Abstract
Both Plasmodium and Babesia species are intraerythrocytic protozoans that infect a wide range of hosts, including humans, and they elicit similar inflammatory responses and clinical manifestations that differ markedly in severity. We recently reported that a rhesus macaque that was chronically infected with Babesia microti was able to control infection with Plasmodium cynomolgi (a parasite of macaques with characteristics very similar to those of Plasmodium vivax) better than naïve monkeys. To confirm this and to investigate the underlying immunopathology, six naïve rhesus monkeys were infected with B. microti. After 24 days, four of these monkeys and four naïve rhesus monkeys were challenged with P. cynomolgi blood-stage parasites. B. microti persisted at low levels in all monkeys, and the clinical parameters were comparable to those of noninfected controls. There was a significant decrease in P. cynomolgi parasitemia in animals coinfected with B. microti compared to the parasitemia in animals infected with P. cynomolgi alone. This decrease in P. cynomolgi parasitemia correlated with increases in the levels of proinflammatory monocytes at the time of P. cynomolgi infection and with higher C-reactive protein (CRP) serum levels 1 week after malaria infection. Therefore, we conclude that ongoing infection with B. microti parasites leads to suppression of malaria infection.
Pathogens rarely infect immunologically naïve hosts. In fact, maturation of the immune system requires antigenic stimulation that begins in the neonatal period and perhaps even during gestation (34). In addition to a history of previous infections, individuals from areas where multiple pathogens are endemic are often coinfected with unrelated organisms. Concrete examples of exacerbated pathology related to coinfection in humans, such as the deleterious effect of schistosomiasis on hepatitis C progression (2, 22), have led to an increased interest in studying heterologous immunity (7). Coinfection of rodents with the apicomplexan, intraerythrocytic parasites Babesia and Plasmodium, the causative agents of babesiosis and malaria in humans, respectively, has been reported to induce cross-protection. Protection against some Plasmodium spp. after a naturally cured or drug-cured infection with Babesia microti (9, 10) was thought to be due to common antigenic determinants for Babesia and Plasmodium spp. (10), although cross-reacting antibody titers were low (11) or could not be detected (9). This suggests that nonspecific factors may also be involved in cross-protection.
Human malaria, an infectious disease vectored by anopheline mosquitoes, is caused by four different species of Plasmodium: Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale. In addition, Plasmodium knowlesi, a simian parasite, is also able to naturally infect humans (12, 21, 43). P. falciparum and P. vivax are the most clinically important Plasmodium species. P. falciparum causes more than 1 million deaths annually in sub-Saharan Africa, and the victims are mainly children under the age of 5 years (16, 44). P. vivax is prevalent in eastern and central Africa and in more temperate climates outside Africa, and it has an enormous socioeconomic impact, particularly in South America and Asia (31). Plasmodium cynomolgi, a simian malaria parasite that has been shown experimentally to infect humans, is phylogenetically and phenotypically closely related to P. vivax, develops hypnozoites (13), and provides a close and relevant biological model for P. vivax (24, 36, 46).
Babesia parasites are vectored by ticks and infect a wide variety of mammals. Awareness is growing of the role of these organisms as zoonotic agents of human diseases in which pyrexia, hemolytic anemia, and hemoglobinuria may be induced (19). A cardinal sign of babesiosis is erythrocyte destruction (48, 49). Recently, human babesiosis caused by B. microti has emerged as a worldwide health threat (15, 19, 29) that can be severe and life threatening (18). Although around 25% of the adults and 50% of the children infected with B. microti are asymptomatic (26), severe babesiosis can occur in patients after splenectomy (38), and other comorbid conditions, like Lyme disease, probably contribute to increased severity of illness (27).
Previously, we showed that in a single rhesus macaque long-term (>5 years) chronic infection with the B. microti MM-1 isolate appeared to control a blood-stage P. cynomolgi infection (45). In the study described here, we investigated this observation more extensively and determined whether short-term infection with B. microti has a similar effect.
MATERIALS AND METHODS
Primates and study design.
Ten female rhesus macaques were selected for this study; all of the monkeys were 6 years old and weighted 5 to 7.5 kg (see Table S1 in the supplemental material). These monkeys were weight matched and assigned to experimental groups as shown in Fig. 1. Animal work was carried out under protocols approved by the Animal Ethics Committee (DEC), following Dutch laws.
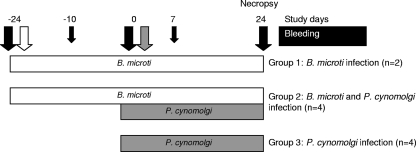
FIG. 1.
Study design. At day −24, six rhesus monkeys were infected with erythrocytes containing B. microti parasites (parasitemia, ∼0.02%) (open arrow). On day 0, four of these monkeys were coinfected with P. cynomolgi together with four naïve monkeys (gray arrow). Twenty-four days later all monkeys were sacrificed, and full necropsies were performed. At several time points (black arrows) blood was drawn.
The study design is shown in Fig. 1. Six monkeys were infected on day −24 with heparinized blood obtained from the monkey previously reported to be chronically infected with B. microti isolate MM-1 (45). Each monkey was inoculated intravenously with 0.5 ml of packed cells containing approximately 5 × 105 B. microti parasites. On day 0, four of these monkeys and four naïve controls were challenged with 1 × 106 P. cynomolgi strain M (8, 39) blood-stage parasites obtained from a parasite donor monkey. This parasite was originally a kind gift from W. E. Collins (CDC, Atlanta, GA). Throughout this study finger prick blood samples were taken every other day (when the majority of the malaria parasites were in the ring stage) to monitor parasitemia by Giemsa-stained thin-film analysis and by PCR. On days −24, −10, 0, 7, and 24, blood was drawn. At the end of the study all animals were euthanized, and full necropsies were performed.
PCR analysis to measure B. microti.
Erythrocytes obtained from whole blood were lysed (catalog no. 158902; Qiagen), and PCR was performed as previously described (41) to specifically amplify hypervariable region V4 of the small-subunit rRNA genes of piroplasms.
Lymphocyte isolation and fluorescence-activated cell sorting (FACS) analysis.
Peripheral blood mononuclear cells (PBMCs) and lymphocytes from the spleens and lymph nodes were isolated using standard procedures. Lymphocytes were obtained from the liver as previously reported (17).
The following antibodies were purchased from BD Pharmingen: CD3-AlexaFluor700, CD4-peridinin chlorophyll protein complex (PerCP) -Cy5.5, CD16- phycoerythrin (PE), CD20- fluorescein isothiocyanate (FITC), CD25- PE, and CD154- FITC. CD14- PE -TexasRed and CD20- PE -TexasRed were obtained from Beckman Coulter. CD8-PacificBlue was purchased from Dako. For detection of selected surface markers, cells were incubated with the appropriate antibodies for 1 5 to 30 min at 4 ° C in the dark. After washing, cells were fixed in 1% paraformaldehyde. Data acquisition and analysis were performed with a FACSAria using FACSDiva 5.0 software (BD Biosciences).
Pathology.
A full necropsy was performed for each animal at the end of the study. Tissues were fixed in 4% buffered formalin and embedded in paraffin for routine histology. Four-micron sections were stained with hematoxylin and eosin. Special staining (Perls' method for iron) was used for detection of hemosiderin in selected tissue samples.
Statistics.
The development of P. cynomolgi parasitemia through time was modeled using nonlinear mixed-effect models (NLME) (24). Differences in clinical parameters were analyzed using one-way analysis of variance (ANOVA), followed by a Bonferroni test. Differences in the average cumulative P. cynomolgi parasitemia data between groups were calculated using an unpaired Student t test, and standard errors are indicated below. C-reactive protein (CRP) levels were log transformed to obtain normality and subsequently analyzed by Student's t test. P values of <0.05 were considered significant, and a 95% confidence interval (95% CI) was calculated.
RESULTS
B. microti infection in rhesus macaques.
To initiate B. microti infection, parasitized erythrocytes from a chronically infected monkey (45) were intravenously inoculated on day −24 into six naïve rhesus monkeys (Fig. 1). All monkeys were PCR positive after 1 week (see Fig. S1 in the supplemental material). Subsequently, B. microti parasites were present in all infected monkeys during the entire study, but the levels were low (<0.1% parasitemia) as analyzed using Giemsa-stained thin blood films (data not shown). These data indicate that the rhesus macaques were infected with B. microti parasites and that these parasites were present throughout the 48-day follow-up period.
Infection with B. microti parasites suppresses blood-stage P. cynomolgi parasitemia.
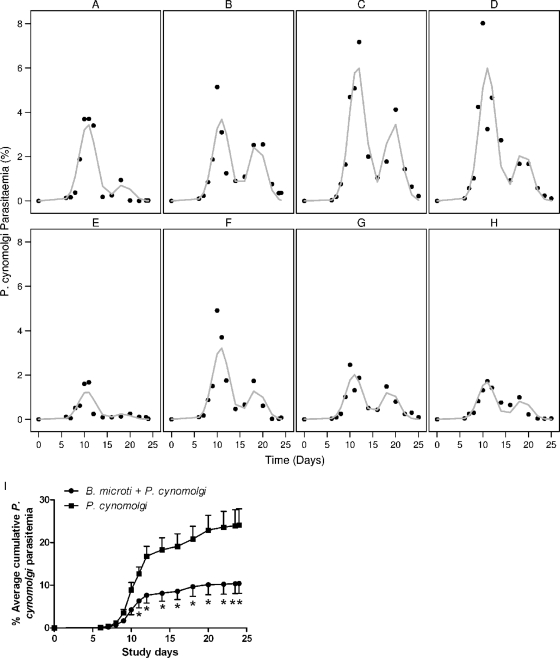
To study whether short-term persistent B. microti infection suppresses P. cynomolgi parasitemia, four B. microti-infected monkeys were coinfected with P. cynomolgi, together with four naïve monkeys. Blood-stage P. cynomolgi parasitemia in rhesus macaques normally follows a characteristic pattern consisting of a first self-curing peak and then recrudescence about 1 week later (24). The P. cynomolgi parasitemia in all infected monkeys displayed this characteristic pattern; however, the monkeys also infected with B. microti had statistically significant lower first-peak levels of parasitemia (2.34%; 95% CI, 0.84 to 3.83%) than the monkeys infected with only P. cynomolgi (P = 0.0025) (Fig. 2A to H). The antimalarial effect was clear when the average cumulative levels of P. cynomolgi parasitemia were examined (P = 0.0143) (Fig. 2I). Thus, persistent infection with B. microti resulted in decreased P. cynomolgi parasitemia. P. cynomolgi infection did not markedly influence B. microti parasitemia; the level of B. microti parasitemia remained low (<0.1%) throughout the study for all infected animals.
FIG. 2.
P. cynomolgi parasitemia. (A to D) P. cynomolgi parasitemia in individual monkeys in the group infected with only P. cynomolgi. (E to H) P. cynomolgi parasitemia in monkeys infected with both B. microti and P. cynomolgi. The first peak was significantly lower for the doubly infected monkeys than for the monkeys infected with only P. cynomolgi (P = 0.0025). The symbols indicate the parasitemia determined in this study, and the lines indicate the NLME-modeled P. cynomolgi parasitemia (24). (I) Average cumulative P. cynomolgi parasitemia. The average cumulative parasitemia was significantly lower for doubly infected monkeys than for monkeys infected with only P. cynomolgi (P = 0.0143). The error bars indicate the standard errors.
P. cynomolgi-induced anemia is not prevented in doubly infected monkeys.
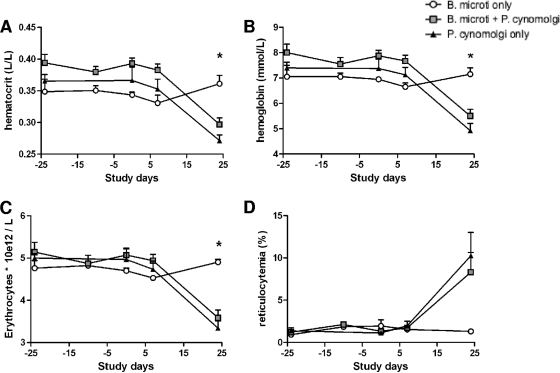
Babesia and Plasmodium are protozoan parasites that infect erythrocytes and result in comparable clinical features, including induction of anemia (25, 37). The hematocrit level, hemoglobin level, erythrocyte count, and percentage of reticulocytes (Fig. 3A to D, respectively) were determined for all groups (monkeys infected with only B. microti, monkeys infected with B. microti and P. cynomolgi, and monkeys infected with only P. cynomolgi). No changes in these parameters were observed in monkeys infected only with B. microti or in the chronically infected monkey (45) (see Table S2 in the supplemental material), indicating that this parasite does not induce anemia in otherwise healthy monkeys. However, in monkeys infected with P. cynomolgi there was a statistically significant decrease in the hematocrit level (P = 0.0029), hemoglobin level (P = 0.0054), and erythrocyte count (P = 0.0025) and there was a trend toward an increase in the percentage of reticulocytes (P = 0.1536) 3 weeks after P. cynomolgi infection. Strikingly, no differences were observed between the group of monkeys infected with only P. cynomolgi and the group of monkeys infected with both parasites. All blood data and cell counts measured during the study are shown in Tables S3 and S4 in the supplemental material; overall the values are in the normal ranges for rhesus macaques (30), and no differences between the groups were observed.
FIG. 3.
B. microti infection does not lead to anemia in rhesus macaques, in contrast to P. cynomolgi infection. Hematocrit levels (A), hemoglobin levels (B), erythrocyte counts (C), and percentages of reticulocytes (D) over time are shown for all groups. On day 24 the hematocrit levels, hemoglobin levels, and erythrocyte counts for doubly infected monkeys and monkeys infected with only P. cynomolgi were significantly lower than the values for B. microti-infected monkeys (P = 0.0029, P = 0.0054, and P = 0.0025, respectively). There was no statistically significant difference between the doubly infected monkeys and the monkeys infected with only P. cynomolgi.
Together, these data indicate that although infection with B. microti in rhesus macaques induces suppression of P. cynomolgi parasitemia, it does not prevent malaria-induced anemia.
Histopathological findings after P. cynomolgi infection.
A typical pathological feature of both Plasmodium and Babesia infections is the presence of marked erythro- and hemosiderophagocytosis and excessive deposition of pigment due to the extreme lysis of erythrocytes and enzymatic transformation of released hemoglobin (1, 25). At the end of this study organs and tissues were collected from all monkeys and examined for pathology.
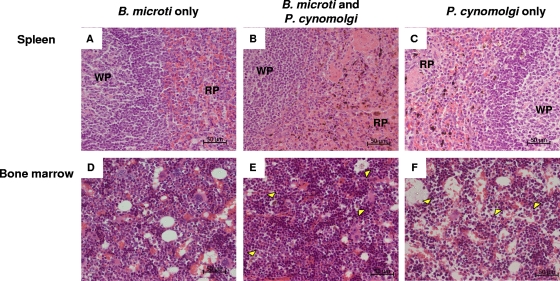
At necropsy all animals, including the monkeys infected with only B. microti, showed mild to moderate enlargement of the spleen (2-fold) (data not shown). After microscopic examination of all parenchymal organs, extensive increases in the amounts of phagocytosed pigmented granular material were observed in several viscera in monkeys infected with P. cynomolgi alone or in combination with B. microti. The spleen, liver, lung, and lymph nodes were the major locations with prominent erythro- and hemosiderophagocytosis and numerous pigmented macrophages. The organs of the gastrointestinal tract (stomach and small and large intestines) showed mild inflammation (gastritis, enteritis, and colitis) with mild edema and congestion. In all malaria-infected monkeys there was yellow-brown granular pigmented material in Kupffer cells in the liver (data not shown). The same pathology was not observed in rhesus macaques infected with only B. microti. Figure 4 shows representative H&E-stained sections of spleen and bone marrow (lymph node and lung sections are not shown).
FIG. 4.
Histopathology of spleen and bone marrow: H&E staining of representative spleen (A to C) and bone marrow (D to F) sections obtained on day 24 from a monkey infected with only B. microti (monkey Ri201046) (A and D), a monkey infected with both parasites (monkey Ri205138) (B and E), and a monkey infected with only P. cynomolgi (monkey Ri201112) (C and F). WP, white pulp; RP, red pulp from the spleen. The arrowheads indicate bone marrow macrophages containing pigment. Magnification, ×40. Bars = 50 μm.
Induction of inflammatory responses by B. microti infection.
Infection with B. microti parasites suppressed P. cynomolgi parasitemia without altering the induction of anemia. To explore the immune responses underlying the suppressive effect on malaria parasitemia, PBMCs were isolated on days −24, 0, and 24, and during necropsy lymphocytes were isolated from the spleen, inguinal lymph nodes, and liver. The percentages of several cell populations present in the different compartments of all monkeys were analyzed.
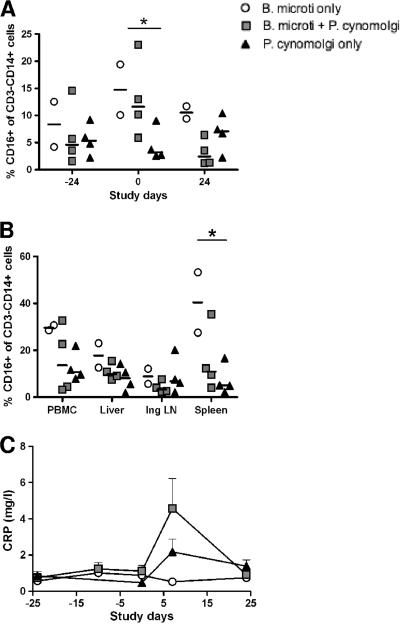
Three weeks after B. microti infection a marked increase in the percentage of activated monocytes (CD3− CD14+ CD16+) in the peripheral blood compartment was evident (Fig. 5A). In monkeys that were then coinfected with P. cynomolgi parasites the percentage of activated monocytes decreased, whereas in monkeys infected with only B. microti a higher percentage of activated monocytes persisted in both the peripheral blood (P = 0.019) and spleen (P = 0.0156) (Fig. 5B).
FIG. 5.
B. microti infection triggers the immune system of rhesus macaques. (A) Percentages of CD3− CD14+ CD16+ monocytes in PBMCs over time. The percentage of CD3− CD14+ CD16+ monocytes in all B. microti-infected monkeys was significantly greater than the percentage in noninfected monkeys (day 0, P = 0.019). (B) Percentages of CD3− CD14+ CD16+ monocytes in organs at the end of the study (P = 0.0156 for a comparison of the percentages of CD3− CD14+ CD16+ cells in the spleens of B. microti-infected monkeys with the percentages in doubly infected monkeys and monkeys infected with only P. cynomolgi). Ing LN, inguinal lymph nodes. (C) Serum CRP levels over time.
A peak in the level of C-reactive protein (CRP), an acute-phase protein that is produced rapidly in response to proinflammatory stimuli and that has binding and functional characteristics suggesting that it has a role in host defense against infection (28), was observed 1 week after malaria infection (Fig. 5C). The CRP levels in the doubly infected monkeys were 0.49-fold greater than those in the monkeys infected with only P. cynomolgi (P = 0.15; 95% CI, P = 0.17 to P = 1.41). Infection with B. microti alone did not increase the CRP levels. Data for serum cytokines and chemokines which were also examined in this study are shown in Fig. S2 in the supplemental material. Gamma interferon (IFN-γ) exhibited a pattern comparable to that of CRP, but, unlike the findings for CRP, the levels were similar in singly and doubly infected monkeys. Furthermore, there were clear decreases in the serum levels of monocyte chemoattractant protein 1 (MCP-1) and macrophage inflammatory protein 1β (MIP-1β) at the time of necropsy for all animals infected with P. cynomolgi.
Together, these data indicate that B. microti activates the immune system at a low level compared to malaria infection, as suggested by the activation of monocytes in the periphery and increased CRP levels following malaria infection.
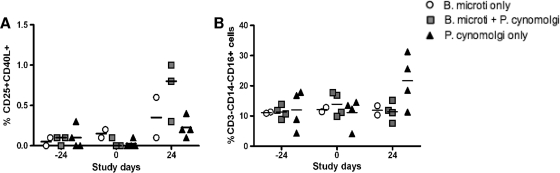
Coinfection with B. microti leads to earlier induction of activated CD4+ T cells after P. cynomolgi infection.
We studied the activation of other immune compartments by determining the levels of activated T and NK cells in peripheral blood over time and in lymphoid organs at the time of necropsy. Figure 6A shows the percentages of activated CD4+ T cells selected by CD25 and CD40L, and Fig. 6B shows the percentages of CD3− CD14+ CD16+ NK cells in peripheral blood. A trend toward more activated CD4+ T cells in the periphery of doubly infected monkeys was observed, whereas in monkeys infected with only P. cynomolgi there was a higher percentage of CD3− CD14+ CD16+ NK cells in the periphery.
FIG. 6.
Doubly infected rhesus monkeys have more activated CD3+ CD4+ T cells, as determined with CD25+ CD40L+. (A) Percentages of CD25+ CD40L+ for CD4+ T cells over time. (B) Percentages of CD16+ for NK cells over time.
DISCUSSION
Previously, we showed that a rhesus monkey chronically infected with B. microti for 5 or more years was better able to control a blood-stage P. cynomolgi infection than naïve rhesus monkeys (45). Here we confirmed that there is a clear interaction between these two parasites and a primate host, even when the prior exposure of B. microti infection was reduced to a 3-week period. In addition, immunological and pathological parameters were investigated in this study.
In general, 3 weeks of infection is sufficient for induction of a full-blown immune response with triggering adaptive immune responses (5, 6, 20). By following the early course of infection with sensitive PCR determinants, we were able to demonstrate that the blood was positive for B. microti 1 week after inoculation (see Fig. S1 in the supplemental material), after which Babesia parasitemia was consistently present at low levels. The persistent low-level B. microti infection in both the doubly infected monkeys and the two monkeys infected with only Babesia suggests that the malaria infection did not have a major effect on the B. microti infection. Development of a quantitative PCR might reveal more subtle changes in parasitemia in subsequent studies.
Although both the peak parasitemia and the cumulative exposure to P. cynomolgi were significantly suppressed in animals previously and concurrently infected with B. microti, the pathology induced by the malaria parasites at the level of both anemia and hypoxia was not reduced. As the first peak of P. cynomolgi infection was still significant (at least 1.67% infected erythrocytes in the doubly infected monkeys), it may not be surprising that pathology, like the decreases in the hematocrit and hemoglobin levels, still occurred. In terms of the protective effects of Babesia coinfection, it would be interesting to investigate whether the pathology in the doubly infected monkeys lasts for a shorter time than the pathology in monkeys infected with only malaria organisms and whether other parameters of pathology are equally unaffected.
The pathological processes that occur following Babesia and Plasmodium infection are complex and incompletely understood. Some evidence indicates that the most important mechanism is excessive production of proinflammatory cytokines. Markedly elevated serum concentrations of tumor necrosis factor (TNF), IFN-γ, interleukin-2 (IL-2), IL-6, E-selectin (expressed in the endothelium), vascular cell adhesion molecule 1 (VCAM-1), and intracellular cell adhesion molecule 1 (ICAM-1) occur during an acute phase of human B. microti infection, and the concentrations return to the baseline levels 1 month after the resolution of infection (40). Here, we observed a marked increase in the level of peripheral blood CD3− CD14+ CD16+ monocytes 3 weeks after B. microti infection. CD3− CD14+ CD16+ monocytes are a unique, distinct population of monocytes (50) with a pattern of surface antigen expression similar to that of tissue macrophages (33, 35). The cytokine expression pattern of this distinct subset of monocytes includes production of high levels of TNF and low levels of IL-10, indicating that these monocytes are so-called proinflammatory monocytes (4, 14). It seems likely that the higher levels of this monocyte subset at the time of P. cynomolgi inoculation into animals exposed to Babesia is partially responsible for the malaria-suppressing effect observed in these monkeys. Due to moderate inflammation induced by B. microti infection, as shown by the increase in the level of CD3− CD14+ CD16+ monocytes, the CRP serum levels were elevated after P. cynomolgi infection in the doubly infected monkeys compared to the levels in monkeys that were infected with only the malaria parasite. CRP is a prototypical acute-phase protein of the innate immune system in humans and nonhuman primates. CRP is able to recognize damaged cells of the host to help with their elimination. It can activate the complement pathway as an opsonic protein or by binding C1q, and by binding Fcγ receptors it can also lead to complement-independent phagocytosis (32, 47). Recently, it has also been shown that CRP can bind differentially to malaria parasite-infected erythrocytes and assist in clearance of these cells from the circulation, implying that it has a potentially important protective role in malaria infection (3). We point out that as this study was focused on measuring effects of an existing Babesia infection on P. cynomolgi blood-stage infection, key cytokine measurements were obtained close to the peak P. cynomolgi parasitemia (day 7 after P. cynomolgi infection). Retrospectively, since at day 14 after B. microti infection (day −10 in Fig. 1 and Fig. S2 in the supplemental material) some effects on cytokine levels were also observed, it would be interesting to compare cytokine levels more closely for days 7 and 14 after infection with B. microti as well as P. cynomolgi (i.e., for days −17 and −10 and days 7 and 14 in Fig. 1).
P. cynomolgi is a parasite that is closely allied with P. vivax, and macaque immunology is closely related to human immunology, suggesting that an interaction between babesiosis and malaria may also occur in humans. There is little information regarding the prevalence of babesia infection worldwide, particularly in countries with underdeveloped health systems where malaria is prevalent. Diagnosis of babesiosis has increased markedly in the past few years, and this disease is considered an emerging disease (23). Given the clear interaction between the related species, we suggest that an effort to better understand the extent of babesia infection in regions where malaria is endemic is warranted. Further research is necessary to obtain a better understanding of the malaria-suppressing effects demonstrated here, with a view toward the development of novel tools to control malaria. It is not inconceivable that the shared characteristics of Babesia and Plasmodium may make genetically modified Babesia an attractive agent for delivery of live antimalaria vaccines. Such thoughts are encouraged by the fact that Babesia has been successfully used as an attenuated vaccine in veterinary applications (42).
In this study, we showed that B. microti remains present at low levels in rhesus monkeys and induces a moderate immune response after a few weeks, as shown by the increase in the level of peripheral blood CD3− CD14+ CD16+ monocytes and the trend toward increased CRP serum levels following subsequent malaria coinfection. Moreover, we observed a clear increase at the end of the study in the level of activated CD3+ CD4+ T cells, as measured by using both CD25 and CD40L, in the doubly infected monkeys, but this increased level was not examined in the singly infected monkeys. Monkeys infected with P. cynomolgi alone had elevated levels of NK cells in the peripheral blood compartment, which was not the case in doubly infected monkeys. These and undoubtedly other factors may have contributed to suppression of P. cynomolgi blood-stage infection.
Supplementary Material
Acknowledgments
We thank the Animal Science Department of BPRC for taking excellent care of the animals and for technical assistance.
Editor: J. H. Adams
Footnotes
Published ahead of print on 4 January 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Alkhalil, A., D. A. Hill, and S. A. Desai. 2007. Babesia and plasmodia increase host erythrocyte permeability through distinct mechanisms. Cell. Microbiol. 9:851-860. [DOI] [PubMed] [Google Scholar]
- 2.Angelico, M., E. Renganathan, C. Gandin, M. Fathy, M. C. Profili, W. Refai, A. De Santis, A. Nagi, G. Amin, L. Capocaccia, F. Callea, M. Rapicetta, G. Badr, and G. Rocchi. 1997. Chronic liver disease in the Alexandria governorate, Egypt: contribution of schistosomiasis and hepatitis virus infections. J. Hepatol. 26:236-243. [DOI] [PubMed] [Google Scholar]
- 3.Ansar, W., S. M. Bandyopadhyay, S. Chowdhury, S. H. Habib, and C. Mandal. 2006. Role of C-reactive protein in complement-mediated hemolysis in malaria. Glycoconj. J. 23:233-240. [DOI] [PubMed] [Google Scholar]
- 4.Belge, K. U., F. Dayyani, A. Horelt, M. Siedlar, M. Frankenberger, B. Frankenberger, T. Espevik, and L. Ziegler-Heitbrock. 2002. The proinflammatory CD14+ CD16+ DR++ monocytes are a major source of TNF. J. Immunol. 168:3536-3542. [DOI] [PubMed] [Google Scholar]
- 5.Brown, W. C. 2001. Molecular approaches to elucidating innate and acquired immune responses to Babesia bovis, a protozoan parasite that causes persistent infection. Vet. Parasitol. 101:233-248. [DOI] [PubMed] [Google Scholar]
- 6.Chen, D., D. B. Copeman, J. Burnell, and G. W. Hutchinson. 2000. Helper T cell and antibody responses to infection of CBA mice with Babesia microti. Parasite Immunol. 22:81-88. [DOI] [PubMed] [Google Scholar]
- 7.Clark, I. A. 2001. Heterologous immunity revisited. Parasitology 122(Suppl.):S51-S59. [PubMed] [Google Scholar]
- 8.Coatney, G. R., H. A. Elder, P. G. Contacos, M. E. Getz, R. Greenland, R. N. Rossan, and L. H. Schmidt. 1961. Transmission of the M strain of Plasmodium cynomolgi to man. Am. J. Trop. Med. Hyg. 10:673-678. [DOI] [PubMed] [Google Scholar]
- 9.Cox, F. E. 1978. Heterologous immunity between piroplasms and malaria parasites: the simultaneous elimination of Plasmodium vinckei and Babesia microti from the blood of doubly infected mice. Parasitology 76:55-60. [DOI] [PubMed] [Google Scholar]
- 10.Cox, F. E., and S. A. Turner. 1970. Antigenic relationships between the malaria parasites and piroplasms of mice as determined by the fluorescent-antibody technique. Bull. World Health Organ. 43:337-340. [PMC free article] [PubMed] [Google Scholar]
- 11.Cox, F. E., and S. A. Turner. 1970. Antibody levels in mice infected with Babesia microti. Ann. Trop. Med. Parasitol. 64:167-173. [DOI] [PubMed] [Google Scholar]
- 12.Cox-Singh, J., T. M. Davis, K. S. Lee, S. S. Shamsul, A. Matusop, S. Ratnam, H. A. Rahman, D. J. Conway, and B. Singh. 2008. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis. 46:165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escalante, A. A., D. E. Freeland, W. E. Collins, and A. A. Lal. 1998. The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome. Proc. Natl. Acad. Sci. U. S. A. 95:8124-8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frankenberger, M., T. Sternsdorf, H. Pechumer, A. Pforte, and H. W. Ziegler-Heitbrock. 1996. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood 87:373-377. [PubMed] [Google Scholar]
- 15.Gorenflot, A., K. Moubri, E. Precigout, B. Carcy, and T. P. Schetters. 1998. Human babesiosis. Ann. Trop. Med. Parasitol. 92:489-501. [DOI] [PubMed] [Google Scholar]
- 16.Guerra, C. A., R. W. Snow, and S. I. Hay. 2006. Mapping the global extent of malaria in 2005. Trends Parasitol. 22:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammond, K. J., D. G. Pellicci, L. D. Poulton, O. V. Naidenko, A. A. Scalzo, A. G. Baxter, and D. I. Godfrey. 2001. CD1d-restricted NKT cells: an interstrain comparison. J. Immunol. 167:1164-1173. [DOI] [PubMed] [Google Scholar]
- 18.Hatcher, J. C., P. D. Greenberg, J. Antique, and V. E. Jimenez-Lucho. 2001. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin. Infect. Dis. 32:1117-1125. [DOI] [PubMed] [Google Scholar]
- 19.Homer, M. J., I. Aguilar-Delfin, S. R. Telford III, P. J. Krause, and D. H. Persing. 2000. Babesiosis. Clin. Microbiol. Rev. 13:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igarashi, I., R. Suzuki, S. Waki, Y. Tagawa, S. Seng, S. Tum, Y. Omata, A. Saito, H. Nagasawa, Y. Iwakura, N. Suzuki, T. Mikami, and Y. Toyoda. 1999. Roles of CD4+ T cells and gamma interferon in protective immunity against Babesia microti infection in mice. Infect. Immun. 67:4143-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jongwutiwes, S., C. Putaporntip, T. Iwasaki, T. Sata, and H. Kanbara. 2004. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg. Infect. Dis. 10:2211-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamal, S., M. Madwar, L. Bianchi, A. E. Tawil, R. Fawzy, T. Peters, and J. W. Rasenack. 2000. Clinical, virological and histopathological features: long-term follow-up in patients with chronic hepatitis C co-infected with S. mansoni. Liver 20:281-289. [DOI] [PubMed] [Google Scholar]
- 23.Kjemtrup, A. M., and P. A. Conrad. 2000. Human babesiosis: an emerging tick-borne disease. Int. J. Parasitol. 30:1323-1337. [DOI] [PubMed] [Google Scholar]
- 24.Kocken, C. H., E. J. Remarque, M. A. Dubbeld, S. Wein, A. van der Wel, R. J. Verburgh, H. J. Vial, and A. W. Thomas. 2009. Statistical model to evaluate in vivo activities of antimalarial drugs in a Plasmodium cynomolgi-macaque model for Plasmodium vivax malaria. Antimicrob. Agents Chemother. 53:421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krause, P. J., J. Daily, S. R. Telford, E. Vannier, P. Lantos, and A. Spielman. 2007. Shared features in the pathobiology of babesiosis and malaria. Trends Parasitol. 23:605-610. [DOI] [PubMed] [Google Scholar]
- 26.Krause, P. J., K. McKay, J. Gadbaw, D. Christianson, L. Closter, T. Lepore, S. R. Telford III, V. Sikand, R. Ryan, D. Persing, J. D. Radolf, and A. Spielman. 2003. Increasing health burden of human babesiosis in endemic sites. Am. J. Trop. Med. Hyg. 68:431-436. [PubMed] [Google Scholar]
- 27.Krause, P. J., S. R. Telford III, A. Spielman, V. Sikand, R. Ryan, D. Christianson, G. Burke, P. Brassard, R. Pollack, J. Peck, and D. H. Persing. 1996. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA 275:1657-1660. [PubMed] [Google Scholar]
- 28.Marnell, L., C. Mold, and T. W. Du Clos. 2005. C-reactive protein: ligands, receptors and role in inflammation. Clin. Immunol. 117:104-111. [DOI] [PubMed] [Google Scholar]
- 29.Matsui, T., R. Inoue, K. Kajimoto, A. Tamekane, A. Okamura, Y. Katayama, M. Shimoyama, K. Chihara, A. Saito-Ito, and M. Tsuji. 2000. First documentation of transfusion-associated babesiosis in Japan. Rinsho Ketsueki 41:628-634. (In Japanese.) [PubMed] [Google Scholar]
- 30.Matsumoto, K., H. Akagi, T. Ochiai, K. Hagino, K. Sekita, Y. Kawasaki, M. A. Matin, and T. Furuya. 1980. Comparative blood values of Macaca mulatta and Macaca fascicularis. Jikken Dobutsu 29:335-340. [PubMed] [Google Scholar]
- 31.Mendis, K., B. J. Sina, P. Marchesini, and R. Carter. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64:97-106. [DOI] [PubMed] [Google Scholar]
- 32.Mold, C., H. Gewurz, and T. W. Du Clos. 1999. Regulation of complement activation by C-reactive protein. Immunopharmacology 42:23-30. [DOI] [PubMed] [Google Scholar]
- 33.Munn, D. H., A. G. Bree, A. C. Beall, M. D. Kaviani, H. Sabio, R. G. Schaub, R. K. Alpaugh, L. M. Weiner, and S. J. Goldman. 1996. Recombinant human macrophage colony-stimulating factor in nonhuman primates: selective expansion of a CD16+ monocyte subset with phenotypic similarity to primate natural killer cells. Blood 88:1215-1224. [PubMed] [Google Scholar]
- 34.Page, K. R., A. L. Scott, and Y. C. Manabe. 2006. The expanding realm of heterologous immunity: friend or foe? Cell. Microbiol. 8:185-196. [DOI] [PubMed] [Google Scholar]
- 35.Passlick, B., D. Flieger, and H. W. Ziegler-Heitbrock. 1989. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74:2527-2534. [PubMed] [Google Scholar]
- 36.Perera, K. L., S. M. Handunnetti, I. Holm, S. Longacre, and K. Mendis. 1998. Baculovirus merozoite surface protein 1 C-terminal recombinant antigens are highly protective in a natural primate model for human Plasmodium vivax malaria. Infect. Immun. 66:1500-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reyers, F., A. L. Leisewitz, R. G. Lobetti, R. J. Milner, L. S. Jacobson, and M. van Zyl. 1998. Canine babesiosis in South Africa: more than one disease. Does this serve as a model for falciparum malaria? Ann. Trop. Med. Parasitol. 92:503-511. [PubMed] [Google Scholar]
- 38.Rosner, F., M. H. Zarrabi, J. L. Benach, and G. S. Habicht. 1984. Babesiosis in splenectomized adults. Review of 22 reported cases. Am. J. Med. 76:696-701. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt, L. H., R. Greenland, and C. S. Genther. 1961. The transmission of Plasmodium cynomolgi to man. Am. J. Trop. Med. Hyg. 10:679-688. [DOI] [PubMed] [Google Scholar]
- 40.Shaio, M. F., and P. R. Lin. 1998. A case study of cytokine profiles in acute human babesiosis. Am. J. Trop. Med. Hyg. 58:335-337. [DOI] [PubMed] [Google Scholar]
- 41.Shayan, P., and S. Rahbari. 2005. Simultaneous differentiation between Theileria spp. and Babesia spp. on stained blood smear using PCR. Parasitol. Res. 97:281-286. [DOI] [PubMed] [Google Scholar]
- 42.Shkap, V., A. J. de Vos, E. Zweygarth, and F. Jongejan. 2007. Attenuated vaccines for tropical theileriosis, babesiosis and heartwater: the continuing necessity. Trends Parasitol. 23:420-426. [DOI] [PubMed] [Google Scholar]
- 43.Singh, B., S. L. Kim, A. Matusop, A. Radhakrishnan, S. S. Shamsul, J. Cox-Singh, A. Thomas, and D. J. Conway. 2004. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363:1017-1024. [DOI] [PubMed] [Google Scholar]
- 44.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voorberg-van der Wel, A., C. H. Kocken, A. M. Zeeman, and A. W. Thomas. 2008. Detection of new Babesia microti-like parasites in a rhesus monkey (Macaca mulatta) with a suppressed Plasmodium cynomolgi infection. Am. J. Trop. Med. Hyg. 78:643-645. [PubMed] [Google Scholar]
- 46.Waters, A. P., D. G. Higgins, and T. F. McCutchan. 1993. Evolutionary relatedness of some primate models of Plasmodium. Mol. Biol. Evol. 10:914-923. [DOI] [PubMed] [Google Scholar]
- 47.Woollard, K. J., D. C. Phillips, and H. R. Griffiths. 2002. Direct modulatory effect of C-reactive protein on primary human monocyte adhesion to human endothelial cells. Clin. Exp. Immunol. 130:256-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright, I. G. 1973. Plasma kallikrein levels in acute Babesia argentina infections in splenectomised and intact calves. Z. Parasitenkd. 41:269-280. [DOI] [PubMed] [Google Scholar]
- 49.Wright, I. G. 1973. Osmotic fragility of erythrocytes in acute Babesia argentina and Babesia bigemina infections in splenectomised Bos taurus calves. Res. Vet. Sci. 15:299-305. [PubMed] [Google Scholar]
- 50.Ziegler-Heitbrock, L. 2007. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J. Leukoc. Biol. 81:584-592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.