Abstract
In rabbit ligated ileal loops, two atypical enteropathogenic Escherichia coli (aEPEC) strains, 3991-1 and 0421-1, intimately associated with the cell membrane, forming the characteristic EPEC attachment and effacement lesion of the brush border, induced a mucous hypersecretion, whereas typical EPEC (tEPEC) strain E2348/69 did not. Using cultured human mucin-secreting intestinal HT29-MTX cells, we demonstrate that apically aEPEC infection is followed by increased production of secreted MUC2 and MUC5AC mucins and membrane-bound MUC3 and MUC4 mucins. The transcription of the MUC5AC and MUC4 genes was transiently upregulated after aEPEC infection. We provide evidence that the apically adhering aEPEC cells exploit the mucins' increased production since they grew in the presence of membrane-bound mucins, whereas tEPEC did not. The data described herein report a putative new virulence phenomenon in aEPEC.
The intestinal mucus offers numerous ecological advantages to bacteria present in the lumen and intestinal epithelium, providing a source of energy by producing the saccharides used for sustained growth by both the indigenous enteric microbiota and pathogens (26). Moreover by producing mucus, goblet cells contribute to the physical and chemical barriers that protect the host against the unwanted intrusion of enterovirulent microorganisms (48). Currently, 17 human mucin-type glycoproteins have been assigned to the MUC gene family, MUC1 and -2, MUC3A, MUC3B, MUC4, MUC5B, MUC5AC, MUC6 to -8, MUC10 to -13, and MUC15 to -17, with the approval of the Human Genome Organization Gene Nomenclature Committee (HUGO/GNC; http://www.hugo-international.org/hugo/) (8, 9, 57). A cluster of four mucin genes (MUC2, MUC5B, MUC5AC, and MUC6) code for secreted mucins. Eight genes (MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC16, and MUC17) that code for membrane-associated mucins have been characterized. In mucin-secreting intestinal cells, the secreted mucins are packaged and stored into large intracellular vesicles (17, 39).
Pathogenic Escherichia coli causes intestinal and extraintestinal diseases (35). There are six well-defined categories of intestinal pathogenic E. coli, each characterized by specific virulence factors that affect a wide range of cellular processes via signaling events. Enteropathogenic E. coli (EPEC), the first pathotype of E. coli to be described, produces characteristic cellular lesions known as “attaching and effacing” (A/E) lesions in the intestinal mucosa after the bacteria have intimately attached to the enterocyte brush border membrane and caused cytoskeletal changes that lead to effacement of the microvilli. The EPEC group is subdivided into typical (tEPEC) and atypical (aEPEC) forms. The aEPEC group consists of a large number of O serogroups (29, 74). These strains have been shown to carry the locus of the enterocyte effacement (LEE) pathogenicity island (PAI), but to lack the EAF (EPEC adherence factor) plasmid that encodes the bundle-forming pilus (BFP) and the Shiga toxin genes (35). LEE encodes the components of a type 3 secretion system (T3SS), an outer membrane adhesive protein (intimin) and its translocated receptor (36), and effector molecules that alter diverse cell signaling processes (19). A large variety of serotypes and genetic virulence properties have been described in aEPEC strains (29).
Several recent epidemiological studies have described increasing isolation of aEPEC in diarrheic feces of children (29). Gomes and coworkers (23) have classified the subclass of aEPEC as an emerging group of pediatric pathogens in Brazil. Among the aEPEC strains isolated in Brazil during an epidemiological study, strain 3991-1, isolated from diarrheic feces, has attracted attention as promoting mucus secretion, a phenotype not previously observed in an EPEC pathotype. To investigate in vitro the production of mucins during aEPEC infection, we used cultured, human, mucin-secreting intestinal HT29-MTX cells (42). The regulation of mucin genes coding for secreted or membrane-bound mucins (13, 28, 68, 79), the production of secreted and membrane-bound mucins (28), MUC5AC mucin exocytosis (28, 67), and the upregulation of mucins by an enteric pathogen (6, 7, 47, 49) have previously been reported for these cells.
MATERIALS AND METHODS
Reagents and antibodies.
Vibrio cholerae neuraminidase was purchased from Sigma (Sigma-Aldrich Chimie SARL, L'Isle d'Abeau Chesnes, France). Oligonucleotide primers were synthesized by Invitrogen. Anti-MUC2 monoclonal antibody (MAb) and rabbit polyclonal anti-MUC3 antibody reactive against deglycosylated MUC3 mucin were from Biomeda (Forster City, CA). Rabbit polyclonal anti-MUC4 antibody was from C. de Bolos (Unitat de Biologica Cellular I Molecular, Institut Municipal d'Investigacio Mèdica, Barcelona, Spain). The anti-MUC5AC MAb 1-13 M1 was from J. Bara (Unité 482, INSERM, Paris, France).
Bacterial strains.
In an epidemiological study carried out in the Federal University of São Paulo (UNIFESP), Brazil, a collection of 59 E. coli strains presenting the eae gene and lacking the EAF and Stx probe sequences have been isolated (76). Among 13 of these aEPEC strains tested in the rabbit ileal loop model, two strains, aEPEC 3991-1 and 0421-1, increased the mucus production. aEPEC strains 3991-1 and 0421-1 were both isolated from children presenting with acute diarrhea in whom none of the other enteropathogens tested for was detected (rotavirus, enterotoxigenic E. coli [ETEC], enterohemorrhagic E. coli [EHEC], enteroinvasive E. coli [EIEC], and Shigella, Salmonella, Yersinia, and Campylobacter species). Strain 3991-1 was nontypeable for O antigens and nonmotile (ONT:H−), and strain 0421-1 had the O101:H33 serotype. Moreover, both strains 3991-1 and 0421-1 carried eae and lacked EAF and stx gene sequences (76), which made them capable of promoting the EPEC-like mobilization of F-actin after interacting for 6 h with HeLa cells (Table 1). EPEC prototype strain E2348/69 was used as a control tEPEC strain (44). All bacterial strains were grown in LB medium at 37°C.
TABLE 1.
Characteristics of bacterial strains
| Strain | Serotype | Origin | FAS testa | Vol of secretion/loop (ml) | Relative mucous aspect of secretion |
|---|---|---|---|---|---|
| HB101 | − | 0 | − | ||
| 3991-1 | NT:H− | Diarrhea | + | 8.5 | ++++ |
| 0421-1 | O101:H33 | Diarrhea | + | 1.5 | ++ |
| E2348/69 | O127:H6 | Diarrhea | + | 0 | − |
Fluorescent actin staining test in undifferentiated HeLa cells and in fully differentiated intestinal Caco-2 cells (37).
Listeria monocytogenes strain EGD-SmR, a streptomycin-resistant derivative of the EGD strain, was grown for 18 h at 37°C in brain heart infusion (BHI) broth with streptomycin at 60 μg/ml (7).
Cell-free spent culture supernatants (CFCSs) from 18-h cultures were obtained by centrifugation at 10,000 × g for 30 min at 4°C. Centrifuged CFCSs were passed through a sterile 0.22-μm-pore filter unit Millex GS (Millipore, Molsheim, France). The filtered CFCSs were then checked to confirm the absence of bacterial colonies by plating on tryptic soy agar (TSA) (Difco) plates.
Rabbit ligated ileal loop assay.
Prior to the assays, six New Zealand White rabbits (weighing 1.8 to 2.5 kg) (4 to 8 weeks of age) were examined for the presence of attaching and effacing E. coli using PCR primers that identify eae genes. The rabbit ligated ileal loop assay has been described previously (73). Briefly, eae+ E. coli-free animals were fasted for 24 h before surgery. The rabbits were sedated and anesthetized by the intramuscular injection of fentanyl (Nilperidol; Cristalia, São Paulo, Brazil) (0.3 ml/kg of body weight) and zolazepam chloridrate (Zoletil; Virbac of Brazil) (0.4 ml/kg of body weight). After ventral laparotomy, the mid-ileum was washed with sterile saline applied with a syringe to eliminate intestinal feces and resident microbiota. In each rabbit, 5-cm ligated ileal loops were constructed and separated by intervening 3-cm ligated loops. An inoculum of 0.3 ml of a bacterial suspension (1 × 108 CFU/ml) or sterile medium (LB) was inoculated into each ligated loop using a 25-gauge needle. At the end of surgery, the animals were kept for 18 h and then killed with intravenous 3% pentobarbital, (Hypnol; Cristalia of Brazil) and zolazepam chloridrate (Virbac of Brazil) (0.4 ml/kg). For each ileal loop, the volume of secretion was measured after excision. The mucous aspect of secretion was scored as follows: ++++, high; +++, medium; ++, weak; and +, very weak. Fragments of ileal loops were excised and fixed for at least 24 h at 4°C in formalin at 10% for standard Giemsa or periodic acid-Schiff (PAS) staining. The fragments were also fixed in 3% glutaraldehyde in 0.1 M sodium cacodylate pH 7.2 buffer for electron microscopy procedures. The animal experiments had been approved by the Ethical Committee of Federal University of Sao Paulo (license no. 1506/05).
Cell lines.
We used the mucin-secreting HT29-MTX cell subpopulation (42) established from the cultured human colonic adenocarcinoma parental HT-29 cell line (16). Cells were routinely grown in Dulbecco's modified Eagle's minimal essential medium (DMEM) (25 mM glucose) (Invitrogen, Cergy, France), supplemented with 10% heat-inactivated (30 min, 56°C) fetal bovine serum (FBS) (Invitrogen) (6).
The HT-29 glc−/+ cell subpopulation used is the HT-29 glc− cell subpopulation (80) established from the cultured human colonic adenocarcinoma parental HT-29 cell line (16) and adapted for culture in the presence of glucose (43). HT-29 glc−/+ cells express the structural and functional characteristics of enterocyte-like cells as a function of the days in culture. Cells were routinely grown in DMEM supplemented with 20% heat-inactivated FBS.
The TC7 clone (Caco-2/TC7) (5) established from the parental human enterocyte-like Caco-2 cell line was used. Cells were routinely grown in Dulbecco's modified Eagle's minimum essential medium (25 mM glucose) (Invitrogen), supplemented with 15% heat-inactivated (30 min, 56°C) fetal calf serum (FCS) (Invitrogen) and 1% nonessential amino acids (47).
For maintenance purposes, cells were passaged weekly using 0.02% trypsin in Ca2+ Mg2+-free PBS containing 3 mM EDTA. Experiments and cell maintenance were carried out at 37°C in an atmosphere of 10% CO2-90% air. The culture medium was changed daily. Cultures were used at late postconfluence (i.e., after 21 days in culture for HT29-MTX cells, and after 15 days in culture for Caco-2/TC7 cells).
Infection of cell lines.
Monolayers of cells prepared in 24-well tissue culture plates (Corning) were washed twice with PBS, and then 1 ml of DMEM was added. For bacterial infection, 20 μl of bacterial suspension (108 CFU/ml) was added, and the cells were incubated for 3 h at 37°C in a 10% CO2-90% air atmosphere. In addition, after infection for 3 h, the cell monolayers were washed 4 times with sterile PBS, and then fresh cell culture medium was added, and the infected cells were subcultured for 3 h at 37°C in a 10% CO2-90% air atmosphere.
Quantification of cell-associated and internalized bacteria.
For the quantitative determination of the cell-associated E. coli, infected cells were washed with sterile phosphate-buffered saline (PBS) and osmotically lysed by adding sterile water. The suspensions were collected and appropriate dilutions plated in TSA (Difco) plates. The colonies were counted after incubating the plates for 18 h at 37°C.
The internalization of E. coli was determined by the quantitative determination of the bacteria located within the infected cell monolayers using the gentamicin protection assay. Infected cells were washed with sterile PBS and incubated for 1 h in a medium containing 100 μg/ml of gentamicin. Bacteria adhering to the cells were rapidly killed, whereas those located within the cells were not. The monolayer was washed once with PBS and then lysed with sterile water. Appropriate dilutions were plated on LB agar to determine the number of viable intracellular bacteria by bacterial colony counts.
TEM.
For transmission electron microscopy (TEM), after fixing for 24 h at 4°C, the ileal fragments were rinsed with 0.1 M cacodylate buffer at pH 7.4 and postfixed in 1% osmium tetroxide. Specimens were then exposed to a graded series of ethanol solutions and to propylene oxide. After being embedded in Araldite resin and being polymerized at 60°C for 48 h, ultrathin sections were stained with 2.0% uranyl acetate aqueous solution and 2.5% lead citrate. The specimens were then examined under a transmission electron microscope (LEO 906E; Zeiss) at 80 kV.
Immunofluorescence and confocal microscopy.
Monolayers of HT29-MTX cells were prepared on glass coverslips, which were placed in 24 well TPP tissue culture plates (ATGC, Marne la Vallée, France). Immunofluorescence labeling was conducted as previously reported (47, 49). Monolayers were fixed with methanol-acetone (vol/vol) for 3 min, and then washed 3 times with PBS. Before MUC3 immunolabeling, the mucins were deglycosylated (3 min, 37°C, 2.25 U Vibrio cholerae neuraminidase in 50 mM sodium acetate-150 mM sodium chloride-100 mM calcium chloride, pH 5.5, for 30 min at 37°C). Monolayers were incubated with the primary antibody for 1 h at room temperature, washed 3 times with PBS, and then incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody for 1 h.
The fluorescent actin staining (FAS) test was conducted as described by Knutton et al. (37) using fluorescein-phalloidin (Molecular Probes, Junction City, OR).
Specimens were mounted using Dako fluorescent mounting medium (Dako Cytomation S.A., Trappes, France) and examined using a confocal laser scanning microscope (CLSM; model LSM 510 Zeiss, equipped with an air-cooled argon ion laser set at 488 nm and a 543-nm and 633-nm helium neon laser; Carl Zeiss, Le Pecq, France) configured with an Axiovert 100 M microscope using a Plan Apochromat 63×/1.40 oil objective. Photographic images were resized and organized using Adobe Photoshop software (San Jose, CA).
MUC5AC IRMA.
A solid-phase double-antibody sandwich immunoradiometric assay (IRMA) (1) was used as previously described (47, 49). Polystyrene stars (Oris Industrie, Saclay, France) were coated with the anti-MUC5AC 1-13 M1 MAb (10 μg/ml in PBS, pH 7.4) by incubation overnight at 37°C, rinsed three times with PBS-1% bovine serum albumin (BSA)-1% Tween 20 overnight at 37°C. After washing several times, the stars were dried at 40°C and then stored at 4°C until use. The M1/MUC5AC mucin standard (10 μg/ml) and the culture media of control unstimulated and listeriolysin O (LLO)-stimulated HT29-MTX cells were serially diluted in PBS-0.1% Tween 20 plus 1 mM NaHCO3. A volume of 300 μl of each dilution was added to M1 MAb-coated stars 1 to 13 and incubated overnight at 37°C. The stars were then washed with PBS-0.1% Tween 20 and incubated with PM7 MAbs previously radiolabeled overnight with 125I (5 × 105 cpm/ml) at 37°C. Subsequently, the stars were washed, and the radioactivity was measured in a gamma counter (Wizard model 147 005). The concentration of mucin in each sample was estimated from the IRMA standard curve obtained with the standard M1/MUC5AC mucin.
qRT-PCR.
Quantitative determination of MUC genes was conducted as previously described (Table 2) (47, 49). RNA was extracted from control and infected HT29-MTX cells by the Trizol method. First-strand cDNA species were synthesized using the 1st-Strand cDNA synthesis kit (Clonetech). The RNA preparations were treated with DNase (Invitrogen) and subjected to treatment with phenol-chloroform, after precipitation with ammonia acetate and ethanol. The preparations were then suspended in 50 μl of nuclease-free water. The quantification and quality analysis of RNA preparations were carried out in LabChip Agilent or in a spectrophotometer, after adequate dilutions in nuclease-free water, subjected to electrophoresis in a 0.8% agarose gel that was observed after staining with ethidium bromide.
TABLE 2.
Oligonucleotides used for MUC qRT-PCR
| Gene | Sequence | Fragment length (bp) | Accession no. | Reference |
|---|---|---|---|---|
| MUC2 | 5′ CTGCACCAAGACCGTCCTCATG | 401 | NM_002457 | 47 |
| 3′ GCAAGGACTGAACAAAGACTCAGAC | ||||
| MUC4 | 5′ CGCGGTGGTGGAGGCGTTCTT | 597 | AJ010901 | 22 |
| 3′ GAAGAATCCTGACAGCCTTCA | ||||
| MUC5AC | 5′ TGATCATCCAGCAGCAGGGCT | 409 | AJ001402 | 47 |
| 3′ CCGAGCTCAGAGGACATATGGG | ||||
| TBP | 5′ GAGAGCCACGAACCACGG | 178 | NM_003194 | |
| 3′ ACATCACAGCTCCCCACCAT |
Four micrograms of RNA was utilized for reverse transcription (RT) using Supersript First-Strand Synthesis for RT-PCR kit (Invitrogen), as described by the manufacturer, and the analysis of real-time quantitative RT-PCR (qRT-PCR) was done using SYBR green in a light cycler thermal cycler (Roche Diagnostics, Meylan, France) technology. For the PCRs, the Roche Plus Fast Master kit (Roche Diagnostics) was utilized under the following operating conditions: 94°C for 6 min, 45 amplification cycles at 95°C for 5 s, annealing at 65°C for 5 s, and extension at 72°C for 7 s. The final extension was 15 min at 72°C. For cDNA amplification, primers for mucin types previously described (22, 47, 49) were utilized (Table 2). The first PCRs were done for the PCR standard, to check the primer quality and to choose a standard gene. The amplification products were subjected to electrophoresis in a 2% agarose gel that was observed after staining with ethidium bromide.
Statistical analysis.
Data are expressed as the mean ± standard deviation [SD] of three separate experiments, with at least three monolayers from three successive passages of cells per experiment. Statistical significance was assessed by Student's t test.
RESULTS
aEPEC strains 3991-1 and 0421-1 promote mucus secretion in rabbit ileal loop.
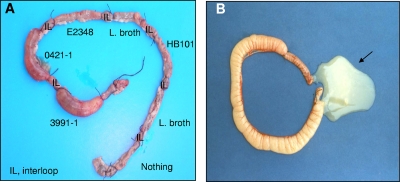
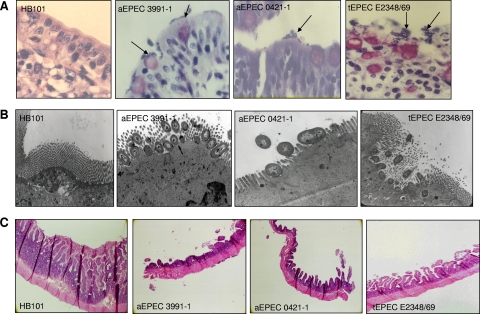
Infection of rabbit ligated ileal loops with aEPEC strains 3991-1 or 0421-1 was accompanied by an increase of fluid secretion 18 h postinfection (Fig. 1A and Table 1). In contrast, infection with tEPEC strain E2348/69 or E. coli laboratory strain HB101 did not result in any increase in fluid secretion (Fig. 1A and Table 1). Excision of rabbit ligated ileal loops infected with aEPEC strain 3991-1 or 0421-1 revealed the mucous aspect of the fluid secretion (Fig. 1B and Table 1). Examination of intestinal tissue specimens after Giemsa staining revealed large clusters of aEPEC 3991-1 and aEPEC 0421-1 bacteria at the brush border of the intestinal epithelium (Fig. 2A). A transmission electron microscopic examination of the infected mucosa showed that aEPEC 3991-1 and aEPEC 0421-1 bacteria were intimately associated with the cell membrane, forming the characteristic EPEC attachment and effacement lesion of the brush border (Fig. 2B). Examination of intestinal tissues specimens in aEPEC 3991-1- and aEPEC 0421-1-infected rabbits after periodic acid-Schiff (PAS) staining, showed an epithelium flattening and an increased production of mucopolysaccharides (Fig. 2C). In contrast, the mucosa of the tEPEC E2348/69-infected rabbit shows no increase of PAS stain (Fig. 2C).
FIG. 1.
aEPEC strains 3991-1 and 0421-1 promote an increase in fluid secretion in rabbit ligated ileal loops. Rabbit ligated ileal loops received LB broth or E. coli HB101, aEPEC 3991-1, aEPEC 0421-1, or tEPEC E2348/69 bacteria as described in Materials and Methods. The photograph in panel A shows that fluid secretion was increased in the presence of aEPEC 3991-1 and to a lesser extent in the presence of aEPEC 0421-1 bacteria, but not in the presence of HB101 and tEPEC E2348/69 bacteria. In panel B, excision of an ileal loop infected with aEPEC 3991-1 bacteria reveals the mucin nature of the increased fluid secretion. The photographs are representative of six rabbits examined.
FIG. 2.
Adhering aEPEC 3991-1 and 0421-1 bacteria formed large clusters of bacteria at the brush border of rabbit intestinal epithelium. In panel A, Giemsa coloration of aEPEC 3991-1- or 0421-1-infected rabbit intestinal epithelium showing the randomly distributed large aEPEC clusters. In panel B, transmission electron microscopy examination of aEPEC 3991-1- or 0421-1-infected rabbit intestinal epithelium showing the adhering bacteria intimately associated with the cell membrane and forming the typical EPEC attachment and effacement lesion of the brush border. In panel C, periodic acid-Schiff coloration of aEPEC 3991-1- or 0421-1-infected rabbit intestinal epithelium showing an increase of PAS staining relative to tEPEC infected. As a negative control, infection was conducted with E. coli HB101 bacteria.
aEPEC strains 3991-1 and 0421-1 increased the secreted and membrane-bound mucins in cultured, fully differentiated, mucin-secreting human intestinal HT29-MTX cells.
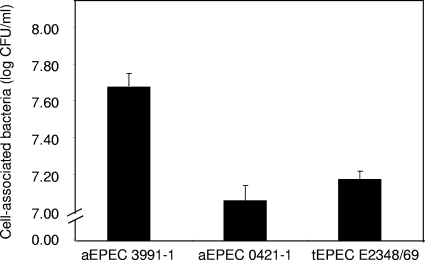
In order to investigate the phenomenon of mucus secretion promoted by aEPEC strains, experiments were conducted using the well-established model of human mucin-secreting intestinal cells, HT29-MTX cells in culture (42), previously used to identify the mechanism of mucus hypersecretion by Listeria monocytogenes (6, 7, 47, 49). Fully differentiated, HT29-MTX cells were apically infected with the strains aEPEC 3991-1, aEPEC 0421-1, and tEPEC E2348/69. Quantitative determination of the cell-associated bacteria revealed that 3 h postinfection, cells of the aEPEC 3991-1 and 0421-1 strains and tEPEC strain E2348/69 had adhered to the apical surface forming brush border of the HT29-MTX cells (Fig. 3). Adhering bacteria of the aEPEC 3991-1 and 0421-1 strains and tEPEC strain E2348/69 were all positively fluorescein-phalloidin labeled as the result of bacterial recruitment of F-actin (not shown).
FIG. 3.
Adhesion of aEPEC 3991-1 and 0421-1 strains and tEPEC strain E2348/69 to fully differentiated, mucus-secreting HT29-MTX cells. Cell monolayers were infected (108 CFU/ml) and incubated at 37°C in a 10% CO2-90% air atmosphere. After 3 h of infection, the cells were lysed with sterile water for the determination of cell-associated bacteria by a colony count assay. Each value shown is the mean ± SD from three experiments (three successive passages of cultured cells) in triplicate. *, P < 0.01 as compared with adhesion at 3 h postinfection for aEPEC 0421-1 and tEPEC E2348/69.
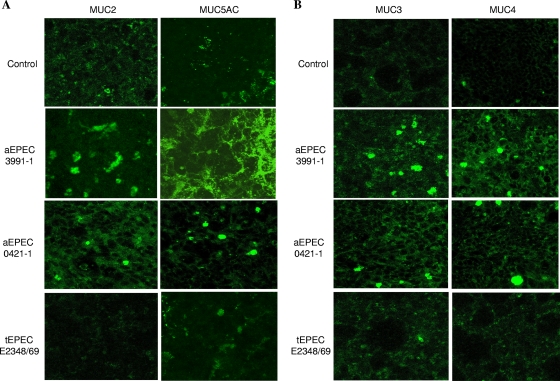
HT29-MTX cells expressed the MUC2 and MUC5AC genes, which code for secreted mucins, and the MUC3 and MUC4 genes, which code for membrane-bound mucins (42, 43). We examined the presence of the MUC2 and MUC5AC secreted mucins in nonpermeabilized control uninfected and aEPEC- or tEPEC-infected HT29-MTX cells by indirect immunofluorescence labeling with specific antibodies followed by a CLSM analysis (Fig. 4A). There was a marked increase in MUC5AC-positive immunoreactivity in aEPEC 3991-1-infected cells compared to control, uninfected cells. MUC5AC formed a dense network that covered much of the cell surface. MUC2-positive immunoreactivity in aEPEC 3991-1-infected cells was greater than in control, uninfected cells. Levels of secreted MUC2 and MUC5AC mucins rose in aEPEC 0421-1-infected cells, but were lower than in aEPEC 3991-1-infected cells, as characterized by several small, randomly distributed patches positive for MUC2 or MUC5AC immunoreactivities. In contrast, no increase in MUC2- or MUC5AC-positive immunoreactivities was observed in tEPEC E2348/69-infected cells.
FIG. 4.
Production of MUC2 and MUC5AC secreted mucins and MUC3 and MUC4 membrane-bound mucins in mucin-secreting HT29-MTX cells infected or not with aEPEC 3991-1 or aEPEC 0421-1 or tEPEC E2348/69 strains. Indirect immunofluorescence labeling was monitored in nonpermeabilized cells with anti-MUC2 MAb, anti-MUC5AC MAb 1-13 M1, anti-MUC3 polyclonal antibody (pAb), or anti-MUC4 pAb and appropriate secondary FITC-conjugated antibodies. Micrographs are representative of two experiments (two successive passages of cultured cells).
The presence of membrane-bound MUC3 and MUC4 mucins was investigated by indirect immunolabeling in control uninfected and aEPEC- or tEPEC-infected HT29-MTX cells (Fig. 4B). There was an increase in MUC3- and MUC4-positive immunoreactivities in aEPEC 3991-1- and 0421-1-infected cells compared to control, uninfected cells. In contrast, MUC3- and MUC4-positive immunoreactivities were not observed tEPEC E2348/69-infected cells.
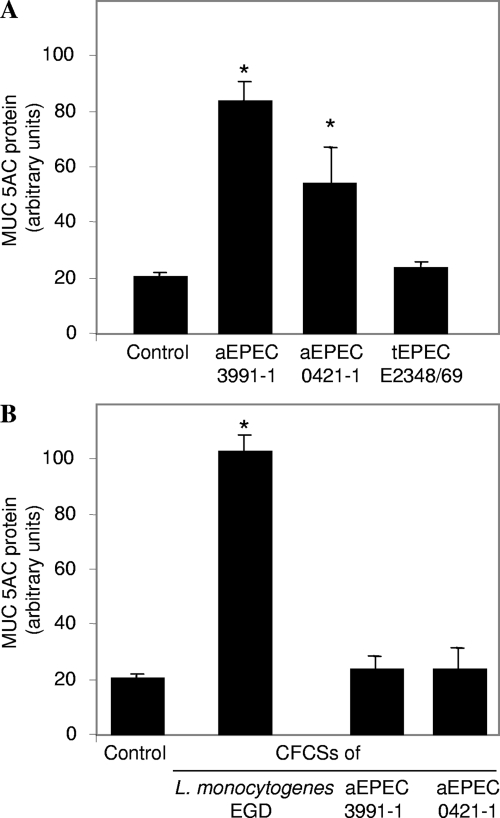
Consistent with the above immunofluorescence analysis, the quantitative determination of secreted MUC5AC mucin by a radioimmunometric assay revealed that there was 6-fold more MUC5AC in aEPEC 3991-1-infected HT29-MTX cells than in control, uninfected cells (Fig. 5A). The increase in MUC5AC mucin production in aEPEC 0421-1-infected cells was lower than that observed in aEPEC 3991-1-infected cells (Fig. 5A). Consistent with the above immunofluorescence analysis, no more MUC5AC mucin was secreted in tEPEC E2348/69-infected cells than in control, uninfected cells (Fig. 5A). We investigated whether secreted products of aEPEC 3991-1 and/or aEPEC 0421-1 strains supported or not the above observed increase of MUC5AC mucin production. To do this, the HT29-MTX cells were subjected to the CFCSs of aEPEC 3991-1 and/or aEPEC 0421-1 strains. As a positive control of MUC5AC production by a bacterially secreted factor, the cells were subjected to the CFCS of the Listeria monocytogenes strain EGD containing the lysteriolysin O toxin inducing the MUC5AC production in HT9-MTX cells (47, 49). No increase in MUC5AC mucin production was observed in cells subjected to aEPEC 3991-1 CFCS or aEPEC 0421-1 CFCS treatment (Fig. 5B). This observation corroborates with the fact that the aEPEC 3991-1 CFCS treatment does not induce fluid hypersecretion when inoculated in rabbit ileal loop (not shown). Collectively, these results showed that the two aEPEC strains after adhesion onto the mucin-secreting cells were able to induce the production of both secreted and membrane-bound mucins, a property not displayed by the prototype tEPEC strain E2348/69.
FIG. 5.
Production of secreted MUC5AC mucin in control mucin-secreting HT29-MTX cells, and in cells infected with aEPEC 3991-1 or aEPEC 0421-1 or tEPEC E2348/69 strains. Culture media of control and infected cells were analyzed for determination of MUC5AC mucin, determined by immunoradiometric assay using the anti-MUC5AC 1-13M1 and PM7 MAbs. In panel A, MUC5AC mucin in control and cells with 3-h bacterial infection cells. In panel B, MUC5AC mucin in cells subjected for 3 h to LB broth (control) or the cell-free spent culture supernatant from an 18-h culture of L. monocytogenes strain EGD or the aEPEC 3991-1, aEPEC 0421-1, or tEPEC E2348/69 strains. L. monocytogenes strain EGD CFCS was used as a positive control for the stimulation of MUC5AC secretion in HT29-MTX by listeriolysin O toxin (47). Each value shown is the mean ± standard error of the mean from three experiments (three successive passages of cultured cells). Statistical analysis was performed with Student's t test. *, P < 0.01 for L. monocytogenes EGD-infected cells compared to control uninfected cells and aEPEC 3991-1 or aEPEC 0421-1-infected cells.
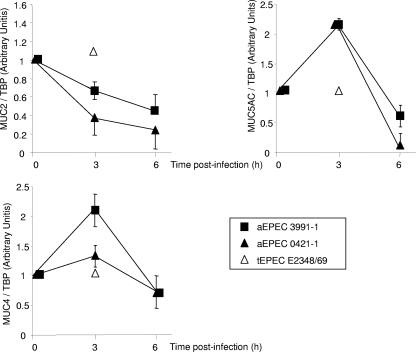
Changes in the transcription of mucin genes in aEPEC 3991-1 or aEPEC 0421-1-infected HT29-MTX cells.
We analyzed the MUC2, MUC5AC, and MUC4 genes by qRT-PCR in control cells and aEPEC 3991-1- or aEPEC 0421-1-infected HT29-MTX cells. As shown in Fig. 6, the transcription of the MUC2 and MUC5AC genes coding for secreted mucins was modified. The MUC5AC gene was transiently upregulated 3 h postinfection and subsequently returned to normal level. Surprisingly, the MUC2 gene was in fact downregulated, showing a decrease as a function of the time postinfection. Like the MUC5AC gene, the MUC4 gene, which codes for a membrane-bound mucin, showed transient upregulation 3 h postinfection. Consistent with the lack of increase in mucin production observed above, transcription of the MUC2, MUC5AC, and MUC4 genes was not modified in HT29-TX cells infected with strain tEPEC E2348/69 (Fig. 6).
FIG. 6.
Determination of MUC2, MUC5AC, and MUC4 mucin mRNA expression in control mucin-secreting HT29-MTX cells and in cells infected with the aEPEC 3991-1, aEPEC 0421-1, or tEPEC E2348/69 strains. qRT-PCR was monitored as described in Materials and Methods. Noninfected HT29-MTX cells have a value of 1. Each value shown is the mean ± standard error of the mean from three experiments (three successive passages of cultured cells).
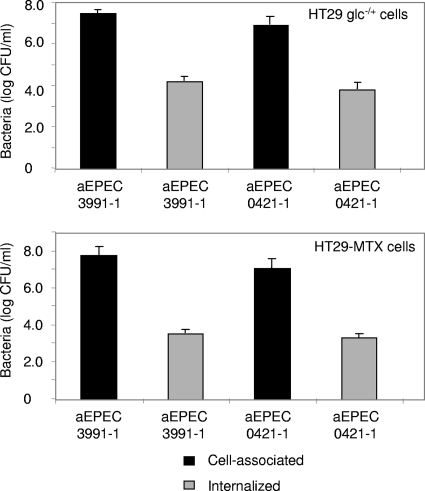
Increased mucin production does not affect cell association or internalization of strains 3991-1 and 0421-1.
Mucus production by intestinal cells has previously been described as a defensive response by the cell against bacterial infection intended to inhibit the penetration into the cell of enteric invasive pathogens (48). In order to find out whether the aEPEC-induced production of mucin is also a cell defense response, we compared the entry of aEPEC 3991-1 and 0421-1 bacteria into cells with different phenotypes: the enterocyte-like HT29 glc−/+ cells and the mucin-secreting HT29-MTX cells. There was no significant difference with regard to the cell association or cell entry of aEPEC 3991-1 and 0421-1 bacteria when the levels of adhering bacteria and those internalized within HT29-MTX and HT-29 glc−/+ cells were compared (Fig. 7).
FIG. 7.
Association of aEPEC 3991-1 and 0421-1 bacterial cells with and internalization within mucin-secreting HT29-MTX cells and enterocyte-like HT-29 glc−/+ cells. Cell monolayers were infected (108 CFU/ml) and incubated for 3 h at 37°C in a 10% CO2-90% air atmosphere. For the determination of cell-associated bacteria, infected cells were washed with sterile PBS and lysed with sterile water. To determine the internalized bacteria, the infected cells were washed with sterile PBS, incubated for 1 h in a medium containing 100 μg/ml of gentamicin to kill extracellular bacteria, and lysed with sterile water. Appropriate dilutions were plated on LB agar to determine the number of viable intracellular bacteria by bacterial colony counts. Each value shown is the mean ± SD of three experiments (three successive passages of cultured cells).
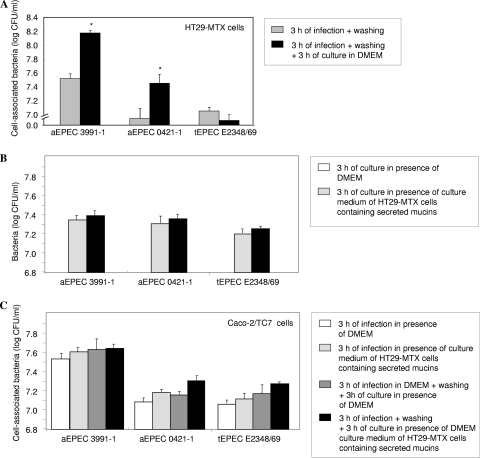
Adhering aEPEC 3991-1 and 0421-1 bacteria, but not tEPEC E2348/69 bacteria, undergo growth at the apical surface of the mucin-secreting HT29-MTX cells.
Mucins have been identified as a source of nutrients for bacterial growth (48). We therefore investigated whether the aEPEC-induced increase in secreted and membrane-bound mucin production had any effect on the growth of aEPEC at the cell surface of cultured human intestinal cells. We examined whether the increase in production of mucins modified the growth of adhering aEPEC 3991-1, aEPEC 0421-1, and tEPEC E2348/69 (Fig. 8A). To do this, mucin-secreting HT29-MTX cells were apically infected for 3 h, and then the cell-adhering bacteria were determined. In parallel, 3-h-infected cells were washed to eliminate any nonadhering bacteria and secreted mucins and were cultured after for 3 h in the presence of DMEM. The levels of adhering aEPEC 3991-1 and aEPEC 0421-1 bacteria were all significantly increased after 3 h of infection plus washing plus 3 h of culture compared to the levels of adhering bacteria after 3 h infection (Fig. 8A). In contrast, no increase in the level of adhering tEPEC E2348/69 bacteria was observed after 3 h of infection plus washing plus 3 h of culture compared with a 3-h infection without subculture (Fig. 8A).
FIG. 8.
Growth of aEPEC 3991-1 and 0421-1 and tEPEC E2348/69 bacteria in the presence of secreted and/or membrane-bound mucins. Cell monolayers were infected (5 × 107 CFU/ml) and incubated at 37°C in a 10% CO2-90% air atmosphere. In panel A, after the secreted mucins had been eliminated by washing from infected HT29-MTX cells, an increased growth of adhering aEPEC 3991-1 and 0421-1 cells, but not of the tEPEC E2348/69 bacteria, develops. Panel B shows growth of aEPEC 3991-1, aEPEC 0421-1, and tEPEC E2348/69 bacteria (2 × 107 CFU/ml) in DMEM or in the presence of DMEM culture medium of ATP-stimulated HT29-MTX cells containing secreted mucins. Panel C shows growth of aEPEC 3991-1 and 0421-1 cells and tEPEC E2348/69 bacteria that adhered to enterocyte-like Caco-2/TC7 cells in the presence or not of HT29-MTX secreted mucins. Each value shown is the mean ± SD of three experiments (three successive passages of cultured cells). *, P < 0.01 as compared to the adhesion 3 h postinfection plus washing.
In the experimental condition described above, the observed increased growth of aEPEC cells adhering onto HT29-MTX cells after the second period of 3 h of culture could result from the presence of membrane-bound or secreted mucins not entirely released during the first 3 h infection. We next investigated whether the secreted mucins alone modified the growth of cells of the aEPEC 3991-1 and 0421-1 strains and tEPEC strain E2348/69. To do this, bacteria were cultured in DMEM or in the presence of DMEM culture medium of ATP-stimulated HT29-MTX cells containing released preformed secreted mucins (65). As shown in Fig. 8B, there was no significant difference between the growth of aEPEC 3991-1 and 0421-1 and tEPEC E2348/69 cells in the presence or not of secreted mucins. This result was confirmed in an additional experiment. Fully differentiated Caco-2/TC7 cells which display the same enterocyte phenotype as HT-29 glc−/+ cells (5, 80), were apically infected for 3 h with the aEPEC 3991-1 and 0421-1 strains or tEPEC E2348/69 strain in the presence of DMEM. In parallel, Caco-2/TC7 cells were infected for 3 h in the presence of the DMEM culture medium of ATP-stimulated HT29-MTX cells containing secreted mucins. In addition, Caco-2 cells were preliminarily apically infected with the strains for 3 h, washed to eliminate the nonadhering bacteria, and then subcultured for 3 h in the presence of DMEM or DMEM culture medium of ATP-stimulated HT29-MTX cells. As shown in Fig. 8C, the addition of secreted mucins during infection of Caco-2/TC7 cells not significantly change the level of the adhering aEPEC 3991-1 and 0421-1 bacteria or tEPEC E2348/69 bacteria compared with infection in the presence of DMEM. Moreover, the addition of secreted mucins in preinfected Caco-2/TC7 cells did not significantly change the level of the adhering aEPEC 3991-1 and 0421-1 or tEPEC E2348/69 bacteria compared with preinfected cells without addition of secreted mucins. Collectively, these results indicate that the growth of the adhering aEPEC 3991-1 and 0421-1 cells, but not tEPEC E2348/69 cells, was positively influenced by the increased production of membrane-bound mucins.
DISCUSSION
The results show that the aEPEC strains studied strongly stimulated the production of the secreted MUC5AC mucin, and slightly increased the production of secreted MUC2 mucin in human mucin-secreting HT29-MTX cells. Moreover, we found that the strains upregulate the MUC5AC gene. Intriguingly, despite an increased production of secreted MUC2 mucin, we found that the MUC2 gene was downregulated. In addition, our results show that the aEPEC strains upregulated the MUC4 gene coding for a membrane-bound mucin which has increased expression at the brush border of infected mucin-secreting HT29-MTX cells. These results obtained in vitro with mucin-secreting human intestinal cells showing the increased production of secreted MUC5AC and MUC2 mucins fits well with the observation that aEPEC strains stimulate mucus secretion in a rabbit ileal loop model. Mucin gene upregulation by Gram-positive and Gram-negative bacteria has been previously reported (54). For example, increased production of MUC2 and MUC5AC secreted mucins has been observed in rabbit ligated ileal loops infected with Shigella flexneri (64) and Pseudomonas aeruginosa upregulates MUC5AC as well as MUC2 secreted mucins in both bronchial explants and cultured airway epithelial cells via an NF-κB and Src-dependent Ras-mitogen-activated protein kinase (MAPK)-pp90rsk pathways (11, 45, 46) or promotes the production of reactive oxygen species (ROS) triggering the release of transforming growth factor alpha (TGF-α), which in turn, upregulates MUC5AC production (78). Untypeable Haemophilus influenzae strongly induces upregulation of MUC5AC mucin via activation of the Toll-like receptor 2-MyD88-dependent p38 pathway (33).
Several toxin-dependent upregulations of intestinal mucins have previously been reported. Vibrio cholerae enterotoxin, by increasing intracellular adenosine 3′,5′-cyclic monophosphate, increases mucus secretion from intestinal goblet cells both in vitro and in vivo (14, 32, 40, 41). The wild-type L. monocytogenes strain EGD increases mucus secretion in vivo (63), with the result that listeriolysin O toxin promotes the increased production of secreted and membrane-bound mucins and the upregulation of MUC genes in cultured mucin-secreting HT29-MTX cells (6, 7, 47). Moreover, Streptococcus pneumoniae pneumolysin has been shown to promote the upregulation of MUC5AC mucin via TLR4-dependent activation of ERK and an IκB kinase-dependent signaling mechanism in human epithelial cells in vitro and in mice in vivo (25). Results reported here showing that CFCSs of aEPEC strains failed to induce any increase of MUC5AC mucin secretion indicate that the mucus-secreting effect on the aEPEC strain did not result from an aEPEC-associated toxin.
Hypersecretion of mucus is not a general trait observed for EPEC infection, although Fagundes-Neto and Scaletsky (15) have reported that surface abnormalities of the small intestinal mucosa of EPEC-infected patients were accompanied by the presence of a mucus pseudomembrane coating the mucosal surface. Recently, it has been reported that the related EPEC and EHEC mouse pathogen Citrobacter rodentium, which infects the apical surfaces of enterocytes and promotes attaching and effacing (A/E) lesions, via the host immune system modulates the function of goblet cells, leading to a dramatic depletion of mucus (2). The nature of the virulence factor of aEPEC strains that increases the secretion of intestinal mucus both in vitro and in vivo remains to be determined. It was noticed that this effect seems to be specific to aEPEC strains, since no increase of secreted mucins develops in human intestinal mucus-secreting cells infected with tEPEC strain E2348/69. On the basis of the results reported here showing that aEPEC intimately attached to the cell membrane of mucin-secreting intestinal HT29-MTX cells triggered an increase in mucin production, we postulate that the observed aEPEC-induced mucin production could be a T3SS-dependent event. Several aEPEC strains promoted the actin cytoskeleton reorganization via the TccP2/EspU-dependent actin-signaling cascade (60). TccP2/EspU, which was first identified in EHEC (20), but is also present in non-O157 EHEC, and in tEPEC and aEPEC isolates, particularly in strains belonging to serogroups O26 (EHEC), O119 (tEPEC), and O55 (aEPEC) (21), displays an Nck-like activity that is essential for promoting actin polymerization (4) and is translocated into the infected cell cytoplasm through the LEE-encoded filamentous T3SS needle. In EPEC/EHEC (19), TSS3-dependent translocation of effector proteins such as Tir, Map, EspF, EspG, EspH, SepZ, and EspB means that these pathogens subvert cell signaling pathways either to modify the polarized organization of the host cells and/or to alter specific intestinal functions. The observation here that a tEPEC strain fails to induce the increase in mucin production suggests that the known tEPEC TSS3-dependent effectors are not involved in the aEPEC-induced increase in mucin production. Interestingly, seven potential TSS3-related effectors, designated NleB to -H with unknown functions and encoded outside the LEE by three uncharacterized PAIs, suggesting that they could act cooperatively with the LEE in pathogenesis, have been identified in EHEC (10). It could be interesting in the future to find out whether the suspected aEPEC, TSS3-dependent effector triggering the mucin upregulation is one of these effectors or not.
Specialized epithelial cells constitute the first line of host innate defense against invading and noninvading microbes by elaborating a range of molecules, including mucins that are involved in pathogen clearance (48). For example, in response to bacterial infection, the Paneth cells discharge antimicrobial peptides into the intestinal luminal space, one of which is the antibacterial peptide cathelicidin, increasing mucus synthesis by activating mitogen-activated protein kinase (71). The mucus coating the intestinal epithelium creates an exclusion barrier effect due to the dense glycosylated layer. Lievin-Le Moal et al. (49) have reported that in HT29-MTX cells the LLO toxin of L. monocytogenes induced an increase in mucin exocytosis that creates a gel of mucins over the apical cell surface, which in turn decreases the cell entry of Listeria monocytogenes. Moreover, it has been reported previously that intestinal mucins inhibit the cell attachment of the rabbit EPEC-related strain RDEC-1 strain (12, 52). A similar inhibition of adhesion by mucus has been observed for the human EPEC strain E2348/69 (69), and it is interesting to note that the probiotic-induced hyperproduction of MUC2 and MUC3 mucins leads to the inhibition of EPEC attachment to host cells (51). In addition, the mucus has a protective role against the deleterious effect of enteric pathogens on intestinal cells, since no increase in TNF-α mRNA occurs in Shigella flexneri-infected HT29-MTX or HT29-FU mucin-secreting cells, in contrast to infected enterocyte-like HT-29 glc− cells (59). It is interesting to note that several gastrointestinal pathogens have developed sophisticated strategies, including specific pathogenic factors, to escape this host defense mechanism. The prototype of these pathogens is Helicobacter pylori, which uses its flagella for growth and motility within the mucus layer (61), reduces gastric mucin synthesis (72) and mucin exocytosis (55), and causes the aberrant production of the gastric mucins MUC1, MUC5AC, and MUC6 (3, 58). It has been demonstrated that one aEPEC strain expressing the intimin subtype omicron was more invasive than tEPEC in undifferentiated, nonintestinal HeLa and Hep-2 cells (30). Consistent with what was previously reported for tEPEC (18), aEPEC strains were invasive in undifferentiated human intestinal Caco-2 cells and weakly invasive in differentiated enterocyte-like cells (66). Intriguingly, the aEPEC 0621-6, 1632-7, and 1871-1 strains showed a significant invasiveness capacity in the differentiated cryptic-like T84 cells in contrast with the typical tEPEC E2348/69 strain (77). Our results indicate that aEPEC-induced increased production of mucins is not a protective cell response against aEPEC infection of the intestinal cells. Indeed, investigating the consequences of the aEPEC-induced hyperproduction of secreted and membrane-bound mucins in mucin-secreting HT29-MTX cells in terms of cell association and internalization, we found that aEPEC strains cell associated and internalized similarly when infection was conducted with the mucin-secreting HT29-MTX cell line and enterocyte-like HT29 glc−/+ or Caco-2/TC7 cell lines.
Despite the known role of mucus in the host's innate defense against invading and noninvading microbes, mucus has also been identified as being a source of nutrients for bacterial growth (48). Yersinia enterocolitica growth was enhanced in the presence of mucus (53). Salmonella and Escherichia coli grew in vitro utilizing glycolytic and gluconeogenic substrates and the lipids derived from mucus as a source of carbon and nitrogen (38, 56). Moreover, growing Lactobacillus reuteri strains in the presence of mucin dramatically improved their mucus-binding activity (34). Macfarlane et al. (50), who examined the role of the mucus layer coating the human large intestine, have concluded that intestinal bacterial populations growing on mucin surfaces are distinct from their planktonic counterparts present in the gut lumen, in particular in terms of colonization. Consistent with this, it has been observed that inoculating the methicillin-resistant Staphylococcus aureus into the colonic mucus layer leads to rapid bacterial growth, facilitating the intestinal colonization of mice (24). In the same way, Campylobacter jejuni utilizes the secreted MUC2 mucin as an environmental cue for modulating the expression of genes with various functions including colonization and pathogenicity (75). Pseudomonas aeruginosa seems to induce a similar induction of mucin production in airway diseases. Via reactive oxygen species, P. aeruginosa upregulates MUC5AC mucin production (78) and utilizes the sputum layer of the cystic fibrosis lung as a carbon and energy source to support its high-density growth during chronic colonization (62). Motility allows Salmonella enterica serovar Typhimurium to benefit from released high-energy nutrients, including galactose-containing glycoconjugates and mucins for enhancing its own growth in the inflamed intestine (70). Harrington et al. (27) have recently reported that the prototype enteroaggregative E. coli strain 042 colonized the intestine of streptomycin-treated mice by a mechanism involving the Pic serine protease autotransporter of Enterobacteriaceae mucinase toxin that favors the bacterial growth in the presence of secreted cecal mucus. In the study reported here, we used two sets of experimental conditions to investigate whether the human secreted and/or membrane-bound mucins play any role in the growth of aEPEC and tEPEC apically infecting enterocyte-like or mucin-secreting human intestinal cells. We observed that in HT29-MTX cells which express mucins into the culture medium and which have an apical surface that forms a brush border expressing membrane-bound mucins, an increase in the levels of adhering aEPEC strains developed under the subculture condition, whereas tEPEC did not. Using the secreted mucins pharmacologically released from HT29-MTX, we observed that these mucins did not modify the levels of aEPEC and tEPEC adhering onto the enterocyte-like Caco-2/TC7 cells. This suggests that aEPEC could possess several enzymatic systems that allow them to break down membrane-bound mucins to generate substrates facilitating bacterial growth. Interestingly, desulfatation activity has been identified in P. aeruginosa, and the desulfatation of mucin increases its susceptibility to degradation by bacterial glycosidases and proteinases, and subsequent deglycosylation facilitates bacterial colonization by increasing the substrates available (31).
In conclusion, we provide evidence that the aEPEC strains studied interact with human mucin-secreting polarized intestinal HT29-MTX cells to induce the increased expression of MUC genes coding for secreted or membrane-bound mucins, stimulate exocytosis of secreted mucins, and elevate the level of membrane-bound mucins at the apical cell surface forming the brush border. In contrast, this effect is not produced by a tEPEC strain. We also demonstrated that the increased production of mucins is not a cell defense response to aEPEC infection, but favors the growth of adhering aEPEC bacteria at the apical cell surface of the mucin-secreting cells. The aEPEC-specific effector that is not a secreted effector, which activates a mucin regulatory signaling pathway within the cells, remains to be identified.
Acknowledgments
We are grateful to R. Amsellem for assistance with cell culture. We thank V. Nicolas (Plateforme Imagerie Cellulaire—IFR141) and C. Deloménie (Plateforme Transcriptome—IFR141) for expert assistance on confocal laser scanning microscopy analysis and qRT-PCR analysis, respectively. We also thank J. R. C. Andrade (State University of Rio de Janeiro) for the transmission electron microscopy images.
This work was supported by institutional French funds from Institut National de la Santé et de la Recherche Médicale (Inserm), Université Paris-Sud 11, and Ministère de la Recherche (to A.L.S.); a postdoctoral position at INSERM (to V.L.-L.M.); and a postdoctoral Pesquisa no Exterior, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (to M.A.M.V.).
Editor: S. M. Payne
Footnotes
Published ahead of print on 11 January 2010.
REFERENCES
- 1.Bara, J., E. Chastre, J. Mahiou, R. L. Singh, M. E. Forgue-Lafitte, E. Hollande, and F. Godeau. 1998. Gastric M1 mucin, an early oncofetal marker of colon carcinogenesis, is encoded by the MUC5AC gene. Int. J. Cancer 75:767-773. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom, K. S., J. A. Guttman, M. Rumi, C. Ma, S. Bouzari, M. A. Khan, D. L. Gibson, A. W. Vogl, and B. A. Vallance. 2008. Modulation of intestinal goblet cell function during infection by an attaching and effacing bacterial pathogen. Infect. Immun. 76:796-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd, J. C., C. K. Yunker, Q. S. Xu, L. R. Sternberg, and R. S. Bresalier. 2000. Inhibition of gastric mucin synthesis by Helicobacter pylori. Gastroenterology 118:1072-1079. [DOI] [PubMed] [Google Scholar]
- 4.Caron, E., V. F. Crepin, N. Simpson, S. Knutton, J. Garmendia, and G. Frankel. 2006. Subversion of actin dynamics by EPEC and EHEC. Curr. Opin. Microbiol. 9:40-45. [DOI] [PubMed] [Google Scholar]
- 5.Chantret, I., A. Rodolosse, A. Barbat, E. Dussaulx, E. Brot-Laroche, A. Zweibaum, and M. Rousset. 1994. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence for glucose-dependent negative regulation. J. Cell Sci. 107:213-225. [DOI] [PubMed] [Google Scholar]
- 6.Coconnier, M. H., E. Dlissi, M. Robard, C. L. Laboisse, J. L. Gaillard, and A. L. Servin. 1998. Listeria monocytogenes stimulates mucus exocytosis in cultured human polarized mucosecreting intestinal cells through action of listeriolysin O. Infect. Immun. 66:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coconnier, M. H., M. Lorrot, A. Barbat, C. Laboisse, and A. L. Servin. 2000. Listeriolysin O-induced stimulation of mucin exocytosis in polarized intestinal mucin-secreting cells: evidence for toxin recognition of membrane-associated lipids and subsequent toxin internalization through caveolae. Cell. Microbiol. 2:487-504. [DOI] [PubMed] [Google Scholar]
- 8.Debailleul, V., A. Laine, G. Huet, P. Mathon, M. C. d'Hooghe, J. P. Aubert, and N. Porchet. 1998. Human mucin genes MUC2, MUC3, MUC4, MUC5AC, MUC5B, and MUC6 express stable and extremely large mRNAs and exhibit a variable length polymorphism. An improved method to analyze large mRNAs. J. Biol. Chem. 273:881-890. [DOI] [PubMed] [Google Scholar]
- 9.Dekker, J., J. W. Rossen, H. A. Buller, and A. W. Einerhand. 2002. The MUC family: an obituary. Trends Biochem. Sci. 27:126-131. [DOI] [PubMed] [Google Scholar]
- 10.Deng, W., J. L. Puente, S. Gruenheid, Y. Li, B. A. Vallance, A. Vazquez, J. Barba, J. A. Ibarra, P. O'Donnell, P. Metalnikov, K. Ashman, S. Lee, D. Goode, T. Pawson, and B. B. Finlay. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. U. S. A. 101:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dohrman, A., S. Miyata, M. Gallup, J. D. Li, C. Chapelin, A. Coste, E. Escudier, J. Nadel, and C. Basbaum. 1998. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim. Biophys. Acta 1406:251-259. [DOI] [PubMed] [Google Scholar]
- 12.Drumm, B., A. M. Roberton, and P. M. Sherman. 1988. Inhibition of attachment of Escherichia coli RDEC-1 to intestinal microvillus membranes by rabbit ileal mucus and mucin in vitro. Infect. Immun. 56:2437-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Homsi, M., R. Ducroc, J. Claustre, G. Jourdan, A. Gertler, M. Estienne, A. Bado, J. Y. Scoazec, and P. Plaisancie. 2007. Leptin modulates the expression of secreted and membrane-associated mucins in colonic epithelial cells by targeting PKC, PI3K, and MAPK pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 293:G365-G373. [DOI] [PubMed] [Google Scholar]
- 14.Epple, H. J., K. M. Kreusel, C. Hanski, J. D. Schulzke, E. O. Riecken, and M. Fromm. 1997. Differential stimulation of intestinal mucin secretion by cholera toxin and carbachol. Pflugers Arch. 433:638-647. [DOI] [PubMed] [Google Scholar]
- 15.Fagundes-Neto, U., and I. C. Scaletsky. 2000. The gut at war: the consequences of enteropathogenic Escherichia coli infection as a factor of diarrhea and malnutrition. Sao Paulo Med. J. 118:21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fogh, J., J. M. Fogh, and T. Orfeo. 1977. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 59:221-226. [DOI] [PubMed] [Google Scholar]
- 17.Forstner, G. 1995. Signal transduction, packaging and secretion of mucins. Annu. Rev. Physiol. 57:585-605. [DOI] [PubMed] [Google Scholar]
- 18.Gabastou, J. M., S. Kerneis, M. F. Bernet-Camard, A. Barbat, M. H. Coconnier, J. B. Kaper, and A. L. Servin. 1995. Two stages of enteropathogenic Escherichia coli intestinal pathogenicity are up and down-regulated by the epithelial cell differentiation. Differentiation 59:127-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garmendia, J., G. Frankel, and V. F. Crepin. 2005. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect. Immun. 73:2573-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garmendia, J., A. D. Phillips, M. F. Carlier, Y. Chong, S. Schuller, O. Marches, S. Dahan, E. Oswald, R. K. Shaw, S. Knutton, and G. Frankel. 2004. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell. Microbiol. 6:1167-1183. [DOI] [PubMed] [Google Scholar]
- 21.Garmendia, J., Z. Ren, S. Tennant, M. A. Midolli Viera, Y. Chong, A. Whale, K. Azzopardi, S. Dahan, M. P. Sircili, M. R. Franzolin, L. R. Trabulsi, A. Phillips, T. A. Gomes, J. Xu, R. Robins-Browne, and G. Frankel. 2005. Distribution of tccP in clinical enterohemorrhagic and enteropathogenic Escherichia coli isolates. J. Clin. Microbiol. 43:5715-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gipson, I. K., S. Spurr-Michaud, R. Moccia, Q. Zhan, N. Toribara, S. B. Ho, A. R. Gargiulo, and J. A. Hill III. 1999. MUC4 and MUC5B transcripts are the prevalent mucin messenger ribonucleic acids of the human endocervix. Biol. Reprod. 60:58-64. [DOI] [PubMed] [Google Scholar]
- 23.Gomes, T. A., K. Irino, D. M. Girao, V. B. Girao, B. E. Guth, T. M. Vaz, F. C. Moreira, S. H. Chinarelli, and M. A. Vieira. 2004. Emerging enteropathogenic Escherichia coli strains? Emerg. Infect. Dis. 10:1851-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gries, D. M., N. J. Pultz, and C. J. Donskey. 2005. Growth in cecal mucus facilitates colonization of the mouse intestinal tract by methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 192:1621-1627. [DOI] [PubMed] [Google Scholar]
- 25.Ha, U., J. H. Lim, H. Jono, T. Koga, A. Srivastava, R. Malley, G. Pages, J. Pouyssegur, and J. D. Li. 2007. A novel role for IkappaB kinase (IKK) alpha and IKKbeta in ERK-dependent up-regulation of MUC5AC mucin transcription by Streptococcus pneumoniae. J. Immunol. 178:1736-1747. [DOI] [PubMed] [Google Scholar]
- 26.Hao, W. L., and Y. K. Lee. 2004. Microflora of the gastrointestinal tract: a review. Methods Mol. Biol. 268:491-502. [DOI] [PubMed] [Google Scholar]
- 27.Harrington, S. M., J. Sheikh, I. R. Henderson, F. Ruiz-Perez, P. S. Cohen, and J. P. Nataro. 2009. The Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin. Infect. Immun. 77:2465-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennebicq-Reig, S., T. Lesuffleur, C. Capon, C. De Bolos, I. Kim, O. Moreau, C. Richet, B. Hemon, M. A. Recchi, E. Maes, J. P. Aubert, F. X. Real, A. Zweibaum, P. Delannoy, P. Degand, and G. Huet. 1998. Permanent exposure of mucin-secreting HT-29 cells to benzyl-N-acetyl-alpha-D-galactosaminide induces abnormal O-glycosylation of mucins and inhibits constitutive and stimulated MUC5AC secretion. Biochem. J. 334:283-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandes, R. T., W. P. Elias, M. A. Vieira, and T. A. Gomes. 2009. An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol. Lett. 297:137-149. [DOI] [PubMed] [Google Scholar]
- 30.Hernandes, R. T., R. M. Silva, S. M. Carneiro, F. A. Salvador, M. C. Fernandes, A. C. Padovan, D. Yamamoto, R. A. Mortara, W. P. Elias, M. R. da Silva Briones, and T. A. Gomes. 2008. The localized adherence pattern of an atypical enteropathogenic Escherichia coli is mediated by intimin omicron and unexpectedly promotes HeLa cell invasion. Cell. Microbiol. 10:415-425. [DOI] [PubMed] [Google Scholar]
- 31.Jansen, H. J., C. A. Hart, J. M. Rhodes, J. R. Saunders, and J. W. Smalley. 1999. A novel mucin-sulphatase activity found in Burkholderia cepacia and Pseudomonas aeruginosa. J. Med. Microbiol. 48:551-557. [DOI] [PubMed] [Google Scholar]
- 32.Jarry, A., D. Merlin, U. Hopfer, and C. L. Laboisse. 1994. Cyclic AMP-induced mucin exocytosis is independent of Cl− movements in human colonic epithelial cells (HT29-Cl. 16E). Biochem. J. 304:675-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jono, H., H. Xu, H. Kai, D. J. Lim, Y. S. Kim, X. H. Feng, and J. D. Li. 2003. Transforming growth factor-beta-Smad signaling pathway negatively regulates nontypeable Haemophilus influenzae-induced MUC5AC mucin transcription via mitogen-activated protein kinase (MAPK) phosphatase-1-dependent inhibition of p38 MAPK. J. Biol. Chem. 278:27811-27819. [DOI] [PubMed] [Google Scholar]
- 34.Jonsson, H., E. Strom, and S. Roos. 2001. Addition of mucin to the growth medium triggers mucus-binding activity in different strains of Lactobacillus reuteri in vitro. FEMS Microbiol. Lett. 204:19-22. [DOI] [PubMed] [Google Scholar]
- 35.Kaper, J. B., J. P. Nataro, and H. L. T. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 36.Kenny, B. 2002. Mechanism of action of EPEC type III effector molecules. Int. J. Med. Microbiol. 291:469-477. [DOI] [PubMed] [Google Scholar]
- 37.Knutton, S., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 57:1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krivan, H. C., D. P. Franklin, W. Wang, D. C. Laux, and P. S. Cohen. 1992. Phosphatidylserine found in intestinal mucus serves as a sole source of carbon and nitrogen for Salmonellae and Escherichia coli. Infect. Immun. 60:3943-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laboisse, C., A. Jarry, J. E. Branka, D. Merlin, C. Bou-Hanna, and G. Vallette. 1995. Regulation of mucin exocytosis from intestinal goblet cells. Biochem. Soc. Trans. 23:810-813. [DOI] [PubMed] [Google Scholar]
- 40.Leitch, G. J. 1988. Cholera enterotoxin-induced mucus secretion and increase in the mucus blanket of the rabbit ileum in vivo. Infect. Immun. 56:2871-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lencer, W. I., F. D. Reinhart, and M. R. Neutra. 1990. Interaction of cholera toxin with cloned human goblet cells in monolayer culture. Am. J. Physiol. 258:G96-G102. [DOI] [PubMed] [Google Scholar]
- 42.Lesuffleur, T., A. Barbat, E. Dussaulx, and A. Zweibaum. 1990. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 50:6334-6343. [PubMed] [Google Scholar]
- 43.Lesuffleur, T., A. Kornowski, C. Augeron, E. Dussaulx, A. Barbat, C. Laboisse, and A. Zweibaum. 1991. Increased growth adaptability to 5-fluorouracil and methotrexate of HT-29 sub-populations selected for their commitment to differentiation. Int. J. Cancer 49:731-737. [DOI] [PubMed] [Google Scholar]
- 44.Levine, M. M., J. P. Nataro, H. Karch, M. M. Baldini, J. B. Kaper, R. E. Black, M. L. Clements, and A. D. O'Brien. 1985. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J. Infect. Dis. 152:550-559. [DOI] [PubMed] [Google Scholar]
- 45.Li, J. D., A. F. Dohrman, M. Gallup, S. Miyata, J. R. Gum, Y. S. Kim, J. A. Nadel, A. Prince, and C. B. Basbaum. 1997. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc. Natl. Acad. Sci. U. S. A. 94:967-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li, J. D., W. Feng, M. Gallup, J. H. Kim, J. Gum, Y. Kim, and C. Basbaum. 1998. Activation of NF-kappaB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 95:5718-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lievin-Le Moal, V., G. Huet, J. P. Aubert, J. Bara, M. E. Forgue-Lafitte, A. L. Servin, and M. H. Coconnier. 2002. Activation of mucin exocytosis and upregulation of MUC genes in polarized human intestinal mucin-secreting cells by the thiol-activated exotoxin listeriolysin O. Cell. Microbiol. 4:515-529. [DOI] [PubMed] [Google Scholar]
- 48.Lievin-Le Moal, V., and A. L. Servin. 2006. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin. Microbiol. Rev. 19:315-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lievin-Le Moal, V., A. L. Servin, and M. H. Coconnier-Polter. 2005. The increase in mucin exocytosis and the upregulation of MUC genes encoding for membrane-bound mucins induced by the thiol-activated exotoxin listeriolysin O is a host cell defence response that inhibits the cell-entry of Listeria monocytogenes. Cell. Microbiol. 7:1035-1048. [DOI] [PubMed] [Google Scholar]
- 50.Macfarlane, S., E. J. Woodmansey, and G. T. Macfarlane. 2005. Colonization of mucin by human intestinal bacteria and establishment of biofilm communities in a two-stage continuous culture system. Appl. Environ. Microbiol. 71:7483-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mack, D. R., S. Michail, S. Wei, L. McDougall, and M. A. Hollingsworth. 1999. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 276:G941-G950. [DOI] [PubMed] [Google Scholar]
- 52.Mack, D. R., and P. M. Sherman. 1991. Mucin isolated from rabbit colon inhibits in vitro binding of Escherichia coli RDEC-1. Infect. Immun. 59:1015-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mantle, M., and C. Rombough. 1993. Growth in and breakdown of purified rabbit small intestinal mucin by Yersinia enterocolitica. Infect. Immun. 61:4131-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNamara, N., and C. Basbaum. 2001. Signaling networks controlling mucin production in response to Gram-positive and Gram-negative bacteria. Glycoconj. J. 18:715-722. [DOI] [PubMed] [Google Scholar]
- 55.Micots, I., C. Augeron, C. L. Laboisse, F. Muzeau, and F. Megraud. 1993. Mucin exocytosis: a major target for Helicobacter pylori. J. Clin. Pathol. 46:241-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miranda, R. L., T. Conway, M. P. Leatham, D. E. Chang, W. E. Norris, J. H. Allen, S. J. Stevenson, D. C. Laux, and P. S. Cohen. 2004. Glycolytic and gluconeogenic growth of Escherichia coli O157:H7 (EDL933) and E. coli K-12 (MG1655) in the mouse intestine. Infect. Immun. 72:1666-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moniaux, N., F. Escande, N. Porchet, J. P. Aubert, and S. K. Batra. 2001. Structural organization and classification of the human mucin genes. Front. Biosci. 6:D1192-D1206. [DOI] [PubMed] [Google Scholar]
- 58.Morgenstern, S., R. Koren, S. F. Moss, G. Fraser, E. Okon, and Y. Niv. 2001. Does Helicobacter pylori affect gastric mucin expression? Relationship between gastric antral mucin expression and H. pylori colonization. Eur. J. Gastroenterol. Hepatol. 13:19-23. [DOI] [PubMed] [Google Scholar]
- 59.Nutten, S., P. Sansonetti, G. Huet, C. Bourdon-Bisiaux, B. Meresse, J. F. Colombel, and P. Desreumaux. 2002. Epithelial inflammation response induced by Shigella flexneri depends on mucin gene expression. Microbes Infect. 4:1121-1124. [DOI] [PubMed] [Google Scholar]
- 60.Ooka, T., M. A. Vieira, Y. Ogura, L. Beutin, R. La Ragione, P. M. van Diemen, M. P. Stevens, I. Aktan, S. Cawthraw, A. Best, R. T. Hernandes, G. Krause, T. A. Gomes, T. Hayashi, and G. Frankel. 2007. Characterization of tccP2 carried by atypical enteropathogenic Escherichia coli. FEMS Microbiol. Lett. 271:126-135. [DOI] [PubMed] [Google Scholar]
- 61.Ottemann, K. M., and A. C. Lowenthal. 2002. Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infect. Immun. 70:1984-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmer, K. L., L. M. Aye, and M. Whiteley. 2007. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 189:8079-8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pron, B., C. Boumaila, F. Jaubert, S. Sarnacki, J. P. Monnet, P. Berche, and J. L. Gaillard. 1998. Comprehensive study of the intestinal stage of listeriosis in a rat ligated ileal loop system. Infect. Immun. 66:747-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Radhakrishnan, P., D. Halagowder, and S. N. Devaraj. 2007. Altered expression of MUC2 and MUC5AC in response to Shigella infection, an in vivo study. Biochim. Biophys. Acta 1770:884-889. [DOI] [PubMed] [Google Scholar]
- 65.Roger, P., J. P. Gascard, J. Bara, V. T. de Montpreville, M. Yeadon, and C. Brink. 2000. ATP induced MUC5AC release from human airways in vitro. Mediators Inflamm. 9:277-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosa, A. C., M. A. Vieira, A. Tibana, T. A. Gomes, and J. R. Andrade. 2001. Interactions of Escherichia coli strains of non-EPEC serogroups that carry eae and lack the EAF and stx gene sequences with undifferentiated and differentiated intestinal human Caco-2 cells. FEMS Microbiol. Lett. 200:117-122. [DOI] [PubMed] [Google Scholar]
- 67.Smirnova, M. G., J. P. Birchall, and J. P. Pearson. 2000. TNF-alpha in the regulation of MUC5AC secretion: some aspects of cytokine-induced mucin hypersecretion on the in vitro model. Cytokine 12:1732-1736. [DOI] [PubMed] [Google Scholar]
- 68.Smirnova, M. G., L. Guo, J. P. Birchall, and J. P. Pearson. 2003. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell. Immunol. 221:42-49. [DOI] [PubMed] [Google Scholar]
- 69.Smith, C. J., J. B. Kaper, and D. R. Mack. 1995. Intestinal mucin inhibits adhesion of human enteropathogenic Escherichia coli to HEp-2 cells. J. Pediatr. Gastroenterol. Nutr. 21:269-276. [DOI] [PubMed] [Google Scholar]
- 70.Stecher, B., M. Barthel, M. C. Schlumberger, L. Haberli, W. Rabsch, M. Kremer, and W. D. Hardt. 2008. Motility allows S. typhimurium to benefit from the mucosal defence. Cell. Microbiol. 10:1166-1180. [DOI] [PubMed] [Google Scholar]
- 71.Tai, E. K., H. P. Wong, E. K. Lam, W. K. Wu, L. Yu, M. W. Koo, and C. H. Cho. 2008. Cathelicidin stimulates colonic mucus synthesis by up-regulating MUC1 and MUC2 expression through a mitogen-activated protein kinase pathway. J. Cell. Biochem. 104:251-258. [DOI] [PubMed] [Google Scholar]
- 72.Tanaka, S., M. Mizuno, T. Maga, F. Yoshinaga, J. Tomoda, J. Nasu, H. Okada, K. Yokota, K. Oguma, Y. Shiratori, and T. Tsuji. 2003. H. pylori decreases gastric mucin synthesis via inhibition of galactosyltransferase. Hepatogastroenterology 50:1739-1742. [PubMed] [Google Scholar]
- 73.Trabulsi, L. R. 1964. Detection of colibacilli associated with infantile diarrheas by the experimental infection of ligated loops of rabbit intestine. Rev. Inst. Med. Trop. Sao Paulo 35:197-203. [PubMed] [Google Scholar]
- 74.Trabulsi, L. R., R. Keller, and T. A. Gomes. 2002. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 8:508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tu, Q. V., M. A. McGuckin, and G. L. Mendz. 2008. Campylobacter jejuni response to human mucin MUC2: modulation of colonization and pathogenicity determinants. J. Med. Microbiol. 57:795-802. [DOI] [PubMed] [Google Scholar]
- 76.Vieira, M. A., J. R. Andrade, L. R. Trabulsi, A. C. Rosa, A. M. Dias, S. R. Ramos, G. Frankel, and T. A. Gomes. 2001. Phenotypic and genotypic characteristics of Escherichia coli strains of non-enteropathogenic E. coli (EPEC) serogroups that carry EAE and lack the EPEC adherence factor and Shiga toxin DNA probe sequences. J. Infect. Dis. 183:762-772. [DOI] [PubMed] [Google Scholar]
- 77.Yamamoto, D., R. T. Hernandes, M. Blanco, L. Greune, M. A. Schmidt, S. M. Carneiro, G. Dahbi, J. E. Blanco, A. Mora, J. Blanco, and T. A. Gomes. 2009. Invasiveness as a putative additional virulence mechanism of some atypical enteropathogenic Escherichia coli strains with different uncommon intimin types. BMC Microbiol. 9:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan, F., W. Li, H. Jono, Q. Li, S. Zhang, J. D. Li, and H. Shen. 2008. Reactive oxygen species regulate Pseudomonas aeruginosa lipopolysaccharide-induced MUC5AC mucin expression via PKC-NADPH oxidase-ROS-TGF-alpha signaling pathways in human airway epithelial cells. Biochem. Biophys. Res. Commun. 366:513-519. [DOI] [PubMed] [Google Scholar]
- 79.Zoghbi, S., A. Trompette, J. Claustre, M. El Homsi, J. Garzon, G. Jourdan, J. Y. Scoazec, and P. Plaisancie. 2006. beta-Casomorphin-7 regulates the secretion and expression of gastrointestinal mucins through a mu-opioid pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 290:G1105-G1113. [DOI] [PubMed] [Google Scholar]
- 80.Zweibaum, A., M. Pinto, G. Chevalier, E. Dussaulx, N. Triadou, B. Lacroix, K. Haffen, J. L. Brun, and M. Rousset. 1985. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J. Cell. Physiol. 122:21-29. [DOI] [PubMed] [Google Scholar]