Abstract
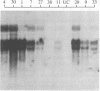
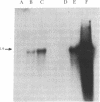
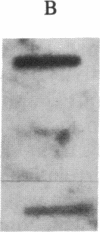
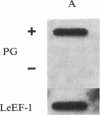
Polygalacturonase [PG; poly(1,4-α-D-galacturonide) glycanhydrolase; EC 3.2.1.15] is expressed in tomato only during the ripening stage of fruit development. PG becomes abundant during ripening and has a major role in cell wall degradation and fruit softening. Tomato plants were transformed to produce antisense RNA from a gene construct containing the cauliflower mosaic virus 35S promoter and a full-length PG cDNA in reverse orientation. The construct was integrated into the tomato genome by Agrobacterium-mediated transformation. The constitutive synthesis of PG antisense RNA in transgenic plants resulted in a substantial reduction in the levels of PG mRNA and enzymatic activity in ripening fruit. The steady-state levels of PG antisense RNA in green fruit of transgenic plants were lower than the levels of PG mRNA normally attained during ripening. However, analysis of transcription in isolated nuclei demonstrated that the antisense RNA construct was transcribed at a higher rate than the tomato PG gene(s). Analysis of fruit from transgenic plants demonstrated a reduction in PG mRNA and enzymatic activity of 70-90%. The reduction in PG activity did not prevent the accumulation of the red pigment lycopene.
Keywords: plant transformation, cauliflower mosaic virus 35S promoter, lycopene accumulation
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. W., Mason S. E., Goelet P. Expression of tobacco mosaic virus coat protein by a cauliflower mosaic virus promoter in plants transformed by Agrobacterium. EMBO J. 1985 Aug;4(8):1921–1926. doi: 10.1002/j.1460-2075.1985.tb03871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua A., Erickson R. P., Hieber V. Antisense RNA inhibits endogenous gene expression in mouse preimplantation embryos: lack of double-stranded RNA "melting" activity. Proc Natl Acad Sci U S A. 1988 Feb;85(3):831–835. doi: 10.1073/pnas.85.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera C. V., Alonso M. C., Johnston P., Phillips R. G., Lawrence P. A. Phenocopies induced with antisense RNA identify the wingless gene. Cell. 1987 Aug 14;50(4):659–663. doi: 10.1016/0092-8674(87)90039-0. [DOI] [PubMed] [Google Scholar]
- Crookes P. R., Grierson D. Ultrastructure of tomato fruit ripening and the role of polygalacturonase isoenzymes in cell wall degradation. Plant Physiol. 1983 Aug;72(4):1088–1093. doi: 10.1104/pp.72.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellapenna D., Alexander D. C., Bennett A. B. Molecular cloning of tomato fruit polygalacturonase: Analysis of polygalacturonase mRNA levels during ripening. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6420–6424. doi: 10.1073/pnas.83.17.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellapenna D., Kates D. S., Bennett A. B. Polygalacturonase Gene Expression in Rutgers, rin, nor, and Nr Tomato Fruits. Plant Physiol. 1987 Oct;85(2):502–507. doi: 10.1104/pp.85.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker J. R., Davis R. W. Inhibition of gene expression in plant cells by expression of antisense RNA. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5372–5376. doi: 10.1073/pnas.83.15.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. J., Pines O., Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- Grierson D., Tucker G. A., Keen J., Ray J., Bird C. R., Schuch W. Sequencing and identification of a cDNA clone for tomato polygalacturonase. Nucleic Acids Res. 1986 Nov 11;14(21):8595–8603. doi: 10.1093/nar/14.21.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: I. ISOLATION OF NUCLEI AND ELIMINATION OF ENDOGENOUS RIBONUCLEASE ACTIVITY. Plant Physiol. 1980 Feb;65(2):305–308. doi: 10.1104/pp.65.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):144–148. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein S. J., Dimaio J., Strand M., Rice D. Stable and heritable inhibition of the expression of nopaline synthase in tobacco expressing antisense RNA. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8439–8443. doi: 10.1073/pnas.84.23.8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K., Imamoto F. Transcriptional control of the endogenous MYC protooncogene by antisense RNA. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7363–7367. doi: 10.1073/pnas.84.21.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]