Abstract
Pseudomonas aeruginosa, an important opportunistic pathogen of humans, exploits epithelial damage to establish infection. We have rigorously explored the role of N-glycoproteins and heparan sulfate proteoglycans (HSPGs) in P. aeruginosa-mediated attachment and subsequent downstream events at the apical (AP) and basolateral (BL) surfaces of polarized epithelium. We demonstrate that the N-glycan chains at the AP surface are necessary and sufficient for binding, invasion, and cytotoxicity to kidney (MDCK) and airway (Calu-3) cells grown at various states of polarization on Transwell filters. Upregulation of N-glycosylation enhanced binding, whereas pharmacologic inhibition of N-glycosylation or infection of MDCK cells defective in N-glycosylation resulted in decreased binding. In contrast, at the BL surface, the HS moiety of HSPGs mediated P. aeruginosa binding, cytotoxicity, and invasion. In incompletely polarized epithelium, HSPG abundance was increased at the AP surface, explaining its increased susceptibility to P. aeruginosa colonization and damage. Using MDCK cells grown as three-dimensional cysts as a model for epithelial organs, we show that P. aeruginosa specifically colocalized with HS-rich areas at the BL membrane but with complex N-glycans at the AP surface. Finally, P. aeruginosa bound to HS chains and N-glycans coated on plastic surfaces, showing the highest binding affinity toward isolated HS chains. Together, these findings demonstrate that P. aeruginosa recognizes distinct receptors on the AP and BL surfaces of polarized epithelium. Changes in the composition of N-glycan chains and/or in the distribution of HSPGs may explain the enhanced susceptibility of damaged epithelium to P. aeruginosa.
Ninety-five percent of all infectious agents enter through mucosal surfaces of the gastrointestinal, genitourinary, and respiratory tracts (reviewed in reference 35). These mucosal surfaces are usually lined by a single layer of epithelial cells, which serves as the primary barrier against the entry of most infectious agents and can be considered a primary component of the innate immune system. Epithelial cells form highly polarized cell layers with apical (AP) and basolateral (BL) surfaces that exhibit distinct protein, lipid, and glycoconjugate compositions. Pseudomonas aeruginosa is a ubiquitous opportunistic pathogen of humans that exploits injured mucosa to cause acute and chronic infections with high morbidity and mortality (reviewed in references 26 and 31). In the setting of epithelial injury and immunocompromise, this Gram-negative pathogen causes serious infections in patients with extensive burns, corneal trauma, or catheter-related bladder injury or in those on ventilators. In addition, P. aeruginosa chronically colonizes the lungs of patients with cystic fibrosis (CF) (4), leading to severe pulmonary damage and death. Despite aggressive antibiotic therapy, the fatality rate for many P. aeruginosa infections is 40%, and new approaches to treatment are even more critical now that antibiotic resistance is widespread among P. aeruginosa isolates.
The first step in establishing P. aeruginosa infection is receptor-mediated binding to the injured epithelium on the AP and/or BL surface, leading to bacterial internalization and/or direct host injury, as well as dissemination to distant tissues and organs. Glycoconjugates, including glycolipids, glycosylated proteins, and proteoglycans, are candidate receptors for P. aeruginosa binding. Their long carbohydrate chains are prominently displayed on the surface, exhibit distinct AP and BL localization, and serve as receptors for many microorganisms (3). For P. aeruginosa, however, conclusive in vitro or in vivo data are missing. For example, the predilection of P. aeruginosa for injured epithelium has been attributed to increased levels of asialo-GM1 on the AP surface of regenerating cells (11, 23, 43, 44), though it remains controversial whether asialo-GM1 and other glycosphingolipids bind P. aeruginosa (13, 49). Furthermore, secreted O-glycoproteins, or mucins, have been associated with the binding of P. aeruginosa to the AP surface (23, 37). N-glycosylated proteins, in which mannose (Man), glucose (Glc), N-acetylglucosamine (GlcN), and fucose are attached to core proteins to form high-mannose, complex, and hybrid N-glycans, are also candidate receptors. For example, the N-glycoproteins CFTR and CD95 have been shown to function as receptors for bacterial binding and internalization (20). However, the role of CFTR as a binding receptor for P. aeruginosa remains controversial (42).
In contrast to N-glycoproteins, which are present at the AP and BL surfaces, heparan sulfate proteoglycans (HSPGs) are preferentially expressed on the BL surface of the polarized epithelium (3) and could serve as BL receptors for P. aeruginosa. HSPGs are heterogeneous structures that are composed of a core protein and one or more covalently attached heparan sulfate (HS) chains. In addition to variability in the number of HS repeating units and the identities of the core proteins, HS chains are further modified by sulfation at the N, 2, 3, and/or 6 position, giving rise to enormous combinatorial diversity. The primary HSPG families include syndecans, transmembrane proteins located at the BL surface; perlecan and agrin, secreted HSPGs associated with the extracellular matrix; and glycosylphosphatidylinositol-anchored glypicans, found at the AP surface. HSPGs are known to mediate binding of various bacterial and viral pathogens (2, 14, 21, 25). They have previously been postulated to modulate adhesion of P. aeruginosa to incompletely polarized epithelial respiratory cells (41) and to the exposed basement membrane of the mouse cornea (9), but direct evidence for this function is lacking.
In this work, we have rigorously explored the role of N-glycan chains of glycoproteins and HS chains of HSPGs in P. aeruginosa infection at the AP and BL surfaces of polarized kidney and airway epithelial cells and how changes in their structure and/or expression affect bacterial binding and downstream events, including internalization and cell damage. Using two-dimensional (2D) and 3D cell cultures, we show that N-glycans are necessary and sufficient for binding, entry, and cytotoxicity at the AP surface of polarized epithelium. Enhanced expression and/or expression of more complex N-glycans, which can occur in damaged epithelium, increases P. aeruginosa infection at the AP surface. We further establish that HS chains of HSPGs are necessary and sufficient to mediate binding, invasion, and cytotoxicity on the BL surface in polarized cells and that sulfation is a critical determinant. Finally, we show that in incompletely polarized cells, a model of tissue injury, HSPGs are upregulated at the AP surface, which leads to enhanced susceptibility to binding and subsequent tissue damage by P. aeruginosa. Together, our results provide a basis for the increased susceptibility of acute or chronically injured tissue to P. aeruginosa infections, and they raise the possibility that well-studied molecules such as N-glycans and HS might be useful therapeutic targets for the treatment of P. aeruginosa infections.
MATERIALS AND METHODS
Bacterial strains and electroporation.
P. aeruginosa strain K (PAK; obtained from J. Mattick, University of Queensland, Brisbane, Australia) was routinely grown with shaking overnight in Luria-Bertani broth (LB broth) at 37°C. To create a plasmid which constitutively produces green fluorescent protein (GFP), the pnpT2-GFP fragment from p519ngfg (33) was excised with HindIII and ExoRI and cloned into the corresponding sites of pUCP20 to yield pnpT2-GFP-pUCP20. This plasmid was introduced into PAK by electroporation with a Bio-Rad Gene Pulser II, with settings of 1.6 V, 25 μF, and 200 Ω. The resulting strain was named PAK-GFP.
2D and 3D cell culture.
MDCK clone II and ConAr MDCK cells obtained from Keith Mostov (University of California, San Francisco, CA) were maintained in minimal essential medium (MEM) (51) supplemented with 5% fetal bovine serum (FBS; Invitrogen) at 37°C with 5% CO2. Calu-3 cells were obtained from the ATCC (Rockville, MD) and maintained in MEM supplemented with 10% FBS and l-glutamate at 37°C with 5% CO2. Cells were grown as 2D monolayers on 12-mm Transwell filters (3-μm pore size; Corning Incorporated). For all experiments, cells were plated under conditions such that they formed confluent monolayers that exhibited basic features of early polarized cells, including polarized distribution of some AP and BL membrane proteins and functional tight junctions that were impermeable to small molecules such as fluorescein isothiocyanate-inulin (FITC-inulin). Cells were grown for different lengths of time to model different states of polarization. For incompletely polarized confluent monolayers, MDCK cells were seeded at 0.7 × 106 cells/well and cultured for 24 h. For well-polarized confluent monolayers, MDCK cells were seeded at 0.3 × 106 cells/well and cultured for 5 days. For incompletely polarized confluent Calu-3 cell monolayers, Calu-3 cells were seeded at 1.5 × 106 cells/well and cultured for 2 days. For well-polarized confluent Calu-3 cell monolayers, Calu-3 cells were seeded at 1 × 106 cells/well and cultured for 7 days.
For some experiments, MDCK or Calu-3 cells grown as 2D cultures on Transwell filters were treated with the following reagents. None of these treatments inhibited bacterial growth (data not shown). To inhibit N-glycosylation, cells were pretreated with 0.1 to 1 μg/ml of tunicamycin (Sigma-Aldrich) for 16 h in MEM supplemented with 5% FBS (MDCK and ConAr cells) or 10% FBS (Calu-3 cells). For competition blocking with glycosaminoglycans, cells were pretreated with 0.1 to 10 μg/ml of heparin or chondroitin sulfate (Sigma-Aldrich) at 37°C for 1 h in serum-free MEM. To remove proteoglycans, cells were treated with 1 to 200 mU of heparinase III (Sigma-Aldrich) or chondroitinase ABC (Sigma-Aldrich) in Hank's buffered salt solution (HBSS) containing 0.1% bovine serum albumin (BSA) at 37°C for 2 h. Proteoglycan desulfation was performed by overnight incubation of cells with 10 mM sodium chlorate in MEM containing 5% FBS (MDCK and ConAr cells) or 10% FBS (Calu-3 cells). Resulfation was accomplished by adding 1 mM sodium sulfate to sodium chlorate-containing medium. Upregulation of N-glycosylation was performed as described previously (46), with the following modifications. Cells were grown in the presence of 1 mM Man, Glc, or Gal (Sigma-Aldrich) in MEM with 5% (MDCK and ConAr cells) or 10% (Calu-3 cells) FBS for 1 week (long upregulation of N-glycosylation) or 1 day (brief upregulation of N-glycosylation). Cells were stained with FITC-concanavalin A (FITC-ConA; Sigma Aldrich) to assess cell surface N-glycosylation. Cell surface proteoglycans were visualized by immunofluorescence staining with HS antibody (10E4; Seikagaku) or with FITC-WFA (a chondroitin sulfate-specific lectin from Wisteria floribunda; Sigma Aldrich).
For 3D cell culture, MDCK or ConAr MDCK cells were grown as cysts in Matrigel-collagen as previously described (39, 52), with the following modifications. A single cell suspension of 4 × 104 cells/ml was added to a solution of buffered, liquefied collagen (3 mg/ml). Two hundred fifty microliters of cells in collagen I was plated in 8-well cover-glass chambers (Nalge Nunc International) that had previously been covered with 100% Matrigel. The Matrigel served as a solid base to which the cysts attached prior to collagen solidification. The collagen was allowed to solidify into a gel by incubation at 37°C prior to the addition of MEM containing 10% FBS. Over the 5-day growth period, individual cells in the collagen matrix proliferated to form cysts, with the AP membrane facing the lumen and the BL membrane facing the surrounding collagen (BL-side-out cysts). For some experiments, the anti-beta-1 antibody AIIB2 (1:100 dilution in the collagen I solution and/or in MEM; a kind gift of Caroline Damsky at the University of California, San Francisco) was included for the entire culture period. Under these conditions, polarized AP-side-out cysts were formed, although the structures were less well organized than those in the BL-side-out cysts (39). For bacterial infections, MDCK cysts grown in Matrigel-collagen were treated with collagenase type VII (Sigma-Aldrich) at 100 U/ml in phosphate-buffered saline (PBS) for 15 min at 37°C to digest the collagen gel. For some experiments, cysts were treated with heparinase III in HBSS containing 0.1% BSA at 37°C for 3 h, washed, and resuspended in serum-free MEM for bacterial infections.
Bacterial adhesion and invasion assays on 2D monolayers.
PAK cells grown overnight in LB broth to stationary phase were diluted in 50 μl of serum-free MEM and added to cells at a multiplicity of infection (MOI) of 20. To infect the AP side of 2D monolayers grown on Transwell filters, the bacteria were added to the AP chamber. For BL infections, the Transwell insert was placed directly onto 50 μl of serum-free MEM containing PAK. After 1 h of infection at 37°C, adhesion and invasion assays were performed as described previously (27). Briefly, for the adhesion assay, cells were washed in PBS to remove nonadherent bacteria and were lysed in 1 ml Ca2+- and Mg2+-free PBS with 0.25% Triton X-100 (Sigma-Aldrich) for 30 min. For invasion assays, cells were first incubated, before lysis, in serum-free MEM containing 0.4 mg/ml amikacin (Fisher Scientific) for 2 h. After lysis, cells were removed from the Transwell filters by gentle scraping. Bacteria were enumerated by plating serial dilutions of cell lysates on LB plates and counting the CFU. All assays were carried out on triplicate wells, and results are reported as averages for three or four experiments. Because of well-established variability in adhesion and invasion assays, data were normalized to 100% of bacterial adhesion or internalization at the AP surface of cultured epithelial cells to allow comparison between experiments performed on different days. Consistent with previous reports, approximately 10% of the PAK inoculum bound to cells, whereas 1 to 2% of the inoculum was internalized (29).
Bacterial infection of 3D cysts.
Prior to bacterial infections, cysts were briefly treated with type VII collagenase (100 U/ml in PBS for 15 min at 37°C), which removed the thin layer of collagen that coated the cysts and allowed the bacteria to access the surface of the cysts. GFP-expressing PAK cells (107) suspended in serum-free MEM were incubated with the cysts for 2 h. Cells were washed with PBS to remove nonbound bacteria and fixed in PBS containing 1% paraformaldehyde at 37°C for 0.5 h. Bacterial adherence and colocalization with specific surface markers are described below.
Immunofluorescence microscopy and image analysis.
HS chains were stained with anti-heparan sulfate antibody (10E4), tight junctions were stained with anti-ZO-1 (R40.76) antibody (a gift from B. Stevenson, University of Edmonton, Alberta, Canada), actin filaments were stained with AlexaFluor594-phalloidin (Invitrogen), and mannose residues were stained with FITC-ConA. AlexaFluor488- or 647-conjugated secondary antibodies were obtained from Invitrogen. Monolayers grown on Transwell filters were fixed in PBS containing 1% paraformaldehyde at 37°C for 0.5 h. After being washed, cells were incubated with primary antibodies overnight at 4°C and, afterwards, with fluorescent secondary antibodies for 2 h at room temperature. Filters were excised and mounted on microscope slides (Fisher Scientific) in mounting medium (Vector Laboratories, Inc.). Cysts grown in eight-well cover-glass chambers were fixed and stained with antibodies directly in chambers. Cysts were incubated with primary antibodies overnight at 4°C and with secondary antibodies for 2 h at room temperature.
Samples were examined with a confocal microscope (LSM 510; Carl Zeiss MicroImaging, Inc.). Images and 3D reconstructions were acquired by and processed in Meta 510 software. Image J analysis was performed on TIFF files.
Bacterial binding to 3D cysts and colocalization with surface markers were quantified using the Image J plug-in Voxel Counter on 3D reconstructions of TIFF images acquired with Meta 510 software. Voxel Counter (ImageJ plug-in) was used to quantify the volume of bound 3D bacterial aggregates, and a minimum volume was set as a threshold to enable automated cell counting using the 3D Object Counter (ImageJ plug-in). Any bacterial aggregate above the threshold was counted as 1. The surface area of membrane regions either enriched with or depleted of HS or N-glycans (determined by staining with an anti-HS antibody or with FITC-ConA, respectively) was measured in pixels by ImageJ, and the number of bacterial aggregates bound was normalized per pixel of each specific surface area. The percentage (compared to the total) of bacterial aggregates bound to each specific region was determined. For each treatment, 40 to 50 cysts were quantified.
In vitro binding assay.
Sugars, N-glycans, and glycosaminoglycans (0.1 to 10 μg in 0.2 ml double-distilled water [ddH2O]; Sigma Aldrich) were added to 96-well plastic plates (Falcon, Becton Dickinson Labware) and incubated overnight at 37°C until evaporated. Wells were washed with ddH2O and blocked in 0.1% BSA for 0.5 h at room temperature. Bound glycans were stained with 1% toluidine blue (Sigma Aldrich), and absorbance was measured at 630 nm. The absorbance values for known concentrations of glycans were used as the standard curve, and the concentration of bound glycans (μg/well) was calculated. Stationary-phase PAK-GFP cells (107 in 0.1 ml ddH2O) were added to wells and incubated for 2 h at room temperature. Nonadherent bacteria were removed by washing with ddH2O. Bound PAK-GFP was quantified using a SpectraMax 340PC plate reader using SOFTmaxPro software (Molecular Devices) at an excitation wavelength (λex) of 480 nm and an emission wavelength (λem) of 530 nm. PAK-GFP bound to noncoated wells was used as a control and subtracted out as background. All assays were carried out on triplicate wells, and results are reported as averages for four experiments.
Cytotoxicity assay.
Epithelial cell cytotoxicity was quantified by colorimetric quantification of lactate dehydrogenase (LDH) release, using a commercially available kit (CytoTox 96 nonradioactive cytotoxicity assay; Promega) according to the manufacturer's instructions and as previously described (17), with the following modifications. For both AP and BL infections, Transwell filters were placed on 50-μl drops of medium only (for AP infections) or medium plus bacteria (for BL infections). Transwell-grown cells were infected with PAK (MOI of 20) for 5 h at the AP or BL surface, and 50 μl of the supernatant was collected from the AP chamber every 1 h. The absorbance of the sample was measured at 490 nm on a SpectraMax 340PC plate reader using SOFTmaxPro software. Results were normalized to the 100% LDH release in infected cells. Assays were carried out on triplicate wells, and the results are reported as averages for three or four experiments.
Transepithelial permeability.
Transwell-grown cells were infected with PAK for 5 h at the AP surface. Nonadherent bacteria were removed by washing with PBS. FITC-inulin (Sigma-Aldrich; 100 μg/ml) in PBS was added to the AP chamber, and PBS alone was added to the BL chamber of Transwell plates. Cells were incubated at 37°C, and 100-μl samples were collected from the BL chamber every 0.5 h. Fluorescence was quantified using a fluorescence plate reader (λex = 480 nm and λem = 530 nm; SpectraMax 340PC plate reader using SOFTmaxPro software). Known dilutions of FITC-inulin were used as a standard curve.
Statistical analysis.
Data are expressed as means ± standard deviations (SD). Statistical significance was estimated by paired Student's t test. Differences were considered to be significant at P values of <0.05.
RESULTS
Modulations of specific enzymes in N-glycosylation pathway affect the expression of N-glycan chains on the cell surface.
Previous studies have shown that P. aeruginosa cytotoxicity is diminished in filter-grown confluent monolayers of concanavalin A-resistant Madin-Darby canine kidney (ConAr MDCK) cells compared to that for wild-type (wt) MDCK cells (1). While the exact defect in the glycosylation pathway in ConAr MDCK cells is not known, there are alterations in the Man core of its N-linked carbohydrates, which impair the formation of more complex N-linked carbohydrate structures (34). We hypothesized that surface-exposed complex N-glycans are important determinants of P. aeruginosa adhesion to an injured epithelium, thus explaining the reduced susceptibility of ConAr MDCK cells to P. aeruginosa-mediated damage. Others have suggested that P. aeruginosa binding and entry into ConAr MDCK cells are diminished because these cells form hyperpolarized monolayers (15).
To test our hypothesis, we utilized MDCK or human airway epithelial (Calu-3) cells grown as confluent monolayers on Transwell filters for various lengths of time. We have shown that under these conditions, MDCK cells form functional tight and adherens junctions within 24 h and show a polarized distribution of many AP and BL markers, such as gp135 and gp58 (28) (see Fig. S1 in the supplemental material). With increasing time in culture, they exhibit an enhanced polarized distribution of markers, such as the predominantly BL surface-expressed epidermal growth factor (EGFR) (see Fig. S1 in the supplemental material). We confirmed that cells grown for 1 day (MDCK cells) or 2 days (Calu-3 cells) formed confluent monolayers with functional tight and adherens junctions, as evidenced by impermeability to apically applied FITC-inulin (see Fig. S1 in the supplemental material), a low-molecular-weight molecule that is not able to diffuse through functional tight junctions (1). Epithelial polarity continued to increase at later times in culture (5 days for MDCK cells and 7 days for Calu-3 cells), as evidenced by an increased polarized distribution of AP and BL markers, such as HSPGs and EGFR (see Fig. 2 and Fig. S1 in the supplemental material). For ease of nomenclature, we refer to cells cultured for 1 day (MDCK cells) or 2 days (Calu-3 cells) as “incompletely polarized” monolayers and those cultured for 5 days (MDCK cells) or 7 days (Calu-3 cells) as “well-polarized” monolayers. We emphasize that under the conditions of our experiments, the term “incompletely polarized monolayers” describes confluent monolayers with functional tight and adherens junctions but with an incompletely polarized distribution of some AP and BL markers.
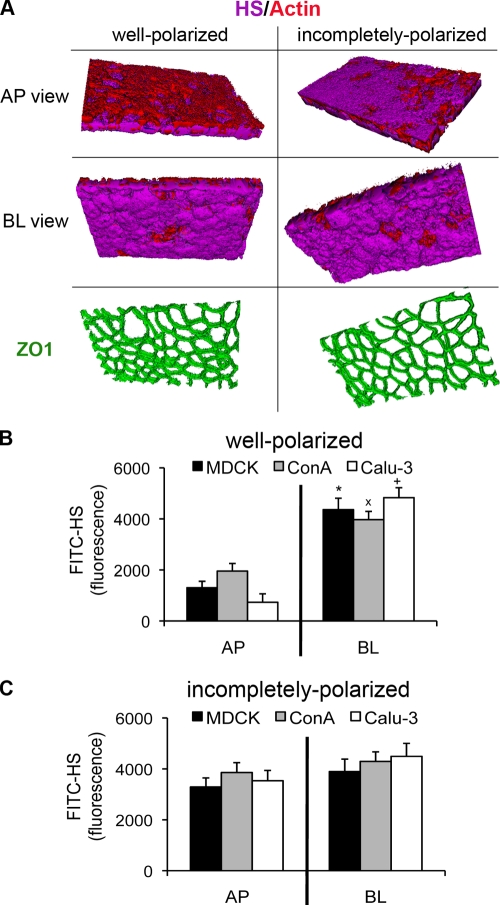
FIG. 2.
HSPGs are expressed on the AP surface in incompletely polarized cells. In well-polarized monolayers grown on Transwell filters, there was a greater amount of HSPGs on the BL surface than on the AP surface. In incompletely polarized monolayers, there was increased expression of HSPGs on the AP surface. (A) 3D reconstruction of z-stack images acquired by confocal microscopy. Shown are AP and BL projections of well-polarized and incompletely polarized Calu-3 cells stained with an antibody against the HS chain of HSPGs (FITC-HS; purple), with phalloidin (red) to visualize actin, and with ZO-1 (green) to visualize tight junctions. (B and C) FITC-HS fluorescence (in arbitrary units) measured in a fluorescence plate reader. Well-polarized (B) or incompletely polarized (C) MDCK, ConAr, and Calu-3 cells were stained with FITC-HS added to the AP or BL surface. Shown are the means ± SD for three separate experiments. *, P < 0.05 compared to AP surface-stained MDCK cells; ×, P < 0.05 compared to AP surface-stained ConAr cells; +, P < 0.05 compared to AP surface-stained Calu-3 cells.
We qualitatively and quantitatively assessed the amount of surface-exposed Man in N-glycan chains in wt MDCK, ConAr MDCK, and Calu-3 cells at various states of polarization by confocal microscopy and fluorescence plate assays using FITC-conjugated ConA, a lectin that specifically recognizes nonreducing terminal α-d-glucosyl and α-d-mannosyl groups. The results obtained with incompletely polarized cells (see Fig. S2A in the supplemental material) were similar to those seen for well-polarized cells (Fig. 1). FITC-ConA bound more efficiently to well-polarized wt MDCK cells than to ConAr MDCK cells at both the AP and BL surfaces (Fig. 1A and B). FITC-ConA binding to the AP and BL surfaces of Calu-3 cells was of the same intensity as binding to wt MDCK cells. We used Man or Glc (a Man precursor) supplementation to complement the defect in ConAr MDCK cells or to augment N-glycosylation in wt MDCK and Calu-3 cells. Addition of these sugars is known to upregulate the activities of phosphomannomutase (PMM) and phosphomannose isomerase (PMI), enzymes that play a critical role in maintaining the supply of d-Man derivatives required for N-glycosylation (46). Man or Glc supplementation restored the binding of FITC-ConA to ConAr MDCK cells (Fig. 1A and B). Increased binding of FITC-ConA to Man residues on N-glycan chains was observed after only 5 to 6 days of treatment with exogenous Man, eliminating the possibility that the exogenous Man simply adhered to the cell surface and facilitated the binding of the lectin. Addition of galactose (Gal), which is not involved in Man synthesis, did not restore binding of FITC-ConA. Moreover, binding of FITC-ConA was enhanced for wt MDCK and Calu-3 cells cultured with Man or Glc (but not Gal) compared to that with untreated cells. We verified that MDCK and Calu-3 cells with normal or enhanced levels of Man were sensitive to killing by ConA added to the growth medium (see Fig. S3 in the supplemental material). ConAr MDCK cells grown in the presence of Man or Glc (but not Gal) lost their normal resistance to the lectin and showed enhanced sensitivity to ConA (see Fig. S3 in the supplemental material).
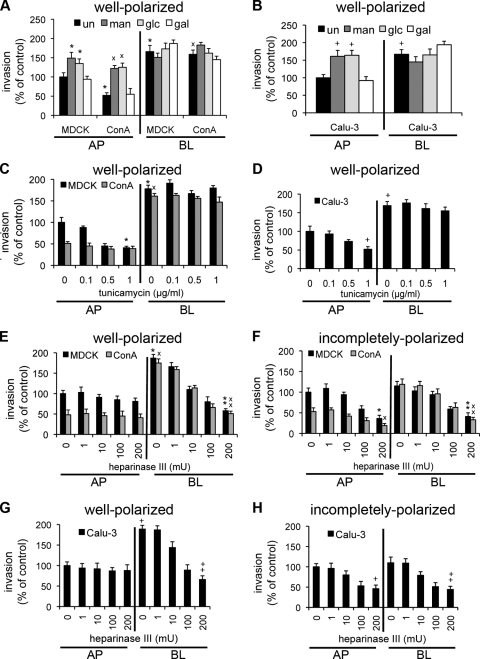
FIG. 1.
P. aeruginosa adhesion to the AP surface is N-glycan dependent. (A and B) Addition of Man or Glc, but not Gal, to the growth medium increases the level of Man on the cell surface. MDCK, ConAr, and Calu-3 cells were cultured in the presence or absence of the indicated sugar. Shown are well-polarized monolayers grown on Transwells that were fixed without permeabilization and stained at either the AP or BL surface with FITC-ConA, which binds to Man in N-glycan chains. ConAr cells have less Man on the AP and BL surfaces than do wt MDCK cells, but the addition of Man or Glc restores FITC-ConA binding to wt levels. The addition of Man or Glc enhances FITC-ConA binding to MDCK and Calu-3 cells at both the AP and BL surfaces. (A) 3D reconstructions of z-stack images acquired by confocal microscopy. Shown are AP and BL projections of well-polarized monolayers. Man is stained with FITC-ConA (green), and actin is stained with phalloidin (red). (B) FITC-ConA fluorescence (in arbitrary units) of well-polarized cells treated with the indicated sugars, as measured in a fluorescence plate reader. Shown are the means ± SD for three separate experiments. *, P < 0.05 compared to AP-side-untreated MDCK cells; ×, P < 0.05 compared to AP-side-untreated ConAr cells; +, P < 0.05 compared to AP-side-untreated Calu-3 cells; **, P < 0.05 compared to BL-side-untreated MDCK cells; ××, P < 0.05 compared to BL-side-untreated ConAr cells; ++, P < 0.05 compared to BL-side-untreated Calu-3 cells. (C and D) Addition of Man or Glc is sufficient to enhance P. aeruginosa binding. Cells were grown in the presence or absence of the indicated sugar, bacteria were added for 1 h to the AP or BL surface of well-polarized cells grown on Transwell filters, and standard adhesion assays were performed. Shown are the means ± SD for three separate experiments. (C) P. aeruginosa binding to well-polarized MDCK and ConAr cells, normalized to AP-side-infected, untreated MDCK cells. (D) P. aeruginosa binding to well-polarized Calu-3 cells, normalized to AP-side-infected, untreated Calu-3 cells. (E and F) Tunicamycin inhibits bacterial binding to the AP surface of wt MDCK and Calu-3 cells in a dose-dependent manner. Cells were pretreated with the indicated amounts of tunicamycin to inhibit N-glycosylation. Bacterial binding was measured as described above. (E) P. aeruginosa binding to well-polarized MDCK and ConAr cells, normalized to AP-side-infected, untreated MDCK cells (point “0”). (F) P. aeruginosa binding to well-polarized Calu-3 cells, normalized to AP-side-infected, untreated Calu-3 cells (point “0”). *, P < 0.05 compared to AP-side-infected, untreated MDCK cells; ×, P < 0.05 compared to AP-side-infected, untreated ConAr cells; +, P < 0.05 compared to AP-side-infected, untreated Calu-3 cells.
Several conclusions that are important for our subsequent work can be drawn from these experiments. First, well-polarized and incompletely polarized ConAr MDCK cells have decreased levels of surface-exposed Man, and thus N-linked glycans, on both the AP and BL surfaces. Second, Man or Glc supplementation is able to restore the cell surface expression of N-glycans in these cells. It is also sufficient to increase the expression of more complex N-glycans on the AP and BL surfaces of wt MDCK and Calu-3 cells. Third, and finally, the surface presentation of N-glycans does not change as cells become more polarized (compare Fig. 1B to Fig. S2A in the supplemental material).
The expression level and structure of N-glycan chains modulate P. aeruginosa binding at the AP surface of well-polarized and incompletely polarized epithelia.
To address the hypothesis that modifications in the expression and structure of N-glycan chains alter interactions of P. aeruginosa with the host epithelium, we assayed the effects of various modifications in N-glycosylation on bacterial adhesion to host epithelial cells. MDCK, ConAr MDCK, and Calu-3 cells were grown on Transwell filters as well-polarized or incompletely polarized monolayers, with or without sugar supplementation. Augmentation of N-glycosylation by addition of Man or Glc (but not Gal) to ConAr MDCK cells increased bacterial binding nearly threefold, to at least wt levels, at the AP surface of well-polarized cells (Fig. 1C). Likewise, addition of Man or Glc (but not Gal) to wt MDCK cells (Fig. 1C) or Calu-3 cells (Fig. 1D) was sufficient to increase bacterial binding at the AP surface. Similar results were observed in incompletely polarized cells (see Fig. S2B and C in the supplemental material). None of these treatments affected bacterial binding at the BL surface of ConAr or wt MDCK cells (Fig. 1C) or Calu-3 cells (Fig. 1D), eliminating the possibility that these treatments nonspecifically affected bacterial binding.
We next pretreated cells with tunicamycin, a well-characterized inhibitor of GlcN phosphotransferase, the enzyme that catalyzes the first step of N-glycoprotein synthesis. In control experiments, we determined that tunicamycin treatment reduced the amounts of N-glycans on the AP and BL cell surfaces of well-polarized and incompletely polarized wt MDCK and Calu-3 cells, to levels roughly similar to those for untreated ConAr MDCK cells (see Fig. S4 in the supplemental material). As expected, tunicamycin treatment had minimal effect on FITC-ConA binding to ConAr MDCK cells, which already express fewer N-glycans on their surfaces. Tunicamycin treatment of well-polarized wt MDCK or Calu-3 cells decreased P. aeruginosa adhesion to the AP surface up to twofold (Fig. 1E and F), in a dose-dependent manner, indicating that N-glycans may function as AP binding receptors. Drug treatment did not further decrease bacterial binding to the AP surface of ConAr MDCK cells (Fig. 1E), which was already decreased approximately twofold compared to that for wt cells, strengthening our conclusion that N-glycosylation is defective in these cells. P. aeruginosa adhesion to the BL surface was not affected in tunicamycin-treated wt or ConAr MDCK cells (Fig. 1E) or in tunicamycin-treated Calu-3 cells (Fig. 1F). Similar results were observed in incompletely polarized cells (see Fig. S2D and E in the supplemental material).
Consistent with previously published results (15, 28), we found that P. aeruginosa bound more efficiently to the AP surface of incompletely polarized cells than to the AP surface of well-polarized cells (see Fig. S5 in the supplemental material). We determined if alteration in the expression of N-glycans in incompletely polarized cells is responsible for the increased binding of P. aeruginosa to the AP surface. Bacterial binding to the AP surface of incompletely polarized cells was twofold higher than binding to well-polarized kidney and lung cells (see Fig. S6A in the supplemental material). Treatment with tunicamycin did not change the binding ratio. These results establish that N-glycans are important determinants of P. aeruginosa binding to the AP surface of polarized epithelium. However, enhanced bacterial binding to the AP surface of incompletely polarized cells is N-glycan independent; otherwise, tunicamycin treatment would have abrogated the increased binding. Together, these results firmly establish that an increase in the abundance and/or length and complexity of N-glycan chains enhances P. aeruginosa binding at the AP surface independent of the state of epithelial polarization.
HSPGs are expressed preferentially at the BL surface of well-polarized epithelium but on both surfaces of incompletely polarized epithelium.
In contrast to N-glycoproteins, which are present at the AP and BL surfaces, HSPGs are preferentially expressed on the BL surface of polarized epithelium (3). We considered the possibilities that (i) HS chains of HSPGs modulate P. aeruginosa attachment to the BL surface of mucosal surfaces and/or (ii) enhanced AP expression of HS explains the increased binding of P. aeruginosa to the AP surface of incompletely polarized epithelium, since the increase is independent of N-glycans.
We first assessed expression of HS qualitatively and quantitatively at the AP and BL surfaces of MDCK cells and Calu-3 cells at various stages of polarization, using a commercially available antibody that recognizes the HS moiety of HSPGs independent of the identity of the core protein. Confocal microscopy revealed that the HS antibody preferentially bound to the BL surface of well-polarized Calu-3 cells (Fig. 2A) as well as to the BL surface of well-polarized wt MDCK and ConAr MDCK cells (see Fig. S1C and D in supplemental material). In contrast, the HS antibody bound approximately equally to the AP and BL surfaces of incompletely polarized cells (Fig. 2A). Both polarization models had functional tight junctions, as evidenced by ZO-1 staining (Fig. 2A) and by the lack of permeability to apically applied FITC-inulin (data not shown). Quantification of bound FITC-conjugated antibody confirmed that HSPGs were largely restricted to the BL surface of well-polarized wt MDCK, ConAr MDCK, and Calu-3 cells (Fig. 2B), whereas in incompletely polarized cells, there were approximately equivalent levels at both the AP and BL surfaces (Fig. 2C). Together, the experiments demonstrate that HS chains of HSPGs fulfill the characteristics predicted for a BL receptor in multiple cell lines. There is preferential presentation of HSPGs at the BL surface of fully polarized cells, while there is increased presentation of HSPGs at the AP surface of incompletely polarized cells.
HS chains of HSPGs contribute to P. aeruginosa binding at the BL surface of well-polarized epithelium and at both surfaces of incompletely polarized epithelium.
We utilized comprehensive approaches to determine the role of HSPGs in bacterial adherence to the AP and BL surfaces of well-polarized and incompletely polarized epithelial monolayers. First, we added excess heparin to block the interaction between P. aeruginosa and HS chains on the cell surface. In well-polarized epithelium, the addition of heparin inhibited bacterial binding in a dose-dependent manner at the BL surface of wt MDCK, ConAr MDCK, and Calu3 cells (Fig. 3A and C). At 10 μg/ml heparin, binding was decreased ∼50% compared to that in cells without the addition of heparin. As expected, exogenous addition of heparin had minimal or no effect on P. aeruginosa binding at the AP surface of well-polarized cells (Fig. 3A and C). The results were distinctly different for incompletely polarized cells. Addition of heparin reduced bacterial adhesion 50% at both the AP and BL surfaces of incompletely polarized wt MDCK (Fig. 3B) and Calu-3 (Fig. 3D) cells. Notably, heparin also decreased bacterial attachment to the AP surface of incompletely polarized ConAr MDCK cells (Fig. 3B). To rule out nonspecific charge effects, we demonstrated that the addition of another highly negatively charged glycosaminoglycan chain, chondroitin sulfate (CS), had no effect on bacterial binding to either surface in any of these cell types (see Fig. S7A to D in the supplemental material).
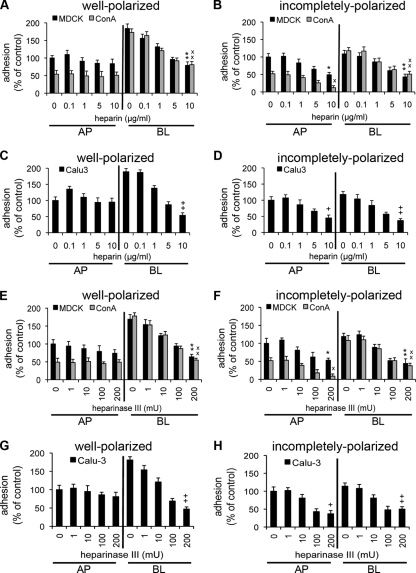
FIG. 3.
HS chains mediate P. aeruginosa adhesion to the BL surface and contribute to binding to the AP surface in incompletely polarized cells. Competitive inhibition with heparin (A to D) or enzymatic removal of HS chains with heparinase III (E to H) inhibits P. aeruginosa binding to the BL surface of well-polarized and incompletely polarized cells and to the AP surface of incompletely polarized cells. Cells were pretreated with the indicated amount of heparin or heparinase III, bacteria were added for 1 h, and standard adhesion assays were performed. Shown are the means ± SD for three separate experiments. P. aeruginosa binding to well-polarized (A) and incompletely polarized (B) MDCK and ConAr cells pretreated with increasing amounts of heparin was determined and normalized to that of AP-side-infected, untreated MDCK cells. P. aeruginosa binding to well-polarized (C) and incompletely polarized (D) Calu-3 cells pretreated with increasing amounts of heparin was determined and normalized to that of AP-side-infected, untreated Calu-3 cells. P. aeruginosa binding to well-polarized (E) and incompletely polarized (F) MDCK and ConAr cells pretreated with increasing doses of heparinase III was determined and normalized to that of AP-side-infected, untreated MDCK cells. P. aeruginosa binding to well-polarized (G) and incompletely polarized (H) Calu-3 cells pretreated with increasing doses of heparinase III was determined and normalized to that of AP-side-infected, untreated Calu-3 cells. *, P < 0.05 compared to AP-side-infected, untreated MDCK cells; ×, P < 0.05 compared to AP-side-infected, untreated ConAr cells; +, P < 0.05 compared to AP-side-infected, untreated Calu-3 cells; **, P < 0.05 compared to BL-side-infected, untreated MDCK cells; ××, P < 0.05 compared to BL-side-infected, untreated ConAr cells; ++, P < 0.05 compared to BL-side-infected, untreated Calu-3 cells.
We further examined the role of HS chains in mediating the interaction between P. aeruginosa and HSPGs in well-polarized and incompletely polarized cells. Pretreatment of cells with heparinase III, an enzyme that cleaves HS chains, reduced bacterial adhesion to the BL surface of well-polarized wt MDCK, ConAr MDCK, and Calu3 cells in a dose-dependent manner, with reductions of up to 50% at 200 mU (Fig. 3E and G). A minimal effect was observed on bacterial binding at the AP surface. In incompletely polarized cells, heparinase III treatment decreased bacterial attachment to both the AP and BL surfaces of all three cell lines (Fig. 3F and H). Enzymatic removal of CS by chondroitinase ABC did not affect bacterial attachment, confirming the specific role of HS in mediating interactions with P. aeruginosa (see Fig. S7E to H in the supplemental material). We qualitatively and quantitatively confirmed the specificity of enzymatic treatment with heparinase III by showing decreased HS antibody staining at the BL surface of well-polarized wt MDCK, ConAr MDCK, and Calu-3 cells and at the AP and BL surfaces of incompletely polarized cells (see Fig. S8 in the supplemental material). Likewise, the efficacy of chondroitinase ABC treatment was confirmed by staining with FITC-conjugated Wisteria floribunda agglutinin (WFA), a lectin that binds to the N-acetylgalactosamine moiety in CS (data not shown). Treatment with chondroitinase ABC did not attenuate the staining of the HS antibody (data not shown).
The sulfate moieties on HS chains are major contributors to their net negative charge and may provide the basis for ionic forces that underlie noncovalent interactions between HSPGs and P. aeruginosa. To study the role of sulfation in bacterial binding, we pretreated wt MDCK, ConAr MDCK, and Calu-3 cells with sodium chlorate, an inhibitor of sulfate adenyltransferase and HS sulfation. As shown in Fig. 4, chemical desulfation of HS reduced bacterial binding over twofold at the BL surface of well-polarized cells and at both surfaces in incompletely polarized cells. Resulfation by addition of sodium sulfate to chlorate-treated cells (53) restored bacterial binding to well-polarized and incompletely polarized epithelia. The efficiencies of desulfation and resulfation were monitored by staining with the anti-HS antibody (data not shown).
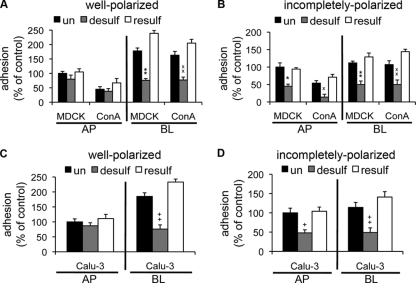
FIG. 4.
P. aeruginosa binding to HS is dependent on sulfation of HS chains. Chemical inhibition of sulfation inhibits P. aeruginosa binding to the BL surface of well-polarized and incompletely polarized cells and to the AP surface of incompletely polarized cells. Cells were pretreated with sodium chlorate for desulfation (desulf) of HS chains, and sulfation was restored (resulf) by treatment with sodium sulfate. Bacteria were added for 1 h, and standard adhesion assays were performed. Shown are the means ± SD for three separate experiments. P. aeruginosa binding to well-polarized (A) and incompletely polarized (B) MDCK and ConAr cells was determined and normalized to that of AP-side-infected, untreated MDCK cells. P. aeruginosa binding to well-polarized (C) and incompletely polarized (D) Calu-3 cells was determined and normalized to AP-side-infected, untreated Calu-3 cells. *, P < 0.05 compared to AP-side-infected, untreated MDCK cells; ×, P < 0.05 compared to AP-side-infected, untreated ConAr cells; +, P < 0.05 compared to AP-side-infected, untreated Calu-3 cells; **, P < 0.05 compared to BL-side-infected, untreated MDCK cells; ××, P < 0.05 compared to BL-side-infected, untreated ConAr cells; ++, P < 0.05 compared to BL-side-infected, untreated Calu-3 cells.
In summary, our results suggest that HS chains with intact sulfate groups play an important role in binding of P. aeruginosa to the BL surface of the polarized epithelium. Importantly, all three treatments that affected HSPGs (addition of excess exogenous heparin, removal of HS chains by digestion with heparinase III, and removal of sulfate residues by chemical desulfation) reduced bacterial binding to the AP surface of incompletely polarized cells approximately two-fold for all three cell lines compared to that for well-polarized cells (see Fig. S6B in the supplemental material). We concluded that the increased presence of HSPGs on the AP surface of incompletely polarized cells accounts at least in part for their increased susceptibility to P. aeruginosa infection.
P. aeruginosa binds directly to isolated HS and N-glycan chains in vitro.
If N-glycans and HS chains serve as receptors for P. aeruginosa binding, then it should be possible to demonstrate direct binding in vitro. We established an in vitro binding affinity assay where informative glycosylated molecules were used to coat 96-well plastic plates at different concentrations, GFP-conjugated bacteria were then added to coated plates, and fluorescence was measured after 1 h. As shown in Fig. 5, P. aeruginosa bound in a dose-dependent manner to HS or to a complex hybrid N-glycan chain [(Gal-GlcN)4Man3(GlcN)2], with the strongest binding to HS. Minimal or no binding was observed with other glycosaminoglycans, such as alternatively sulfated CS chains or nonsulfated hyaluronic acid (HA). These results suggest that (i) P. aeruginosa binds to specific sulfated sequences in HS chains and (ii) the anionic charge provided by sulfate groups is necessary for the interaction. Moreover, no binding was observed for wells coated with GlcN, which is one of the sugar residues present in N-glycan chains. Our findings suggest that single sugars are not sufficient and that recognition of specific ordered combinations of sugar sequences along the N-glycan chain are required for bacterial adhesion. Together, these results strongly indicate that HS and N-glycans can function as binding receptors for P. aeruginosa.
FIG. 5.

P. aeruginosa directly binds in vitro to isolated HS and N-glycan chains in a dose-dependent manner. Ninety-six-well plastic plates were coated overnight with increasing concentrations of the indicated molecules. GFP-conjugated bacteria were added for 1 h, and fluorescence was quantified in a fluorescence plate reader. Control bacterial binding to noncoated wells was set as a background, and the percentage of binding above that of the control is indicated. Shown are means ± SD for four separate experiments. HS, heparan sulfate; CS-4, 4-0-sulfated chondroitin sulfate; CS-6, 6-0-sulfated chondroitin sulfate; HA, hyaluronic acid; GlcN, N-acetylglucosamine.
P. aeruginosa-induced host cell injury or internalization into host cells is mediated by binding to N-glycans at the AP surface or to HS at the BL surface.
Following binding to epithelial cells, P. aeruginosa is able to enter into cells and/or, at later time points, cause type III secretion-dependent cytotoxicity. In order to study the extent of host cell damage after bacterial attachment to N-glycans or HS, variously treated cells were incubated with bacteria for 5 h, and standard LDH release assays (which measure cell damage) and FITC-inulin monolayer permeability assays (which measure the integrity of tight junctions) were performed. We first studied the role of N-glycans by pretreating cells with tunicamycin to inhibit N-glycosylation or by exogenous addition of Man to increase N-glycosylation. At up to 5 h postinfection, after AP-side addition of bacteria to tunicamycin-treated cells, LDH release was reduced 50 to 70% in both well-polarized and incompletely polarized wt MDCK and Calu-3 cells (Fig. 6; see Fig. S9A to H in the supplemental material). In contrast, Man supplementation augmented bacterium-induced cytotoxicity at the AP surface of well-polarized and incompletely polarized monolayers, as evidenced by increased LDH release. Bacterium-induced cell death correlated well with the loss of the epithelial barrier, as measured by FITC-inulin diffusion through the epithelial monolayer (data not shown; see Fig. S10 in the supplemental material). As expected, ConAr MDCK cells were resistant to bacterial killing at the AP surface, and tunicamycin treatment did not further decrease LDH release (Fig. 6; see Fig. S9I to L in the supplemental material) or affect FITC-inulin diffusion (data not shown). However, incubation with Man increased the sensitivity of ConAr MDCK cells to bacterium-induced injury at the AP surface (Fig. 6). Consistent with our binding data (Fig. 1), cytotoxicity induced by bacteria added to the BL surface was unaffected by tunicamycin treatment or Man supplementation (Fig. 6; see Fig. S9 in the supplemental material).
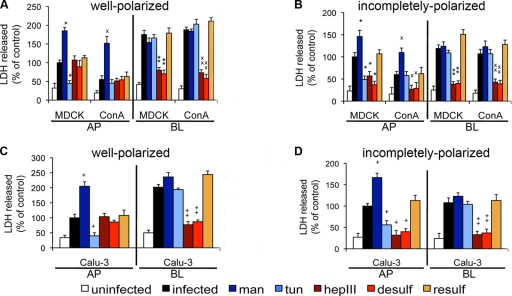
FIG. 6.
Bacterial cytotoxicity is mediated upon P. aeruginosa binding to N-glycans on the AP surface of polarized cells, to HSPGs on the BL surface of polarized cells, and to both molecules on the AP surface of incompletely polarized cells. Cells were pretreated with mannose (man) to upregulate N-glycosylation, with tunicamycin (tun) (32) to inhibit N-glycosylation, with heparinase III (hepIII) to remove HS chains, with sodium chlorate for desulfation (desulf), or with sodium sulfate for resulfation (resulf). Host cell treatments are color coded as follows: modifications of HSPGs are shown in shades of red and modifications of N-glycans are shown in shades of blue. Bacteria were added for 5 h, and standard LDH release assays were performed. Shown are the means ± SD for four separate experiments. Percentages of LDH release in well-polarized (A) and incompletely polarized (B) MDCK and ConAr cells were determined and normalized to that for AP-side-infected, untreated MDCK cells. Percentages of LDH release in well-polarized (C) and incompletely polarized (D) Calu-3 cells were determined and normalized to AP-side-infected, untreated Calu-3 cells. *, P < 0.05 compared to AP-side-infected, untreated MDCK cells; ×, P < 0.05 compared to AP-side-infected, untreated ConAr cells; +, P < 0.05 compared to AP-side-infected, untreated Calu-3 cells; **, P < 0.05 compared to BL-side-infected, untreated MDCK cells; ××, P < 0.05 compared to BL-side-infected, untreated ConAr cells; ++, P < 0.05 compared to BL-side-infected, untreated Calu-3 cells.
Next, we investigated the role of HS chains of HSPGs in promoting host cell injury upon P. aeruginosa binding. Heparinase III digestion or HSPG desulfation decreased LDH release upon BL-side addition of bacteria to well-polarized wt MDCK, ConAr MDCK, and Calu-3 cells (Fig. 6A and C; see Fig. S9 in the supplemental material). Importantly, these treatments also decreased cytotoxicity at the AP surface of incompletely polarized cells (Fig. 6B and D; see Fig. S9 in the supplemental material). Resulfation of chemically desulfated HS chains restored cytotoxicity when bacteria were added to the BL surface of well-polarized cells or to both surfaces of incompletely polarized cells (Fig. 6A to D). As expected, decreased cytotoxicity correlated with decreased FITC-inulin diffusion (data not shown; see Fig. S10 in the supplemental material).
In order to examine the roles of N-glycans and HS chains in P. aeruginosa entry into host cells, variously pretreated cells were infected for 1 h and standard bacterial internalization assays were performed. Bacterial internalization was reduced at the AP membrane of well-polarized ConAr MDCK cells compared to that with wt MDCK cells, but internalization at the BL surface was equivalent in the two cell lines (Fig. 7A and C). It was restored to wt levels in ConAr MDCK cells supplemented with Man or Glc (Fig. 7A). An increase in bacterial internalization at the AP surface was observed in well-polarized wt MDCK or Calu-3 cells expressing more complex N-glycans after supplementation with Man or Glc (Fig. 7A and B). Addition of Man or Glc did not enhance internalization at the BL surface of wt MDCK, ConAr MDCK, or Calu-3 cells (Fig. 7A and B). Inhibition of N-glycosylation with tunicamycin reduced bacterial entry at the AP surface of well-polarized MDCK and Calu-3 cells but did not further decrease bacterial internalization into ConAr MDCK cells (Fig. 7C and D). Tunicamycin had no effect on P. aeruginosa internalization from the BL surface of any of these cell types. Similar results were observed in incompletely polarized monolayers (data not shown). Finally, enzymatic cleavage of HS by heparinase III reduced bacterial invasion in a dose-dependent manner on both the AP and BL surfaces of incompletely polarized cells but only at the BL surface of well-polarized cells (Fig. 7E to H). Chondroitinase ABC treatment had no effect on invasion under any of these conditions (data not shown).
FIG. 7.
P. aeruginosa internalization is dependent on bacterial binding to N-glycans on the AP surface of well-polarized cells, to HSPGs on the BL surface of well-polarized cells, and to both molecules at the AP surface of incompletely polarized cells. Cells were pretreated with the indicated sugars to upregulate N-glycosylation (A and B), with the indicated concentrations of tunicamycin to inhibit N-glycosylation (C and D), or with the indicated amounts of heparinase III to remove HS chains (E to H). Bacteria were added for 1 h, and standard invasion assays were performed. Shown are the means ± SD for three separate experiments. (A and B) Man and Glc are sufficient to enhance bacterial invasion of MDCK, ConAr, and Calu-3 cells from the AP surface. (A) P. aeruginosa internalization in well-polarized MDCK and ConAr cells, normalized to AP-side-infected, untreated MDCK cells. (B) P. aeruginosa internalization in well-polarized Calu-3 cells, normalized to AP-side-infected, untreated cells. (C and D) Tunicamycin inhibits invasion from the AP surface of well-polarized MDCK and Calu-3 cells in a dose-dependent manner. (C) P. aeruginosa internalization in well-polarized MDCK and ConAr cells, normalized to AP-side-infected, untreated MDCK cells. (D) P. aeruginosa internalization in well-polarized Calu-3 cells, normalized to AP-side-infected, untreated cells. (E to H) Heparinase III treatment inhibits invasion from the BL surface of well-polarized and incompletely polarized MDCK, ConAr, and Calu-3 cells and from the AP surface of incompletely polarized cells. P. aeruginosa binding to well-polarized (E) and incompletely polarized (F) MDCK and ConAr cells was determined and normalized to that of AP-side-infected, untreated MDCK cells. P. aeruginosa binding to well-polarized (G) and incompletely polarized (H) Calu-3 cells was determined and normalized to that of AP-side-infected, untreated cells. *, P < 0.05 compared to AP-side-infected, untreated MDCK cells; ×, P < 0.05 compared to AP-side-infected, untreated ConAr cells; +, P < 0.05 compared to AP-side-infected, untreated Calu-3 cells; **, P < 0.05 compared to BL-side-infected, untreated MDCK cells; ××, P < 0.05 compared to BL-side-infected, untreated ConAr cells; ++, P < 0.05 compared to BL-side-infected, untreated Calu-3 cells.
Importantly, bacterial internalization at the AP surface of incompletely polarized cells was twofold higher than that in well-polarized cells (see Fig. S6C in the supplemental material). This twofold difference was still observed after tunicamycin treatment, again indicating that the enhanced internalization observed at the AP surface of incompletely polarized cells is not due to upregulation of N-glycans at the AP surface. However, pretreatment of incompletely polarized cells with heparinase III reduced bacterial internalization at the AP surface to the levels observed in well-polarized cells. Together, these data confirm that P. aeruginosa-induced binding and subsequent host injury and entry into well-polarized epithelium are mediated by bacterial binding to HS chains at the BL surface and to N-glycans at the AP surface. In contrast, these events are mediated by HS at the BL surface and by both HS and N-glycans at the AP surface in incompletely polarized epithelium. An increase in the abundance and complexity of N-glycan chains enhances P. aeruginosa binding at the AP surface of both well-polarized and incompletely polarized epithelia. However, the enhancement of bacterial internalization on the AP surface of incompletely polarized cells compared to that of well-polarized cells is HS dependent.
P. aeruginosa colocalizes with N-glycans at the AP membrane or with HS chains at the BL membrane of 3D cysts.
MDCK cells grown in collagen or Matrigel form highly polarized 3D clonal cysts comprised of a layer of well-polarized cells surrounding a simple lumen, with the AP side facing the lumen and the BL side facing the surrounding collagen (BL-side-out cysts). This system recapitulates the organization of simple epithelial tissues (5). It also affords the possibility of studying microbe interactions with the BL surface of the mucosal barrier in the absence of a Transwell filter. If an antibody to the extracellular domain of integrin is present during growth, cysts with opposite polarity are formed, in which the AP surface faces outward (AP-side-out cysts), allowing direct comparison with BL-side-out cysts (52). These cysts, while less well organized, are as well polarized as BL-side-out cysts. We used this model to test whether P. aeruginosa binding to the outside surface of highly polarized BL-side-out and AP-side-out cysts occurs at HSPG-rich or N-glycan-rich regions. Without additional manipulations, the 3D cysts are not representative of incompletely polarized epithelium.
We first examined the distributions of HS chains and N-glycans in cysts. An antibody to HS exhibited uniform staining of the outer membrane of BL-side-out cysts, consistent with the known polarized distribution of HSPGs in the BL membrane (Fig. 8A, left panel). In AP-side-out cysts, it showed a chicken-wire-like pattern of staining of the inner BL membrane (Fig. 8A, right panel). Heparinase III treatment of BL-side-out cysts resulted in patchy staining of HS compared to that in untreated cysts, affording us the possibility of correlating bacterial binding to HS-rich patches (Fig. 8C and D). N-glycans, revealed by staining with FITC-ConA, were found on the BL surface of BL-side-out cysts and on both surfaces of AP-side-out cysts (Fig. 8B). Staining of the luminal surface of BL-side-out cysts with FITC-ConA was not detectable, since the lectin could not penetrate through well-polarized cells. As expected, ConAr MDCK BL-side-out (Fig. 8E and F) and AP-side-out (Fig. 8I) cysts exhibited decreased staining with FITC-ConA compared to wt MDCK cells, with a patchy distribution of N-glycans. Brief Man supplementation of ConAr MDCK cysts resulted in increased surface FITC-ConA staining (Fig. 8J). However, the uniform staining seen in wt MDCK cysts was not achieved because the sugar was added for a shorter time (1 day) than that for complete supplementation (1 week). Together, these studies suggest that the distributions of N-glycans and HSPGs are similar in MDCK cells grown as 3D cysts or as well-polarized 2D monolayers.
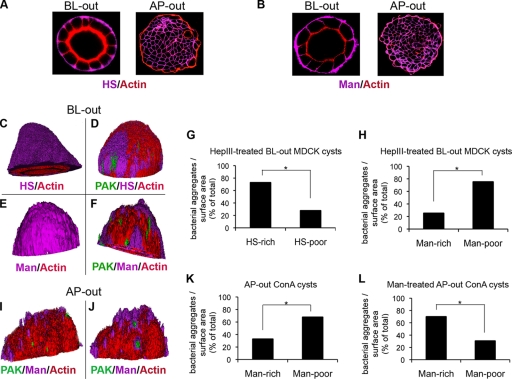
FIG. 8.
P. aeruginosa colocalizes with HS-rich patches on the exposed BL membrane and with N-glycan-rich patches on the exposed AP membrane of highly polarized 3D cysts. (A and B) Representative x-y confocal sections of BL-side-out or AP-side-out MDCK cysts cultured for 5 days and stained with anti-HS antibody (HS; purple) or ConA (Man; purple) and with phalloidin (actin; red). (A) BL-side-out (left) and AP-side-out (right) MDCK cysts show preferential localization of HSPGs to the BL surface of the cysts. (B) BL-side-out (left) and AP-side-out (right) MDCK cysts show localization of Man to both the AP and BL surfaces of the cysts. (C to F) 3D reconstructions of z-stack images acquired by confocal microscopy. HS is stained with an anti-HS antibody (HS; purple), Man is stained with ConA (Man; purple), and actin is stained with phalloidin (actin; red). (C) BL-side-out MDCK cysts show uniform staining of HS at the exposed BL membrane. (D) MDCK cysts treated with heparinase III and infected with GFP-PAK cells for 2 h (green) show nonuniform patchy HS staining, and the bacteria bind preferentially to HS-rich areas (purple). (E) BL-side-out wt MDCK cysts show uniform staining of Man at the exposed BL membrane. (F) BL-side-out ConAr MDCK cysts briefly treated with Man (to induce expression of more complex N-glycans) and infected with GFP-PAK cells for 2 h (green) show nonuniform patchy Man staining, and the bacteria do not bind preferentially to Man-rich areas (purple). (G) Quantification of GFP-PAK bound to HS-rich versus HS-poor patches in BL-side-out MDCK cysts pretreated with heparinase III. (H) Quantification of bacteria bound to Man-rich versus Man-poor patches in BL-side-out ConAr cysts briefly treated with Man. (I and J) 3D reconstructions of z-stack images of ConAr MDCK cysts acquired by confocal microscopy. Man is stained with ConA (purple), and actin is stained with phalloidin (red). (I) AP-side-out ConAr cysts infected with PAK-GFP cells show nonuniform patchy staining of Man at the exposed AP membrane, and the bacteria do not bind preferentially to Man-rich areas. (J) ConAr cysts briefly treated with Man and infected with GFP-PAK cells (green) show nonuniform patchy Man staining, and the bacteria bind preferentially to Man-rich areas (purple). (K) Quantification of GFP-PAK bound to Man-rich versus Man-poor patches in AP-side-out ConAr cysts. (L) Quantification of GFP-PAK bound to Man-rich versus Man-poor patches in AP-side-out ConAr cysts briefly treated with Man. *, P < 0.01.
We used heparinase III-treated BL-side-out MDCK cell cysts and Man-supplemented AP-side-out ConAr MDCK cysts to ask whether P. aeruginosa binding to cysts correlated with HS- or N-glycan-rich regions at the BL or AP surface. Since BL-side-out cysts show a uniform distribution of HSPGs on the BL surface, we briefly exposed these cysts to heparinase III and then correlated the binding of GFP-expressing P. aeruginosa with HS-rich or HS-poor patches. For comparison, we determined the association of binding to Man-rich regions by using cysts formed from ConAr MDCK cells or ConAr MDCK cells briefly supplemented with Man (1 day), where N-glycosylation is upregulated but not restored to wt levels. Under these conditions, a nonuniform distribution of N-glycans was observed, as determined by binding of FITC-ConA. As shown in Fig. 8G, significantly more GFP-expressing bacteria colocalized to HS-rich patches (70%) than to HS-poor patches (30%) in BL-side-out wt MDCK cysts. Only 25% of P. aeruginosa organisms bound to Man-rich patches expressed on the BL membrane of BL-side-out ConAr MDCK cysts (data not shown) or ConAr MDCK cysts grown briefly in the presence of Man (Fig. 8H).
We also tested whether bacteria bound preferentially to Man-rich regions on the AP surface of AP-side-out cysts by comparing binding to AP-side-out ConAr MDCK cysts grown in the absence or presence of supplemental Man. As shown in Fig. 8K, only ∼30% of PAK-GFP colocalized with Man-rich regions in AP-side-out ConAr MDCK cysts. When the cysts were supplemented briefly with Man, the fraction of P. aeruginosa bacteria colocalizing with more complex N-glycans increased to 70% (compare panels K and L). In summary, in 3D cysts, P. aeruginosa binds preferentially to more complex N-glycans on the AP surface or to HS chains of HSPGs on the BL surface, supporting our hypothesis that these molecules are specific binding partners for P. aeruginosa.
DISCUSSION
Successful opportunistic pathogens, of which P. aeruginosa is a prime example, exploit specific niches in the host in order to facilitate attachment, colonization, damage, and dissemination. Our work was inspired by the fact that long carbohydrate chains of various glycoconjugates, including HSPGs and N-glycoproteins, could potentially serve as bacterial receptors since they are prominent cell surface-exposed structures at the mucosal epithelium. We extensively characterized the distribution of HSPGs and the structure of N-glycan chains in cultured epithelial cells grown at various states of polarization. Since P. aeruginosa requires preexisting epithelial damage and the loss of at least some degree of polarity in order to cause disease, these in vitro epithelial cell culture systems recapitulate important aspects of human infections and serve as a useful model to further dissect mechanisms of disease. Using comprehensive and multifaceted approaches, we demonstrated that complex N-glycan chains are necessary and sufficient to mediate P. aeruginosa binding at the AP surface, whereas the HS moieties of HSPGs mediate binding at the BL surface of the polarized epithelium. During epithelial injury and dedifferentiation, HSPG presentation at the AP surface is increased, explaining at least in part the predilection of this important pathogen for such injured tissues. Changes in the composition of N-glycan chains and/or in the polarized segregation of HSPGs could contribute to the pathogenesis of acute and chronic diseases.
We first focused on N-glycosylation, as increased or altered expression of N-glycans could result in enhanced susceptibility to P. aeruginosa infections in the setting of acute or chronic injury. Using chemical and enzymatic inhibitors, we showed that N-glycans are important contributors to P. aeruginosa binding, entry, and damage at the AP surface of airway and kidney cells grown at various states of polarity as confluent 2D monolayers. ConAr MDCK cells, which are defective in N-glycosylation, were particularly informative in identifying the role of N-glycans in P. aeruginosa binding and subsequent internalization and host injury. Although the specific defect in these cells is not known, the fact that N-glycosylation could be restored by growing these cells in the presence of excess Man or Glc suggested that they are defective in the activities of PMM and PMI enzymes (46). When P. aeruginosa was added to the AP surface of ConAr MDCK cells, decreased binding, entry, and cytotoxicity were observed compared to those in wt cells, whereas no difference was observed at the BL surface. While it has previously been suggested that ConAr MDCK cells form more highly polarized monolayers, which leads to resistance to P. aeruginosa infection (15), we found no evidence for altered polarity in these cells under our experimental conditions. Importantly, when ConAr MDCK cells were grown in the presence of Man or Glc, P. aeruginosa binding, entry, and cytotoxicity were restored to wild-type levels at the AP surface. We could also enhance the binding and subsequent damage at the AP surface of wt MDCK and airway epithelial cells grown in the presence of excess Man or Glc. We demonstrated increased colocalization of bacteria with more complex N-glycan patches on the AP membrane of highly organized 3D cysts grown in the presence of Man to upregulate N-glycosylation. Finally, P. aeruginosa preferentially bound in vitro to a mixture of complex N-glycans over an individual sugar. Together, these results suggest that N-glycan chains of one or more N-glycosylated proteins at the AP surface serve as important receptors for AP binding of P. aeruginosa. The N-glycosylated molecule is unlikely to be the previously identified receptors CD95 (Fas receptor), integrin, and fibronectin, as these molecules are preferentially expressed at the BL surface (50). Likewise, it is unlikely to be CFTR, since similar results were obtained with Calu-3 cells, which express high levels of CFTR at the AP surface, and wt MDCK cells, which express very little CFTR (18). Future studies will be aimed at identifying this important molecule. In summary, N-glycans and particular enzymes in the N-glycosylation pathway could be potential targets for novel therapeutic approaches in treatment of P. aeruginosa infections. Most importantly, simple sugars could be used to either modify the course of the disease or competitively inhibit bacterial binding and subsequent infection, a line of treatment currently being investigated.
While these results identified N-glycan chains as AP receptors, they did not reveal the identity of the BL receptor or the receptor that is upregulated in incompletely polarized cells. We therefore examined whether HSPGs, which are abundantly expressed on the BL surface (3), can serve as specific BL receptors for P. aeruginosa. We demonstrated that HSPGs are upregulated in incompletely polarized cells and lose their polarized distribution. Competitive inhibition, enzymatic removal, or desulfation of HS chains decreased P. aeruginosa binding, entry, and cytotoxicity at the BL surface of well-polarized cells and at both surfaces in incompletely polarized cells. We corroborated these results by showing that P. aeruginosa binds to HS in vitro, with the highest affinity among all tested compounds, and that it preferentially binds to HS-rich patches on the BL surface of 3D cysts.
If N-glycans can function as binding receptors and are found on both the AP and BL surfaces, why do they not contribute to P. aeruginosa binding to the BL surface? First, the identity of the protein core that is N-glycosylated may also contribute to binding specificity and the BL N-glycoproteins may not function as binding receptors or may not be readily accessible. In addition, or alternatively, the binding affinity for HSPGs may be higher than that for N-glycan chains (as suggested by our in vitro data). While our results demonstrate that AP N-glycans can function as receptors, in healthy hosts with well-polarized intact mucosal barriers P. aeruginosa does not cause disease. This observation likely reflects the importance of local defense mechanisms, such as a functional innate immune system. However, upregulation of AP HSPGs, particularly in the context of altered local innate immune responses, may offer an explanation for the increased binding and subsequent damage seen when P. aeruginosa infects incompletely polarized but intact monolayers, such as might be seen during regenerating epithelium. In more severe injury, when the monolayer is disrupted, increased access to HSPGs on the BL surface may also contribute to enhanced susceptibility to infection. Interestingly, it has been shown that inhibition of shedding of the HSPG syndecan-1 or degradation of the shed HS chains attenuates mouse lung infection (40). These results suggest an additional possible role for secreted HSPGs in mediating microbial virulence. They also indirectly suggest that P. aeruginosa binds to HS chains on syndecan-1, thus supporting the work presented here.
Flagella and type IV pili are the predominant P. aeruginosa adhesins. Studies are under way to determine their binding specificities toward N-glycans and HSPGs and whether they play different roles in adhesion at the AP versus BL surface. Previous studies have suggested that glycosphingolipids may serve as AP receptors for type IV pili (10, 47), although these findings remain controversial (13). Flagellar components have been shown to bind to Lewis X derivatives that are found on secreted mucins (48). Moreover, Toll-like receptor 5, predominantly found on the AP surface, has been found to bind flagellin (24). Finally, there can be flagellum- and pilus-independent bacterial binding to the cell surface, since two different lectins have been described for P. aeruginosa, specifically binding to either Fuc or Gal, sugars present in both N- and O-glycan chains (8, 22).
There is a great deal of potential to use heparin or heparin-like structures as drugs to treat a wide range of disorders, including respiratory diseases (12, 30). Although heparin has been in use as an anticoagulant for over 70 years, heparan sulfate-like structures are attracting considerable interest as a source of new therapeutics, since it has been proposed that the main purpose of heparin is in a defensive mechanism at sites of tissue injury against invading bacteria and other foreign materials (36). HSPGs are attractive as therapeutic targets since a wide range of viral and bacterial pathogens are known to bind to HS chains (2, 14, 21, 25), and our work further shows that the sulfate moieties of HSPGs function as P. aeruginosa-binding receptors. Drugs that disrupt HSPG-pathogen interactions may be an effective strategy for preventing or treating acute P. aeruginosa infections, particularly as inhaled therapy for pneumonia.
An important recent advance in understanding epithelial cell biology is the ability to grow epithelial cells as 3D cysts in a collagen-Matrigel matrix, which recapitulates the development and structural organization of tubular structures such as the lung alveolus. (5). While there are a few reports of studies of the interactions of pathogens with epithelial cells grown as aggregates (7, 38), to the best of our knowledge our study is the first that examines the interaction of bacteria with epithelial cells grown as organized 3D cysts. This approach required that we overcome several technical challenges. First, the cysts had to be treated briefly with collagenase in order to expose their surface. Second, bacterial binding had to be quantified by direct confocal microscopy, as bacteria also bind avidly to the collagen-Matrigel matrix. Third, during the 7 days it takes for mature cysts to form, the cells become highly polarized, and bacterial binding is limited. In addition, N-glycans and HSPGs are expressed at high levels in a highly polarized manner, with uniform distributions on the AP and BL sides, respectively. Therefore, we either exposed the BL surface of BL-side-out cysts to heparinase III (to correlate binding to HS-rich domains) or utilized ConAr MDCK cells (to correlate binding to Man-rich domains). Altogether, cysts provide a promising avenue for further study of host-pathogen interactions in vitro in a biologically relevant context, particularly as they relate to microbes that infect tubular or luminal structures. Future directions will include observing these events by time-lapse confocal microscopy to delineate the temporal and spatial events that occur during bacterial attachment, internalization, and host damage.
While our work examined epithelial cells in various states of polarization as a model for acute or chronic epithelial injury, upregulation of N-glycosylation and/or altered expression or localization of HSPGs may be specifically relevant to the chronic P. aeruginosa infections that result in end-stage lung disease in patients with CF. CFTR itself is a highly glycosylated protein, and there are published data that suggest that the sugar composition of membrane glycoproteins and secreted mucins is altered in CF, though the exact mechanism remains unknown (45). We are currently testing whether N-glycosylation or HSPGs are upregulated in lung tissues from patients with CF.
Another possible way in which upregulation of HSPG expression could affect both chronic and acute P. aeruginosa infections is through enhancing the binding and dimerization of growth factors and their receptors as well as by stabilizing these receptors. Of particular relevance are the epidermal growth factor (EGF) and its receptor, EGFR. EGFR activation is important in wound repair. In addition, EGFR has been shown to be hyperphosphorylated in CF patients (6). HSPGs also bind and stabilize the activity of interleukin-8 (IL-8), which may play a role in IL-8-driven hyperinflammatory responses in CF (16, 19). Thus, upregulation of HSPGs during acute and chronic epithelial injury may amplify P. aeruginosa-induced changes in growth factor signaling. In preliminary studies, we have found that P. aeruginosa activates EGFR in an HS-dependent manner.
The studies presented here on the pathogenesis of P. aeruginosa infections provide key insights into how changes in the presentation of AP and BL molecules contribute to acute and chronic P. aeruginosa infections. The use of cultured epithelial cells grown as 3D cysts provides a new and powerful in vitro tool that has the potential to offer better insights into understanding the complex mechanism(s) involved in the establishment of the infection and in subsequent organ damage. Our data show an important role for HS chains and N-glycans in mediating microbial adherence, entry, and cytotoxicity and provide a basis for designing therapeutic strategies based on these interactions. The use of combined therapeutic strategies that target N-glycosylation, HSPG synthesis, or polarized segregation of these molecules may provide powerful new therapeutic approaches to correct the abnormal epithelial architecture that occurs in the setting of chronic lung disease, including CF, in order to improve clinical outcomes. In summary, our work establishes fertile ground for better understanding the connection between specific physiological alterations in various acute and chronic disorders and the predilection to infections by P. aeruginosa.
Supplementary Material
Acknowledgments
This work was supported by the Elizabeth Nash Memorial Fellowship of the Cystic Fibrosis Research Inc. (I.B.) and by the National Institutes of Health (P01 AI053194 [J.N.E. and K.M.], R01 AI065902 [J.N.E.], and R01 DK067153 [K.M.]).
We thank Armando Lemus for his kind gift of pnpT2-GFP-pUCP20. We thank members of our laboratories for experimental suggestions and for advice and comments on the paper.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 14 December 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Apodaca, G., M. Bomsel, R. Lindstedt, J. Engel, D. Frank, K. Mostov, and J. Wiener-Kronish. 1995. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation defective host cells are resistant to bacterial killing. Infect. Immun. 63:1541-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avirutnan, P., L. Zhang, N. Punyadee, A. Manuyakorn, C. Puttikhunt, W. Kasinrerk, P. Malasit, J. P. Atkinson, and M. S. Diamond. 2007. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathog. 3:e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop, J. R., M. Schuksz, and J. D. Esko. 2007. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446:1030-1037. [DOI] [PubMed] [Google Scholar]
- 4.Borkowski, T., B. J. Van Dyke, K. Schwarzenberger, V. MacFarland, A. Farr, and M. Udey. 1994. Expression of E-cadherin by murine dendritic cells: E-cadherin as a dendritic cell-differentiation antigen characteristic of epidermal Langerhans cells and related cells. Eur. J. Immunol. 24:2767-2774. [DOI] [PubMed] [Google Scholar]
- 5.Bryant, D. M., and K. E. Mostov. 2008. From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell. Biol. 9:887-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgel, P. R., D. Montani, C. Danel, D. J. Dusser, and J. A. Nadel. 2007. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax 62:153-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carterson, A. J., K. Honer zu Bentrup, C. M. Ott, M. S. Clarke, D. L. Pierson, C. R. Vanderburg, K. L. Buchanan, C. A. Nickerson, and M. J. Schurr. 2005. A549 lung epithelial cells grown as three-dimensional aggregates: alternative tissue culture model for Pseudomonas aeruginosa pathogenesis. Infect. Immun. 73:1129-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chemani, C., A. Imberty, S. de Bentzmann, M. Pierre, M. Wimmerova, B. P. Guery, and K. Faure. 2009. Role of LecA and LecB lectins in Pseudomonas aeruginosa-induced lung injury and effect of carbohydrate ligands. Infect. Immun. 77:2065-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L. D., and L. D. Hazlett. 2000. Perlecan in the basement membrane of corneal epithelium serves as a site for P. aeruginosa binding. Curr. Eye Res. 20:260-267. [PubMed] [Google Scholar]
- 10.Comolli, J. C., A. R. Hauser, L. Waite, C. B. Whitchurch, J. S. Mattick, and J. N. Engel. 1999. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect. Immun. 67:3625-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comolli, J. C., L. L. Waite, K. E. Mostov, and J. N. Engel. 1999. Pili binding to asialo-GM1 on epithelial cells can mediate cytotoxicity or bacterial internalization by Pseudomonas aeruginosa. Infect. Immun. 67:3207-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coombe, D. R., and W. C. Kett. 2005. Heparan sulfate-protein interactions: therapeutic potential through structure-function insights. Cell Mol. Life Sci. 62:410-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emam, A., A. R. Yu, H. J. Park, R. Mahfoud, J. Kus, L. L. Burrows, and C. A. Lingwood. 2006. Laboratory and clinical Pseudomonas aeruginosa strains do not bind glycosphingolipids in vitro or during type IV pili-mediated initial host cell attachment. Microbiology 152:2789-2799. [DOI] [PubMed] [Google Scholar]
- 14.Fleckenstein, J. M., J. T. Holland, and D. L. Hasty. 2002. Interaction of an outer membrane protein of enterotoxigenic Escherichia coli with cell surface heparan sulfate proteoglycans. Infect. Immun. 70:1530-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleiszig, S. M., D. J. Evans, N. Do, V. Vallas, S. Shin, and K. E. Mostov. 1997. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun. 65:2861-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frevert, C. W., M. G. Kinsella, C. Vathanaprida, R. B. Goodman, D. G. Baskin, A. Proudfoot, T. N. Wells, T. N. Wight, and T. R. Martin. 2003. Binding of interleukin-8 to heparan sulfate and chondroitin sulfate in lung tissue. Am. J. Respir. Cell Mol. Biol. 28:464-472. [DOI] [PubMed] [Google Scholar]
- 17.Garrity-Ryan, L., S. Shafikhani, P. Balachandran, L. Nguyen, J. Oza, T. Jakobsen, J. Sargent, X. Fang, S. Cordwell, M. A. Matthay, and J. N. Engel. 2004. The ADP ribosyltransferase domain of Pseudomonas aeruginosa ExoT contributes to its biological activities. Infect. Immun. 72:546-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerçeker, A. A., T. Zaidi, P. Marks, D. E. Golan, and G. B. Pier. 2000. Impact of heterogeneity within cultured cells on bacterial invasion: analysis of Pseudomonas aeruginosa and Salmonella enterica serovar Typhi entry into MDCK cells by using a green fluorescent protein-labeled cystic fibrosis transmembrane conductance regulator receptor. Infect. Immun. 68:861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goger, B., Y. Halden, A. Rek, R. Mosl, D. Pye, J. Gallagher, and A. J. Kungl. 2002. Different affinities of glycosaminoglycan oligosaccharides for monomeric and dimeric interleukin-8: a model for chemokine regulation at inflammatory sites. Biochemistry 41:1640-1646. [DOI] [PubMed] [Google Scholar]
- 20.Grassmé, H., S. Kirschnek, J. Riethmueller, Andrea Riehle, G. von Kürthy, F. Lang, M. Weller, and E. Gulbins. 2000. CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science 290:527-530. [DOI] [PubMed] [Google Scholar]
- 21.Hess, D. J., M. J. Henry-Stanley, S. L. Erlandsen, and C. L. Wells. 2006. Heparan sulfate proteoglycans mediate Staphylococcus aureus interactions with intestinal epithelium. Med. Microbiol. Immunol. 195:133-141. [DOI] [PubMed] [Google Scholar]
- 22.Imberty, A., M. Wimmerova, E. P. Mitchell, and N. Gilboa-Garber. 2004. Structures of the lectins from Pseudomonas aeruginosa: insight into the molecular basis for host glycan recognition. Microbes Infect. 6:221-228. [DOI] [PubMed] [Google Scholar]
- 23.Imundo, L., J. Barasch, A. Prince, and Q. Al-Awqati. 1995. Cystic fibrosis epithelial cells have a receptor for pathogenic bacteria on their apical surface. Proc. Natl. Acad. Sci. USA 92:3019-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacchieri, S. G., R. Torquato, and R. R. Brentani. 2003. Structural study of binding of flagellin by Toll-like receptor 5. J. Bacteriol. 185:4243-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, K. M., R. C. Kines, J. N. Roberts, D. R. Lowy, J. T. Schiller, and P. M. Day. 2009. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J. Virol. 83:2067-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazmierczak, B., K. Mostov, and J. Engel. 2001. Interaction of bacterial pathogens with polarized epithelium. Annu. Rev. Microbiol. 55:407-435. [DOI] [PubMed] [Google Scholar]
- 27.Kazmierczak, B. I., T. S. Jou, K. Mostov, and J. N. Engel. 2001. Rho GTPase activity modulates Pseudomonas aeruginosa internalization by epithelial cells. Cell. Microbiol. 3:85-98. [DOI] [PubMed] [Google Scholar]
- 28.Kazmierczak, B. I., K. Mostov, and J. N. Engel. 2004. Epithelial cell polarity alters Rho-GTPase responses to Pseudomonas aeruginosa. Mol. Biol. Cell 15:411-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kierbel, A., A. Gassam, K. Mostov, and J. Engel. 2005. The phosphoinositol-3-kinase-protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol. Biol. Cell 16:2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lever, R., and C. P. Page. 2002. Novel drug development opportunities for heparin. Nat. Rev. Drug Discov. 1:140-148. [DOI] [PubMed] [Google Scholar]
- 31.Mandell, G. L., J. E. Bennett, and R. Dolin. 2005. Principles and practice of infectious diseases, 6th ed. Churchill Livingstone Inc., New York, NY.
- 32.Marjomaki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of echovirus 1 in caveolae. J. Virol. 76:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthysse, A. G., S. Stretton, C. Dandie, N. C. McClure, and A. E. Goodman. 1996. Construction of GFP vectors for use in gram-negative bacteria other than Escherichia coli. FEMS Microbiol. Lett. 145:87-94. [DOI] [PubMed] [Google Scholar]
- 34.Meiss, H. K., R. F. Green, and E. J. Rodriguez-Boulan. 1982. Lectin-resistant mutants of polarized epithelial cells. Mol. Cell. Biol. 2:1287-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mostov, K., T. Su, and M. ter Beest. 2003. Polarized epithelial membrane traffic: conservation and plasticity. Nat. Cell Biol. 5:287-293. [DOI] [PubMed] [Google Scholar]
- 36.Nader, H. B., S. F. Chavante, E. A. dos-Santos, T. W. Oliveira, J. F. de-Paiva, S. M. Jeronimo, G. F. Medeiros, L. R. de-Abreu, E. L. Leite, J. F. de-Sousa-Filho, R. A. Castro, L. Toma, I. L. Tersariol, M. A. Porcionatto, and C. P. Dietrich. 1999. Heparan sulfates and heparins: similar compounds performing the same functions in vertebrates and invertebrates? Braz. J. Med. Biol. Res. 32:529-538. [DOI] [PubMed] [Google Scholar]
- 37.Nichols, G. E., T. Shiraishi, and W. W. J. Young. 1988. Polarity of neutral glycolipids, gangliosides, and sulfated lipids in MDCK epithelial cells. J. Lipid Res. 29:1205-1213. [PubMed] [Google Scholar]
- 38.Nickerson, C. A., T. J. Goodwin, J. Terlonge, C. M. Ott, K. L. Buchanan, W. C. Uicker, K. Emami, C. L. LeBlanc, R. Ramamurthy, M. S. Clarke, C. R. Vanderburg, T. Hammond, and D. L. Pierson. 2001. Three-dimensional tissue assemblies: novel models for the study of Salmonella enterica serovar Typhimurium pathogenesis. Infect. Immun. 69:7106-7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Brien, L. E., T. S. Jou, A. L. Pollack, Q. Zhang, S. H. Hansen, P. Yurchenco, and K. E. Mostov. 2001. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 3:831-838. [DOI] [PubMed] [Google Scholar]
- 40.Park, P. W., G. B. Pier, M. T. Hinkes, and M. Bernfield. 2001. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature 411:98-102. [DOI] [PubMed] [Google Scholar]
- 41.Plotkowski, M. C., A. O. Costa, V. Morandi, H. S. Barbosa, H. B. Nader, S. de Bentzmann, and E. Puchelle. 2001. Role of heparan sulphate proteoglycans as potential receptors for non-piliated Pseudomonas aeruginosa adherence to non-polarised airway epithelial cells. J. Med. Microbiol. 50:183-190. [DOI] [PubMed] [Google Scholar]
- 42.Plotkowski, M. C., S. de Bentzmann, S. H. Pereira, J. M. Zahm, O. Bajolet-Laudinat, P. Roger, and E. Puchelle. 1999. Pseudomonas aeruginosa internalization by human epithelial respiratory cells depends on cell differentiation, polarity, and junctional complex integrity. Am. J. Respir. Cell Mol. Biol. 20:880-890. [DOI] [PubMed] [Google Scholar]
- 43.Prince, A. 1992. Adhesins and receptors of Pseudomonas aeruginosa associated with infection of the respiratory tract. Microb. Pathog. 13:251-260. [DOI] [PubMed] [Google Scholar]
- 44.Ramphal, R., C. Carnoy, S. Fievre, J. C. Michalski, and N. Houdret. 1991. Pseudomonas aeruginosa recognizes carbohydrate chains containing type 1 (Gal β1-3GlcNac) or type 2 (Gal β1-4GlcNac) disaccharide units. Infect. Immun. 59:700-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhim, A. D., L. I. Stoykova, A. J. Trindade, M. C. Glick, and T. F. Scanlin. 2004. Altered terminal glycosylation and the pathophysiology of CF lung disease. J. Cyst. Fibros. 3(Suppl. 2):95-96. [DOI] [PubMed] [Google Scholar]
- 46.Rush, J. S., K. Panneerselvam, C. J. Waechter, and H. H. Freeze. 2000. Mannose supplementation corrects GDP-mannose deficiency in cultured fibroblasts from some patients with congenital disorders of glycosylation (CDG). Glycobiology 10:829-835. [DOI] [PubMed] [Google Scholar]
- 47.Saiman, L., and A. Prince. 1993. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J. Clin. Invest. 92:1875-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scharfman, A., S. K. Arora, P. Delmotte, E. Van Brussel, J. Mazurier, R. Ramphal, and P. Roussel. 2001. Recognition of Lewis X derivatives present on mucins by flagellar components of Pseudomonas aeruginosa. Infect. Immun. 69:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schroeder, T. H., T. Zaidi, and G. B. Pier. 2001. Lack of adherence of clinical isolates of Pseudomonas aeruginosa to asialo-GM(1) on epithelial cells. Infect. Immun. 69:719-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan, K. H., and W. Hunziker. 2003. Compartmentalization of Fas and Fas ligand may prevent auto- or paracrine apoptosis in epithelial cells. Exp. Cell Res. 284:283-290. [DOI] [PubMed] [Google Scholar]
- 51.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166-1174. [DOI] [PubMed] [Google Scholar]
- 52.Yu, W., A. Datta, P. Leroy, L. E. O'Brien, G. Mak, T. S. Jou, K. S. Matlin, K. E. Mostov, and M. M. Zegers. 2005. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol. Biol. Cell 16:433-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zweegman, S., J. Van Den Born, A. M. Mus, F. L. Kessler, J. J. Janssen, T. Netelenbos, P. C. Huijgens, and A. M. Drager. 2004. Bone marrow stromal proteoglycans regulate megakaryocytic differentiation of human progenitor cells. Exp. Cell Res. 299:383-392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.