Abstract
Capsular polysaccharides (CP) of serotypes 5 (CP5) and 8 (CP8) are major Staphylococcus aureus virulence factors. Previous studies have shown that salicylic acid (SAL), the main aspirin metabolite, affects the expression of certain bacterial virulence factors. In the present study, we found that S. aureus strain Reynolds (CP5) cultured with SAL was internalized by MAC-T cells in larger numbers than strain Reynolds organisms not exposed to SAL. Furthermore, the internalization of the isogenic nonencapsulated Reynolds strain into MAC-T cells was not significantly affected by preexposure to SAL. Pretreatment of S. aureus strain Newman with SAL also enhanced internalization into MAC-T cells compared with that of untreated control strains. Using strain Newman organisms, we evaluated the activity of the major cap5 promoter, which was significantly decreased upon preexposure to SAL. Diminished transcription of mgrA and upregulation of the saeRS transcript, both global regulators of CP expression, were found in S. aureus cultured in the presence of SAL, as ascertained by real-time PCR analysis. In addition, CP5 production by S. aureus Newman was also decreased by treatment with SAL. Collectively, our data demonstrate that exposure of encapsulated S. aureus strains to low concentrations of SAL reduced CP production, thus unmasking surface adhesins and leading to an increased capacity of staphylococci to invade epithelial cells. The high capacity of internalization of the encapsulated S. aureus strains induced by SAL pretreatment may contribute to the persistence of bacteria in certain hosts.
Staphylococcus aureus is an opportunistic pathogen that causes both community-acquired and life-threatening nosocomial infections (35). Although S. aureus can colonize mucosal surfaces of healthy humans, it is also a major cause of skin and soft tissue infections and has the invasive potential to cause severe infections, including osteomyelitis, endocarditis, and bacteremia with metastatic complications (35). The pathogenicity of S. aureus depends upon successful adaptation of the microorganism to the host and the coordinated expression of virulence factors. S. aureus is usually surrounded by a thin capsule, and capsular polysaccharides (CP) of serotypes 5 (CP5) and 8 (CP8) are the most prevalent ones in clinical isolates from humans (40). S. aureus CP5 and CP8 are antiphagocytic and positively contribute to the virulence of this pathogen (27, 40). Production of CP5 (or CP8) may be deeply modified by different global regulators (agr, arlRS, saeRS, and sarA) and transcriptional factors (mgrA, σB) (30, 31, 34, 55, 59). A recent report has shown that the sbcDC locus mediates repression of CP5 production as a part of the SOS response in S. aureus (5). Furthermore, the expression of CP5 (7) is highly sensitive to diverse environmental signals, such as iron concentration, specific nutrients, CO2 concentration, or subinhibitory concentrations of ciprofloxacin and mitomycin C (5, 17, 26, 40).
Aspirin is a nonsteroidal anti-inflammatory agent that is regularly taken by hundreds of millions of individuals worldwide due to its known analgesic and cardiovascular protective activities. The main aspirin biometabolite, salicylic acid (SAL), affects the expression of bacterial virulence factors (45). A study performed using a rabbit model of endocarditis demonstrated that SAL causes a reduction in S. aureus virulence (23, 24). Biofilm formation and attachment of S. aureus strain 8325 to the Arabidopsis thaliana root surface were disrupted by a low concentration of SAL (48). It has also been shown that the effects of SAL on S. aureus include activation of the sigB operon via both rsbU-dependent and -independent mechanisms (24, 42). Moreover, growth of S. aureus in the presence of SAL reduced bacterial susceptibility to multiple antimicrobials (16, 44, 46, 47).
S. aureus can adhere to and invade nonprofessional phagocytic cells (9, 12, 54). The relevance of this finding is that intracellular S. aureus may be a source of persistent or recurrent infection (60). Results from our laboratory demonstrated that the production of CP5 (or CP8) reduced the internalization of S. aureus into epithelial cells (4, 57). Growth of Klebsiella pneumoniae, a capsule-containing microorganism, in the presence of SAL also resulted in reduced synthesis of CP (6, 7). In this study, we evaluated whether SAL negatively affects CP5 expression in S. aureus, thus enhancing the capacity of S. aureus to invade epithelial cells.
MATERIALS AND METHODS
Bacterial isolates and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were stored in Trypticase soy broth (TSB) (Difco, Detroit, MI) with 20% glycerol at −20°C until use. S. aureus was routinely cultured at 37°C and 200 rpm for 18 h in Casamino Acids-yeast extract-glycerophosphate (CYGP) broth without glucose (CYGPw) (38). When necessary, SAL was added to the culture medium at 50 μg/ml (0.36 mM). For cell invasion experiments, bacterial cells were collected by centrifugation, washed with sterile saline solution, and suspended in invasion medium (see below) to a density of ca. 107 CFU/ml.
TABLE 1.
Staphylococcus aureus strains and plasmids used in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| S. aureus strains | ||
| Newman | Clinical isolate (ATCC 25904); CP5 producer | 8 |
| RN6390 | Laboratory strain related to 8325-4 | 22 |
| Reynolds (CP5) | Capsular polysaccharide serotype 5 strain Reynolds | 62 |
| Reynolds (CP−) | Reynolds (CP5) isogenic mutant that does not express CP5 | 62 |
| ALC2547 | mgrA mutant of Newman (ΔmgrA::ermC) | 20 |
| ALC4483 | Newman sae single-deletion mutant | 28 |
| ALC1842 | Strain Newman with pALC1766 | 59 |
| ALC5163 | Strain Newman with pALC4991 | 28 |
| ALC6141 | Strain Newman with pALC2566 | 19 |
| ALC3257 | Strain Newman with pALC1484 | 33 |
| Plasmids | ||
| pALC1484 | Derivative of pSK236 containing the promoterless gfpuvr gene preceded by an S. aureus ribosome binding site | 33 |
| pALC1766 | Derivative of pALC1484 containing the main cap5 promoter driving the expression of the gfpuvr gene | 59 |
| pALC4991 | Derivative of pALC1484 containing the sae P3 promoter driving the expression of the gfpuvr gene | 28 |
| pALC2566 | Derivative of pALC1484 containing the mgrA promoter driving the expression of the gfpuvr gene | 19 |
Antigenic extracts.
Bacterial extracts from wild-type Newman and mgrA and saeRS isogenic mutant strains (Table 1) were prepared as previously described (56). Briefly, S. aureus was cultured for 24 h at 37°C on Columbia agar (Difco) supplemented with 2% NaCl, to enhance CP production, plus 0.36 or 2 mM SAL. Controls were cultured in the same medium with no SAL added. The colonies from one plate were harvested in 1 ml of 10 mM phosphate-buffered saline (PBS) (0.15 M NaCl, pH 7.2), and the cell suspensions were autoclaved for 1 h at 121°C. Bacteria were pelleted by centrifugation at 10,000 × g, and the supernatants containing the cell extracts were passed through 0.45-μm filters and stored at −20°C. Bacterial extracts from isogenic Reynolds (CP5) and Reynolds (CP−) (nonencapsulated) reference strains of S. aureus were used as positive and negative controls, respectively.
CP expression by immunoprecipitation.
CP5 expression in S. aureus Newman was determined by immunoprecipitation in the presence of 0.36 or 2 mM SAL as previously described (21). Briefly, a 1% agarose gel was prepared on a glass slide, and wells were punched in a circular fashion around a central hole. Absorbed type 5 antiserum (25) was added to the central well, and serial dilutions of bacterial extracts [including known serotype 5 Reynolds (CP5) and nonencapsulated Reynolds (CP−) extracts as controls] were applied to the outer wells. Immunodiffusion was conducted in a moist chamber at room temperature. After 24 h, the precipitin lines were examined by staining with Coomassie brilliant blue.
Cell culture.
The established bovine mammary epithelial cell line MAC-T (18) was generously provided by Nexia Biotechnologies (Quebec, Canada). MAC-T cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL), insulin (5 μg/ml), hydrocortisone (5 μg/ml), penicillin (100 U/ml), and streptomycin sulfate (100 μg/ml) (Sigma Chemical Co., St. Louis, MO). Prior to each experiment, MAC-T cells were seeded at 1.5 × 105 cells/well in 24-well tissue culture plates and grown for 24 h at 37°C with 5% CO2.
Internalization assays.
Internalization assays were performed as described previously (4). Briefly, confluent MAC-T cell monolayers (approximately 2.5 × 105 cells/well) were inoculated with S. aureus suspended in fresh growth medium without antibiotics (invasion medium) to a multiplicity of infection (MOI) of 40. Plates were subjected to centrifugation at 1,000 × g for 20 min to deposit bacteria on the monolayer surface and to synchronize the bacterial internalization into cells. After incubation for 1 h at 37°C under 5% CO2, the wells were washed with PBS, and then 1 ml of invasion medium supplemented with 25 μg of lysostaphin (Sigma) was added to each well to kill extracellular bacteria. Incubation of cocultures with lysostaphin proceeded for an additional 2 h at 37°C with 5% CO2. Supernatants were then collected and plated on Trypticase soy agar (TSA) to verify 100% bacterial killing by lysostaphin. The monolayer was washed with sterile PBS, treated for 5 min at 37°C with 100 μl of 0.25% trypsin-0.1% EDTA (Gibco BRL), and lysed by the addition of 900 μl of 0.025% Triton X-100 (USB, Cleveland, OH) in sterile distilled water to release intracellular staphylococci. The CFU number was determined by quantitative plating on TSA. MAC-T cell viability was evaluated by trypan blue exclusion.
Transcriptional fusion studies.
After overnight culture, S. aureus strains harboring different recombinant plasmids (Table 1) were diluted 1:100 and grown with or without 50 μg/ml SAL at 37°C with shaking in CYGPw broth. Aliquots were transferred hourly to microtiter plates and assessed for cell density (optical density at 650 nm [OD650]) and fluorescence for 9 h in an FL600 fluorescence spectrophotometer (BioTek Instruments, Winooski, VT). Promoter activities were plotted as mean fluorescence/OD650 ratios to minimize variations due to changes in cell density between experiments, using the average values for triplicate readings.
Real-time PCR.
Bacterial RNA was extracted using Trizol (Gibco BRL) and 0.1-mm silica beads in a reciprocating shaker (Biospec, Bartlesville, OK) according to the manufacturer's instructions. RNA was subjected to DNase treatment, using a Turbo DNAfree kit (Ambion, Austin, TX) according to the manufacturer's protocol. cDNA synthesis was performed with a Transcriptor first-strand cDNA synthesis kit (Roche, Basel, Switzerland), using random hexamer primers. Quantitative real-time PCR was performed using LightCycler FastStart DNA Master SYBR green I (Roche) equipment and kits. cDNA was subjected to real-time PCR using the following primers: cap5K-f, 5′-CCA GTG AAT TGT TTG CAA CG-3′; cap5K-r, 5′-CAT TTT CCC AAT AAA TGT TGA AAG-3′; mgrA-f, 5′-GGG ATG AAT CTC CTG TAA AC-3′; mgrA-r, 5′-GCT GAA GCG ACT TTG TCA GA-3′; saeRS-f, 5′-ATG CTA ATA CCG TGA ATG TCC A-3′; saeRS-r, 5′-TGG CCG TTA AAC CAC ATT AAA-3′ (3.1-, 2.4-, and 2.0-kb sae transcripts were amplified using these primers); gyrB-f, 5′-GGT GCT GGG CAA ATA CAA GT-3′; and gyrB-r, 5′-TGG GAT ACC ACG TCC GTT AT-3′. The gyrB gene was used as a calibrator and as an internal control to normalize data. Cycling conditions were 95°C for 10 min followed by 45 cycles of 95°C for 10 s, 55°C for 10 s, and 72°C for 15 s and 1 cycle of 40°C for 30 s. The number of copies of each sample transcript was determined with the aid of LightCycler software. The −ΔΔCT value represents the difference in threshold cycle (CT) between the target and control (gyrB) genes treated with SAL minus the difference in CT between the untreated target and control genes (29).
Statistical analysis.
Nonparametric data were analyzed with the Mann-Whitney test, using GraphPad software (version 4.0; GraphPad Prism). P values of <0.05 were considered significant.
RESULTS
Effect of SAL on internalization of encapsulated S. aureus.
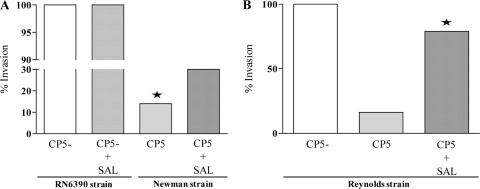
Previous studies have reported a reduction in staphylococcal adherence when bacteria are cultured in the presence of SAL (10, 36, 43). In the present study, we investigated whether S. aureus strains Newman and RN6390 treated with SAL exhibited a reduced ability to invade epithelial cells. S. aureus Newman organisms express CP5, whereas S. aureus RN6390 cells do not express CP5 due to a mutation in the essential capE5 gene (61). The SAL concentration (0.36 mM) used in the experiments did not affect the growth of the S. aureus strains used in this study (data not shown) and represents the levels achievable in human serum after ingestion of aspirin in pharmacological (antithrombotic) doses (11, 37). Two hours after addition of lysostaphin to the infected MAC-T cells, the number of intracellular CFU of S. aureus RN6390 cells pretreated with SAL did not differ significantly compared with that of cells without SAL pretreatment (Fig. 1A). Preexposure of S. aureus Newman to SAL significantly enhanced the internalization of the bacteria into MAC-T cells compared with that of untreated bacteria (Fig. 1A). This finding was consistent with previous results from our laboratory demonstrating that S. aureus strains with no capsule or reduced capsule formation displayed an increased ability to invade MAC-T cells compared with those that express either CP5 or CP8, possibly due to unmasking of surface adhesins beneath the capsule (4, 57). The differences observed in the present study were likely attributed to the influence of SAL on CP expression. To test this hypothesis, we performed a similar study with S. aureus Reynolds (CP5) and the isogenic nonencapsulated Reynolds (CP−) derivative under the conditions described above. As shown in Fig. 1B, Reynolds (CP5) organisms pretreated with SAL were internalized by the MAC-T cells in significantly higher numbers than those of Reynolds (CP5) organisms not exposed to SAL. Moreover, the invasiveness of the nonencapsulated derivative of the Reynolds strain was not significantly affected by preexposure to SAL (data not shown). Taken together, our results demonstrated that encapsulated strains of S. aureus pretreated with SAL exhibited an increased capacity for invasion of MAC-T epithelial cells, probably due to decreased CP expression.
FIG. 1.
Effect of SAL on internalization of S. aureus into MAC-T epithelial cells. Confluent MAC-T cells were tested for staphylococcal invasion. Each bar represents the “percentage of invasion,” defined as the median of intracellular CFU/ml (n = 9 to 12) from S. aureus strains related to the median of intracellular CFU/ml from S. aureus RN6390 or Reynolds (CP−) without SAL treatment (100% invasion). The asterisks represent significant differences between strain Newman treated and not treated with SAL (P = 0.0001) (A) and between Reynolds (CP5) organisms treated and not treated with SAL (P = 0.0089; Mann-Whitney test) (B). Comparison of Reynolds (CP5) treated with SAL and Reynolds (CP−) showed no significant differences.
cap expression is diminished by SAL treatment.
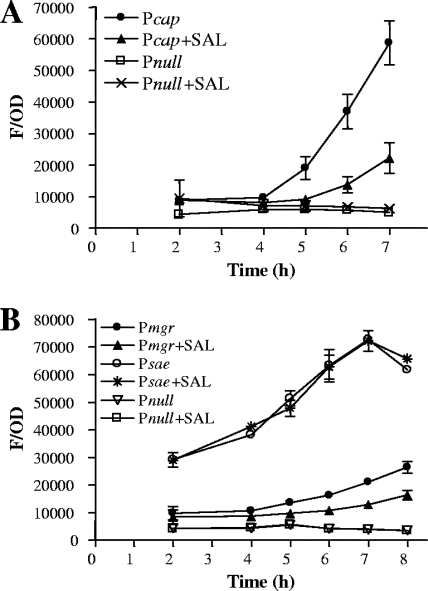
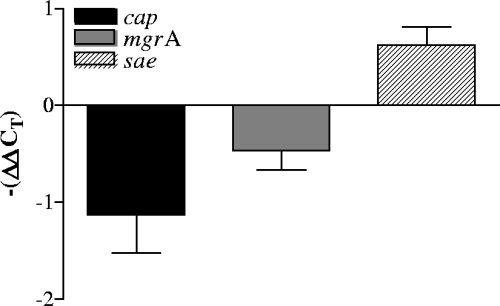
The effect of SAL on cap5 promoter activity was ascertained by expression of the gfpuvr reporter gene driven by the major cap5 promoter. As shown in Fig. 2A, SAL induced a significant decrease in the activity of the strain Newman cap5 promoter. The promoter activities of saeRS and mgrA, two known regulators involved in down- and upmodulation of CP expression, respectively, were also measured after SAL exposure. These assays established that treatment with SAL reduced the mgrA promoter activity (Fig. 2B). However, the main sae promoter (28) was not affected by SAL treatment at the time point studied (Fig. 2B). Furthermore, real-time PCR experiments were performed to estimate the quantities of cap5K, saeRS, and mgrA transcripts formed under the effects of SAL. Our results showed that the levels of expression of cap5K and mgrA transcripts decreased in S. aureus organisms treated with SAL. In contrast, SAL treatment increased the level of saeRS transcript expression (Fig. 3). These results demonstrate that pretreatment of S. aureus Newman organisms with SAL decreased transcription of cap5 and mgrA, an upregulator of CP expression. Decreased transcription of cap5 and mgrA was accompanied by enhanced expression of saeRS, a downregulator of CP expression, thus contributing to an overall decrease in CP expression upon SAL exposure.
FIG. 2.
Effect of SAL on cap5 (A), mgrA (B), and sae P3 (B) promoter activities. Expression of gfp driven by the target promoters was measured during the growth cycle, and fluorescence values were expressed as green fluorescent protein fluorescence related to the OD650 (F/OD) in order to minimize variations in fluorescence due to varying cell density. The data represent the arithmetic means ± standard deviations for triplicate measurements from three or four independent experiments.
FIG. 3.
Real-time PCR analysis of cap, mgrA, and sae transcription. Changes in gene expression are shown as fold changes [−(ΔΔCT)] following growth with 50 μg/ml of SAL to an OD650 of 1.4. The data represent the means ± standard deviations for duplicate measurements from three independent experiments.
SAL reduces phenotypic CP expression.
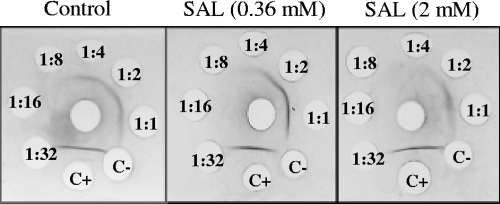
The effect of SAL on CP production was also evaluated by immunoprecipitation, using monospecific antiserum to CP5. This method was suitable for semiquantitative estimation of the amount of CP produced using twofold serial dilutions of CP extracts (32). A high concentration of SAL (2 mM) was used to investigate the effect on CP expression. The results revealed a dose-dependent decrease of CP5 expression, caused by SAL, in S. aureus Newman organisms (Fig. 4). The highest CP5 expression level was seen in the control (bacteria not treated with SAL). These results showed that CP5 production by the Newman strain organisms was decreased by treatment with SAL.
FIG. 4.
Immunodiffusion analysis of CP5 extracts. Thirty microliters of monospecific antiserum to serotype 5 was added to the center well. Thirty-microliter samples of undiluted and twofold serially diluted (indicated inside the well) CP5 extracts of Newman strain organisms, cultured or not with different concentrations of SAL, were added to the outer wells. Precipitin lines were visualized after staining with Coomassie brilliant blue. C+, positive control [undiluted extract of S. aureus Reynolds (CP5) strain]; C−, negative control [undiluted extract of S. aureus Reynolds (CP−) strain]. Precipitin lines were visible up to a 1:4 dilution of capsular extracts in the control without SAL, up to a 1:2 dilution at a 0.36 mM SAL concentration, and at only a 1:1 dilution at the highest SAL concentration (2 mM).
DISCUSSION
Previous results from our laboratory demonstrated that nonencapsulated S. aureus strains were internalized more efficiently into MAC-T cells than were their encapsulated (CP5+ or CP8+) counterparts (4, 57). In the present study, we demonstrated that pretreatment of encapsulated (CP5+) S. aureus strain Newman organisms with SAL increased the ability of the bacteria to invade MAC-T cells. This observation correlated with a diminished production of CP5. Our experiments were conducted in medium without glucose to avoid the repression of CP production (52). Besides, the concentration of SAL (0.36 mM) used matched the antiplatelet concentration achievable in human plasma (11, 37). It was recently reported that S. aureus 8325-4 (nonencapsulated) (61) cultured in the presence of SAL exhibited reduced adhesion to human umbilical vein endothelial cells (43). In our studies performed with strain RN6390 (an 8325 strain derivative), we were unable to confirm these experimental results. This discrepancy, however, can be explained by the different cell line used and, perhaps more importantly, the major differences in the experimental design and bacterial growth conditions utilized.
Internalization of S. aureus into mammalian cells depends upon fibronectin bridging between fibronectin binding proteins (FnBPs) and the integrin α5β1 (53). Kupferwasser et al. (24) described that pretreatment of several S. aureus strains with SAL (0.36 mM) resulted in low levels of adhesion to cell matrix proteins, such as fibronectin and fibrinogen. In that study, the reduction in matrix binding protein expression on the surface of S. aureus represented composite pooled data derived from S. aureus laboratory strains RN6390, COL, Newman, and ISP479C as well as from several clinical (methicillin-resistant S. aureus [MRSA]) S. aureus isolates. Grundmeier et al. (15) have shown that both fnbA and fnbB of S. aureus Newman contain a centrally located point mutation resulting in a stop codon. This causes a truncation of both FnBPs at the end of the C domain. Consequently, this led to deficient adherence of strain Newman compared with that of S. aureus strains with wild-type FnBPs. Our results also confirmed that strain Newman (7 × 104 CFU/ml) organisms were internalized into MAC-T cells at lower rates than those of strain RN6390 (4.9 × 105 CFU/ml) organisms. However, when both strains were cultured in the presence of SAL, only the internalization capability of strain Newman was affected. Moreover, the increased invasion of MAC-T cells after SAL treatment of Newman organisms may suggest that another factor(s) besides CP may be affected by SAL, thus improving cellular internalization (2). We are now conducting experiments to evaluate this hypothesis. The preliminary results of SDS-PAGE analysis of Newman, AH12 (Newman eap mutant), and complemented strain AH12pCXEap surface proteins revealed increased Eap expression after SAL treatment in strain Newman (L. P. Alvarez et al., unpublished data). Thus, the increased invasion observed after SAL treatment may be due not only to the diminished expression of CP but also to an increased expression of certain adhesins, such as Eap.
The low CP5 production by S. aureus induced by SAL treatment was also seen at the transcriptional level. Previous studies showed that expression of the cap operon is mainly driven by the major promoter located at the beginning of the operon (41, 51). The expression of the major cap5 promoter was thus monitored with a cap5 promoter-gfp+ reporter gene fusion by measuring fluorescence emission. Indeed, the activity of the cap5 promoter was diminished by exposure to SAL. Downregulation of CP5 by SAL was also confirmed by analyzing the cap5K (CP5-specific region) mRNA, using real-time PCR. Blickwede et al. (3) observed that mRNA expression of cap5A was decreased in S. aureus strain Newman after treatment with subinhibitory concentrations of florfenicol (an antibiotic used in veterinary medicine), indicating that cap expression can be affected by different pharmacological agents. In the present study, when the expression of CP5 was evaluated by immunoprecipitation using monospecific antiserum to CP5, we observed a dose-dependent reduction of CP5 production by S. aureus Newman after SAL treatment. In a study on Gram-negative bacteria, Domenico et al. (6) observed a decrease of K. pneumoniae CP production by SAL, in a concentration-dependent manner. In particular, at 0.21 mM SAL, CP was reduced nearly 60%. In both cases, SAL exerted its effect on CP production at serum concentrations normally attained after ingestion of aspirin at doses within the therapeutic range.
Expression of the cap operon and CP expression in S. aureus are known to be under multiple levels of control and affected by several environmental stimuli (49). Previous studies have shown that the global regulators agr, mgrA, arlRS, σB, sarA, and saeRS control CP production at the transcriptional level (30, 31, 34, 55, 59). It should be noted that the results from several previous reports are contradictory. In this regard, the sae locus has been shown to repress the cap5 genes (55). In contrast, Rogasch et al. (50) did not find any influence of SaeRS on the transcription of the cap operon in S. aureus strain Newman or COL. On the other hand, the sbcD and sbcC genes were involved in the repression of CP5 production through an arl-mgr-dependent pathway at subinhibitory concentrations of ciprofloxacin or mitomycin C (5). As expected, we observed that after SAL treatment of S. aureus strain Newman, the activity of the mgrA promoter was diminished. In agreement with this finding, the level of mgrA transcript, as determined by real-time PCR, was also decreased after SAL exposure. Similarly, Riordan et al. (49) demonstrated that salicylate induction downregulated mgrA transcripts. In addition, the mgrA mutant of the Newman strain did not express CP under any of the conditions studied here (data not shown). Several studies identified four overlapping transcripts (3.1-, 2.4-, 2.0-, and 0.7-kb mRNAs) of the sae locus (1, 39, 55). The main sae promoter (28), located upstream of saeP, is strongly autoregulated and also repressed by SigB in strains 8325-4, ISP479R, UAMS-1, and COL (13). This sae promoter appears to be activated by H2O2 and subinhibitory concentrations of α-defensins (13). Conversely, the main sae promoter did not respond to high salt concentrations (1). We did not observe any effect on the main sae promoter's activity by SAL treatment of S. aureus Newman strain organisms at the time points studied. However, the level of sae transcripts (3.1-, 2.4-, and 2.0-kb transcripts included) increased after SAL exposure. Previous studies showed that the sae transcripts T3 (3.1 kb), T1 (2.4 kb), and T4 (0.7 kb) were highly expressed in strain Newman compared with those in strain 8325-4 (14, 55).
Since the sae system of the Newman strain is not activated by agr or repressed by sigB (13), we suggest that the diminished CP5 production by S. aureus observed upon SAL exposure may be caused mainly by high expression of saeRS and reduced expression of mgrA. The relevance of our findings is underscored by the fact that aspirin, a main source of salicylic acid in the human host, is being taken by millions of human beings worldwide without medical prescription and is widely prescribed for defined purposes, including prevention of cardiac infarction (58). Therefore, modulation of the invasive capacity of bacteria may play a role in pathogenesis, prophylaxis, and treatment of staphylococcal infections in certain at-risk populations colonized with S. aureus. It should not be forgotten, however, that nonencapsulated S. aureus strains have a greater ability to invade epithelial cells and may persist for long periods inside the host. Furthermore, persistent or recurrent infection may be favored by SAL administration.
Acknowledgments
We are grateful to Lorena Medina for her expert technical assistance. We also thank the anonymous reviewers, whose comments and constructive criticisms helped us to improve the manuscript. We thank Jean C. Lee (Channing Laboratory, Brigham & Women's Hospital, Harvard Medical School, Boston, MA) for generously providing S. aureus strains Reynolds (CP5) and Reynolds (CP−).
This work was supported in part by grants from ANPCyT (PICT 06/00991), CONICET (PIP 5933), and Universidad de Buenos Aires (UBACyT M-070 and M-406), Buenos Aires, Argentina.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 14 December 2009.
REFERENCES
- 1.Adhikari, R. P., and R. P. Novick. 2008. Regulatory organization of the staphylococcal sae locus. Microbiology 154:949-959. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez, L. P., M. S. Barbagelata, A. L. Cheung, D. O. Sordelli, and F. R. Buzzola. 2008. Abstr. XII Int. Cong. Bacteriol. Appl. Microbiol., abstr. BP-17.
- 3.Blickwede, M., R. Goethe, C. Wolz, P. Valentin-Weigand, and S. Schwarz. 2005. Molecular basis of florfenicol-induced increase in adherence of Staphylococcus aureus strain Newman. J. Antimicrob. Chemother. 56:315-323. [DOI] [PubMed] [Google Scholar]
- 4.Buzzola, F. R., L. P. Alvarez, L. P. Tuchscherr, M. S. Barbagelata, S. M. Lattar, L. Calvinho, and D. O. Sordelli. 2007. Differential abilities of capsulated and noncapsulated Staphylococcus aureus isolates from diverse agr groups to invade mammary epithelial cells. Infect. Immun. 75:886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Z., T. T. Luong, and C. Y. Lee. 2007. The sbcDC locus mediates repression of type 5 capsule production as part of the SOS response in Staphylococcus aureus. J. Bacteriol. 189:7343-7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domenico, P., S. Schawartz, and B. A. Cunha. 1989. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect. Immun. 57:3778-3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domenico, P., D. R. Landolphi, and B. A. Cunha. 1991. Reduction of capsular polysaccharide and potentiation of aminoglycoside inhibition in gram-negative bacteria by bismuth subsalicylate. J. Antimicrob. Chemother. 28:801-810. [DOI] [PubMed] [Google Scholar]
- 8.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 6:95-107. [DOI] [PubMed] [Google Scholar]
- 9.Dziewanowska, K., A. R. Carson, J. M. Patti, C. F. Deobald, K. W. Bayles, and G. A. Bohach. 2000. Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: role in internalization by epithelial cells. Infect. Immun. 68:6321-6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farber, F. B., and R. C. Bone. 1992. The use of nonsteroidal anti-inflammatory drugs to prevent adherence of Staphylococcus epidermidis to medical polymers. J. Infect. Dis. 166:861-865. [DOI] [PubMed] [Google Scholar]
- 11.Farthing, D., L. Gehr, T. H. Karnes, D. Sica, T. Gehr, T. Larus, C. Farthing, and L. Xi. 2007. Effects of salicylic acid on post-ischaemic ventricular function and purine efflux in isolated mouse hearts. Biomarkers 12:623-634. [DOI] [PubMed] [Google Scholar]
- 12.Garzoni, C., P. Francois, A. Huyghe, S. Couzinet, C. Tapparel, Y. Charbonnier, A. Renzoni, S. Lucchini, D. P. Lew, P. Vaudaux, W. L. Kelley, and J. Schrenzel. 2007. A global view of Staphylococcus aureus whole genome expression upon internalization in human epithelial cells. BMC Genomics 8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geiger, T., C. Goerke, M. Mainiero, D. Kraus, and C. Wolz. 2008. The virulence regulator Sae of Staphylococcus aureus: promoter activities and response to phagocytosis-related signals. J. Bacteriol. 190:3419-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goerke, C., U. Fluckiger, A. Steinhuber, V. Bisanzio, M. Ulrich, M. Bischoff, J. M. Patti, and C. Wolz. 2005. Role of Staphylococcus aureus global regulators sae and σB in virulence gene expression during device-related infection. Infect. Immun. 73:3415-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundmeier, M., M. Hussain, P. Becker, C. Heilmann, G. Peters, and B. Sinha. 2004. Truncation of fibronectin-binding proteins in Staphylococcus aureus strain Newman leads to deficient adherence and host cell invasion due to loss of the cell wall anchor function. Infect. Immun. 72:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafson, J. E., P. V. Candelaria, S. A. Fisher, J. P. Goodridge, T. M. Lichocik, T. M. McWilliams, C. T. D. Price, F. G. O'Brien, and W. B. Grubb. 1999. Growth in the presence of salicylate increases fluoroquinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 43:990-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbert, S., S. W. Newell, C. Lee, K. P. Wieland, B. Dassy, J. M. Fournier, C. Wolz, and G. Döring. 2001. Regulation of Staphylococcus aureus type 5 and type 8 capsular polysaccharides by CO2. J. Bacteriol. 183:4609-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyunth, H., G. Robitaille, and J. D. Turner. 1991. Establishment of bovine mammary epithelial cells (MAC-T): an in vitro model for bovine lactation. Exp. Cell Res. 197:191-199. [DOI] [PubMed] [Google Scholar]
- 19.Ingavale, S. S., W. van Wamel, and A. L. Cheung. 2003. Characterization of RAT, an autolysis regulator in Staphylococcus aureus. Mol. Microbiol. 48:1451-1466. [DOI] [PubMed] [Google Scholar]
- 20.Ingavale, S. S., W. van Wamel, T. T. Luang, C. Y. Lee, and A. L. Cheung. 2005. Rat/MgrA, a regulator of autolysis, is a regulator of virulence genes in Staphylococcus aureus. Infect. Immun. 73:1423-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karakawa, W. W., J. M. Fournier, W. F. Vann, R. Arbeit, R. S. Schneerson, and J. B. Robbins. 1985. Method for the serological typing of the capsular polysaccharides of Staphylococcus aureus. J. Clin. Microbiol. 22:445-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kullik, I., P. Giachino, and T. Fuchs. 1998. Deletion of the alternative sigma factor sigmaB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 180:4814-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kupferwasser, L. I., M. R. Yeaman, S. M. Shapiro, C. C. Nast, P. M. Sullam, S. G. Filler, and A. S. Bayer. 1999. Acetylsalicylic acid reduces vegetation bacterial density, hematogenous bacterial dissemination, and frequency of embolic events in experimental Staphylococcus aureus endocarditis through antiplatelet and antibacterial effects. Circulation 99:2791-2797. [DOI] [PubMed] [Google Scholar]
- 24.Kupferwasser, L. I., M. R. Yeaman, C. C. Nast, D. Kupferwasser, Y. Q. Xiong, M. Palma, A. L. Cheung, and A. S. Bayer. 2003. Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathways in Staphylococcus aureus. J. Clin. Invest. 112:222-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, J. C., M. J. Liu, J. Parsonnet, and R. D. 1990. Expression of type-8 capsular polysaccharide and production of toxic shock syndrome toxin-1 are associated among vaginal isolates of Staphylococcus aureus. J. Clin. Microbiol. 28:2612-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, J. C., S. Takeda, P. J. Livolsi, and L. C. Paoletti. 1993. Effects of in vitro and in vivo growth conditions on expression of type-8 capsular polysaccharide by Staphylococcus aureus. Infect. Immun. 61:1853-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, C. Y., and J. C. Lee. 2006. Staphylococcal capsule, p. 456-463. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, DC.
- 28.Li, D., and A. L. Cheung. 2008. Repression of hla by rot is dependent on sae in Staphylococcus aureus. Infect. Immun. 76:1068-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 30.Luong, T., S. Sau, M. Gomez, J. C. Lee, and C. Y. Lee. 2002. Regulation of Staphylococcus aureus capsular polysaccharide expression by agr and sarA. Infect. Immun. 70:444-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luong, T. T., and C. Y. Lee. 2006. The arl locus positively regulates Staphylococcus aureus type 5 capsule via an mgrA-dependent pathway. Microbiology 152:3123-3131. [DOI] [PubMed] [Google Scholar]
- 32.Ma, J., J. Cocchiaro, and J. C. Lee. 2004. Evaluation of serotypes of Staphylococcus aureus strains used in the production of a bovine mastitis bacterin. J. Dairy Sci. 87:178-182. [DOI] [PubMed] [Google Scholar]
- 33.Manna, A., and A. L. Cheung. 2001. Characterization of sarR, a modulator of sar expression in Staphylococcus aureus. Infect. Immun. 69:885-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meier, S., C. Goerke, C. Wolz, K. Seidl, D. Homerova, B. Schulthess, J. Kormanec, B. Berger-Bächi, and M. Bischoff. 2007. σB and the σB-dependent arlRS and yabJ-spoVG loci affect capsule formation in Staphylococcus aureus. Infect. Immun. 75:4562-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreillon, P., Y. I. Que, and M. P. Glauser. 2005. Staphylococcus aureus, p. 2321-2351. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious disease. Elsevier-Churchill Livingstone, Philadelphia, PA.
- 36.Muller, E., J. Al-Attar, A. G. Wolff, and B. F. Farber. 1998. Mechanism of salicylate-mediated inhibition of biofilm in Staphylococcus epidermidis. J. Infect. Dis. 177:501-503. [DOI] [PubMed] [Google Scholar]
- 37.Needs, C. J., and P. M. Brooks. 1985. Clinical pharmacokinetics of the salicylates. Clin. Pharmacokinet. 10:164-177. [DOI] [PubMed] [Google Scholar]
- 38.Novick, R. P. 1991. Genetic systems in staphylococci. Methods Enzymol. 204:587-636. [DOI] [PubMed] [Google Scholar]
- 39.Novick, R. P., and D. Jiang. 2003. The staphylococcal saeRS system coordinates environmental signals with agr quorum sensing. Microbiology 149:2709-2717. [DOI] [PubMed] [Google Scholar]
- 40.O'Riordan, K., and J. C. Lee. 2004. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17:218-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouyang, S., S. Sau, and C. Y. Lee. 1999. Promoter analysis of the cap8 operon, involved in the type 8 capsular polysaccharide production in Staphylococcus aureus. J. Bacteriol. 181:2492-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palma, M., A. Bayer, L. I. Kupferwasser, T. Joska, M. R. Yeaman, and A. L. Cheung. 2006. Salicylic acid activates sigma factor B by rsbU-dependent and -independent mechanisms. J. Bacteriol. 188:5896-5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park, W. B., S. H. Kim, J. H. Cho, J. H. Bang, H. B. Kim, N. J. Kim, M. D. Oh, and K. W. Choe. 2006. Effect of salicylic acid on invasion of human vascular endothelial cells by Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 49:56-61. [DOI] [PubMed] [Google Scholar]
- 44.Price, C. T. D., F. G. O'Brien, B. P. Shelton, J. R. Warmington, W. B. Grubb, and J. E. Gustafson. 1999. Effects of salicylate and related compounds on fusidic acid MICs in Staphylococcus aureus. J. Antimicrob. Chemother. 44:57-64. [DOI] [PubMed] [Google Scholar]
- 45.Price, C. T. D., I. R. Lee, and J. E. Gustafson. 2000. The effects of salicylate on bacteria. Int. J. Biochem. Cell Biol. 32:1029-1043. [DOI] [PubMed] [Google Scholar]
- 46.Price, C. T. D., and J. E. Gustafson. 2001. Increases in the mutation frequency at which fusidic acid-resistant Staphylococcus aureus arise with salicylate. J. Med. Microbiol. 50:104-106. [DOI] [PubMed] [Google Scholar]
- 47.Price, C. T. D., G. W. Kaatz, and J. E. Gustafson. 2002. The multidrug efflux pump NorA is not required for salicylate-induced reduction in drug accumulation by Staphylococcus aureus. Int. J. Antimicrob. Agents 20:212-219. [DOI] [PubMed] [Google Scholar]
- 48.Prithiviraj, B., H. P. Bais, A. K. Jha, and J. M. Vivanco. 2005. Staphylococcus aureus pathogenicity on Arabidopsis thaliana is mediated either by a direct effect of salicylic acid on the pathogen or by SA-dependent, NPR1-independent host responses. Plant J. 42:417-432. [DOI] [PubMed] [Google Scholar]
- 49.Riordan, J. T., A. Muthaiyan, W. van Borréis, C. T. Price, J. E. Gram, B. J. Wilkinson, and J. E. Gustafson. 2007. Response of Staphylococcus aureus to salicylate challenge. J. Bacteriol. 189:220-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogasch, K., V. Rühmling, J. Pané-Farré, D. Höper, C. Weinberg, S. Fuchs, M. Schmudde, B. M. Bröker, C. Wolz, M. Hecker, and S. Engelmann. 2006. Influence of the two-component system saeRS on global gene expression in two different Staphylococcus aureus strains. J. Bacteriol. 188:7742-7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sau, S., J. Sun, and C. Y. Lee. 1997. Molecular characterization and transcriptional analysis of type 8 capsule genes in Staphylococcus aureus. J. Bacteriol. 179:1614-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seidl, K., M. Stucki, M. Ruegg, C. Goerke, C. Wolz, L. Harris, B. Berger-Bächi, and M. Bischoff. 2006. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 50:1183-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinha, B., P. P. François, O. Nüsse, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. P. Lew, M. Herrmann, and K. H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1:101-117. [DOI] [PubMed] [Google Scholar]
- 54.Sinha, B., and M. Herrmann. 2005. Mechanism and consequences of invasion of endothelial cells by Staphylococcus aureus. Thromb. Haemost. 94:266-277. [DOI] [PubMed] [Google Scholar]
- 55.Steinhuber, A., C. Goerke, M. G. Bayer, G. Doring, and C. Wolz. 2003. Molecular architecture of the regulatory locus sae of Staphylococcus aureus and its impact on expression of virulence factors. J. Bacteriol. 185:6278-6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tollersrud, T., K. Kenny, A. J. Reitz, and J. C. Lee. 2000. Genetic and serologic evaluation of capsule production by bovine mammary isolates of Staphylococcus aureus and other Staphylococcus spp. from Europe and the United States. J. Clin. Microbiol. 38:2998-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuchscherr, L. P. N., F. R. Buzzola, L. P. Alvarez, R. Caccuri, J. C. Lee, and D. O. Sordelli. 2005. Capsule-negative Staphylococcus aureus induces chronic experimental mastitis in mice. Infect. Immun. 73:7932-7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vane, J. R., and R. M. Botting. 1992. The history of aspirin, p. 3-34. In J. R. Vane and R. M. Botting (ed.), Aspirin and other salicylates. Chapman and Hall, London, United Kingdom.
- 59.van Wamel, W., Y. Q. Xiong, A. S. Bayer, M. R. Yeaman, C. C. Nast, and A. L. Cheung. 2002. Regulation of Staphylococcus aureus type 5 capsular polysaccharides by agr and sarA in vitro and in an experimental endocarditis model. Microb. Pathog. 33:73-79. [DOI] [PubMed] [Google Scholar]
- 60.von Eiff, C., G. Peters, and K. Becker. 2006. The small colony variant (SCV) concept—the role of staphylococcal SCVs in persistent infections. Injury 37(Suppl. 2):S26-S33. [DOI] [PubMed] [Google Scholar]
- 61.Wann, E. R., B. Dassy, J. M. Fournier, and T. J. Foster. 1999. Genetic analysis of the cap5 locus of Staphylococcus aureus. FEMS Microbiol. Lett. 170:97-103. [DOI] [PubMed] [Google Scholar]
- 62.Watts, J. A., D. Ke, Q. Wang, A. Pillay, A. Nicholson-Weller, and J. C. Lee. 2005. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect. Immun. 73:3502-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]