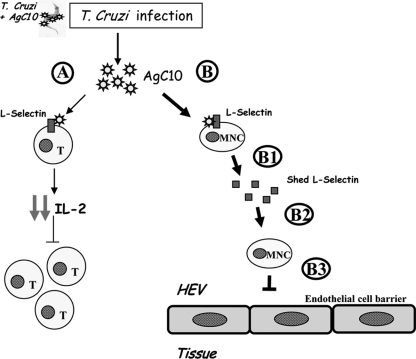
Our results show that the T. cruzi mucin, AgC10, specifically inhibited adhesion of U397LAM and not U937 to immobilized P-selectin, and this was reproducible in a second model of TNF-α-activated HUVEC. In both assays, adhesion of U937LAM treated with AgC10 was reduced to the same extent as cells treated with a blocking MAb to L-selectin. Interestingly, binding of U937LAM to P-selectin was preserved when L-selectin shedding was inhibited by RO 31-9790, indicating that AgC10 not only can bind to L-selectin, as previously described for T cells and macrophages (2, 7), but also can induce its shedding. More important, and physiologically relevant, is the fact that these results were corroborated with human primary monocytes which, upon T. cruzi infection, can encounter circulating AgC10 in the bloodstream that binds to L-selectin, affecting their recruitment to the tissue. Monocytes can rapidly shed L-selectin when activated, so when encountering AgC10, monocytes could also shed L-selectin in a direct or indirect way mediated by AgC10 occupancy of surface L-selectin (Fig. 6). This finding suggests that AgC10 directly interferes with the adhesive function of L-selectin. The facts that regulation of cell surface expression of L-selectin involves its shedding from the cell surface (15, 17, 31) and that the AgC10 inhibitory effect on U937LAM in L-selectin-mediated adhesion is increased after longer periods of incubation supported the idea that AgC10 could modulate the surface expression of L-selectin. In fact, the decreased surface expression of L-selectin upon AgC10 treatment is prevented when cell were cultured in the presence of an inhibitor of L-selectin shedding. Longer periods of incubation with AgC10 could not be tested when using human primary monocytes due to the rapid loss of L-selectin that monocytes undergo when activated at 37°C (31). This supports the idea that the effect of AgC10 in monocyte recruitment can be mediated by direct binding of AgC10 to monocytes (Fig. 6B) as well as by inducing L-selectin shedding (Fig. 6B, B1).
FIG. 6.
Model by which AgC10 interferes with L-selectin-mediated adhesion. Upon infection, T. cruzi can release AgC10 that is bound to its surface. Released AgC10 can bind to L-selectin in T cells (A), resulting in decreased interleukin-2 (IL-2) production and consequent T-cell proliferation (2). (B) Interestingly, AgC10 can bind to L-selectin in monocytes (MNC) and, at the same time, induce L-selectin shedding (B1). The monocytes lacking L-selectin at the cell surface (B2) cannot fully interact with activated endothelium in the high endothelial venules (HEV) (B3). This results in loss of monocyte recruitment into the tissues. This model represents a new mechanism used by T. cruzi to evade the immune response.
Interestingly, L-selectin was shown to be required for leukocyte extravasation migrating from the bloodstream to the sites of infection by Leishmania, another related protozoan parasite (18). On the other hand, the mechanism of action of AgC10 resembles that induced by polysaccharides from Cryptoccocus neoformans (8, 9), for which it has been reported that the capsular glucuronoxylomannan (GXM) or galactoxylomannan (GalXM) is able to inhibit T-cell activation. Moreover, this capsular material is released to the medium during the infection, and it causes L-selectin shedding from the leukocyte surface. In this regard, these polysaccharides strongly resemble AgC10, which shares with them all those properties. Nonetheless, we have gone further to show that this mimicry has functional consequences, reducing monocyte adhesion under flow conditions. This is the first description of such a mechanism by a pathogen-derived product. AgC10 is a highly sialylated O glycoprotein (mucin). Gly-CAM-1, the only known ligand of L-selectin, is also sialylated. Thus, this similarity may allow AgC10 to bind carbohydrate structures of L-selectin.
The molecular mechanism by which AgC10 induces L-selectin shedding from the cell surface of U937LAM cells is presently unknown. However, we found that AgC10 binds to L-selectin in macrophages (7) and T cells (2), affecting their activation by inhibiting events proximal to TcR triggering, including tyrosine phosphorylation in T cells and mitogen-activated protein kinase p38 activation in macrophages. L-selectin can be shed from the cell surface by activation of some T-cell surface molecules, such as CD4, via activation of metalloproteases as has been described for HIV-1-infected cells. Thus, ligation of CD4 by T-cell-tropic HIV-1 NL4-3 induces metalloproteinase-dependent L-selectin (CD62L) shedding on resting CD4+ T cells (23). Thus, it is possible that AgC10 acts by a similar mechanism by inducing a protease in monocytes.
In summary, this is the first description of a molecule from T. cruzi or any other parasite having inhibitory properties on cell trafficking, and it adds a new potential mechanism used by T. cruzi to evade the immune response.
Supplementary Material
Acknowledgments
This work was supported by grants from Fulbright-MECD to P.A.; National Institutes of Health to F.W.L. (HL53993 and HL36028) and P.A. (HL094706); and FIS (PI040993), Ministerio de Ciencia e Innovacion (SAF2005-02220 and SAF2007-61716), European Union (Eicosanox and ChagasEpiNet) CSIC-CONICET, BSCH/UAM, and Comunidad de Madrid (S-SAL-0159/2006, RED RECAVA RD06/0014/1013 and RED RICET RD06/0021/0016) to M.F.
Editor: J. H. Adams
Footnotes
Published ahead of print on 11 January 2010.
Supplemental data for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Alcaide, P., and M. Fresno. 2004. AgC10, a mucin from Trypanosoma cruzi, destabilizes TNF and cyclooxygenase-2 mRNA by inhibiting mitogen-activated protein kinase p38. Eur. J. Immunol. 34:1695-1704. [DOI] [PubMed] [Google Scholar]
- 2.Alcaide, P., and M. Fresno. 2004. The Trypanosoma cruzi membrane mucin AgC10 inhibits T cell activation and IL-2 transcription through L-selectin. Int. Immunol. 16:1365-1375. [DOI] [PubMed] [Google Scholar]
- 3.Allport, J. R., H. T. Ding, A. Ager, D. A. Steeber, T. F. Tedder, and F. W. Luscinskas. 1997. L-selectin shedding does not regulate human neutrophil attachment, rolling, or transmigration across human vascular endothelium in vitro. J. Immunol. 158:4365-4372. [PubMed] [Google Scholar]
- 4.Alon, R., R. C. Fuhlbrigge, E. B. Finger, and T. A. Springer. 1996. Interactions through L-selectin between leukocytes and adherent leukocytes nucleate rolling adhesions on selectins and VCAM-1 in shear flow. J. Cell Biol. 135:849-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burleigh, B. A., and N. W. Andrews. 1995. The mechanisms of Trypanosoma cruzi invasion of mammalian cells. Annu. Rev. Microbiol. 49:175-200. [DOI] [PubMed] [Google Scholar]
- 6.Burleigh, B. A., and A. M. Woolsey. 2002. Cell signalling and Trypanosoma cruzi invasion. Cell. Microbiol. 4:701-711. [DOI] [PubMed] [Google Scholar]
- 7.de Diego, J., C. Punzon, M. Duarte, and M. Fresno. 1997. Alteration of macrophage function by a Trypanosoma cruzi membrane mucin. J. Immunol. 159:4983-4989. [PubMed] [Google Scholar]
- 8.Ellerbroek, P. M., D. J. Lefeber, R. van Veghel, J. Scharringa, E. Brouwer, G. J. Gerwig, G. Janbon, A. I. Hoepelman, and F. E. Coenjaerts. 2004. O-acetylation of cryptococcal capsular glucuronoxylomannan is essential for interference with neutrophil migration. J. Immunol. 173:7513-7520. [DOI] [PubMed] [Google Scholar]
- 9.Ellerbroek, P. M., L. H. Ulfman, A. I. Hoepelman, and F. E. Coenjaerts. 2004. Cryptococcal glucuronoxylomannan interferes with neutrophil rolling on the endothelium. Cell. Microbiol. 6:581-592. [DOI] [PubMed] [Google Scholar]
- 10.Gallatin, W. M., I. L. Weissman, and E. C. Butcher. 1983. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature 304:30-34. [DOI] [PubMed] [Google Scholar]
- 11.Girones, N., H. Cuervo, and M. Fresno. 2005. Trypanosoma cruzi-induced molecular mimicry and Chagas' disease. Curr. Top. Microbiol. Immunol. 296:89-123. [DOI] [PubMed] [Google Scholar]
- 12.Girones, N., C. I. Rodriguez, E. Carrasco-Marin, R. F. Hernaez, J. L. de Rego, and M. Fresno. 2001. Dominant T- and B-cell epitopes in an autoantigen linked to Chagas' disease. J. Clin. Invest. 107:985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goetz, D. J., H. Ding, W. J. Atkinson, G. Vachino, R. T. Camphausen, D. A. Cumming, and F. W. Luscinskas. 1996. A human colon carcinoma cell line exhibits adhesive interactions with P-selectin under fluid flow via a PSGL-1-independent mechanism. Am. J. Pathol. 149:1661-1673. [PMC free article] [PubMed] [Google Scholar]
- 14.Goni, O., P. Alcaide, and M. Fresno. 2002. Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1(+))CD11b(+)immature myeloid suppressor cells. Int. Immunol. 14:1125-1134. [DOI] [PubMed] [Google Scholar]
- 15.Jung, T. M., and M. O. Dailey. 1990. Rapid modulation of homing receptors (gp90MEL-14) induced by activators of protein kinase C. Receptor shedding due to accelerated proteolytic cleavage at the cell surface. J. Immunol. 144:3130-3136. [PubMed] [Google Scholar]
- 16.Kansas, G. S. 1996. Selectins and their ligands: current concepts and controversies. Blood 88:3259-3287. [PubMed] [Google Scholar]
- 17.Kishimoto, T. K., M. A. Jutila, E. L. Berg, and E. C. Butcher. 1989. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science 245:1238-1241. [DOI] [PubMed] [Google Scholar]
- 18.Leon, B., and C. Ardavin. 2008. Monocyte migration to inflamed skin and lymph nodes is differentially controlled by L-selectin and PSGL-1. Blood 111:3126-3130. [DOI] [PubMed] [Google Scholar]
- 19.Lim, Y. C., L. Henault, A. J. Wagers, G. S. Kansas, F. W. Luscinskas, and A. H. Lichtman. 1999. Expression of functional selectin ligands on Th cells is differentially regulated by IL-12 and IL-4. J. Immunol. 162:3193-3201. [PubMed] [Google Scholar]
- 20.Lim, Y. C., M. W. Wakelin, L. Henault, D. J. Goetz, T. Yednock, C. Cabanas, F. Sanchez-Madrid, A. H. Lichtman, and F. W. Luscinskas. 2000. Alpha4beta1-integrin activation is necessary for high-efficiency T-cell subset interactions with VCAM-1 under flow. Microcirculation 7:201-214. [PubMed] [Google Scholar]
- 21.Lopes, M. F., and G. A. dos Reis. 1994. Trypanosoma cruzi-induced immunosuppression: blockade of costimulatory T-cell responses in infected hosts due to defective T-cell receptor-CD3 functioning. Infect. Immun. 62:1484-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luscinskas, F. W., G. S. Kansas, H. Ding, P. Pizcueta, B. E. Schleiffenbaum, T. F. Tedder, and M. A. Gimbrone, Jr. 1994. Monocyte rolling, arrest and spreading on IL-4-activated vascular endothelium under flow is mediated via sequential action of L-selectin, beta 1-integrins, and beta 2-integrins. J. Cell Biol. 125:1417-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marschner, S., B. A. Freiberg, A. Kupfer, T. Hunig, and T. H. Finkel. 1999. Ligation of the CD4 receptor induces activation-independent down-regulation of L-selectin. Proc. Natl. Acad. Sci. U. S. A. 96:9763-9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McEver, R. P. 2002. P-selectin and PSGL-1: exploiting connections between inflammation and venous thrombosis. Thromb. Haemost. 87:364-365. [PubMed] [Google Scholar]
- 25.McEver, R. P. 1995. Regulation of function and expression of P-selectin. Agents Actions Suppl. 47:117-119. [DOI] [PubMed] [Google Scholar]
- 26.Motran, C., A. Gruppi, C. M. Vullo, M. C. Pistoresi-Palencia, and H. M. Serra. 1996. Involvement of accessory cells in the Trypanosoma cruzi-induced inhibition of the polyclonal response of T lymphocytes. Parasite Immunol. 18:43-48. [DOI] [PubMed] [Google Scholar]
- 27.Patel, K. D., S. L. Cuvelier, and S. Wiehler. 2002. Selectins: critical mediators of leukocyte recruitment. Semin. Immunol. 14:73-81. [DOI] [PubMed] [Google Scholar]
- 28.Rosen, S. D. 2004. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 22:129-156. [DOI] [PubMed] [Google Scholar]
- 29.Serbina, N. V., T. Jia, T. M. Hohl, and E. G. Pamer. 2008. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 26:421-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw, S. K., P. S. Bamba, B. N. Perkins, and F. W. Luscinskas. 2001. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J. Immunol. 167:2323-2330. [DOI] [PubMed] [Google Scholar]
- 31.Smalley, D. M., and K. Ley. 2005. L-selectin: mechanisms and physiological significance of ectodomain cleavage. J. Cell. Mol. Med. 9:255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spertini, O., A. S. Cordey, N. Monai, L. Giuffre, and M. Schapira. 1996. P-selectin glycoprotein ligand 1 is a ligand for L-selectin on neutrophils, monocytes, and CD34+ hematopoietic progenitor cells. J. Cell Biol. 135:523-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tangemann, K., A. Bistrup, S. Hemmerich, and S. D. Rosen. 1999. Sulfation of a high endothelial venule-expressed ligand for L-selectin. Effects on tethering and rolling of lymphocytes. J. Exp. Med. 190:935-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanowitz, H. B., L. V. Kirchhoff, D. Simon, S. A. Morris, L. M. Weiss, and M. Wittner. 1992. Chagas' disease. Clin. Microbiol. Rev. 5:400-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tedder, T. F., D. A. Steeber, A. Chen, and P. Engel. 1995. The selectins: vascular adhesion molecules. FASEB J. 9:866-873. [PubMed] [Google Scholar]
- 36.Thornhill, M. H., and D. O. Haskard. 1990. IL-4 regulates endothelial cell activation by IL-1, tumor necrosis factor, or IFN-gamma. J. Immunol. 145:865-872. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.