Abstract
The control of malaria is challenged by resistance of Plasmodium falciparum to multiple drugs. New combination regimens are now advocated for the treatment of uncomplicated falciparum malaria, but the extent of resistance to newer agents is incompletely understood. We measured the in vitro sensitivity of P. falciparum parasites cultured from children enrolled in a drug efficacy trial in Kampala, Uganda, from 2006 to 2008. Sensitivities were measured by comparing levels of histidine-rich protein-2 in parasites incubated with different concentrations of drugs with those in untreated controls. The cultured parasites exhibited a wide range of sensitivities to chloroquine (CQ); monodesethylamodiaquine (MDAQ), the major active metabolite of amodiaquine; and quinine (QN). Mean 50% inhibitory concentration (IC50) results were above standard cutoffs for resistance for CQ and MDAQ. Parasites were generally sensitive to dihydroartemisinin (DHA), lumefantrine (LM), and piperaquine (PQ). For CQ, MDAQ, and QN but not the other drugs, activities against individual strains were highly correlated. We also assessed known resistance-mediating polymorphisms in two putative transporters, pfcrt and pfmdr1. When parasites that were least and most sensitive to each drug were compared, the pfmdr1 86Y mutation was significantly more common in parasites that were most resistant to CQ and MDAQ, and the pfmdr1 D1246Y mutation was significantly more common in parasites that were most resistant to MDAQ and QN. In summary, we demonstrated in parasites from Kampala a range of sensitivities to older drugs; correlation of sensitivities to CQ, MDAQ, and QN; and good activity against nearly all strains for DHA, LM, and PQ.
Resistance of Plasmodium falciparum to available drugs remains a major challenge to the control of malaria. Older drugs, including the aminoquinolines chloroquine (CQ) and amodiaquine (AQ) and the antifolate sulfadoxine/pyrimethamine (SP), are already seriously compromised, with unacceptable levels of treatment failure in most of Africa (61). In the setting of increasing drug resistance, the WHO has recommended artemisinin-based combination therapy (ACT) for the treatment of uncomplicated falciparum malaria (42). The commonly used ACTs in Africa are artemether/lumefantrine (AM/LM), artesunate/amodiaquine (AS/AQ), and dihydroartemisinin/piperaquine (DHA/PQ), each containing an artemisinin combined with a longer-acting drug. These ACTs have shown excellent efficacy for the treatment of malaria in Africa. However, there is concern that heavy use of ACTs will offer strong selective pressure for parasites with diminished sensitivity to the drugs. This development may seriously jeopardize the efficacy of ACTs.
Multiple recent studies in Africa have demonstrated excellent efficacy of AM/LM, AS/AQ, and DHA/PQ for the treatment of falciparum malaria (8, 18, 29, 30, 34, 46, 63, 64). Clinical trials provide our primary means of assessing antimalarial drug efficacy, but they offer only an indirect measure of the sensitivity of parasites to drugs because outcomes can be affected by multiple factors independent of drug sensitivity, including compliance with treatment regimens, drug absorption, pharmacokinetics, antimalarial immunity, and human genetic polymorphisms. In addition, clinical trials are not a sensitive means of identifying early selection of parasites with diminished drug sensitivity, as moderate decreases in sensitivity may have limited impact on clinical outcomes.
The sensitivity of malaria parasites to drugs can be evaluated directly using parasites cultured in vitro. Systems for the culture of P. falciparum are well established, although the adaptation of parasites from an active infection to culture remains somewhat problematic. Thus, information on the in vitro drug sensitivity of cultured parasites is limited. Available studies have shown a wide range of sensitivities to older drugs. Parasites with diminished sensitivity to CQ, AQ, and SP are commonly seen (61). For newer drugs, including the active artemisinin metabolite DHA and the ACT partner drugs LM and PQ, African studies have suggested good sensitivity of most parasites (4, 6, 10, 48, 49). However, there is reason for concern that increasing use of ACTs and monotherapies may select for parasites with resistance to important newer agents. First, one study demonstrated parasites with markedly diminished sensitivity to artemether from French Guiana and Senegal (28). Second, recent data from Cambodia have shown diminished responsiveness to artesunate and prolonged parasite clearance times, suggesting the emergence of parasites with diminished sensitivity to artemisinins (15, 39, 54). Third, poor in vitro sensitivity to AQ and its active metabolite monodesethylamodiaquine (MDAQ) has already been demonstrated in Africa (55), parasite resistance-mediating polymorphisms predicted poor response to treatment with AQ and were selected by prior AQ therapy (12, 13, 23, 25, 27, 43), and use of an AQ-containing regimen selected for parasites with diminished in vitro drug sensitivity in subsequent new infections (36). Fourth, treatment with AM/LM selected in subsequent infections for parasites with polymorphisms that may lead to diminished drug responsiveness (14, 27, 31, 57). Fifth, PQ has a history of widespread resistance after broad use as monotherapy some decades ago in China (9). African studies have generally demonstrated good sensitivity of African parasites to piperaquine, but some parasites with in vitro 50% inhibitory concentrations (IC50s) over 100 nM have been identified (6, 10, 35).
In some cases, parasite genetic polymorphisms that mediate decreased drug responsiveness are known. The K76T polymorphism in the putative transporter pfcrt is the key mediator of resistance to CQ (11, 21) and also impacts response to AQ (13, 23). Mutations in another putative transporter, pfmdr1, appear to decrease sensitivity to CQ, AQ, and QN, and some of the same mutations may increase sensitivity to other drugs, including mefloquine and halofantrine, both of which are related to LM, and artemisinin. Increases in pfmdr1 copy number decrease in vitro sensitivity to mefloquine, halofantrine, LM, QN, and artemisinin (56) and have clearly been associated with mefloquine treatment failure (50). However, some pfmdr1 polymorphisms (S1034C and N1042D) and increases in pfmdr1 gene copy number are generally not seen in Africa (14, 25, 59), and our understanding of the importance of the pfmdr1 polymorphisms that are common in Africa (N86Y, Y184F, and D1246Y) is incomplete.
In order to better characterize the sensitivity of P. falciparum from Kampala, Uganda, to relevant antimalarial drugs, we collected parasites causing uncomplicated malaria in a cohort of children enrolled in a drug efficacy trial, determined the sensitivity of these parasites to six key antimalarial drugs, and searched for associations between sensitivities to different drugs and between in vitro drug sensitivity and parasite genotypes. We found that parasites in Kampala exhibit a broad range of drug sensitivities, especially to CQ, MDAQ, and QN; that sensitivities to these three drugs, but not other tested drugs, were tightly correlated; and that polymorphisms in pfmdr1 were associated with but did not fully explain resistance to these drugs.
MATERIALS AND METHODS
Clinical trial.
Blood samples containing malaria parasites were collected from a cohort of children in the Mulago III parish of Kampala enrolled in a longitudinal comparison of the efficacies against uncomplicated malaria of AQ/SP, AS/AQ, and AM/LM (18). In this trial, patients were offered all medical care through the study clinic, and so use of antimalarial drugs outside the study protocol was uncommon (18). Samples were collected for parasite culture between August 2006 and May 2008. The clinical trial and analysis of cultured parasites were approved by the Uganda National Council of Science and Technology, the Makerere University Research and Ethics Committee, and the University of California, San Francisco Committee on Human Research.
Collection and culture of parasites.
At the time of diagnosis of a new episode of malaria, and before the initiation of therapy, blood was collected in heparinized tubes, and transported within 30 min to our laboratory. Giemsa-stained thin blood smears were examined, parasitemia was determined, and if P. falciparum infection and the lack of other plasmodial species were confirmed, culture was initiated. Blood was centrifuged, plasma and buffy coat were removed, and the erythrocyte pellet was washed twice with RPMI 1640 medium at 37°C. Parasites were diluted with 2% group O uninfected erythrocytes to obtain a density of 0.05%, to avoid any influence of an inoculum effect on assay results. Aliquots (200 μl) were then cultured in 10 ml RPMI 1640 medium supplemented with 25 mM HEPES, 0.2% NaHCO3, 0.1 mM hypoxanthine, 100 μg/ml gentamicin, and 0.5% Albumax II serum substitute to produce a packed cell volume of ∼2%.
Measurement of in vitro drug sensitivity.
Sensitivities were measured for CQ (Sigma-Aldrich), MDAQ (BD Gentest), QN (Sigma-Aldrich), DHA (Dafra Pharma), LM (Porton International), and PQ (Porton International). Multiple wells of a 96-well culture plate were predosed with seven duplicate 2-fold serial dilutions of each drug of interest (CQ, 15.7 to 1,002 nM; MDAQ, 6.25 to 400 nM; QN, 15.4 to 985 nM; DHA, 0.13 to 8.5 nM; LM, 0.2 to 12.5 nM; PQ, 1.6 to 100 nM). Wells were dried in an incubator and stored at 4°C in sealed plastic bags. Wells without drug served as controls. For each parasite sample, 200-μl aliquots of cultured parasites, prepared as described above, were added to each well and maintained at 5% CO2 at 37°C for 72 h. After the incubation, a blood smear was prepared to confirm healthy growth of controls and determine the parasitemia. Samples were then frozen (−20°C overnight) and thawed before analysis. Drug sensitivity was assessed using an enzyme-linked immunosorbent assay (ELISA) that quantifies parasite histidine-rich protein-2 (HRP-2) (40) to compare levels of this protein in treated and control cultures. Samples were diluted (1:4 to 1:10; the same dilution for each sample in an experiment) in water, and 100 μl of each hemolyzed sample preparation was added to an ELISA plate precoated with mouse anti-HRP-2 IgM capture antibody (Immunology Consultant Laboratory) and incubated at room temperature for 1 h. Plates were then washed four times with the kit washing solution, incubated with 100 μl of secondary antibody (horseradish peroxidase-conjugated anti-mouse IgG) for 1 h at room temperature, washed again four times, incubated with 100 μl of 3,3′,5,5′-tetramethylbenzidine single-solution chromogen (Zymed) in the dark for 5 to 10 min, and then incubated with 50 μl of 1 M sulfuric acid to stop the reactions. The A450 was then measured for each well with a VersaMax 340 spectrophotometer (Molecular Devices). Optical density values were fitted to normal curves based on serial dilutions of HRP-2 standards and a four-parameter curve model (Softmax Pro 2.1.1; Molecular Devices), and IC50s were calculated based on a nonlinear regression model, with attention to test validity based on adequate readings above background, as previously described (3).
Analysis of parasite polymorphisms associated with drug resistance.
At the time of each new diagnosis of malaria, blood was also spotted onto filter paper (Whatman 3MM) for molecular studies. To identify polymorphisms most likely to impact upon drug sensitivity, we selected parasite samples with the highest and lowest levels of drug sensitivity for analysis. For the drugs with wide ranges of sensitivity (CQ, MDAQ, and QN), 20 samples were selected for each category; smaller numbers were studied for LM and PQ based on the limited numbers of samples with remarkably diminished drug sensitivity. DHA was not considered in this analysis, as all parasites had similarly low IC50s. DNA was isolated by Chelex extraction (47). We evaluated polymorphisms at the pfcrt allele (K76T) and the pfmdr-1 allele (N86Y, Y184F, and D1246Y), all of which have previously shown association with varied drug response, by amplification of flanking sequences by PCR, digestion with sequence-specific restriction endonucleases, and evaluation of digested fragments by agarose gel electrophoresis, all as previously described (11, 19).
Statistical analysis.
Inhibitory concentrations (IC50s) for antimalarials were calculated using a polynomial regression model and HN-NonLin software, which is available at http://malaria.farch.net (38). Correlations between drugs were evaluated by Pearson correlation. Associations between drug sensitivities and specific parasite polymorphisms were evaluated using the Fisher exact test. For all statistical tests, the significance level was set at P ≤ 0.05.
RESULTS
In vitro sensitivity of freshly isolated P. falciparum to six antimalarial drugs in Kampala.
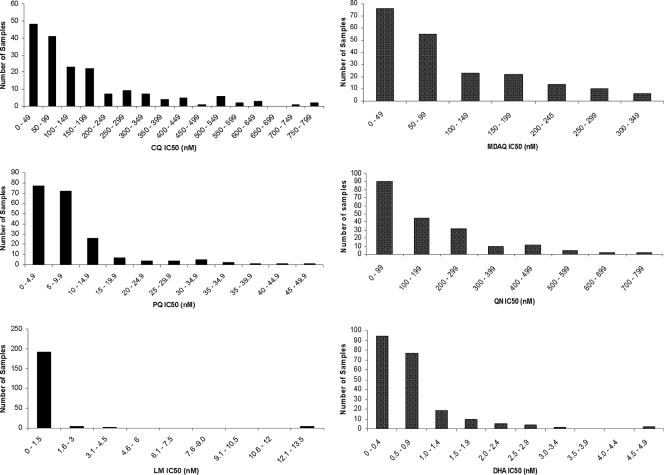
A cohort study of Ugandan children (18) allowed us the opportunity to assess the in vitro drug sensitivity of P. falciparum parasites causing uncomplicated malaria. Blood was collected from children presenting with malaria between August 2006 and May 2008. Erythrocytes were washed and placed into culture, and in vitro drug sensitivity was assessed using an ELISA to quantify HRP-2. A total of 241 parasite samples were set up for analysis. The IC50 determination was successful for 181 (75%) samples for CQ, 206 (86%) for MDAQ, 199 (83%) for PQ, 196 (81%) for QN, 200 (83%) for LM, and 212 (88%) for DHA (Fig. 1). Failure to obtain results for particular samples was due to poor in vitro growth, culture contamination, or failure to achieve adequate fit on a log dose-response curve. We found a wide range of sensitivities to CQ, MDAQ, and QN, with geometric mean IC50s above typical cutoffs (7) for resistance for CQ (100 nM) and MDAQ (60 nM) (Table 1). The geometric mean IC50 for PQ was 6.1 nM, and the maximum IC50 determined, 46.2 nM, is generally considered within the drug-sensitive range (6). For QN, the geometric mean IC50 of 94 nM was well below a previously assigned arbitrary cutoff for resistance (600 nM [53]), but the IC50 for QN was >200 nM in 31% of samples, >400 nM in 9.7% of samples, and >600 nM in 2% of samples, suggesting potential for true drug resistance in Ugandan parasites. For DHA, the major active metabolite of artemisinins, ranges in IC50 were narrower. Mean IC50s were subnanomolar for DHA, and all samples had IC50s of <5 nM. For LM the geometric mean IC50 was also subnanomolar, but 2% of tested samples had IC50s of >12 nM. Sensitivities of control parasite lines previously characterized as CQ sensitive (D6 and HB3) and CQ resistant (W2 and K1) are shown in Table 2.
FIG. 1.
Sensitivity of clinical samples to CQ, MDAQ, PQ, QN, LM, and DHA. Parasites were placed into culture and sensitivities were assessed by comparing levels of HRP-2 with those of untreated controls.
TABLE 1.
In vitro sensitivity of fresh clinical isolates of P. falciparuma
| Drug | No. of isolates | IC50 (geometric mean, nM) | 95% confidence interval | Range |
|---|---|---|---|---|
| CQ | 181 | 101.1 | 86.8-117.7 | 15.6-767.0 |
| MDAQ | 206 | 66.4 | 58.1-76.0 | 6.5-311.6 |
| PQ | 199 | 6.1 | 5.5-6.8 | 1.6-46.2 |
| QN | 196 | 94.4 | 80.4-110.8 | 15.4-760.9 |
| LM | 200 | 0.51 | 0.46-0.56 | 0.19-12.5 |
| DHA | 212 | 0.55 | 0.50-0.61 | 0.13-4.8 |
Standard cutoffs for drug sensitivity are 100 nM for CQ, 60 nM for MDAQ, and 600 nM for QN.
TABLE 2.
Drug sensitivities of control strains
| Straina | Sensitivityb |
|||||
|---|---|---|---|---|---|---|
| CQ | MDAQ | PQ | QN | LUM | DHA | |
| W2 (MRA4-157) | 630.6 | 267.7 | 14.5 | 490.8 | 0.45 | 0.41 |
| D6 (MRA4-285) | 47.8 | 30.9 | 6.26 | 106.9 | 1.82 | 0.79 |
| HB3 (MRA4-155) | 61.8 | 29.8 | 10.5 | 236.1 | 1.41 | 0.71 |
| K1 (MRA4-159) | Not done | 262.1 | 12.5 | 276.0 | 0.31 | 0.49 |
MR4 reference numbers are in parentheses.
Results are shown as IC50 (nM).
Correlations between sensitivities to different antimalarial drugs.
It was of interest to assess relationships between sensitivities measured for different drugs. Sensitivities to CQ, MDAQ, and QN were tightly correlated (Table 3). Inverse correlations were seen between sensitivities to CQ, MDAQ, and QN and those to LM and DHA, although not all comparisons reached statistical significance (Table 3). No correlation was seen between sensitivity to PQ, a bisquinoline related to CQ and MDAQ, and that to any of the other tested antimalarials.
TABLE 3.
Correlations between sensitivities to different antimalarial drugs
| Drug | Correlationa |
||||
|---|---|---|---|---|---|
| CQ | MDAQ | PQ | QN | LM | |
| MDAQ | 0.594 (<0.0001) | ||||
| PQ | 0.121 (0.15) | 0.113 (0.14) | |||
| QN | 0.406 (<0.0001) | 0.564 (<0.0001) | −0.0563 (0.48) | ||
| LM | −0.122 (0.13) | −0.153 (0.047) | 0.112 (0.12) | −0.153 (0.055) | |
| DHA | −0.161 (0.036) | −0.219 (0.0022) | 0.0673 (0.38) | −0.125 (0.091) | 0.0189 (0.80) |
Values shown are Pearson's correlations, with P values in parentheses.
Polymorphisms in pfcrt and pfmdr1 and in vitro drug sensitivity.
We evaluated associations between known polymorphisms in the putative drug transporters pfcrt and pfmdr1 and in vitro sensitivity. In this analysis we studied the samples with the highest and lowest sensitivities to the five drugs with a fairly broad range of sensitivities. For pfcrt, all samples contained the pfcrt 76T mutation that has been strongly associated with resistance to CQ (11, 21). Consistent with other recent studies (17, 22), this mutation remains fixed in parasites in Uganda. Polymorphisms in pfmdr1 may impact sensitivity to multiple drugs, with, most notably, the 86Y mutation associated with diminished clinical or in vitro sensitivity to aminoquinolines (13, 62) but increased sensitivity to other drugs, including mefloquine, halofantrine, and LM (35, 45, 62). In our analysis, the three pfmdr1 polymorphisms that are common in Africa were not tightly linked to in vitro drug sensitivity (Table 4). Nonetheless, the strains that were most resistant to CQ, MDAQ, and QN all had a greater likelihood of harboring the 86Y and 1246Y mutations than did the strains that were most drug sensitive, although not all differences were statistically significant (Table 4).
TABLE 4.
Associations between pfmdr1 polymorphisms and drug sensitivitya
| Drug | n | IC50 (nM) | No. of strains with polymorphism: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N86Y |
D1246Y |
Y184F |
||||||||||||
| WT | M | MIX | P | WT | M | MIX | P | WT | M | MIX | P | |||
| CQ | 20 | 15.7-49.0 | 5 | 9 | 6 | 0.007 | 7 | 10 | 3 | 0.41 | 18 | 2 | 0 | 1.0 |
| 20 | 324-767 | 0 | 20 | 0 | 2 | 8 | 10 | 17 | 2 | 1 | ||||
| MDAQ | 20 | 6.3-19.0 | 6 | 12 | 2 | 0.019 | 6 | 8 | 6 | 0.006 | 15 | 5 | 0 | 0.18 |
| 20 | 199-312 | 0 | 17 | 3 | 0 | 15 | 5 | 19 | 1 | 0 | ||||
| QN | 20 | 15.4-98.8 | 6 | 11 | 3 | 0.22 | 10 | 6 | 4 | 0.029 | 16 | 4 | 0 | 0.34 |
| 20 | 380-761 | 2 | 14 | 4 | 3 | 12 | 5 | 19 | 1 | 0 | ||||
| LM | 4 | 0.19-0.23 | 2 | 1 | 1 | 0.14 | 2 | 1 | 1 | 1.0 | 4 | 0 | 0 | 1.0 |
| 4 | 12.5 | 0 | 4 | 0 | 2 | 2 | 0 | 4 | 0 | 0 | ||||
| PQ | 18 | 1.6-2.0 | 5 | 11 | 2 | 1.0 | 5 | 9 | 4 | 1.0 | 13 | 4 | 1 | 0.69 |
| 18 | 19.6-38.9 | 4 | 12 | 2 | 4 | 10 | 4 | 15 | 3 | 0 | ||||
For each drug, the stated number of strains (n) with the highest and lowest IC50s was analyzed for polymorphisms in pfmdr1. For each polymorphism, the number of strains with the wild-type (WT), mutant (M), or mixed (MIX) genotype is shown. P values are based on the two-tailed Fisher exact test comparing the prevalence of pure wild-type and pure mutant alleles. For all significant comparisons, significance was also seen when the prevalence of wild-type alleles was compared with that of combined mixed and mutant alleles.
DISCUSSION
We made use of a drug efficacy trial in Kampala to acquire a large number of P. falciparum samples for the evaluation of in vitro sensitivity to the drugs that are presently most important for the treatment of falciparum malaria in Africa. We found that sensitivities of parasites to CQ, MDAQ, and QN varied greatly, while most parasites remained highly sensitive to the newer agents DHA, LM, and PQ. There were strong correlations between sensitivities to CQ, MDAQ, and QN, but not other tested drugs. Well-known polymorphisms in the putative drug transporter pfmdr1 were associated with decreased sensitivity to CQ, MDAQ, and QN, although these polymorphisms did not fully account for alterations in drug sensitivity. Our results suggest that antimalarial drug resistance is complex, with multiple mediators of resistance and associations for resistance between some but not other relevant drugs. Importantly, our results further suggest that resistance to newer agents, including the components of the ACTs AM/LM and DHA/PQ, is not yet well established in Kampala.
Other groups have performed evaluations of the in vitro sensitivity of P. falciparum to commonly used antimalarial drugs at a number of locations in Africa. Consistent with our findings, parasites commonly respond poorly to CQ (53). However, it is difficult to assign a precise cutoff for in vitro resistance for CQ. In our study in Uganda, where the antimalarial efficacy of CQ is poor (16) and the resistance-mediating pfcrt 76T mutation is universal, 49% of parasites had IC50s for CQ below 100 nM, the generally accepted cutoff for drug sensitivity (53). Other studies have also identified CQ IC50s well below 100 nM in many parasites with the pfcrt 76T mutation (5, 58). Some differences between results may be explained by different methodologies used to measure IC50s, although the HRP-2 assay that we used correlated well with other standard assays in prior studies (41). Our results suggest that, using an HRP2-based assay, the cutoff for in vitro resistance to CQ might be lowered. AQ often retains efficacy against CQ-resistant parasites, probably due to improved potency over CQ, but in vitro resistance to AQ and MDAQ has been described in Africa (32, 55). Our study showed that parasites with IC50s above the accepted cutoff for resistance to MDAQ are common in Kampala (58% of tested samples had IC50s of >60 nM). Clinical and in vitro resistance to QN has been seen in Southeast Asia but not clearly in Africa (61). With moves away from older therapies but limited availability of ACTs in many areas, QN is increasingly used as a first-line therapy for uncomplicated malaria. This development, and the fact that compliance with QN regimens is particularly poor, suggests that there will be increasing pressure for the selection of parasites with diminished sensitivity to QN. Indeed, we found that, although mean IC50s for QN were quite low, a good number of parasites had much higher IC50s. It is uncertain whether diminished parasite sensitivity may explain the surprisingly poor efficacy of QN in some recent studies in Africa (1, 2, 51). QN failures have generally been attributed to poor compliance with this drug, which must be taken three times per day for a week, but a recent effectiveness study, which demonstrated success for only 64% of QN treatments, did not identify a strong association between treatment compliance and clinical responses (1).
For newer drugs now very relevant for antimalarial treatment, relatively little information on parasite drug sensitivity is available. Available ACTs include artesunate, artemether, or DHA as the artemisinin component, but artesunate and artemether are both rapidly metabolized to DHA, so sensitivity to DHA is relevant for the three ACTs now widely advocated in Africa. Most studies have shown parasites to be universally highly sensitive to artemisinins in Africa. An exception is a study that demonstrated IC50s against artemether above 100 nM in South America and as high as 45 nM in Senegal (28). In our studies, all parasites were highly sensitive to DHA. Sensitivity to LM has been little studied. We found LM to be highly active against cultured parasites, but a small number of parasites were considerably less sensitive. PQ was used heavily in China after it replaced CQ for the treatment of falciparum malaria in 1978, followed by the emergence of PQ-resistant parasites, documented by high rates of clinical treatment failure and poor in vitro sensitivity in the 1980s (9). In Africa, reports from Madagascar (10), Cameroon (6), and Kenya (33) have shown generally good in vitro activity of PQ, but some parasites with IC50s above 100 nM have been seen. In our study PQ sensitivity was good, but a small number of parasites were much less sensitive, possibly identifying parasites selected for decreased drug sensitivity by repeated use of the drug in our cohort study. Considering clinical outcomes, AM/LM (18, 34) and DHA/PQ (29, 30, 63) have recently demonstrated outstanding treatment efficacy against falciparum malaria in Africa, suggesting that, at present, resistance is not widespread. However, recrudescences after treatment do occur, and it remains to be seen if decreased sensitivity to LM or PQ will increasingly impact treatment responses.
Parasite polymorphisms that mediate diminished sensitivity to some drugs are now well characterized. We searched for associations between sequences at key alleles in the putative drug transporters pfcrt and pfmdr1 and extremes of drug sensitivity. Evaluations of pfcrt were not instructive, as the key pfcrt 76T mutation remains universal in Kampala. Considering pfmdr1, we found that the least sensitive parasites had a greater prevalence of mutant alleles at positions 86 and 1246 for CQ, MDAQ, and QN. Notably, in prior studies, pfmdr1 86Y, and in some studies also 1246Y, predicted AQ treatment failure or was selected by prior therapy with a regimen including AQ (12, 13, 23, 26, 27, 43, 64). However, in our in vitro studies, associations between these markers and decreased drug sensitivity were fairly weak, and it is likely that additional parasite factors, including other polymorphisms in pfcrt, pfmdr1, or other genes, contribute to resistant phenotypes. Two drugs are of particular interest in this evaluation. For the other aminoquinoline studied, PQ, mutant alleles at pfmdr1 86 and 1246 were as prevalent in the most sensitive as in the least sensitive parasites. These results suggest that the diminished response of parasites to PQ has been mediated by polymorphisms other than the well-characterized polymorphisms in pfmdr1 that affect responses to CQ and AQ. Further, parasites in Kampala were generally highly sensitive to PQ. Thus, heavy use of CQ and AQ has apparently not selected for parasites with diminished sensitivity to PQ. For QN, our data showed a correlation between sensitivity to this drug and to the aminoquinolines CQ and MDAQ. These data contrast with those from Asia, where resistance appears to be mediated primarily by alterations in the copy number of pfmdr1 and sensitivity to QN follows a trend opposite that for CQ (increasing copy number leads to increased sensitivity to CQ but decreased sensitivity to QN [60]). Thus, in Africa, where increases in copy number of pfmdr1 have rarely been identified (14, 25, 59), decreased sensitivity to QN appears to be mediated by the same factors that diminish sensitivity to CQ and AQ. Diminished responsiveness to QN may also be mediated, in part, by polymorphisms in another gene encoding a predicted transporter, pfnhe1 (20, 24, 37). Studies to characterize impacts of multiple polymorphisms on QN sensitivity are under way.
It would also be of interest to characterize associations between in vitro drug sensitivity and treatment outcomes. Although it seems logical that diminished in vitro drug sensitivity should worsen treatment outcomes, it has been surprisingly difficult to show such a correlation (61). In our study of leading combination regimens, treatment failures were uncommon. With the regimen that failed most often, AQ/SP, associations between treatment outcomes and in vitro sensitivity to MDAQ were not apparent (36), although, of note, we did not assess sensitivities to the antifolate components of SP.
In considering our results, it is important to realize that most infections in Kampala are polyclonal and that our results were based on assessment of sensitivities of the subset of parasites that survived the first two days of in vitro culture. Other studies have shown the complexity of infection to decrease over short-term culture (44) and the prevalence of certain genotypes to frequently differ pre- and postculture (52). Thus, certain features that impacted treatment outcomes may not have been apparent in our cultured parasites.
Our results offer some reason for optimism regarding new ACT regimens, as parasites in Uganda were generally highly sensitive to the important ACT components DHA, LM, and PQ. However, parasites were commonly fairly resistant to MDAQ, the active metabolite of AQ, which is a component of the ACT AS/AQ. Correlations were seen between sensitivities to CQ, MDAQ, and QN, and many parasites had fairly high IC50s for QN. The results thus suggest that CQ and AQ should not be used to treat malaria in Uganda; that the ACT AS/AQ may have limited efficacy; that QN, which is increasingly used to treat uncomplicated malaria, would best not be used for this indication; and that the ACTs AM/LM and DHA/PQ are highly active against malaria parasites now circulating in Uganda.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI52142, AI075045, and TW01506) and the Doris Duke Charitable Foundation. P.J.R. is a Doris Duke Charitable Foundation Distinguished Clinical Scientist.
We thank the participants in the clinical trial from which samples were collected, their parents and guardians, and our clinical study team. We thank Harald Noedl, Medical University of Vienna, for valuable advice regarding drug sensitivity assays. Control P. falciparum strains were from the Malaria Research and Reference Reagent Resource Center.
Footnotes
Published ahead of print on 11 January 2010.
REFERENCES
- 1.Achan, J., J. K. Tibenderana, D. Kyabayinze, F. Wabwire Mangen, M. R. Kamya, G. Dorsey, U. D'Alessandro, P. J. Rosenthal, and A. O. Talisuna. 2009. Effectiveness of quinine versus artemether-lumefantrine for treating uncomplicated falciparum malaria in Ugandan children: randomised trial. BMJ 339:b2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam, I., I. Salih, and M. I. Elbashir. 2005. Quinine for the treatment of uncomplicated Plasmodium falciparum malaria in eastern Sudan. Trans. R. Soc. Trop. Med. Hyg. 99:736-738. [DOI] [PubMed] [Google Scholar]
- 3.Basco, L. 2007. Field application of in vitro assays for the sensitivity of human malaria parasites to antimalarial drugs. World Health Organization, Geneva, Switzerland.
- 4.Basco, L. K., J. Bickii, and P. Ringwald. 1998. In vitro activity of lumefantrine (benflumetol) against clinical isolates of Plasmodium falciparum in Yaounde, Cameroon. Antimicrob. Agents Chemother. 42:2347-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basco, L. K., and P. Ringwald. 2001. Analysis of the key pfcrt point mutation and in vitro and in vivo response to chloroquine in Yaounde, Cameroon. J. Infect. Dis. 183:1828-1831. [DOI] [PubMed] [Google Scholar]
- 6.Basco, L. K., and P. Ringwald. 2003. In vitro activities of piperaquine and other 4-aminoquinolines against clinical isolates of Plasmodium falciparum in Cameroon. Antimicrob. Agents Chemother. 47:1391-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basco, L. K., and P. Ringwald. 2007. Molecular epidemiology of malaria in Cameroon. XXIV. Trends of in vitro antimalarial drug responses in Yaounde, Cameroon. Am. J. Trop. Med. Hyg. 76:20-26. [PubMed] [Google Scholar]
- 8.Bukirwa, H., A. Yeka, M. R. Kamya, A. Talisuna, K. Banek, N. Bakyaita, J. B. Rwakimari, P. J. Rosenthal, F. Wabwire-Mangen, G. Dorsey, and S. G. Staedke. 2006. Artemisinin combination therapies for treatment of uncomplicated malaria in Uganda. PLoS Clin. Trials 1:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, T. M., T. Y. Hung, I. K. Sim, H. A. Karunajeewa, and K. F. Ilett. 2005. Piperaquine: a resurgent antimalarial drug. Drugs 65:75-87. [DOI] [PubMed] [Google Scholar]
- 10.Deloron, P., J. Le Bras, J. A. Ramanamirija, and P. Coulanges. 1985. Plasmodium falciparum in Madagascar: in vivo and in vitro sensitivity to seven drugs. Ann. Trop. Med. Parasitol. 79:357-365. [DOI] [PubMed] [Google Scholar]
- 11.Djimde, A., O. K. Doumbo, J. F. Cortese, K. Kayentao, S. Doumbo, Y. Diourte, A. Dicko, X. Z. Su, T. Nomura, D. A. Fidock, T. E. Wellems, C. V. Plowe, and D. Coulibaly. 2001. A molecular marker for chloroquine-resistant falciparum malaria. N. Engl. J. Med. 344:257-263. [DOI] [PubMed] [Google Scholar]
- 12.Djimde, A. A., B. Fofana, I. Sagara, B. Sidibe, S. Toure, D. Dembele, S. Dama, D. Ouologuem, A. Dicko, and O. K. Doumbo. 2008. Efficacy, safety, and selection of molecular markers of drug resistance by two ACTs in Mali. Am. J. Trop. Med. Hyg. 78:455-461. [PubMed] [Google Scholar]
- 13.Dokomajilar, C., Z. M. Lankoande, G. Dorsey, I. Zongo, J. B. Ouedraogo, and P. J. Rosenthal. 2006. Roles of specific Plasmodium falciparum mutations in resistance to amodiaquine and sulfadoxine-pyrimethamine in Burkina Faso. Am. J. Trop. Med. Hyg. 75:162-165. [PubMed] [Google Scholar]
- 14.Dokomajilar, C., S. L. Nsobya, B. Greenhouse, P. J. Rosenthal, and G. Dorsey. 2006. Selection of Plasmodium falciparum pfmdr1 alleles following therapy with artemether-lumefantrine in an area of Uganda where malaria is highly endemic. Antimicrob. Agents Chemother. 50:1893-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dondorp, A. M., F. Nosten, P. Yi, D. Das, A. P. Phyo, J. Tarning, K. M. Lwin, F. Ariey, W. Hanpithakpong, S. J. Lee, P. Ringwald, K. Silamut, M. Imwong, K. Chotivanich, P. Lim, T. Herdman, S. S. An, S. Yeung, P. Singhasivanon, N. P. Day, N. Lindegardh, D. Socheat, and N. J. White. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorsey, G., M. R. Kamya, G. Ndeezi, J. N. Babirye, C. R. Phares, J. E. Olson, E. T. Katabira, and P. J. Rosenthal. 2000. Predictors of chloroquine treatment failure in children and adults with falciparum malaria in Kampala, Uganda. Am. J. Trop. Med. Hyg. 62:686-692. [DOI] [PubMed] [Google Scholar]
- 17.Dorsey, G., M. R. Kamya, A. Singh, and P. J. Rosenthal. 2001. Polymorphisms in the Plasmodium falciparum pfcrt and pfmdr-1 genes and clinical response to chloroquine in Kampala, Uganda. J. Infect. Dis. 183:1417-1420. [DOI] [PubMed] [Google Scholar]
- 18.Dorsey, G., S. Staedke, T. D. Clark, D. Njama-Meya, B. Nzarubara, C. Maiteki-Sebuguzi, C. Dokomajilar, M. R. Kamya, and P. J. Rosenthal. 2007. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA 297:2210-2219. [DOI] [PubMed] [Google Scholar]
- 19.Duraisingh, M. T., P. Jones, I. Sambou, L. von Seidlein, M. Pinder, and D. C. Warhurst. 2000. The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisinin. Mol. Biochem. Parasitol. 108:13-23. [DOI] [PubMed] [Google Scholar]
- 20.Ferdig, M. T., R. A. Cooper, J. Mu, B. Deng, D. A. Joy, X. Z. Su, and T. E. Wellems. 2004. Dissecting the loci of low-level quinine resistance in malaria parasites. Mol. Microbiol. 52:985-997. [DOI] [PubMed] [Google Scholar]
- 21.Fidock, D. A., T. Nomura, A. K. Talley, R. A. Cooper, S. M. Dzekunov, M. T. Ferdig, L. M. Ursos, A. B. Sidhu, B. Naude, K. W. Deitsch, X. Z. Su, J. C. Wootton, P. D. Roepe, and T. E. Wellems. 2000. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6:861-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis, D., S. L. Nsobya, A. Talisuna, A. Yeka, M. R. Kamya, R. Machekano, C. Dokomajilar, P. J. Rosenthal, and G. Dorsey. 2006. Geographic differences in antimalarial drug efficacy in Uganda are explained by differences in endemicity and not by known molecular markers of drug resistance. J. Infect. Dis. 193:978-986. [DOI] [PubMed] [Google Scholar]
- 23.Happi, C. T., G. O. Gbotosho, O. A. Folarin, O. M. Bolaji, A. Sowunmi, D. E. Kyle, W. Milhous, D. F. Wirth, and A. M. Oduola. 2006. Association between mutations in Plasmodium falciparum chloroquine resistance transporter and P. falciparum multidrug resistance 1 genes and in vivo amodiaquine resistance in P. falciparum malaria-infected children in Nigeria. Am. J. Trop. Med. Hyg. 75:155-161. [PubMed] [Google Scholar]
- 24.Henry, M., S. Briolant, A. Zettor, S. Pelleau, M. Baragatti, E. Baret, J. Mosnier, R. Amalvict, T. Fusai, C. Rogier, and B. Pradines. 2009. Plasmodium falciparum Na+/H+ exchanger 1 transporter is involved in reduced susceptibility to quinine. Antimicrob. Agents Chemother. 53:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmgren, G., A. Bjorkman, and J. P. Gil. 2006. Amodiaquine resistance is not related to rare findings of pfmdr1 gene amplifications in Kenya. Trop. Med. Int. Health 11:1808-1812. [DOI] [PubMed] [Google Scholar]
- 26.Holmgren, G., J. P. Gil, P. M. Ferreira, M. I. Veiga, C. O. Obonyo, and A. Bjorkman. 2006. Amodiaquine resistant Plasmodium falciparum malaria in vivo is associated with selection of pfcrt 76T and pfmdr1 86Y. Infect. Genet. Evol. 6:309-314. [DOI] [PubMed] [Google Scholar]
- 27.Humphreys, G. S., I. Merinopoulos, J. Ahmed, C. J. Whitty, T. K. Mutabingwa, C. J. Sutherland, and R. L. Hallett. 2007. Amodiaquine and artemether-lumefantrine select distinct alleles of the Plasmodium falciparum mdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob. Agents Chemother. 51:991-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jambou, R., E. Legrand, M. Niang, N. Khim, P. Lim, B. Volney, M. T. Ekala, C. Bouchier, P. Esterre, T. Fandeur, and O. Mercereau-Puijalon. 2005. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet 366:1960-1963. [DOI] [PubMed] [Google Scholar]
- 29.Kamya, M. R., A. Yeka, H. Bukirwa, M. Lugemwa, J. B. Rwakimari, S. G. Staedke, A. O. Talisuna, B. Greenhouse, F. Nosten, P. J. Rosenthal, F. Wabwire-Mangen, and G. Dorsey. 2007. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: a randomized trial. PLoS Clin. Trials 2:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karema, C., C. I. Fanello, C. van Overmeir, J. P. van Geertruyden, W. van Doren, D. Ngamije, and U. D'Alessandro. 2006. Safety and efficacy of dihydroartemisinin/piperaquine (Artekin) for the treatment of uncomplicated Plasmodium falciparum malaria in Rwandan children. Trans. R. Soc. Trop. Med. Hyg. 100:1105-1111. [DOI] [PubMed] [Google Scholar]
- 31.Martensson, A., J. Stromberg, C. Sisowath, M. I. Msellem, J. P. Gil, S. M. Montgomery, P. Olliaro, A. S. Ali, and A. Bjorkman. 2005. Efficacy of artesunate plus amodiaquine versus that of artemether-lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, Tanzania. Clin. Infect. Dis. 41:1079-1086. [DOI] [PubMed] [Google Scholar]
- 32.Menard, D., D. Djalle, A. Manirakiza, F. Yapou, V. Siadoua, S. Sana, M. D. Matsika-Claquin, M. Nestor, and A. Talarmin. 2005. Drug-resistant malaria in Bangui, Central African Republic: an in vitro assessment. Am. J. Trop. Med. Hyg. 73:239-243. [PubMed] [Google Scholar]
- 33.Muangnoicharoen, S., D. J. Johnson, S. Looareesuwan, S. Krudsood, and S. A. Ward. 2009. Role of known molecular markers of resistance in the antimalarial potency of piperaquine and dihydroartemisinin in vitro. Antimicrob. Agents Chemother. 53:1362-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutabingwa, T. K., D. Anthony, A. Heller, R. Hallett, J. Ahmed, C. Drakeley, B. M. Greenwood, and C. J. Whitty. 2005. Amodiaquine alone, amodiaquine+sulfadoxine-pyrimethamine, amodiaquine+artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet 365:1474-1480. [DOI] [PubMed] [Google Scholar]
- 35.Mwai, L., S. M. Kiara, A. Abdirahman, L. Pole, A. Rippert, A. Diriye, P. Bull, K. Marsh, S. Borrmann, and A. Nzila. 2009. In vitro activity of piperaquine, lumefantrine, and dihydroartemisinin in Kenyan Plasmodium falciparum isolates and polymorphisms in Pfcrt and Pfmdr1. Antimicrob. Agents Chemother. 53:5069-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nawaz, F., S. Nsobya, M. Kiggundu, M. Joloba, and P. J. Rosenthal. 2009. Selection of parasites with diminished drug sensitivity by amodiaquine-containing antimalarial regimens in Uganda. J. Infect. Dis. 200:1650-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nkrumah, L. J., P. M. Riegelhaupt, P. Moura, D. J. Johnson, J. Patel, K. Hayton, M. T. Ferdig, T. E. Wellems, M. H. Akabas, and D. A. Fidock. 2009. Probing the multifactorial basis of Plasmodium falciparum quinine resistance: evidence for a strain-specific contribution of the sodium-proton exchanger PfNHE. Mol. Biochem. Parasitol. 165:122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noedl, H., J. Bronnert, K. Yingyuen, B. Attlmayr, H. Kollaritsch, and M. Fukuda. 2005. Simple histidine-rich protein 2 double-site sandwich enzyme-linked immunosorbent assay for use in malaria drug sensitivity testing. Antimicrob. Agents Chemother. 49:3575-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noedl, H., Y. Se, K. Schaecher, B. L. Smith, D. Socheat, and M. M. Fukuda. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359:2619-2620. [DOI] [PubMed] [Google Scholar]
- 40.Noedl, H., P. Teja-Isavadharm, and R. S. Miller. 2004. Nonisotopic, semiautomated Plasmodium falciparum bioassay for measurement of antimalarial drug levels in serum or plasma. Antimicrob. Agents Chemother. 48:4485-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noedl, H., W. H. Wernsdorfer, R. S. Miller, and C. Wongsrichanalai. 2002. Histidine-rich protein II: a novel approach to malaria drug sensitivity testing. Antimicrob. Agents Chemother. 46:1658-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nosten, F., and N. J. White. 2007. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 77:181-192. [PubMed] [Google Scholar]
- 43.Nsobya, S. L., C. Dokomajilar, M. Joloba, G. Dorsey, and P. J. Rosenthal. 2007. Resistance-mediating Plasmodium falciparum pfcrt and pfmdr1 alleles after treatment with artesunate-amodiaquine in Uganda. Antimicrob. Agents Chemother. 51:3023-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nsobya, S. L., M. Kiggundu, M. Joloba, G. Dorsey, and P. J. Rosenthal. 2008. Complexity of Plasmodium falciparum clinical samples from Uganda during short-term culture. J. Infect. Dis. 198:1554-1557. [DOI] [PubMed] [Google Scholar]
- 45.Pickard, A. L., C. Wongsrichanalai, A. Purfield, D. Kamwendo, K. Emery, C. Zalewski, F. Kawamoto, R. S. Miller, and S. R. Meshnick. 2003. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob. Agents Chemother. 47:2418-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piola, P., C. Fogg, F. Bajunirwe, S. Biraro, F. Grandesso, E. Ruzagira, J. Babigumira, I. Kigozi, J. Kiguli, J. Kyomuhendo, L. Ferradini, W. Taylor, F. Checchi, and J. P. Guthmann. 2005. Supervised versus unsupervised intake of six-dose artemether-lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: a randomised trial. Lancet 365:1467-1473. [DOI] [PubMed] [Google Scholar]
- 47.Plowe, C. V., A. Djimde, M. Bouare, O. Doumbo, and T. E. Wellems. 1995. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am. J. Trop. Med. Hyg. 52:565-568. [DOI] [PubMed] [Google Scholar]
- 48.Pradines, B., P. Hovette, T. Fusai, H. L. Atanda, E. Baret, P. Cheval, J. Mosnier, A. Callec, J. Cren, R. Amalvict, J. P. Gardair, and C. Rogier. 2006. Prevalence of in vitro resistance to eleven standard or new antimalarial drugs among Plasmodium falciparum isolates from Pointe-Noire, Republic of the Congo. J. Clin. Microbiol. 44:2404-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pradines, B., A. Tall, T. Fusai, A. Spiegel, R. Hienne, C. Rogier, J. F. Trape, J. Le Bras, and D. Parzy. 1999. In vitro activities of benflumetol against 158 Senegalese isolates of Plasmodium falciparum in comparison with those of standard antimalarial drugs. Antimicrob. Agents Chemother. 43:418-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Price, R. N., A. C. Uhlemann, A. Brockman, R. McGready, E. Ashley, L. Phaipun, R. Patel, K. Laing, S. Looareesuwan, N. J. White, F. Nosten, and S. Krishna. 2004. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 364:438-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pukrittayakamee, S., A. Chantra, S. Vanijanonta, R. Clemens, S. Looareesuwan, and N. J. White. 2000. Therapeutic responses to quinine and clindamycin in multidrug-resistant falciparum malaria. Antimicrob. Agents Chemother. 44:2395-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Purfield, A., A. Nelson, A. Laoboonchai, K. Congpuong, P. McDaniel, R. S. Miller, K. Welch, C. Wongsrichanalai, and S. R. Meshnick. 2004. A new method for detection of pfmdr1 mutations in Plasmodium falciparum DNA using real-time PCR. Malar. J. 3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ringwald, P., J. Bickii, and L. K. Basco. 1996. In vitro activity of antimalarials against clinical isolates of Plasmodium falciparum in Yaounde, Cameroon. Am. J. Trop. Med. Hyg. 55:254-258. [DOI] [PubMed] [Google Scholar]
- 54.Rogers, W. O., R. Sem, T. Tero, P. Chim, P. Lim, S. Muth, D. Socheat, F. Ariey, and C. Wongsrichanalai. 2009. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar. J. 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasi, P., A. Abdulrahaman, L. Mwai, S. Muriithi, J. Straimer, E. Schieck, A. Rippert, M. Bashraheil, A. Salim, J. Peshu, K. Awuondo, B. Lowe, M. Pirmohamed, P. Winstanley, S. Ward, A. Nzila, and S. Borrmann. 2009. In vivo and in vitro efficacy of amodiaquine against Plasmodium falciparum in an area of continued use of 4-aminoquinolines in east Africa. J. Infect. Dis. 199:1575-1582. [DOI] [PubMed] [Google Scholar]
- 56.Sidhu, A. B., A. C. Uhlemann, S. G. Valderramos, J. C. Valderramos, S. Krishna, and D. A. Fidock. 2006. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J. Infect. Dis. 194:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sisowath, C., J. Stromberg, A. Martensson, M. Msellem, C. Obondo, A. Bjorkman, and J. P. Gil. 2005. In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine (Coartem). J. Infect. Dis. 191:1014-1017. [DOI] [PubMed] [Google Scholar]
- 58.Thomas, S. M., O. Ndir, T. Dieng, S. Mboup, D. Wypij, J. H. Maguire, and D. F. Wirth. 2002. In vitro chloroquine susceptibility and PCR analysis of pfcrt and pfmdr1 polymorphisms in Plasmodium falciparum isolates from Senegal. Am. J. Trop. Med. Hyg. 66:474-480. [DOI] [PubMed] [Google Scholar]
- 59.Uhlemann, A. C., M. Ramharter, B. Lell, P. G. Kremsner, and S. Krishna. 2005. Amplification of Plasmodium falciparum multidrug resistance gene 1 in isolates from Gabon. J. Infect. Dis. 192:1830-1835. [DOI] [PubMed] [Google Scholar]
- 60.Valderramos, S. G., and D. A. Fidock. 2006. Transporters involved in resistance to antimalarial drugs. Trends Pharmacol. Sci. 27:594-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wongsrichanalai, C., A. L. Pickard, W. H. Wernsdorfer, and S. R. Meshnick. 2002. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2:209-218. [DOI] [PubMed] [Google Scholar]
- 62.Woodrow, C. J., and S. Krishna. 2006. Antimalarial drugs: recent advances in molecular determinants of resistance and their clinical significance. Cell. Mol. Life Sci. 63:1586-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zongo, I., G. Dorsey, N. Rouamba, C. Dokomajilar, Y. Sere, P. J. Rosenthal, and J. B. Ouedraogo. 2007. Randomized comparison of amodiaquine plus sulfadoxine-pyrimethamine, artemether-lumefantrine, and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Burkina Faso. Clin. Infect. Dis. 45:1453-1461. [DOI] [PubMed] [Google Scholar]
- 64.Zongo, I., G. Dorsey, N. Rouamba, H. Tinto, C. Dokomajilar, R. T. Guiguemde, P. J. Rosenthal, and J. B. Ouedraogo. 2007. Artemether-lumefantrine versus amodiaquine plus sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Burkina Faso: a randomised non-inferiority trial. Lancet 369:491-498. [DOI] [PubMed] [Google Scholar]