Abstract
Although most Dot/Icm-translocated effectors of Legionella pneumophila are not required for intracellular proliferation, the eukaryotic-like ankyrin effectors, AnkH and AnkJ are required for intracellular proliferation. In this report, we show that the IcmSW chaperones are essential for translocation of AnkJ but not AnkH. The 10 C-terminal residues and the ANK domains of AnkH and AnkJ are required for translocation. Our data indicate that the two ANK domains of AnkH are critical domains required for the function of the effector in intracellular replication of L. pneumophila. The ankH and ankJ mutants are severely defective in intrapulmonary proliferation in mice. Expression of AnkH and AnkJ fusions within HEK293 cells show a punctuate distribution in the cytosol but no association with endocytic vesicles, the Golgi apparatus or the endoplasmic reticulum. Interestingly, the defect in intracellular proliferation of the ankH or ankJ mutants is rescued in HEK293 cells expressing the respective protein. We conclude that AnkH and AnkJ are effectors translocated by the Dot/Icm system by distinct mechanisms and modulate distinct cytosolic processes in the host cell.
The gram-negative intracellular bacterial pathogen Legionella pneumophila is found ubiquitously in aquatic environments, where it replicates within a wide range of protozoan hosts (2, 18). Once inhaled by humans in aerosolized contaminated water, L. pneumophila replicates in human alveolar macrophages and causes Legionnaires' disease or a less severe flulike symptoms designated Pontiac fever (19, 29).
L. pneumophila is equipped with many sophisticated mechanisms that allow it to survive and replicate within the host cell by creating a specialized endoplasmic reticulum (ER)-like compartment known as the Legionella-containing vacuole (LCV) (16, 23, 26, 42, 43). Within the LCV, L. pneumophila utilizes its specialized Dot/Icm type IVB secretion system (TFSS) machinery to export a cohort of >200 effectors into the host cell cytosol that are essential to modulate various cellular processes, such as interception of ER-to-Golgi vesicle traffic, evasion of endocytic traffic, and triggering pro- and antiapoptotic processes (1, 13, 15, 16, 27, 28, 32, 40, 45). Likewise, L. pneumophila is able to translocate into the host cell cytosol specific Dot/Icm substrates before its internalization (12, 35, 39). The IcmS and IcmW chaperones facilitate translocation of few Dot/Icm effectors (7, 10, 12, 36).
In silico analyses of four L. pneumophila genomes (Corby, Paris, Lens, and Philadelphia-1) have revealed the presence of many eukaryotic-like genes, which have been suggested to be acquired through horizontal gene transfer (3, 11, 14). Among the genes encoding eukaryotic-like proteins in L. pneumophila is a family of at least 11 proteins containing ankyrin eukaryotic-like domains (Ank) (4, 11, 14, 22). The ankyrin domain (ANK) is a 33-amino-acid structural motif and is the most common protein domain in the eukaryotic kingdom, where it functions as a scaffold to mediate protein-protein interactions that play essential roles in various eukaryotic cellular processes, ranging from regulation of transcription, signaling, cytoskeleton, and cell cycle regulation (3, 5, 8, 34). Therefore, it is predicted that the L. pneumophila ankyrin proteins may mimic or interfere with various cellular processes to remodel the host cell into a proliferative niche.
Thus far, most of Dot/Icm-exported substrates reported have little or no detectable role in intracellular proliferation, suggesting a possible functional redundancy among them (16). Strikingly, of the known Dot/Icm effectors, only loss of SdhA, SidJ or AnkB effectors results in a severe intracellular growth defect. The sdhA mutant is defective only in macrophages (31), but the sidJ mutant (32) and ankB mutant (4, 39) are defective in human macrophages and protozoa. Furthermore, two L. pneumophila ankyrin proteins (AnkH and AnkJ) play a significant role in intracellular replication of L. pneumophila in human macrophages and in protozoa (22), indicating that they modulate cellular processes that are highly conserved through evolution from protozoa to mammals.
The AnkH and AnkJ proteins that possess two and three ANK domains, respectively, have been reported to be delivered into host cytosol (13, 37). However, their mechanism of translocation and more importantly the role of the eukaryotic-like ANK domains of AnkH and AnkJ in the intracellular proliferation of L. pneumophila and in translocation of AnkH and AnkJ proteins remains unknown.
In the present study, we show that three of the L. pneumophila ankyrins are delivered into infected cells by a IcmSW-dependent mechanism. The AnkH and AnkJ are essential in vivo for in intrapulmonary proliferation in the mouse model. The ANK domains and the last 10 C-terminal residues of AnkH and AnkJ are required for translocation and for intracellular replication. Importantly, the expression of AnkH and AnkJ in HEK293 cells rescues the intracellular growth defect of the respective effector mutant, indicating modulation of distinct cytosolic processes by the two effectors to enable intracellular proliferation.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, and media.
The parental L. pneumophila serogroup 1 strain AA100/130b (ATCC BAA-74) and its isogenic dotA, icmS, and icmW mutant strains have been described previously (46). Escherichia coli strain DH5α was used as surrogate to clone the Cya fusion constructs. L. pneumophila samples were grown from frozen stocks on buffered charcoal-yeast extract (BCYE) agar at 37°C or in buffered yeast extract (BYE) broth at 37°C with shaking (17) for 3 days. The plates and broth used for the cultivation of the L. pneumophila wild-type (WT) strains expressing Cya fusion proteins were supplemented with 5 μg of chloramphenicol/ml, whereas the mutants expressing Cya fusion proteins were supplemented with 5 μg of chloramphenicol and 50 μg of kanamycin/ml. The plates used for the cultivation of E. coli strains were supplemented with 50 μg of chloramphenicol/ml on Luria-Bertani (LB) agar plates or broth at 37°C with 5% of CO2 or in LB broth at 37°C with shaking.
DNA manipulations and Cya reporter constructs.
Transfections, restriction enzyme digestions, and DNA manipulation were performed as previously described (38). Restriction enzymes and T4 DNA ligase were purchased from Promega (Madison, WI). L. pneumophila chromosomal DNA was prepared by using a Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN). Plasmid preparations were performed with the Bio-Rad Quantum miniprep kit. Electroporations were performed with a Bio-Rad Gene Pulser, as recommended by the manufacturer's specifications. Purification of DNA fragments from agarose gels for subcloning was carried out with a QIAquick gel purification kit (Qiagen, Valencia, CA). The primers (see Table 2) used to amplify the coding sequences of L. pneumophila ank genes utilized to engineer Cya-Ank or GFP-Ank constructs by PCR were from Integrated DNA Technologies, Inc. (Coralville, IA). The coding sequences of L. pneumophila ank genes were fused to the C-terminal of adenylate cyclase (Cya) of B. pertussis using plasmid pcya-ralF, which is a derivative of pMMB207M45NT (35). All of the L. pneumophila ank gene PCR products were digested by using BamHI and PstI. The pCya-RalF plasmid was digested with BamHI and PstI to release the ralF gene. Digested products were ligated by using T4 DNA ligase. The resulting Cya-Ank reporter constructs are displayed in Table 2. For in-frame deletion of the ANK domains and C-terminal of AnkH or AnkJ, we used L. pneumophila ank genes cloned into the plasmid vector pBC-SK+ as previously described (22). To generate domain mutant alleles of ankH and ankJ, an inverse PCR strategy was used with pBCSK+ harboring the ankH and ankJ genes as a template. Briefly, phosphorylated primers (Table 1) were designed to hybridize adjacent to DNA encoding the ANK domains or C termini, and then the entire plasmid lacking the domain of interest was PCR amplified using Phusion DNA polymerase (Finnzymes). The resulting PCR product was then treated with DpnI restriction to remove residual template DNA from the reaction and allowed to religate by using T4 DNA ligase. The ligation products were transformed into E. coli DH5α. Domain deletions of ankH and ankJ were verified by DNA sequencing to ensure the integrity of the ankH and ankJ reading frames. Recombinant plasmids were then electroporated into the L. pneumophila.
TABLE 2.
Cya-Ank reporter constructs and primers used to generate ank fusions
| Construct | Primera |
Restriction site | |
|---|---|---|---|
| Orientation | Sequence (5′-3′) | ||
| pCya-ankB | F | GGATCCTTATGAAAAAGAATTTTTTTTCTG | BamHI |
| R | CTGCAGTTAACAAACAAGGCACTTGCT | PstI | |
| pCya-ankC | F | CCCGGATCCTTATGGATTTTGTAAGTGAAATGAA | BamHI |
| R | CCCCTGCAGTTACTATTTTAGGACAACTCGT | PstI | |
| pCya-ankD | F | CCCGGATCCTTATGTTGACTCCTCCGCCTGACT | BamHI |
| R | CCCCTGCAGTTAGTCCTGAGGATTTTCTTTA | PstI | |
| pCya-ankG | F | CCCGGATCCTTCTGAATTCATTATGGATAGC | BamHI |
| R | CCCCTGCAGTTATTTCATACCAAAACGAG | PstI | |
| pCya-ankH | F | CCCGGATCCTTATGAGTATTGCAAAC | BamHI |
| R | CCCCTGCAGTTATAGGCCTGTCGCAACAGGAT | PstI | |
| pCya-ankI | F | CCCGGATCCTTATGATTATTTTATATGATTTT | BamHI |
| R | CCCCTGCAGTTAAAAAAACTTGCTTTCAAGTGTGAT | PstI | |
| pCya-ankJ | F | CCCGGATCCTTGTGATTAAAATGGGTAGA | BamHI |
| R | CCCCTGCAGTTAAAGTGCGTTTTTAGGGGTATCTA | PstI | |
| pCya-ankK | F | CCCGGATCCTTATGCCTAGAGTTTATAATCTTA | BamHI |
| R | CCCCTGCAGTTAGATTTTATTCTTTGATAGTGATA | PstI | |
| pCya-ankN | F | CCCGGATCCTTGGTAAAAATTATGCC | BamHI |
| R | CCCCTGCAGTTACTACCATTTTAATTTCAAG | PstI | |
| pCya-ankQ | F | CCCGGATCCTTATGCTTATGGCCG | BamHI |
| R | CCCCTGCAGTTATGCTTATGGCCGCAACAA | PstI | |
Restriction sites are indicated in boldface. Orientation: F, forward; R, reverse.
TABLE 1.
ANK deletion constructs and primers used to generate ANK deletions in ankH and ankJ
| Construct | Primera |
|
|---|---|---|
| Orientation | Sequence (5′-3′) | |
| ankHΔA1 | F | CCAGACGTCACAGGACGC |
| R | TTCATCGATATCATCCAAAG | |
| ankHΔA2 | F | TACACTCGTAATGGTCTTTG |
| R | GACGTCTGGCTTGTTGATAT | |
| ankHΔ1391-1401 | F | TTAATTAGGATTAATCCCACAATCATCCAGAATT |
| R | TAACCCGTAAAGGAAATAATTTATT | |
| ankJΔA1 | F | TGATGCTATTTTCATTTC |
| R | TACGCCCCCCCCACTTATG | |
| ankJΔA2 | F | GGGGGGGGCGTACTGATT |
| R | CCTATCAACTATCACAAAG | |
| ankJΔA3 | F | ATAGTTGATAGGATAATC |
| R | CTGGTGAATAGCACCAATAT | |
| ankJΔ797-807 | F | TTATTCTTCAAAACGACTCTCTGGAAC |
| R | GGAGAAATACCTCCTTCAAGAA | |
All primers are 5′-phosphorylated. Orientation: F, forward; R, reverse.
The various pBCSK+ vectors harboring the mutant ankH and ankJ alleles were used as templates to generate Cya fusions. The primers listed in Table 2 were used in PCR to amplify the mutant ankH and ankJ alleles, and the resulting PCR products were cloned into pCR2.1 via topoisomerization as described by the manufacturer (Invitrogen Corp., Carlsbad, CA). The mutant ankH and ankJ alleles were then subcloned into the BamHI-PstI sites of pcya-ralF (35), resulting in replacement of the ralF gene with ankH and ankJ alleles in frame with cya. Recombinant plasmids were electroporated into the L. pneumophila.
To generate the mammalian fusion constructs, open reading frames of L. pneumophila ankH and ankJ were cloned into the mammalian expression vectors pAcGFP (Sigma) or p3×Flag-CMV (Sigma) using the primers displayed in Table 3 to generate green fluorescent protein (GFP) or 3×Flag-AnkH or AnkJ fusion constructs.
TABLE 3.
GFP/3×Flag-Ank fusion constructs and primers used to generate ank fusions
| Construct | Primera |
Restriction site | |
|---|---|---|---|
| Orientation | Sequence (5′-3′) | ||
| p3×Flag-ankH | F | AGATCTGATGAGTATTGCAAACGATA | BglII |
| R | GGATCCTTATAGGCCTGTCGCAACAGGATT | BamH | |
| p3×Flag-ankJ | F | AAGCTTGTGATTAAAATGGGTAGA | HindIII |
| R | AGATCTTTAAAGTGCGTTTTTAGGGGTATC | BglII | |
| pAcGFP-ankH | F | AGATCTGATGAGTATTGCAAACGATA | BglII |
| R | TCTAGATTATAGGCCTGTCGCAACAGGATT | XbaI | |
| pAcGFP-ankJ | F | AGATCTGTGATTAAAATGGGTAGA | BglII |
| R | TCTAGATTAAAGTGCGTTTTTAGGGGTATC | XbaI | |
Restriction sites are indicated in boldface. Orientation: F, forward; R, reverse.
Translocation assay.
For adenylate cyclase (Cya) activity assays, differentiated U937 cells monolayers grown in 24-well plates were infected with various strains of L. pneumophila at a multiplicity of infection (MOI) of 50 for 1 h at 37°C. For the cytochalasin D treatment assay, U937 cells were treated with 1 μg of cytochalasin D/ml for 30 min. Cells were then infected for 30 min, washed three times extensively with 1× phosphate-buffered saline to remove extracellular bacteria, and subsequently lysed in 200 μl of 0.25% dodecyltrimethylammonium bromide in assay buffer. Cell lysates were processed for the detection of intracellular cyclic AMP (cAMP) using an Amersham cAMP enzyme immunoassay kit (GE Healthcare, Piscataway, NJ), as recommended by the manufacturer.
Macrophage culture and L. pneumophila intracellular replication analysis.
Isolation and preparation of the human monocyte-derived macrophages (hMDMs) was carried out as we previously described (41). The hMDMs and U937 cells were maintained in RPMI 1640 tissue culture medium (Gibco-BRL) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco-BRL). The cells were cultured under a humidified atmosphere containing 5% CO2 and 95% air at 37°C. For intracellular proliferation studies, infections of macrophages were performed as we described previously (22). Briefly, cells were infected with various strains of at an MOI of 10 for 1 h, followed by treatment with 50 μg of gentamicin/ml for 1 h to kill extracellular bacteria, and this was considered the zero time point. The intracellular proliferation was assessed by plating and enumerating the CFU at 24 and 48 h postinfection. Alternatively, the intracellular replication was examined after 10 h postinfection by single cell analysis using laser scanning microscopy as previously described (22).
Infection of A/J mice with L. pneumophila.
Female pathogen-free, 6- to 8-week-old A/J mice were used for infection by intratracheal inoculation as described previously (6, 20). The L. pneumophila strain AA100, ankH and ankJ mutants were grown on BCYE agar plates for 72 h. Three mice per strain were used for the intrapulmonary proliferation assay, and three mice per dose were used for the survival assay. The mice were inoculated intratracheally with 50 μl containing a bacterial dose of 106 for intrapulmonary proliferation assay and a dose of 107, 108, 8 × 108, or 109 for the survival assay as described previously (6, 20). At 2 h, 24 h, and 48 h, 72 h, and 7 days postinoculation, the mice were humanely euthanized, the lungs were removed, and the bacteria were cultured on BCYE agar for 72 h as described previously (6, 20). For the survival assay, the mice were observed for 3 days postinoculation.
Expression of AnkH and AnkJ in mammalian cells.
The human renal epithelial cell line HEK293 cells or HEK293T cells were grown on circular glass coverslips (Fisher) pretreated with 0.1 mg of poly-d-lysine/ml in 24-well culture plates at a concentration of 5 × 104 cells/ml in Dulbecco modified Eagle medium containing 10% FBS overnight. The subconfluent culture of HEK293T and HEK293 cells were transiently transfected by using calcium phosphate method with the mammalian expression vectors pAcGFP (Sigma) or BAP3×Flag (Sigma) and constructs of AnkH or AnkJ fusion GFP or 3×Flag for 18 h. In the present study, HEK293T cells were transiently transfected with GFP or 3×Flag constructs to examine their subcellular distribution. Since HEK293T are not suitable for generating stable transfected cells, therefore we used HEK293 cells. Transiently transfected HEK293 cells were used to generate stably transfected HEK293 cells. Briefly, 1/10-diluted transiently transfected HEK293 cells were grown in the presence of 1.4 mg of Geneticin (G418; Sigma)/ml for 15 days, and every other 3 days the medium was replaced by a new one containing 1.4 mg of Geneticin/ml. After 15 days, clones were picked and screened by confocal laser scanning microscopy and Western blot analysis for expression of the AnkH or AnkJ fusion protein. To study colocalization of AnkH and AnkJ with late endosomal, nuclear, lysosomal, cis- and trans-Golgi apparatus and ER markers, we labeled transfected cells with the primary antibodies anti-Lamp2, anti-cathepsin D, anti-GM130 (cis), anti-Golgi 58k (trans), or anti-KDEL. The anti-LAMP-2 (H4B4) monoclonal antibody (developed by J. T. August and J. E. K. Hildreth) was obtained from the Developmental Studies Hybridoma Bank (University of Iowa). To label the lysosomes, transfected cells were incubated with mouse monoclonal anti-cathepsin D antibody (BD Transduction, Franklin Lakes, NJ). Mouse anti-KDEL monoclonal antibody was purchased from StressGen Biotechnologies (Ann Arbor, MI). Rabbit anti-GM130 was obtained from Santa Cruz Biotechnology, Inc., and monoclonal anti-Golgi 58k was purchased from Sigma. The polyclonal anti-COX (mitochondria marker) was obtained fro Cell Signaling, and monoclonal antibodies anti-actin and anti-tubulin were obtained from Sigma. Primary antibodies were detected by Alexa Fluor 555-conjugated donkey anti-mouse or anti-rabbit IgG (Molecular Probes). The cells were examined by using an Olympus Fluoview1000 laser scanning confocal microscopy as described previously (41). On average, 8 to 15 0.2-mm serial Z sections of each image were captured and stored for further analyses by using Adobe Design Premium CS3.
Western blot assay.
To detect the Cya-Ank hybrid proteins in L. pneumophila WT, dotA, icmS, or icmW strains harboring empty vector or Cya-Ank constructs, bacteria were grown for 3 days on BCYE in the presence of appropriate antibiotics prior to infection of U937 cells. A total of 5 × 108 bacteria were isolated by centrifugation and lysed with B-per bacterial protein extraction reagent (Thermo Scientific, Waltham, MA), followed by boiling in SDS-PAGE sample buffer. Proteins were transferred onto nitrocellulose membrane, and the Cya-Ank hybrid proteins were detected by using monoclonal anti-M45 antibody at 1:50 dilution (4). After incubation with a secondary antibody conjugated to horseradish peroxidase, signals were detected by the Supersignal West Femto maximum sensitivity substrate (Thermo Scientific, Waltham, MA).
Statistical analysis.
All experiments were performed in triplicate at least three times, and the data shown are representative of one experiment. To analyze for statistical significant differences between different sets of data, a two-tailed Student t test was used, and the P value was obtained.
RESULTS
Potential translocation of L. pneumophila ankyrins upon bacterial attachment to the macrophage.
Potential role of the IcmSW chaperon in translocation of the Ank proteins was evaluated by using adenylate cyclase assays. The data showed that L. pneumophila AnkD, AnkG, and AnkJ are translocated by the Dot/Icm machinery in an IcmSW-dependent manner, whereas the translocation of AnkH, AnkI, AnkK, and AnkN is independent of the IcmSW complex (data not shown). Western blots confirmed equivalent expression of all of the proteins in the icmS and IcmW mutants (data not shown).
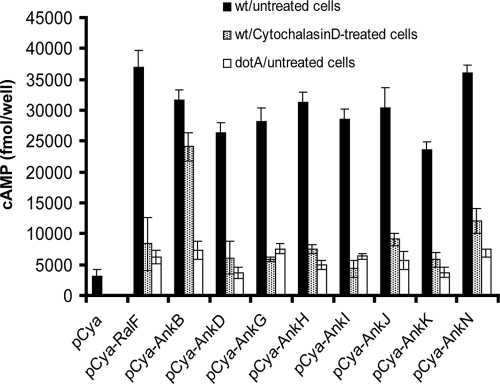
Some Dot/Icm effectors, such as LepA and LepB, are translocated into the host cell upon contact of L. pneumophila with the host cell prior to bacterial internalization (12, 35). We have recently shown that AnkB is exported into host cell by extracellular bacteria (39). Therefore, we examined whether the other Ank proteins are translocated into macrophages prior to bacterial internalization. Prior to infection, U937 cells were treated for 30 min with 1 μg of cytochalasin D/ml to prevent phagocytosis. The cells were infected for 30 min with the WT strain or the dotA mutant harboring Cya-Ank fusion constructs or the empty vector. In addition, we infected cells with the WT strain or the dotA mutant expressing Cya-RalF or Cya-AnkB fusion protein as controls. As expected, the WT strain translocated significantly the RalF into untreated cells compared to the empty vector (Student t test, P < 0.0001) (Fig. 1). The results showed that the AnkB controls was efficiently translocated by attached extracellular bacteria as indicated by comparable level of cAMP in cytochalasin D-treated and untreated cell (Student t test, P > 0.5) (Fig. 1). In contrast, the levels of cAMP were significantly different between cytochalasin D-treated and untreated cells infected with WT strain harboring any of the tested Cya-Ank reporters (Student t test, P < 0.0001) (Fig. 1).
FIG. 1.
Potential translocation of L. pneumophila Ankyrin proteins by attached extracellular bacteria. Untreated or cytochalasin D-treated U937 cells were infected with the WT strain of L. pneumophila expressing the indicated Cya hybrid proteins. After 30 min of infection, cultured cells were lysed and cAMP was quantified by enzyme-linked immunosorbent assay (ELISA) and the amount of cAMP is indicated as fmol/well. The experiment was performed three times. The data points are the average of cAMP concentration for one representative experiment performed in triplicate. Error bars represent standard deviations of triplicate samples.
The C termini of L. pneumophila AnkH and AnkJ are essential for translocation.
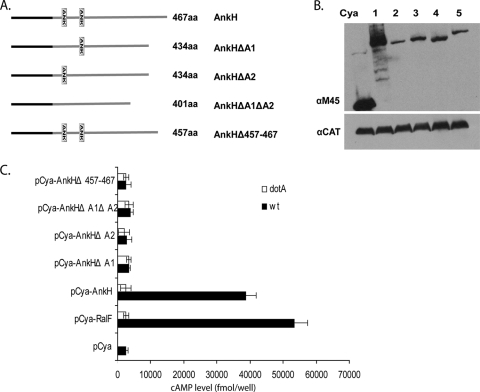
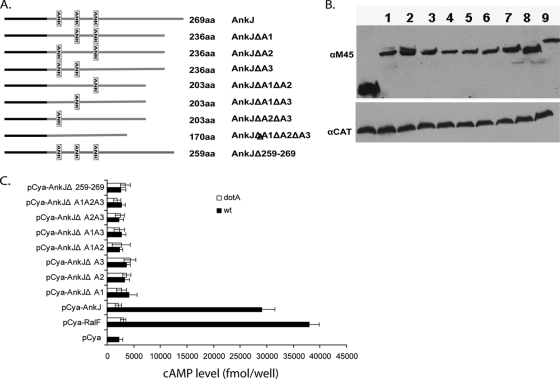
In addition to the existence of a C terminus secretion motifs, Nagai et al. have shown that some but not all Dot/Icm substrates harbor conserved hydrophobic residues in the C terminus (30, 33, 35). Since AnkH and AnkJ bear hydrophobic residues at the −4 or −5 positions in the C terminus (see Fig. S1 in the supplemental material), we tested whether the C-terminal region of AnkH and AnkJ proteins were required for their translocation. In-frame deletion of the last 10 residues of AnkH and AnkJ were constructed and fused to Cya (Fig. 2A and Fig. 3A). U937 cells were infected with the WT strain or the dotA mutant expressing the truncated AnkH and AnkJ reporter fusions. Our results showed significantly low levels of cAMP in cells infected with the WT strain expressing C-terminal deletion of AnkH and AnkJ with >10-fold fewer compared to cells infected with the WT strain expressing full-length AnkH and AnkJ (Student t test, P < 0.0001) (Fig. 2C and 3C). Thus, the C terminus of AnkH and AnkJ is required for translocation into the host cell.
FIG. 2.
The ANK repeats and the C terminus of L. pneumophila AnkH are required for delivery into host cell. (A) Organization of the AnkH and different ANK domain or C terminus deletions. Each protein size and truncation is displayed. (B) Immunoblots of whole-cell bacterial extracts expressing indicated Cya hybrid proteins from the WT, probed with a monoclonal antibody specific to the M45 epitope and reprobed with anti-CAT as a loading control. The numbers represent the different truncated Cya-AnkH hybrid proteins: Cya-AnkH (lane 1), Cya-AnkHΔA1 (lane 2), Cya-AnkHΔA2 (lane 3), Cya-AnkHΔA1ΔA2 (lane 4), and Cya-AnkJΔ457-467 (lane 5). (C) U937 cells were infected with the WT strain or the dotA mutant of L. pneumophila expressing the indicated Cya hybrid proteins. After 1 h of infection, cultured cells were lysed, and the cAMP was quantified by ELISA. The amount of cAMP is indicated as fmol/well. The experiment was performed three times, and the data are the average of cAMP concentration for one representative experiment performed in triplicate. Error bars represent standard deviations of triplicate samples.
FIG. 3.
The ANK repeats and the C terminus of L. pneumophila AnkJ are required for delivery into host cell. (A) Organization of the AnkJ and different ANK repeats or C terminus in-frame deletions. Each protein size and different truncation is displayed. (B) Immunoblots of whole-cell bacterial extracts expressing the indicated Cya hybrid were probed with monoclonal antibody specific to the M45 epitope and reprobed with anti-CAT as a loading control. The numbers represent the different Cya-AnkJ truncated hybrid proteins: Cya-AnkJ (lane 1), Cya-AnkJΔA1 (lane 2), Cya-AnkJΔA2 (lane 3), Cya-AnkJΔA3 (lane 4), Cya-AnkJΔA1ΔA2 (lane 5), Cya-AnkJΔA1ΔA3 (lane 6), Cya-AnkJΔA2 ΔA3 (lane 7), Cya-AnkJΔA1ΔA2 ΔA3 (lane 8), and Cya-AnkJΔ259-269 (lane 9). (C) U937 cells were infected with the WT strain or the dotA mutant of L. pneumophila expressing the indicated Cya hybrid proteins. After 1 h of infection, cultured cells were lysed, and the cAMP was quantified by ELISA. The amount of cAMP is indicated as fmol/well. The experiment was performed three times, and the data are the average of the cAMP concentration for one representative experiment performed in triplicate. Error bars represent standard deviations of triplicate samples.
The ANK domains of L. pneumophila are essential for the translocation of AnkH and AnkJ.
To test whether the ANK domains of AnkH and AnkJ proteins could be required for translocation of the two proteins into macrophages, single, double, or triple in-frame deletions of the ANK domains of AnkH and AnkJ were fused to Cya (Fig. 2A and 3A). Immunoblot analysis revealed equivalent expression of the truncated proteins (Fig. 2B and 3B). The U937 cells were infected for 1 h with the WT strain or the dotA isogenic mutant expressing each of the truncated AnkH or AnkJ fusion proteins, and the intracellular cAMP level was determined in cell lysates. The level of cAMP in cells infected with L. pneumophila harboring the ANK domains deletion was significantly lower with >10-fold less for truncated AnkH and >7-fold less for truncated AnkJ compared to the full-length fusion or the positive control (Student t test, P < 0.0001) (Fig. 2C and 3C). Taken together, these data indicate that the ANK domains of AnkH and AnkJ are essential for their translocation into the host cell. This is the first example of the role of the eukaryotic-like domains in translocation of Dot/Icm effectors into the host cell. It is also possible that the reduced translocation may be due to a mild reduction in the protein level of the variant proteins.
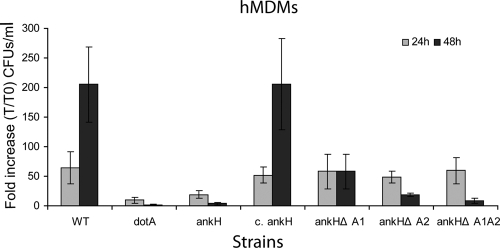
Role of the ANK domains of AnkH and AnkJ in intracellular growth of L. pneumophila.
We have previously shown that AnkH and AnkJ are required for intracellular replication of L. pneumophila (22). The aforementioned role of ANK domains of AnkH and AnkJ in translocation of AnkH and AnkJ proteins into host cytosol prompted us to examine their role in intracellular replication. Therefore, we performed single, double, or triple in-frame deletion of the ANK domains of AnkH and AnkJ. The ankH and ankJ mutants were transcomplemented with the full-length genes or the corresponding engineered ANK domain in-frame deletion constructs. The hMDMs were infected with L. pneumophila WT strain; the dotA, ankH, or ankJ mutant; and ankH and ankJ mutants transcomplemented with the various constructs. At 24 h postinfection, there was an increase in the number of bacteria in all strains ranging from 10- to 65-fold, with an increase in number of bacteria for the WT strain and the ankH mutant harboring all different constructs compared to dotA and the ankH mutant (Student t test, P < 0.001) (Fig. 4). However, at 48 h postinfection there was a significant reduction in the CFU of the ankH mutant transcomplemented with AnkHΔA1, AnkHΔA2 or AnkHΔA1ΔA2 compared to mutant complemented with the full-length gene with a >100-fold increase (Student t test, P < 0.0001). As expected, the dotA and the ankH mutants did not grow in hMDMs (Fig. 4). Because of inconsistent results in multiple experiments, data from the ankJ mutant transcomplemented with engineered ANK deletion constructs are not shown. Our results demonstrate that the two ANK domains of AnkH play a vital role in intracellular proliferation of L. pneumophila. It is also possible that the reduced intracellular growth may be due to a reduced translocation of the truncated proteins.
FIG. 4.
The ANK repeats of AnkH are indispensable for intracellular growth of L. pneumophila. Monolayers of hMDMs were infected with the L. pneumophila WT strain, the dotA or ankH mutants complemented with full-length AnkH, or constructs with in-frame deletions of the ANK domains. The infection was carried out in triplicate with an MOI of 10 for 1 h, followed by 1 h of gentamicin treatment to kill extracellular bacteria. At 24 and 48 h, L. pneumophila-infected cells were lysed and plated onto BCYE plates for CFU enumeration. The WT strain was used as a positive control and dotA mutant strain as a negative control. The results are represented as the fold increase (T/T0). The experiment was performed three times. The data points are the average of one representative experiment performed in triplicate. Error bars represent standard deviations of triplicate samples.
L. pneumophila ankH and ankJ mutants are attenuated in intrapulmonary replication in mice.
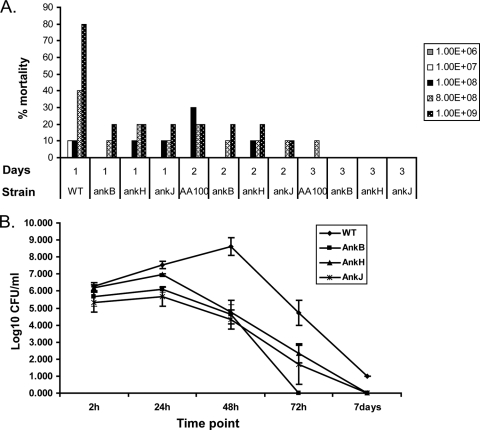
The AnkH and AnkJ effectors are essential for intracellular proliferation of L. pneumophila within hMDMs and protozoa (22), but whether the two effectors are required for the infection in vivo in animal models is not known. The AnkB attenuated mutant was used as a negative control (39). To determine whether the mutation in ankH or ankJ caused a decrease in mortality in the A/J mouse model, we infected mice intratracheally (6, 9, 20) with doses of 107 to 109 CFU. By the first day postinfection with a high dose of 109 CFU there was a mortality rate of 80% in mice infected by the WT strain compared to the mortality rate of 20% in mice infected by an ankB, ankH, or ankJ mutant at the similar dose (Fig. 5A) (Student t test, P < 0.001). These data show that the two Ank effectors contribute to lethality of Legionnaires' disease in the mice model of the disease, a finding consistent with their role in intracellular proliferation within cultured macrophages.
FIG. 5.
The L. pneumophila ankH and ankJ mutants are defective in the A/J mice model. (A) Groups of 30 mice were infected with 107, 108, 8 × 108, or 109 CFU of the L. pneumophila WT strain or the ankH or ankJ mutant compared to the ankB mutant. At 1, 2, 3, 4, and 5 days postinfection, mortality was determined. After 3 days there was no mortality. (B) A/J mice were infected with 106 CFU of L. pneumophila WT strain or the ankH or ankJ mutant and compared to the ankB mutant. At 1, 2, 3, and 7 days postinfection, three mice were sacrificed, and the lungs were collected for CFU enumeration.
To investigate whether AnkH and AnkJ are required for intrapulmonary proliferation of L. pneumophila, we infected A/J mice with 106 of the L. pneumophila WT strain or the ankH or ankJ mutant. Multiplication of L. pneumophila in the lungs of infected mice was assessed by CFU enumeration after 24 h, 48 h, 72 h, and 7 days postinfection. At 48 and 72 h postinfection, the CFU counts of L. pneumophila recovered from mice infected with the ankH or the ankJ mutant were significantly lower than for the WT strain (Student t test, P < 0.001) with at least a 1,000- and a 100-fold fewer bacteria, respectively, recovered from the lungs for both mutants (Fig. 5B). The ankB attenuated mutant (39), which was used as a control, was severely defective in intrapulmonary replication. These data show that the ankH and ankJ mutants are defective in intrapulmonary proliferation and mice mortality, a finding consistent with their in vitro intracellular growth defect in cultured macrophages (22). Taken together, our data show that the two Ank effectors are required for intrapulmonary proliferation and lethality of L. pneumophila in the mice model of Legionnaires' disease.
Expression and trafficking of AnkH and AnkJ in mammalian cells.
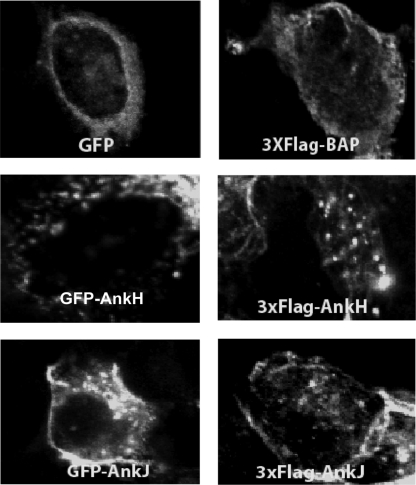
Ectopic expression of bacterial proteins in eukaryotic cells has been an important strategy to study localization of bacterial effector proteins translocated into host cell and may provide key insights into the function of the effectors (13, 30, 37). Therefore, GFP-tagged or 3×Flag-tagged AnkH and AnkJ were constructed and transiently or stably expressed in HEK293T or HEK293 cells to study their subcellular distribution and trafficking in mammalian cells. Constructs of GFP or bacterial alkaline phosphatase (BAP) fusion was used as a negative control. Transient transfection of HEK293T cells with plasmids harboring GFP-tagged or 3×Flag-tagged ankH and ankJ using calcium phosphate yielded 70 to 75% transfection efficiency. Transient or stable expression of AnkH and AnkJ were not toxic to the HEK293 cells (data not shown). Transient or stable expression of GFP-tagged or 3×Flag-tagged AnkH and AnkJ were distributed in the cytoplasm with punctuate distribution, but neither of the two effectors was detected in the nucleus (Fig. 6), suggesting that AnkH and AnkJ were associated with host cell vesicles. Nevertheless, these punctuate structures did not colocalize with L. pneumophila.
FIG. 6.
Subcellular localization of AnkH and AnkJ. HEK293T cells were transiently transfected with the empty vector pAcGFP, the pAcGFP-AnkH or pAcGFP-AnkJ fusion constructs, or with pBAP-3×Flag, and pAnkH-3×Flag or pAnkJ-3×Flag fusion constructs for 18 h. After transfection, localization of AnkH and AnkJ fusion proteins was examined by confocal laser scanning microscopy. Similar results were obtained in stable transfections (data not shown).
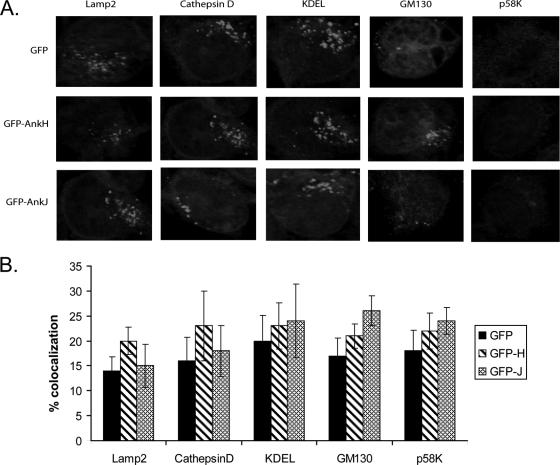
We utilized laser scanning confocal microscopy to determine trafficking and potential colocalization of the two effectors with endosomal, lysosomal, Golgi, ER, microfilament, microtubule, mitochondrial, and nuclear compartments using the specific markers Lamp2, cathepsin D, GM130 or P58-k, KDEL, actin, tubulin, mitochondrial protein, and nuclear dye (DAPI [4′,6′-diamidino-2-phenylindole]), respectively. The data showed that there were no significant differences in association of the above markers with GFP-AnkH or GFP-AnkJ fusion proteins compared to a GFP negative control (13 to 20%) (Student t test, P > 0.5), indicating that these proteins are not associated with endosomal, lysosomal, ER, or Golgi vesicles (Fig. 7). We conclude that despite the punctuate distribution of the two Ank effectors in mammalian cells, they do not colocalize with any subcellular compartment.
FIG. 7.
Colocalization of AnkH and AnkJ with endosomal, lysosomal, Golgi, and ER compartments. Stable transfected HEK293 cells expressing GFP, the GFP-AnkH or GFP-AnkJ fusion proteins were fixed and stained with anti-Lamp2, anti-cathepsinD, anti-KDEL, anti-GM130, and anti-p58k antibodies. (A) Their association with endosomal, lysosomal, and ER compartments was assessed by confocal laser scanning microscopy. (B) Quantitation of colocalization. The results shown are representative of three independent experiments performed in triplicate. The data represent means ± the standard deviations.
L. pneumophila ankH and ankJ mutants are rescued in HEK293 cells expressing AnkH- and AnkJ-GFP fusion proteins.
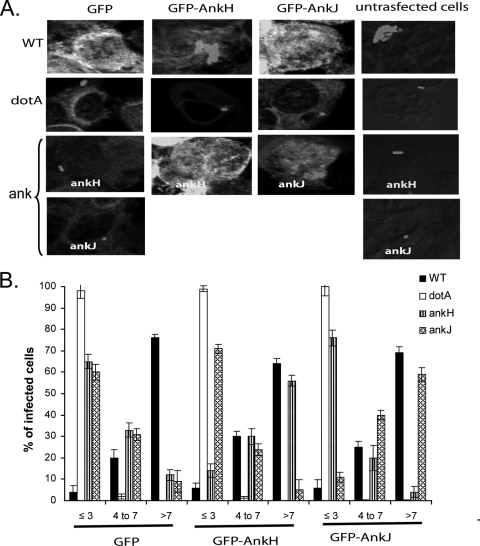
Since L. pneumophila ankH and ankJ mutants exhibited intracellular growth defect and the AnkH and AnkJ are translocated into host cells by the Dot/Icm system, we examined whether stable HEK293 cells expressing L. pneumophila AnkH or AnkJ fusion proteins could rescue the respective mutant for the defect in intracellular proliferation. Therefore, HEK293 cells with stable expression of GFP, GFP-AnkH, or GFP-AnkJ were infected with L. pneumophila WT or the dotA, ankH, or ankJ mutant. After 10 h postinfection, infected cells were labeled with anti-L. pneumophila antibody to evaluate the intracellular replication by single-cell analysis using confocal microscopy. The data showed that by 10 h postinfection, ∼70% of the WT strain-infected cells harbored more than seven bacteria/cell in HEK293 cells expressing GFP, GFP-AnkH, or GFP-AnkJ. In contrast, the dotA mutant did not replicate with one to three bacteria/infected cell. Similar to the WT-infected cells expressing GFP-AnkH or GFP-AnkJ, ∼60% of the ankH mutant-infected cells and ankJ mutant-infected cells harbored more than seven bacteria (Fig. 8). In contrast, the ankH and ankJ mutants did not replicate in HEK293 cells expressing GFP alone with >60% of the ankH and ankJ mutant-infected cells harbored ≤3 bacteria/cell (Fig. 8). The ankH mutant was not rescued in cells expressing ankJ or vice versa (data not shown). These data show that the ankH and ankJ mutants are rescued in mammalian cells expressing L. pneumophila AnkH and AnkJ, respectively. Collectively, our data indicate that AnkH and AnkJ modulate distinct processes in the host cell cytosol, a finding consistent with their distinct structure and mode of export.
FIG. 8.
L. pneumophila ankH or ankJ mutants are rescued within HEK293 cells expressing AnkH or AnkJ-GFP fusion proteins, respectively. HEK293 cells with stable expression of GFP and AnkH- and AnkJ-GFP fusion proteins were infected with the WT strain or the dotA, ankH, or ankJ mutant at an MOI of 5. (A) At 10 h postinfection, the cells were stained for laser scanning confocal microscopy analysis. (B) The percentages of infected cells harboring ≤3, 4 to 7, and >7 bacteria per cell were determined based on analyses of 100 infected cells. The results shown are representative of three independent experiments performed in triplicate. The data represent means ± the standard deviations.
DISCUSSION
The ANK domains are the most abundant domain in the eukaryotic kingdom, where they function as scaffold to mediate protein-protein interactions required for various eukaryotic cellular processes, ranging from regulation of transcription, signaling, cytoskeleton, and cell cycle regulation (5, 8, 34). Recently, genomic analyses have shown that L. pneumophila genome encodes a large family of eukaryotic-like ankyrin proteins (4, 11, 14, 22). It is thought that these proteins have been acquired by L. pneumophila through horizontal gene transfer through coevolution with its natural protozoan hosts to perhaps mimic or interfere with host cell processes to establish a replicative niche within host cells. In addition to AnkB, the AnkH and AnkJ proteins play a significant role in intracellular replication of L. pneumophila in human macrophages and protozoa (22), indicating that these Dot/Icm-translocated effectors modulate cellular processes that are highly conserved through evolution from protozoa to mammals. Importantly, our data show that the AnkH and AnkJ Dot/Icm-translocated effectors are essential for intrapulmonary proliferation in vivo in A/J mice. These data are consistent with the role of the two effectors in intracellular growth of L. pneumophila in macrophages (22). To our knowledge, AnkH, AnkJ, and AnkB are the first demonstration for an essential role of Dot/Icm-translocated effectors in intrapulmonary proliferation in animal models.
In several other pathogens, such as Agrobacterium, Bordetella, Helicobacter, Anaplasma, Coxiella, and Brucella spp., the TFSS is essential for delivery of host cell-modulating effectors (3). In agreement with two different studies (13, 37), our data show that seven L. pneumophila Ank proteins are delivered into the host cytosol. In contrast to de Filipe et al. study (13), using a different strategy our data show that AnkG/LegA7 is part of the cohort of Dot/Icm-translocated effectors. Consistent with the L. pneumophila Ank proteins being translocated by the Dot/Icm secretion system, there is a recent report of 13 Dot/Icm-translocated ankyrin proteins of Coxiella burnetii when expressed in L. pneumophila as a surrogate host (37, 44). Moreover, Anaplasma phagocytophilum encodes an ankyrin protein (AnkA), which is translocated by the TFSS into the host cell cytosol and nucleus (21, 24, 25). Given the rising number of translocated effector proteins by L. pneumophila into the host cell and its large spectrum of environmental hosts, it is possible that L. pneumophila selectively deploy a specific set of effectors that best promote its survival and proliferation within a specific host cell in the environment or in humans during infection.
The components of the Dot/Icm TFSS engage some of its effector proteins through a recognition of a translocation signal predicted to be at the C terminus of some Dot/Icm substrates (10, 35). Our data show that deletion of the last 10 residues of AnkH and AnkJ abrogates their translocation, indicating that their translocation signal is located at the C terminus (4, 12, 35).
The deletion of the ANK domains in AnkH and AnkJ reduced substantially their translocation, suggesting that the ANK domains actively participate in their translocation. Two explanations appear to validate the involvement of the ANK domains in AnkH and AnkJ proteins delivery into host cytosol. First, the ANK domains may participate in folding or unfolding of the AnkH or AnkJ proteins for suitable presentation of their C-terminal translocation signal to the Dot/Icm components. Second, the ANK domains may be involved in interaction between AnkH or AnkJ proteins and the Dot/Icm components in order to be properly delivered into host cells, where they interfere with host cell processes to facilitate bacterial proliferation. However, it is possible that the ANK deletion proteins are translocated into host cell and that the enzymatic activity of adenylate cyclase (Cya) is inhibited due to misfolding of the truncated protein, with Cya thereby becoming inaccessible to the activating host calmodulin. In addition, possible misfolding of the in-frame deletion of the Ank protein may have rendered it unrecognizable to be delivered by the Dot/Icm system.
Interestingly, expression of AnkH and AnkJ in mammalian cells show a punctate distribution throughout the cytosol, but our data indicate no association of AnkH or AnkJ with endosomal, lysosomal, ER, mitochondrial, and Golgi vesicles or actin and tubulin. These data are consistent with our previous findings that the phagosomes harboring the L. pneumophila ankH and ankJ mutants are trafficked in a manner similar to those harboring the WT strain (22), suggesting that these proteins are not involved in the trafficking of the LCV or recruitment of the ER to the LCV. It is unlikely that the punctuate distribution of proteins in mammalian cells is due to protein aggregation, since the ectopically expressed protein is functional in trans-rescue of the mutants for the defect in intracellular proliferation. Subcellular localization, as well as the host cell targets of the Ank effectors still to be identified.
The complementation of the ankH mutant by in-frame deletions of the ANK domains of AnkH does not restore its intracellular growth defect (22). However, deletion of the ANK domains of AnkH abrogates its translocation, indicating that the role of AnkH in intracellular replication requires its ANK domains that are also indispensable for its translocation. Whether translocation of the in-frame deletion of the Ank protein would render it functional in the host cell cytosol is not known.
Remarkably, our data indicate that ankH and ankJ mutants exhibit a severe intrapulmonary replication defect resulting in less mortality compared to the WT strain. This is consistent with our previous ex vivo results in human macrophages and alveolar epithelial cells (22) and corroborates a high rate of survival of animals infected by the ankH or ankJ mutants. AnkH, AnkJ, and AnkB are the first demonstration of the role of Dot/Icm effectors in the development of Legionnaires' disease in animal models (39).
Interestingly, when HEK293 cells expressing AnkH and AnkJ are infected with the ankH and ankJ mutants, the intracellular growth defect of the respective mutant is rescued. These data completely support the findings that the AnkH and AnkJ are translocated into the host cell cytosol to modulate distinct cytosolic processes needed to sustain the intracellular proliferation of the ankH and ankJ mutants. This is similar to AnkB where ectopic expression of a Dot/Icm effector in mammalian cells can rescue the growth of an effector mutant (39).
In summary, our data show that 3 L. pneumophila Ank proteins are delivered into the host cells in an IcmSW complex-dependent manner, and none of the seven translocated Ank proteins tested are delivered into the host cell by attached extracellular bacteria. Furthermore, our data indicate that the ANK domains and the C terminus of the AnkH and AnkJ are indispensable for translocation into the host cell, which is essential for intracellular proliferation of L. pneumophila. Ectopic expression in mammalian cells and colocalization studies show that AnkH and AnkJ proteins are distributed in punctuate structures throughout the cytosol and are not associated with nuclear, endosomal, lysosomal, Golgi, or ER compartments. Our data show that the L. pneumophila ankH and ankJ mutants are rescued for their intracellular growth defect in HEK293 cells expressing the respective effector.
Supplementary Material
Editor: J. N. Weiser
Footnotes
Published ahead of print on 22 December 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Abu-Zant, A., S. Jones, R. Asare, J. Suttles, C. Price, J. Graham, and Y. A. Kwaik. 2007. Anti-apoptotic signaling by the Dot/Icm secretion system of Legionella pneumophila. Cell Microbiol. 9:246-264. [DOI] [PubMed]
- 2.Abu Kwaik, Y., L.-Y. Gao, B. J. Stone, C. Venkataraman, and O. S. Harb. 1998. Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl. Environ. Microbiol. 64:3127-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Khodor1, S., C. T. Price, A. Kalia, and Y. Abu Kwaik. Functional diversity of ankyrin repeats in microbial proteins. Trends Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 4.Al-Khodor, S., C. T. Price, F. Habyarimana, A. Kalia, and Y. Abu Kwaik. 2008. A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol. Microbiol. 70:908-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amstutz, P., H. K. Binz, P. Parizek, M. T. Stumpp, A. Kohl, M. G. Grutter, P. Forrer, and A. Pluckthun. 2005. Intracellular kinase inhibitors selected from combinatorial libraries of designed ankyrin repeat proteins. J. Biol. Chem. 280:24715-24722. [DOI] [PubMed] [Google Scholar]
- 6.Asare, R., M. Santic, I. Gobin, M. Doric, J. Suttles, J. Graham, C. Price, and Y. A. Kwaik. 2007. Genetic susceptibility to Legionella longbeachae and caspase activation within mice and human macrophages is distinct from L. pneumophila. Infect. Immun. 75:1933-1945. [DOI] [PMC free article] [PubMed]
- 7.Bardill, J. P., J. L. Miller, and J. P. Vogel. 2005. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol. Microbiol. 56:90-103. [DOI] [PubMed] [Google Scholar]
- 8.Batchelor, A. H., D. E. Piper, F. C. de la Brousse, S. L. McKnight, and C. Wolberger. 1998. The structure of GABPα/β: an ETS domain-ankyrin repeat heterodimer bound to DNA. Science 279:1037-1041. [DOI] [PubMed] [Google Scholar]
- 9.Brieland, J., P. Freeman, R. Kunkel, C. Chrisp, M. Hurley, J. Fantone, and C. Engleberg. 1994. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice: a murine model of human Legionnaires' disease. Am. J. Pathol. 145:1537-1546. [PMC free article] [PubMed] [Google Scholar]
- 10.Cambronne, E. D., and C. R. Roy. 2007. The Legionella pneumophila IcmSW complex interacts with multiple Dot/Icm effectors to facilitate type IV translocation. PLoS Pathog. 3:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cazalet, C., C. Rusniok, H. Bruggemann, N. Zidane, A. Magnier, L. Ma, M. Tichit, S. Jarraud, C. Bouchier, F. Vandenesch, F. Kunst, J. Etienne, P. Glaser, and C. Buchrieser. 2004. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 36:1165-1173. [DOI] [PubMed] [Google Scholar]
- 12.Chen, J., M. Reyes, M. Clarke, and H. A. Shuman. 2007. Host cell-dependent secretion and translocation of the LepA and LepB effectors of Legionella pneumophila. Cell Microbiol. 9:1660-1671. [DOI] [PubMed] [Google Scholar]
- 13.De Felipe, K. S., R. T. Glover, X. Charpentier, O. R. Anderson, M. Reyes, C. D. Pericone, and H. A. Shuman. 2008. Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog. 4:e1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Felipe, K. S., S. Pampou, O. S. Jovanovic, C. D. Pericone, S. F. Ye, S. Kalachikov, and H. A. Shuman. 2005. Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J. Bacteriol. 187:7716-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derre, I., and R. R. Isberg. 2004. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect. Immun. 72:3048-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ensminger, A. W., and R. R. Isberg. 2009. Legionella pneumophila Dot/Icm translocated substrates: a sum of parts. Curr. Opin. Microbiol. 12:67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feeley, J. C., R. J. Gibson, G. W. Gorman, N. C. Langford, J. K. Rasheed, D. C. Mackel, and W. B. Baine. 1979. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J. Clin. Microbiol. 10:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fields, B. S., G. N. Sanden, J. M. Barbaree, W. E. Morrill, R. M. Wadowsky, E. H. White, and J. C. Feeley. 1989. Intracellular multiplication of Legionella pneumophila in amoebae isolated from hospital hot water tanks. Curr. Microbiol. 18:131-137. [Google Scholar]
- 19.Fraser, D. W., T. R. Tsai, W. Orenstein, W. E. Parkin, H. J. Beecham, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, J. E. McDade, C. C. Shepard, and P. S. Brachman. 1977. Legionnaires' disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297:1189-1197. [DOI] [PubMed] [Google Scholar]
- 20.Gao, L. Y., B. J. Stone, J. K. Brieland, and Y. Abu Kwaik. 1998. Different fates of Legionella pneumophila pmi and mil mutants within macrophages and alveolar epithelial cells. Microb. Pathog. 25:291-306. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Garcia, J. C., K. E. Rennoll-Bankert, S. Pelly, A. M. Milstone, and J. S. Dumler. 2009. Silencing of host cell CYBB gene expression by the nuclear effector AnkA of the intracellular pathogen Anaplasma phagocytophilum. Infect. Immun. 77:2385-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habyarimana, F., S. Al-Khodor, A. Kalia, J. E. Graham, C. T. Price, M. T. Garcia, and Y. A. Kwaik. 2008. Role for the ankyrin eukaryotic-like genes of Legionella pneumophila in parasitism of protozoan hosts and human macrophages. Environ. Microbiol. 10:1460-1474. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz, M. A. 1983. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158:2108-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ijdo, J., A. C. Carlson, and E. L. Kennedy. 2007. Anaplasma phagocytophilum AnkA is tyrosine-phosphorylated at EPIYA motifs and recruits SHP-1 during early infection. Cell Microbiol. 9:1284-1296. [DOI] [PubMed] [Google Scholar]
- 25.Ijdo, J. W., A. C. Carlson, and E. L. Kennedy. 2007. Anaplasma phagocytophilum AnkA is tyrosine-phosphorylated at EPIYA motifs and recruits SHP-1 during early infection. Cell Microbiol. 9:1284-1296. [DOI] [PubMed] [Google Scholar]
- 26.Isberg, R. R., T. J. O'Connor, and M. Heidtman. 2009. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 7:13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagan, J. C., and C. R. Roy. 2002. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 4:945-954. [DOI] [PubMed] [Google Scholar]
- 28.Kagan, J. C., M. P. Stein, M. Pypaert, and C. R. Roy. 2004. Legionellae subvert the functions of rab1 and sec22b to create a replicative organelle. J. Exp. Med. 199:1201-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufmann, A. F., J. E. McDade, C. M. Patton, J. V. Bennett, P. Skaliy, J. C. Feeley, D. C. Anderson, M. E. Potter, V. F. Newhouse, M. B. Gregg, and P. S. Brachman. 1981. Pontiac fever: isolation of the etiologic agent (Legionella pneumophila) and demonstration of its mode of transmission. Am. J. Epidemiol. 114:337-347. [DOI] [PubMed] [Google Scholar]
- 30.Kubori, T., A. Hyakutake, and H. Nagai. 2008. Legionella translocates an E3 ubiquitin ligase that has multiple U-boxes with distinct functions. Mol. Microbiol. 67:1307-1319. [DOI] [PubMed] [Google Scholar]
- 31.Laguna, R. K., E. A. Creasey, Z. Li, N. Valtz, and R. R. Isberg. 2006. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc. Natl. Acad. Sci. U. S. A. 103:18745-18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, Y., and Z. Q. Luo. 2007. The Legionella pneumophila effector SidJ is required for efficient recruitment of endoplasmic reticulum proteins to the bacterial phagosome. Infect. Immun. 75:592-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo, Z. Q., and R. R. Isberg. 2004. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. U. S. A. 101:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosavi, L. K., T. J. Cammett, D. C. Desrosiers, and Z. Y. Peng. 2004. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 13:1435-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagai, H., E. D. Cambronne, J. C. Kagan, J. C. Amor, R. A. Kahn, and C. R. Roy. 2005. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc. Natl. Acad. Sci. U. S. A. 102:826-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ninio, S., D. M. Zuckman-Cholon, E. D. Cambronne, and C. R. Roy. 2005. The Legionella IcmS-IcmW protein complex is important for Dot/Icm-mediated protein translocation. Mol. Microbiol. 55:912-926. [DOI] [PubMed] [Google Scholar]
- 37.Pan, X., A. Luhrmann, A. Satoh, M. A. Laskowski-Arce, and C. R. Roy. 2008. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science 320:1651-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pomerantsev, A. P., and V. M. Pavlov. 1999. pCSE4 plasmid for cloning of promoter-containing DNA fragments in Francisella tularensis. Vestn. Ross Akad. Med. Nauk. 12:29-32. (In Russian.) [PubMed] [Google Scholar]
- 39.Price, C. T. 2009. Acquisition of poly-ubiquitinated proteins by Legionella pneumophila containing phagosome requires a bacterial F-box containing ankyrin effector. PLoS Pathog. 5:e1000704.20041211 [Google Scholar]
- 40.Ragaz, C., H. Pietsch, S. Urwyler, A. Tiaden, S. S. Weber, and H. Hilbi. 2008. The Legionella pneumophila phosphatidylinositol-4 phosphate-binding type IV substrate SidC recruits endoplasmic reticulum vesicles to a replication-permissive vacuole. Cell Microbiol. 10:2416-2433. [DOI] [PubMed] [Google Scholar]
- 41.Santic, M., M. Molmeret, and Y. Abu Kwaik. 2005. Maturation of the Legionella pneumophila-containing phagosome into a phagolysosome within gamma interferon-activated macrophages. Infect. Immun. 73:3166-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swanson, J. A., and S. C. Baer. 1995. Phagocytosis by zippers and triggers. Trends Cell Biol. 5:89-93. [DOI] [PubMed] [Google Scholar]
- 43.Tilney, L. G., O. S. Harb, P. S. Connelly, C. G. Robinson, and C. R. Roy. 2001. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J. Cell Sci. 114:4637-4650. [DOI] [PubMed] [Google Scholar]
- 44.Voth, D. E., D. Howe, P. A. Beare, J. P. Vogel, N. Unsworth, J. E. Samuel, and R. A. Heinzen. 2009. The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J. Bacteriol. 191:4232-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber, S. S., C. Ragaz, K. Reus, Y. Nyfeler, and H. Hilbi. 2006. Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog. 2:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zink, S. D., L. Pedersen, N. P. Cianciotto, and Y. Abu Kwaik. 2002. The Dot/Icm type IV secretion system of Legionella pneumophila is essential for the induction of apoptosis in human macrophages. Infect. Immun. 70:1657-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.