Abstract
Mycobacterium avium subsp. paratuberculosis (basonym M. paratuberculosis) is the causative agent of paratuberculosis, a chronic enteritis of ruminants. To control the considerable economic effect that paratuberculosis has on the livestock industry, a vaccine that induces protection with minimal side effects is required. We employed transposon mutagenesis and allelic exchange to develop three potential vaccine candidates, which were then tested for virulence with macrophages, mice, and goats. All three models identified the WAg906 mutant as being the most attenuated, but some differences in the levels of attenuation were evident among the models when testing the other strains. In a preliminary mouse vaccine experiment, limited protection was induced by WAg915, as evidenced by a reduced bacterial load in spleens and livers 12 weeks following intraperitoneal challenge with M. paratuberculosis K10. While we found macrophages and murine models to be rapid and cost-effective alternatives for the initial screening of M. paratuberculosis mutants for attenuation, it appears necessary to do the definitive assessment of attenuation with a ruminant model.
Paratuberculosis, or Johne's disease, is caused by Mycobacterium avium subsp. paratuberculosis (basonym M. paratuberculosis). The disease is transmitted via the fecal-oral route and causes chronic granulomatous enteritis in ruminants. Infection normally occurs very early in life, but clinical disease in cattle rarely manifests itself until animals are more than 2 years old, and often much older. In most herds, only a minority of the infected animals ever becomes clinically diseased, but subclinically affected animals can shed organisms and act as a source of infection. The clinical stages in cattle are characterized by diarrhea, weight loss, reduced milk production, and the presence of large numbers of bacteria shed in the feces (33, 52). Considerable economic losses occur worldwide due to this reduced milk production and to premature disposal of diseased animals (42). There is also concern that M. paratuberculosis may be a cause of Crohn's disease in humans.
Vaccines for paratuberculosis have been available since 1926, but they induce suboptimal levels of protection and have adverse side effects. Substantial granulomatous lesions often occur at the site of inoculation in animals (37) and in accidental self-injection cases with humans (51). The vaccines also interfere with current cellular immune assays for bovine tuberculosis (25, 30), restricting their potential use in many countries. However, an aqueous live attenuated M. paratuberculosis vaccine has been shown to cause less interference with a comparative cervical skin test and blood tests for tuberculosis than do the currently available oil-adjuvanted Johne's disease vaccines (30).
The advances of mycobacterial molecular biology and genome sequencing in the last 15 years have enabled programs aimed at developing new, improved M. paratuberculosis vaccines. Live attenuated strains of M. paratuberculosis with vaccine potential have been produced by transposon mutagenesis (8) and, more recently, by allelic exchange (34).
Various animal models of paratuberculosis have been investigated for the study of virulence and vaccine efficacy, but none appears ideal (5, 20). Mice are cost effective, present some histological and immunological features similar to those of ruminants, and can serve as a preliminary screening model for vaccine candidates (39, 41), but they do not develop disease typical of that seen with ruminants with paratuberculosis (9, 41). In comparison, goats are much more costly but provide a direct homologue of the disease in cattle and other ruminants, which is beneficial for pathogenesis and vaccine efficacy studies (21, 32).
In infected animals, M. paratuberculosis resides and multiplies within macrophages. Its survival depends on the balance between apoptosis and the ability of M. paratuberculosis to multiply and evade the host's immune response. Several studies have reported apoptosis of M. paratuberculosis-infected macrophages (1, 16, 45, 46), although the mechanisms involved have not been extensively investigated. A major extrinsic mediator of apoptosis is tumor necrosis factor alpha (TNF-α). The anti-inflammatory cytokine interleukin-10 (IL-10) is responsible for the suppression of various cytokines, including TNF-α, and thus has downstream effects on apoptosis. Elevated levels of IL-10 have been found in cows with clinical paratuberculosis (23) and with M. paratuberculosis-infected macrophages (45, 47). IL-10 acts to promote the growth of M. paratuberculosis within macrophages (45) by suppressing the inflammatory immune responses (7) and decreasing phagosome acidification (52).
In this study, we produced and characterized allelic exchange and transposon mutants of M. paratuberculosis and investigated their survival and the levels of apoptosis and IL-10 production in macrophages infected with the strains. The virulence of three of the vaccine candidates was assessed in mice and goats, and a preliminary investigation was carried out with mice to assess their vaccine potential.
MATERIALS AND METHODS
Bacterial strains.
M. paratuberculosis strains used in this study are listed in Table 1. The wild-type strain K10, whose genome has been sequenced (28), and the New Zealand cattle strain 989 (11) were used to develop M. paratuberculosis recombinants. Both wild-type and mutant strains of M. paratuberculosis were grown in Middlebrook 7H9 (Difco) broth enriched with 10% oleic acid-albumin-dextrose-catalase (OADC), 0.5% glycerol, 0.05% Tween 80, and 2 μg/ml mycobactin (Allied Monitor). When required, hygromycin B (50 μg/ml) or kanamycin (20 μg/ml) was added to liquid or solid media.
TABLE 1.
M. paratuberculosis strains used in this study
| M. paratuberculosis strain | Source | Reference |
|---|---|---|
| K10 | Cattle, Minnesota | 28 |
| 989 | Cattle, New Zealand | 8 |
| WAg906 | Transposon mutant of 989 | 8a |
| WAg913 | Transposon mutant of 989 | This study |
| WAg915 | Allelic exchange mutant of K10 | This study |
WAg906 was designated TM58 in that study.
M. paratuberculosis strains WAg906, which is an auxotroph, and WAg913, which grows on glycerol but not pyruvate as a carbon source, were developed by transposon mutagenesis and screened on appropriate media as described previously by Cavaignac et al. (8). WAg906 was designated TM58 in that paper, and WAg913 was developed in a fashion identical to that for mutants in that study that grew with glycerol as a carbon source. WAg915, which contains an inactivated ppiA gene, was derived from parent M. paratuberculosis strain K10 by allelic exchange, using methods described by Bardarov et al. (2). Briefly, a hygromycin resistance gene was inserted into the StyI site of a 1.2-kb fragment of M. paratuberculosis DNA containing the 0.55-kb ppiA gene and flanking regions. This construct was cloned into pYUB572, a plasmid designed for phagemid construction. The pYUB572 construct was inserted into the temperature-sensitive phage pHAE159 and transduced into K10. Hygromycin-resistant colonies were selected at the nonpermissive temperature for the phagemid of 39°C, and the expected allelic exchange mutant was characterized by PCR and Southern blotting.
Animals.
BALB/c mice of 4 to 7 weeks of age bred within the institution were used for testing both virulence and vaccine potential. Recently weaned Boer goats were obtained from two properties where there was no evidence of infection with M. paratuberculosis, based on flock history and fecal culturing. The animals were housed and fed ad libitum on hay and sheep nuts. Blood was collected from healthy, rising 1-year-old steers from a research herd with no history of paratuberculosis. The steers had negative fecal culture and negative gamma interferon (IFN-γ) tests to Johnin and avian purified protein derivative (PPD) (AsureQuality, New Zealand). Animal ethics approval for all animal experiments was obtained from a local institutional ethics committee.
Preparation of bovine blood-derived macrophages and infection.
Blood was collected from the jugular vein of cattle in heparinized Vacutainer tubes (Becton Dickinson), and peripheral blood mononuclear cells (PBMC) were isolated with Lymphoprep (Axis-Shield) as previously described (38). Briefly, equal volumes of blood were mixed with phosphate-buffered saline (PBS), and 35 ml blood-PBS was underlaid with 15 ml lymphoprep and centrifuged. PBMC were removed and washed three times. Approximately 5 × 105 PBMC were plated into each well of 96-well tissue culture plates in RPMI 1640 (Invitrogen) containing 25 mM HEPES (Invitrogen) supplemented with 2% fetal bovine serum (Invitrogen) and 4 mM l-glutamine (Invitrogen) for 3 h at 37°C in 5% CO2. Nonadherent cells were removed, and the adhered cells were washed twice. Monocytes were replenished and cultured with supplemented RPMI 1640 media containing 25 mM HEPES, 10% heat-inactivated fetal bovine serum, and 4 mM l-glutamine. The cells were incubated for 5 days to allow for the differentiation of macrophages.
Differentiated macrophages were infected on day 5 at a multiplicity of infection of 5:1 for 4 h. Exogenous bacteria were removed, and macrophages were washed. This was defined as the day zero time point. Macrophages were maintained in culture for various periods of time prior to the assessment of bacterial survival. Macrophages were lysed with 0.1% Triton-X (Sigma), and bacterial counts were obtained by plating the lysates at appropriate dilutions onto quad plates (Becton Dickinson) containing 7H11 agar (Difco) supplemented with 10% OADC, 0.5% glycerol, 0.05% Tween 80, and 2 μg/ml mycobactin.
Apoptosis.
Bovine monocyte-derived macrophages were grown and infected in a chamber slide (Nunc) as described above. After 24 h of infection, the supernatant was removed and used for IL-10 enzyme-linked immunosorbent assay (ELISA) studies, and the remaining cells were fixed with 4% paraformaldehyde at room temperature for 20 min. After the slide was washed with PBS, 5 μg/ml Hoechst stain (Invitrogen) was added for 10 min. The slide was washed with PBS and examined by a fluorescence microscope (Olympus) with excitation at 350 nm and emission at 461 nm. At least 200 cells were counted in 4 random fields of view of each duplicate well on the slide, and the percentage of fluorescence was calculated. Staurosporine was added at 500 nM to some wells as a positive control, and uninfected cells were also examined for fluorescence.
IL-10 ELISA.
Maxisorp 96-flat-well, black plates (Nunc) were coated with anti-bovine IL-10 (Serotec) and incubated overnight at room temperature. Plates were blocked with 1 mg/ml casein in PBS, washed, incubated with 50 μl undiluted culture supernatants collected 24 h after infection, and washed again. The detection monoclonal antibody CC320-biotin (Serotec) was added at 1 μg/ml, and horseradish peroxidase-conjugated streptavidin was added at a 1:2,500 dilution for 1 h at room temperature. Following the final washing step, tetramethylbenzidine substrate was added, the reaction was stopped by the addition of H2SO4, and the absorbance values were read on a microtiter plate reader. Macrophages pretreated with 100 U IFN-γ and 10 μg lipopolysaccharide were used as a positive control.
Real-time PCR.
After infection of macrophages with a M. paratuberculosis strain for 24 h, total RNA was extracted using Trizol and converted into first-strand cDNA using Transcriptor (Roche). The IL-10 primers used for the real-time PCR were TGCTGGATGACTTTAAGGGTTACC and TCATTTCCGACAAGGCTTGG, and the primers for the B2 microglobulin housekeeping gene were TTACCTGAACTGCTATGTGTATGG and GCTGTACTGATCCTTGCTGTTG. Real-time reactions were performed in 25-μl volumes with SYBR green Supermix (Stratagene), 2 μM each primer, and equal amounts of cDNA. The PCR was performed in an MX3000 (Stratagene) real-time machine under the following conditions: 95°C for 10 min, 40 cycles of 95°C for 30 s, and 60°C for 1 min, followed by a dissociation run. Results were analyzed by the threshold cycle (2−ΔΔCT) method as described previously (29).
Animal infections.
For animal experiments, the inoculum was prepared by growing the M. paratuberculosis strains to log phase in enriched Middlebrook 7H9 broth and concentrating the cultures based on an optical density at 600 nm (OD600) to approximately 5 × 108/ml for mice and 2 × 108/ml for goats. The virulence of mutants in mice was based on the procedure described by Shin et al. (39). In summary, recently weaned BALB/c mice aged 4 to 7 weeks were inoculated by intraperitoneal injection with approximately 108 CFU in 0.2 ml PBS. Mice were sacrificed 3, 6, and 12 weeks postinoculation, and spleens and livers were collected for bacteriological examination. Spleens, livers, and whole bodies were weighed postmortem. The level of organ colonization by M. paratuberculosis strains was determined by plate counts using 7H11 medium (Difco) supplemented with 10% albumin dextrose complex, 0.5% glycerol, 2 μg/ml mycobactin J (Allied Monitor, IN), and an antibiotic cocktail (10 μg/ml amphotericin B, 200 units/ml polymyxin B, 50 μg/ml ticarcillin, and 10 μg/ml trimethoprim). The virulence of mutants in goats was determined by inoculating approximately 2 × 109 CFU in 10 ml PBS into the jugular vein. Infection was monitored by fecal culturing carried out every 8 weeks (50). In one experiment, goats were sacrificed 10 months after infection and 6 intestinal tissues were collected; and in the other two experiments, goats were sacrificed 6 months after infection and 10 intestinal tissues were collected. Bacteriological examination was performed using Bactec 12B (Becton Dickinson, Sparks, MD) liquid medium and by following a procedure described previously (17).
A vaccination trial was performed by inoculating separate groups of 5 to 7 mice subcutaneously with approximately 2 × 106 CFU of three different M. paratuberculosis mutants in 0.2 ml PBS. Seven weeks later, the inoculated mice as well as a noninoculated control group were challenged by intraperitoneal injection with 2 × 108 CFU of M. paratuberculosis K10. One set of groups was sacrificed at 6 weeks postchallenge, and the remainder at 12 weeks postchallenge. The numbers of M. paratuberculosis CFU from spleens and livers were determined by plate counts, as described above.
Statistical analysis.
Statistical analysis of the results from the log-transformed bacterial counts, immunological assays, and vaccination study was conducted using Fisher's protected least-significant-difference analysis of variance (ANOVA) for pair-wise comparison of multigrouping data sets. Differences with a P value of <0.05 were considered significant. The binary logistic regression model was used to analyze the apoptosis data. Differences in apoptosis levels of macrophages infected with mutants with a P value of<0.05 were considered significant compared to levels for wild-type-infected macrophages.
RESULTS
Characterization of mutants.
WAg906 was previously identified as having a transposon inserted into a hypothetical protein gene with homology to M. tuberculosis Rv1645c (8). With the sequencing of the M. paratuberculosis genome (28), this gene has been designated MAP1566 and is 190 bp upstream of modB, a gene involved in molybdate transport. The transposon insertion site in WAg913 was identified by cloning and sequencing the restriction fragment from the WAg913 genome that contained the kanamycin resistance gene by methods described previously (8). The transposon had inserted into the hypothetical protein gene MAP0053c, which is 120 bp upstream of glnQ, a gene involved in glutamine transport. The ppiA gene, which is an iron-regulated peptidyl-prolyl cis-trans-isomerase was targeted to create the ppiA knockout strain WAg915. It was characterized by appropriate PCR and Southern blot hybridization, using methods similar to those described for Mycobacterium bovis (44).
Survival of mutants in macrophages.
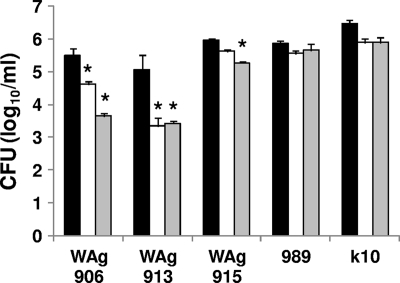
Survival of the M. paratuberculosis mutants within macrophages was determined at days 0, 7, and 14 (Fig. 1). The wild-type strains 989 and K10 had similar survivals. Both strains had a decrease in bacterial load at day 7 compared to day 0, but the bacterial numbers were similar at 7 and 14 days (Fig. 1). In contrast, a significant decrease in survival of over 1.5 logs from day 0 to day 14 was evident for WAg906 and WAg913. The K10-derived ppiA knockout strain (WAg915) showed a small but significant decrease of 0.7 log by day 14.
FIG. 1.
Survival of mutant M. paratuberculosis strains within bovine macrophages. Macrophages were harvested for bacterial culture at day 0 (black bar), day 7 (white bar), and day 14 (gray bar). The data are shown as the mean number of CFU (±standard error [SE]), and the significance of the difference between the mutant strains and their parent wild type was determined by least-significant-difference ANOVA (*, P < 0.05).
Apoptosis and IL-10 production.
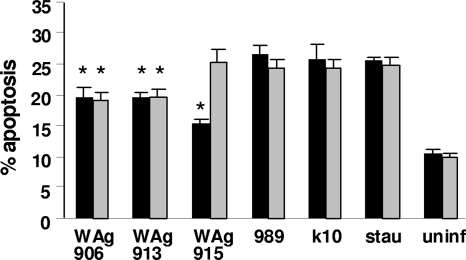
The levels of apoptosis varied between macrophages infected with different M. paratuberculosis strains. Infection with wild-type strains resulted in approximately 25% apoptosis, compared to 19% for both WAg906 and WAg913 (Fig. 2). There was little variability between the levels of apoptosis in macrophages isolated from the two animals, except for WAg915, which showed apoptosis levels of 15% and 25% for the macrophages of the two animals tested.
FIG. 2.
Apoptosis of bovine macrophages infected with M. paratuberculosis strains. Macrophages from the two animals used are represented by black and gray bars; stau, staurosporine-positive control; uninf, uninfected macrophages. The data are representative of 1 of 2 experiments. Data are shown as mean percent apoptosis (±SE); *, significantly different from wild type (P < 0.05).
There was no significant difference in the amounts of IL-10 produced from macrophages infected with the wild-type or mutant M. paratuberculosis strains, except for WAg915, which produced a significantly lower level (Fig. 3). There were also no significant differences in the levels of IL-10 gene expression from macrophages infected with the different M. paratuberculosis strains (data not shown).
FIG. 3.
Production of IL-10 by bovine macrophages infected with M. paratuberculosis strains. Data are shown as the mean of IL-10 production (±SE) from macrophages of two animals indicated by black and gray bars; pos, positive control; uninf, uninfected; *, significantly different from wild type (P < 0.05).
Survival of mutants in animals.
The effect of the mutations on the virulence of M. paratuberculosis was assessed with mice and goats. In mice, the bacterial load was determined for the liver and spleen at 3, 6, and 12 weeks following infection (Fig. 4), except in the case of WAg915 at 3 weeks, in which the plates were overgrown by contaminants. Each strain had bacterial loads in the spleen and liver at 12 weeks that were within 0.5 logs of each other, with the exception of WAg915, for which the number of bacteria in the spleen was 0.9 logs higher than that in the liver. While all three mutants had lower survival than their parent wild-type strains, the difference was most marked for WAg906 and was significant (P < 0.05) only for WAg906 and WAg915.
FIG. 4.
Persistence of mutant M. paratuberculosis strains in mice. The data represent the mean CFU values (±standard deviation) from the spleens (A) and livers (B) of 5 mice. The growth of mutant strains WAg906 (▴), WAg913 (×) and WAg915 (○) was compared to that of the wild-type strains 989 (▪) and K10 (□). *, significantly different from wild type (P < 0.05).
Three separate experiments were conducted with goats, and the results are shown in Table 2. In the experiment in which only 6 tissues were sampled, these came from the ileocecal valve and its draining lymph node, the ileum approximately 50 cm from the ileocecal valve and its draining lymph node, and the jejunum and the draining lymph node. In the other two experiments, these tissues were also sampled as well as an additional sample of jejunum and the draining lymph node, the spleen, and a bronchial lymph node. The number of tissues positive for M. paratuberculosis for each animal was determined, and a mean number of positive tissues per group was calculated (Table 2). Both 989 and K10 wild-type strains had high percentages of tissues (92.5 to 100%) positive for M. paratuberculosis. In contrast, the percentage of infected tissues was much lower for the three mutants, and there were no positive tissues for the seven animals infected with WAg906. M. paratuberculosis was not isolated from the feces of any of the goats infected with mutant strains of M. paratuberculosis. In contrast, M. paratuberculosis was isolated from the feces of all goats inoculated with M. paratuberculosis K10 and 989.
TABLE 2.
Percent M. paratuberculosis-positive tissues from goats infected with wild-type and mutant strains in three separate experiments
| Strain | Parent | Mean % positive for M. paratuberculosis for 10 tissues/ animal (no. of goats) | Mean % positive for M. paratuberculosis for 10 tissues/ animal (no. of goats) | Mean % positive for M. paratuberculosis for 6 tissues/ animal (no. of goats) |
|---|---|---|---|---|
| K10 | 97.5 (4) | |||
| 989 | 92.5 (4) | 100 (3) | ||
| WAg906 | 989 | 0 (5) | 0 (2) | |
| WAg913 | 989 | 0 (4) | 4 (4) | |
| WAg915 | K10 | 15 (4) |
Mouse vaccination.
No significant differences were observed with the number of organ CFU of the vaccine groups in the animals sacrificed 6 weeks postchallenge (Table 3). The mean numbers of CFU of the spleens and livers were less than those of the unvaccinated animals for both WAg906 and WAg915 and were statistically significant for WAg915 for the livers at 12 weeks (P < 0.05) and almost significant for the spleens (P = 0.052) (Table 3). No significant differences were observed with the organ weights of the animals sacrificed 6 weeks postchallenge, but in the animals sacrificed 12 weeks postchallenge, the mean spleen weights ± standard deviation for the animals vaccinated with WAg906 (0.099 ± 0.007 g) and WAg915 (0.103 ± 0.013 g) were significantly less than those for the unvaccinated controls (0.120 ± 0.014 g).
TABLE 3.
Bacterial loads in the spleens and livers of mice following vaccination trial
| Vaccination groups | Mean no. of organ CFU (log10/ml)a |
|||
|---|---|---|---|---|
| Spleen |
Liver |
|||
| 6 wk | 12 wk | 6 wk | 12 wk | |
| Unvaccinated | 4.17 ± 0.82 | 4.97 ± 0.87 | 5.11 ± 0.57 | 6.01 ± 0.84 |
| WAg906 | 4.08 ± 0.46 | 4.63 ± 0.56 | 4.75 ± 0.47 | 5.69 ± 0.70 |
| WAg913 | 4.20 ± 0.33 | 5.16 ± 0.23 | 4.78 ± 0.44 | 5.90 ± 0.46 |
| WAg915 | 3.51 ± 0.88 | 4.19 ± 0.75 | 4.45 ± 0.77 | 5.07 ± 0.59b |
Values are means ± standard deviation (5 to 7 mice per group) for organ CFU (log10/ml) at 6 and 12 weeks following challenge with K10.
Significantly different (P < 0.05) from noninoculated controls determined by least-significant-difference ANOVA.
DISCUSSION
The availability of an improved vaccine against paratuberculosis would provide a cost-effective tool for the control of this disease. As a major step toward this goal, we have produced and screened mutant strains of M. paratuberculosis produced by transposon mutagenesis and allelic exchange and tested them for their survival and virulence in macrophages and animals. We decided to develop live attenuated candidate vaccines because it has been found that in the case of the related mycobacterial disease, tuberculosis, such candidates possess all the immunogenic proteins necessary to display stronger and longer immune stimulation without the virulent properties which cause disease (35, 26). For this study, we produced several thousand transposon mutants and screened them to identify a small number of mutants that were likely avirulent. Transposon mutants were investigated because such mutants can be more easily produced than allelic exchange mutants and have previously been shown to have vaccine potential against disease caused by the related mycobacteria M. tuberculosis and M. bovis (14, 22). Targeting specific genes thought to be responsible for the virulence of bacterial strains has also proven successful in the development of tuberculosis vaccine candidates (24, 31). The ppiA gene was targeted because in previous unpublished work we discovered that such a mutation in M. bovis produced an avirulent strain with vaccine properties similar to those of the human tuberculosis vaccine Mycobacterium bovis BCG. This enzyme is considered important for protein folding and thought to participate in cell signaling, cell surface recognition, chaperoning, and heat shock responses (19).
Macrophages have proven to be a rapid and efficient screening tool for M. paratuberculosis vaccine candidates (38) because they identify attenuated strains of Mycobacterium spp. by reduced strain survival (15, 36, 38). The survival of the bacterium is dependent on the ability of the host macrophage to kill and the ability of the bacterium to overcome the killing mechanisms, including interfering with the intricate process of apoptosis. Evidence suggests that virulent mycobacteria are capable of inducing host cell apoptosis (1, 6) as well as antiapoptotic mechanisms, which are important for bacterial virulence (43, 53). The ability of macrophages to kill intracellular M. paratuberculosis is strain dependent, with some M. paratuberculosis strains being able to interfere with macrophage mechanisms more successfully than others (18). In this study, both the WAg906 and WAg913 mutants were severely attenuated within macrophages, and this decrease in survival correlated with a significant decrease in apoptosis compared to that for wild-type strains. The ppiA mutant, WAg915, survived better in macrophages than the other two mutants and caused a lower level of apoptosis than did its parent wild-type strain in the macrophages from only one of the two animals. This indicates that apoptosis correlates with the survival of M. paratuberculosis strains in this macrophage model and that in this model WAg906 and WAg913 are more attenuated than WAg915. However, repetition of this experiment using macrophages from more animals is required to better determine the levels of apoptosis induced, particularly in the case of WAg915.
Several of the techniques that pathogenic mycobacteria use to limit apoptosis have been associated with the overexpression of IL-10 (48, 49). This anti-inflammatory cytokine suppresses TH1-type responses, necessary for the control of mycobacterial infections. M. paratuberculosis has been reported to induce early and persistent IL-10 expression that correlates with suppression of apoptosis (45). As macrophages exhibit different immunological responses following infection with different mycobacterial strains (3, 47), we investigated whether this increase in IL-10 could be used to distinguish M. paratuberculosis strains of different virulence levels. There were no significant differences in IL-10 gene expression levels of macrophages infected with the different M. paratuberculosis strains, and although there were minor differences detected in the production of IL-10, it did not generally correlate with the attenuation of M. paratuberculosis strains in the models.
While several animal models have been assessed and compared for the study of potential vaccine candidates for paratuberculosis (4, 27, 39, 40), only one study has assessed the virulence in mice of selected transposon mutants of M. paratuberculosis (39), and a vaccine study of M. paratuberculosis mutants has yet to be reported. In this study, we assessed attenuation of the mutants in mice using proposed guidelines (20) based on the methods of Shin et al. (39). Intravenous inoculation of goats for the assessment of virulence of M. paratuberculosis mutants was used in order to simulate the parenteral administration of a live vaccine rather than reproduce natural infection with M. paratuberculosis. Results from both the mouse and goat models indicate that WAg906 is the most attenuated of the three mutants. However, different strains appeared more or less attenuated relative to each other, depending on which animal model was used, which has also been found for the M. tuberculosis complex (12).
The attenuation of the transposon mutants was attributed to the insertion of the transposon into hypothetical proteins (MAP1566 and MAP0053c) in WAg906 and WAg913, respectively. The gene disrupted in WAg906, the most attenuated candidate from this study, encodes an uncharacterized protein and is positioned within a group of molybdate (mod) genes encoding transport proteins. It is unknown whether this gene is part of the mod operon, but one of the downstream genes within this operon, modD, encodes a secreted protein antigen with promising serodiagnostic applications (10). The WAg913 hypothetical protein is upstream of glnQ, which is involved in glutamine transport and may be within the same operon. It is clear that both hypothetical proteins affect the in vivo survival of M. paratuberculosis, but further genetic and functional investigation of these mutants is required to clearly understand their role.
The preliminary study that examined the vaccine potential of two of the M. paratuberculosis mutants identified some protection at 12 weeks postchallenge induced by the ppiA mutant WAg915 based on the mean number of CFU of M. paratuberculosis present in the liver and spleen. No protection was induced in this study by the more attenuated strain WAg906, indicating the need for an effective live paratuberculosis vaccine to retain sufficient viability in vivo to produce a protective immune response. A similar situation has been observed with strains of the M. tuberculosis complex, in which strains that are too attenuated induce little vaccine protection (14). It must be emphasized that the vaccine results reported are preliminary, and more confident conclusions about the vaccine potential of these mutants can be attained only by repeating the study with mice, by including one or more of the current or older paratuberculosis vaccines for comparison, and then by carrying out a vaccine study with ruminants.
This is the first study directly comparing animal models and macrophages for the assessment of attenuation of mutant strains of M. paratuberculosis. With the exception of WAg906, which was attenuated in macrophages and both animal models of virulence, the mutants exhibited different levels of attenuation in the different models. At this stage, it is unclear what significance some of these differences will have on the effectiveness of the mutants as vaccine candidates, as there have been no previous investigations correlating degree of attenuation of M. paratuberculosis strains with vaccine potential. However, the finding that WAg906 did not provide any protection in a limited vaccine experiment in mice, whereas some protection was provided by the less attenuated strain WAg915, indicates that the degree of attenuation of a candidate vaccine is likely to be a critical factor in inducing protection.
Further study of the immunological responses of mutants may also help to indicate the most likely vaccine candidate. The macrophage model proved to be a rapid, inexpensive way of identifying attenuated strains that are at least moderately attenuated in the goat model. This suggests that macrophages could play an important role in screening potential M. paratuberculosis vaccine candidates, the best of which could then be investigated with an animal model. Alternative methods of selecting attenuated strains that might also prove useful include signature tag mutagenesis (13) or preselection of mutants from a transposon library using bioinformatic criteria, followed by virulence assessment with animals (39). It was evident that while the mouse model for virulence correlated imperfectly with the goat model, it has the advantages of enabling much-shorter-term experiments to identify potential vaccine candidates. Overall, the results of this study indicate that while macrophages and mice can be used to narrow down the number of mutant strains to be tested for attenuation, ultimately the strains can be fully assessed only in a ruminant model.
Acknowledgments
This work was supported by the New Zealand Foundation for Research Science and Technology and Dairy NZ.
We thank Bill Jacobs (Albert Einstein College of Medicine, NY) for the conditionally replicating phagemids used for transposon mutagenesis and allelic exchange.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 28 December 2009.
REFERENCES
- 1.Allen, S., J. Sotos, M. J. Sylte, and C. J. Czuprynski. 2001. Use of Hoechst 33342 staining to detect apoptotic changes in bovine mononuclear phagocytes infected with Mycobacterium avium subsp. paratuberculosis. Clin. Diagn. Lab. Immunol. 8:460-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardarov, S., J. Kriakov, C. Carriere, S. Yu, C. Vaamonde, R. A. McAdam, B. R. Bloom, G. F. Hatfull, and W. R. Jacobs, Jr. 1997. Conditionally replicating mycobacteriophages: a system for transposon delivery to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 94:10961-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basler, T., R. Geffers, S. Weiss, P. Valentin-Weigand, and R. Goethe. 2008. Mycobacterium avium subspecies induce differential expression of proinflammatory mediators in a murine macrophage model: evidence for enhanced pathogenicity of Mycobacterium avium subspecies paratuberculosis. Immunobiology 213:879-888. [DOI] [PubMed] [Google Scholar]
- 4.Begg, D. J., and J. F. Griffin. 2005. Vaccination of sheep against M. paratuberculosis: immune parameters and protective efficacy. Vaccine 23:4999-5008. [DOI] [PubMed] [Google Scholar]
- 5.Begg, D. J., and R. J. Whittington. 2008. Experimental animal infection models for Johne's disease, an infectious enteropathy caused by Mycobacterium avium subsp. paratuberculosis. Vet. J. 176:129-145. [DOI] [PubMed] [Google Scholar]
- 6.Briken, V., and J. L. Miller. 2008. Living on the edge: inhibition of host cell apoptosis by Mycobacterium tuberculosis. Future Microbiol. 3:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buza, J. J., H. Hikono, Y. Mori, R. Nagata, S. Hirayama, G. Aodon, A. M. Bari, Y. Shu, N. M. Tsuji, and E. Momotani. 2004. Neutralization of interleukin-10 significantly enhances gamma interferon expression in peripheral blood by stimulation with Johnin purified protein derivative and by infection with Mycobacterium avium subsp. paratuberculosis in experimentally infected cattle with paratuberculosis. Infect. Immun. 72:2425-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavaignac, S. M., S. J. White, G. W. de Lisle, and D. M. Collins. 2000. Construction and screening of Mycobacterium paratuberculosis insertional mutant libraries. Arch. Microbiol. 173:229-231. [DOI] [PubMed] [Google Scholar]
- 9.Chiodini, R. J., and C. D. Buergelt. 1993. Susceptibility of Balb/c, C57/B6 and C57/B10 mice to infection with Mycobacterium paratuberculosis. J. Comp. Pathol. 109:309-319. [DOI] [PubMed] [Google Scholar]
- 10.Cho, D., S. J. Shin, A. M. Talaat, and M. T. Collins. 2007. Cloning, expression, purification and serodiagnostic evaluation of fourteen Mycobacterium paratuberculosis proteins. Protein Expr. Purif. 53:411-420. [DOI] [PubMed] [Google Scholar]
- 11.Collins, D. M., D. M. Gabric, and G. W. de Lisle. 1990. Identification of two groups of Mycobacterium paratuberculosis strains by restriction endonuclease analysis and DNA hybridization. J. Clin. Microbiol. 28:1591-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins, D. M., R. P. Kawakami, B. M. Buddle, B. J. Wards, and G. W. de Lisle. 2003. Different susceptibility of two animal species infected with isogenic mutants of Mycobacterium bovis identifies phoT as having roles in tuberculosis virulence and phosphate transport. Microbiology 149:3203-3212. [DOI] [PubMed] [Google Scholar]
- 13.Collins, D. M., B. Skou, S. White, S. Bassett, L. Collins, R. For, K. Hurr, G. Hotter, and G. W. de Lisle. 2005. Generation of attenuated Mycobacterium bovis strains by signature-tagged mutagenesis for discovery of novel vaccine candidates. Infect. Immun. 73:2379-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins, D. M., T. Wilson, S. Campbell, B. M. Buddle, B. J. Wards, G. Hotter, and G. W. De Lisle. 2002. Production of avirulent mutants of Mycobacterium bovis with vaccine properties by the use of illegitimate recombination and screening of stationary-phase cultures. Microbiology 148:3019-3027. [DOI] [PubMed] [Google Scholar]
- 15.Cosma, C. L., K. Klein, R. Kim, D. Beery, and L. Ramakrishnan. 2006. Mycobacterium marinum Erp is a virulence determinant required for cell wall integrity and intracellular survival. Infect. Immun. 74:3125-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coussens, P. M., C. B. Pudrith, K. Skovgaard, X. Ren, S. P. Suchyta, J. R. Stabel, and P. M. Heegaard. 2005. Johne's disease in cattle is associated with enhanced expression of genes encoding IL-5, GATA-3, tissue inhibitors of matrix metalloproteinases 1 and 2, and factors promoting apoptosis in peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 105:221-234. [DOI] [PubMed] [Google Scholar]
- 17.de Lisle, G. W., G. F. Yates, and H. Montgomery. 2003. The emergence of Mycobacterium paratuberculosis in farmed deer in New Zealand: a review of 619 cases. N. Z. Vet. J. 51:58-62. [DOI] [PubMed] [Google Scholar]
- 18.Gollnick, N. S., R. M. Mitchell, M. Baumgart, H. K. Janagama, S. Sreevatsan, and Y. H. Schukken. 2007. Survival of Mycobacterium avium subsp. paratuberculosis in bovine monocyte-derived macrophages is not affected by host infection status but depends on the infecting bacterial genotype. Vet. Immunol. Immunopathol. 120:93-105. [DOI] [PubMed] [Google Scholar]
- 19.Henriksson, L. M., P. Johansson, T. Unge, and S. L. Mowbray. 2004. X-ray structure of peptidyl-prolyl cis-trans isomerase A from Mycobacterium tuberculosis. Eur. J. Biochem. 271:4107-4113. [DOI] [PubMed] [Google Scholar]
- 20.Hines, M. E., II, J. R. Stabel, R. W. Sweeney, F. Griffin, A. M. Talaat, D. Bakker, G. Benedictus, W. C. Davis, G. W. de Lisle, I. A. Gardner, R. A. Juste, V. Kapur, A. Koets, J. McNair, G. Pruitt, and R. H. Whitlock. 2007. Experimental challenge models for Johne's disease: a review and proposed international guidelines. Vet. Microbiol. 122:197-222. [DOI] [PubMed] [Google Scholar]
- 21.Hines, M. E., II, S. Stiver, D. Giri, L. Whittington, C. Watson, J. Johnson, J. Musgrove, M. Pence, D. Hurley, C. Baldwin, I. A. Gardner, and S. Aly. 2007. Efficacy of spheroplastic and cell-wall competent vaccines for Mycobacterium avium subsp. paratuberculosis in experimentally-challenged baby goats. Vet. Microbiol. 120:261-283. [DOI] [PubMed] [Google Scholar]
- 22.Jackson, M., S. W. Phalen, M. Lagranderie, D. Ensergueix, P. Chavarot, G. Marchal, D. N. McMurray, B. Gicquel, and C. Guilhot. 1999. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect. Immun. 67:2867-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalifeh, M. S., and J. R. Stabel. 2004. Effects of gamma interferon, interleukin-10, and transforming growth factor beta on the survival of Mycobacterium avium subsp. paratuberculosis in monocyte-derived macrophages from naturally infected cattle. Infect. Immun. 72:1974-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khare, S., M. K. Hondalus, J. Nunes, B. R. Bloom, and L. Garry Adams. 2007. Mycobacterium bovis DeltaleuD auxotroph-induced protective immunity against tissue colonization, burden and distribution in cattle intranasally challenged with Mycobacterium bovis Ravenel S. Vaccine 25:1743-1755. [DOI] [PubMed] [Google Scholar]
- 25.Kohler, H., H. Gyra, K. Zimmer, K. G. Drager, B. Burkert, B. Lemser, D. Hausleithner, K. Cubler, W. Klawonn, and R. G. Hess. 2001. Immune reactions in cattle after immunization with a Mycobacterium paratuberculosis vaccine and implications for the diagnosis of M. paratuberculosis and M. bovis infections. J. Vet. Med. B Infect. Dis. Vet. Public Health 48:185-195. [DOI] [PubMed] [Google Scholar]
- 26.Lee, J. S., R. Krause, J. Schreiber, H. J. Mollenkopf, J. Kowall, R. Stein, B. Y. Jeon, J. Y. Kwak, M. K. Song, J. P. Patron, S. Jorg, K. Roh, S. N. Cho, and S. H. Kaufmann. 2008. Mutation in the transcriptional regulator PhoP contributes to avirulence of Mycobacterium tuberculosis H37Ra strain. Cell Host Microbe 3:97-103. [DOI] [PubMed] [Google Scholar]
- 27.Lei, L., B. L. Plattner, and J. M. Hostetter. 2008. Live Mycobacterium avium subsp. paratuberculosis and a killed-bacterium vaccine induce distinct subcutaneous granulomas, with unique cellular and cytokine profiles. Clin. Vaccine Immunol. 15:783-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, L., J. P. Bannantine, Q. Zhang, A. Amonsin, B. J. May, D. Alt, N. Banerji, S. Kanjilal, and V. Kapur. 2005. The complete genome sequence of Mycobacterium avium subspecies paratuberculosis. Proc. Natl. Acad. Sci. U. S. A. 102:12344-12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 30.Mackintosh, C. G., R. E. Labes, and J. F. Griffin. 2005. The effect of Johne's vaccination on tuberculin testing in farmed red deer (Cervus elaphus). N. Z. Vet. J. 53:216-222. [DOI] [PubMed] [Google Scholar]
- 31.Mitsuyama, M., and D. N. McMurray. 2007. Tuberculosis: vaccine and drug development. Tuberculosis (Edinb.) 87(Suppl. 1):S10-S13. [DOI] [PubMed] [Google Scholar]
- 32.Munjal, S. K., B. N. Tripathi, and O. P. Paliwal. 2005. Progressive immunopathological changes during early stages of experimental infection of goats with Mycobacterium avium subspecies paratuberculosis. Vet. Pathol. 42:427-436. [DOI] [PubMed] [Google Scholar]
- 33.Olsen, I., G. Sigurgardottir, and B. Djonne. 2002. Paratuberculosis with special reference to cattle. A review. Vet. Q. 24:12-28. [DOI] [PubMed] [Google Scholar]
- 34.Park, K. T., J. L. Dahl, J. P. Bannantine, R. G. Barletta, J. Ahn, A. J. Allen, M. J. Hamilton, and W. C. Davis. 2008. Demonstration of allelic exchange in the slow-growing bacterium Mycobacterium avium subsp. paratuberculosis, and generation of mutants with deletions at the pknG, relA, and lsr2 loci. Appl. Environ. Microbiol. 74:1687-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto, R., B. M. Saunders, L. R. Camacho, W. J. Britton, B. Gicquel, and J. A. Triccas. 2004. Mycobacterium tuberculosis defective in phthiocerol dimycocerosate translocation provides greater protective immunity against tuberculosis than the existing bacille Calmette-Guerin vaccine. J. Infect. Dis. 189:105-112. [DOI] [PubMed] [Google Scholar]
- 36.Rampini, S. K., P. Selchow, C. Keller, S. Ehlers, E. C. Bottger, and P. Sander. 2008. LspA inactivation in Mycobacterium tuberculosis results in attenuation without affecting phagosome maturation arrest. Microbiology 154:2991-3001. [DOI] [PubMed] [Google Scholar]
- 37.Reddacliff, L., J. Eppleston, P. Windsor, R. Whittington, and S. Jones. 2006. Efficacy of a killed vaccine for the control of paratuberculosis in Australian sheep flocks. Vet. Microbiol. 115:77-90. [DOI] [PubMed] [Google Scholar]
- 38.Scandurra, G. M., M. Young, G. W. de Lisle, and D. M. Collins. 2009. A bovine macrophage screening system for identifying attenuated transposon mutants of Mycobacterium avium subsp. paratuberculosis with vaccine potential. J. Microbiol. Methods 77:58-62. [DOI] [PubMed] [Google Scholar]
- 39.Shin, S. J., C. W. Wu, H. Steinberg, and A. M. Talaat. 2006. Identification of novel virulence determinants in Mycobacterium paratuberculosis by screening a library of insertional mutants. Infect. Immun. 74:3825-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh, S. V., P. K. Singh, A. V. Singh, J. S. Sohal, V. K. Gupta, and V. S. Vihan. 2007. Comparative efficacy of an indigenous ‘inactivated vaccine’ using highly pathogenic field strain of Mycobacterium avium subspecies paratuberculosis ‘Bison type’ with a commercial vaccine for the control of Capri-paratuberculosis in India. Vaccine 25:7102-7110. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka, S., M. Sato, T. Taniguchi, and Y. Yokomizo. 1994. Histopathological and morphometrical comparison of granulomatous lesions in BALB/c and C3H/HeJ mice inoculated with Mycobacterium paratuberculosis. J. Comp. Pathol. 110:381-388. [DOI] [PubMed] [Google Scholar]
- 42.van Schaik, G., C. H. Kalis, G. Benedictus, A. A. Dijkhuizen, and R. B. Huirne. 1996. Cost-benefit analysis of vaccination against paratuberculosis in dairy cattle. Vet. Rec. 139:624-627. [PubMed] [Google Scholar]
- 43.Velmurugan, K., B. Chen, J. L. Miller, S. Azogue, S. Gurses, T. Hsu, M. Glickman, W. R. Jacobs, Jr., S. A. Porcelli, and V. Briken. 2007. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 3:e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wards, B. J., G. W. de Lisle, and D. M. Collins. 2000. An esat6 knockout mutant of Mycobacterium bovis produced by homologous recombination will contribute to the development of a live tuberculosis vaccine. Tuber. Lung Dis. 80:185-189. [DOI] [PubMed] [Google Scholar]
- 45.Weiss, D. J., O. A. Evanson, C. de Souza, and M. S. Abrahamsen. 2005. A critical role of interleukin-10 in the response of bovine macrophages to infection by Mycobacterium avium subsp. paratuberculosis. Am. J. Vet. Res. 66:721-726. [DOI] [PubMed] [Google Scholar]
- 46.Weiss, D. J., O. A. Evanson, M. Deng, and M. S. Abrahamsen. 2004. Gene expression and antimicrobial activity of bovine macrophages in response to Mycobacterium avium subsp. paratuberculosis. Vet. Pathol. 41:326-337. [DOI] [PubMed] [Google Scholar]
- 47.Weiss, D. J., O. A. Evanson, A. Moritz, M. Q. Deng, and M. S. Abrahamsen. 2002. Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. Infect. Immun. 70:5556-5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss, D. J., O. A. Evanson, and C. D. Souza. 2005. Expression of interleukin-10 and suppressor of cytokine signaling-3 associated with susceptibility of cattle to infection with Mycobacterium avium subsp. paratuberculosis. Am. J. Vet. Res. 66:1114-1120. [DOI] [PubMed] [Google Scholar]
- 49.Weiss, D. J., and C. D. Souza. 2008. Review paper: modulation of mononuclear phagocyte function by Mycobacterium avium subsp. paratuberculosis. Vet. Pathol. 45:829-841. [DOI] [PubMed] [Google Scholar]
- 50.Whittington, R. J., I. Marsh, S. McAllister, M. J. Turner, D. J. Marshall, and C. A. Fraser. 1999. Evaluation of modified BACTEC 12B radiometric medium and solid media for culture of Mycobacterium avium subsp. paratuberculosis from sheep. J. Clin. Microbiol. 37:1077-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Windsor, P. A., R. Bush, I. Links, and J. Eppleston. 2005. Injury caused by self-inoculation with a vaccine of a Freund's complete adjuvant nature (Gudair) used for control of ovine paratuberculosis. Aust. Vet. J. 83:216-220. [DOI] [PubMed] [Google Scholar]
- 52.Woo, S. R., and C. J. Czuprynski. 2008. Tactics of Mycobacterium avium subsp. paratuberculosis for intracellular survival in mononuclear phagocytes. J. Vet. Sci. 9:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woo, S. R., J. A. Heintz, R. Albrecht, R. G. Barletta, and C. J. Czuprynski. 2007. Life and death in bovine monocytes: the fate of Mycobacterium avium subsp. paratuberculosis. Microb. Pathog. 43:106-113. [DOI] [PubMed] [Google Scholar]