Abstract
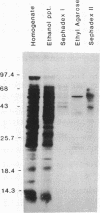
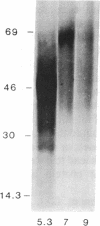
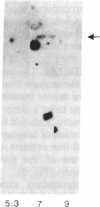
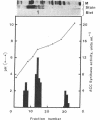
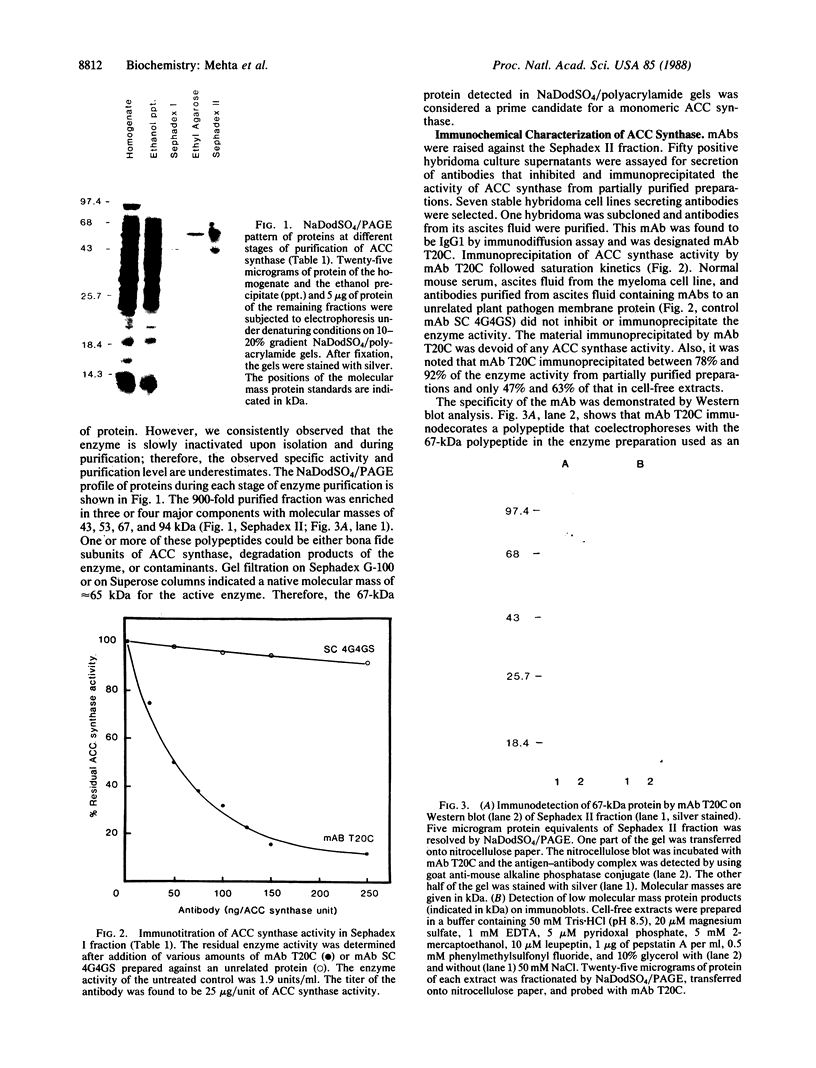
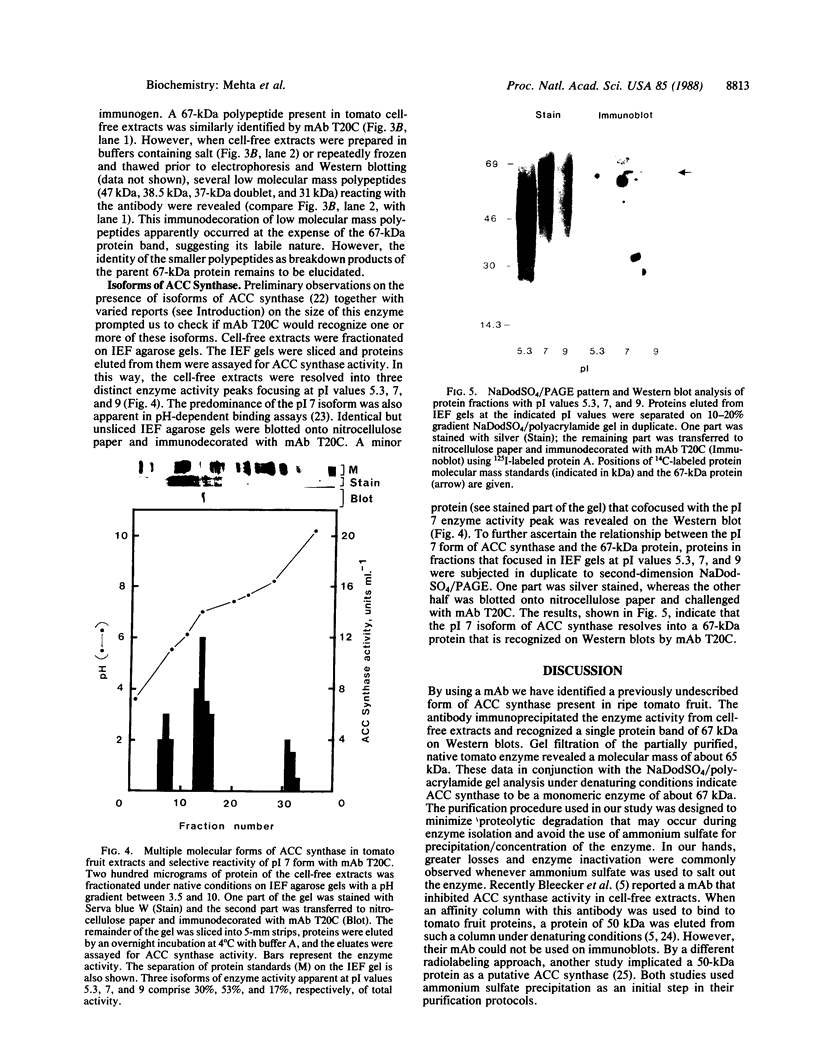
1-Aminocyclopropane-1-carboxylic acid (ACC) synthase (EC 4.4.1.14) is a key enzyme regulating ethylene biosynthesis in higher plants. A monoclonal antibody (mAb T20C) that immunoprecipitates the ACC synthase activity from tomato pericarp tissue extracts revealed that mAb T20C immunodecorates an ≈67-kDa polypeptide. On isoelectric focusing gels, ACC synthase activity in cell-free preparations was resolved into three distinct activity peaks with pI values 5.3, 7, and 9. mAb T20C specifically recognized the pI 7 form of the enzyme on electrophoretic transfer (Western) blots. When analyzed by sodium dodecyl sulfate gel electrophoresis under reducing conditions, the eluted pI 7 form was confirmed to migrate as a polypeptide of 67 kDa. The 67-kDa pI 7 isoform is a previously undescribed form of ACC synthase.
Keywords: ethylene, 1-aminocyclopropane-1-carboxylic acid synthase, hormones, Lycopersicon esculentum L.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleecker A. B., Kenyon W. H., Somerville S. C., Kende H. Use of monoclonal antibodies in the purification and characterization of 1-aminocyclopropane-1-carboxylate synthase, an enzyme in ethylene biosynthesis. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7755–7759. doi: 10.1073/pnas.83.20.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broglie K. E., Gaynor J. J., Broglie R. M. Ethylene-regulated gene expression: molecular cloning of the genes encoding an endochitinase from Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6820–6824. doi: 10.1073/pnas.83.18.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Pick U., Hoffman-Falk H., Edelman M. The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the "proteinaceous shield" regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Parham P. Monoclonal antibodies against HLA products and their use in immunoaffinity purification. Methods Enzymol. 1983;92:110–138. doi: 10.1016/0076-6879(83)92012-8. [DOI] [PubMed] [Google Scholar]
- Privalle L. S., Graham J. S. Radiolabeling of a wound-inducible pyridoxal phosphate-utilizing enzyme: evidence for its identification as ACC synthase. Arch Biochem Biophys. 1987 Mar;253(2):333–340. doi: 10.1016/0003-9861(87)90186-x. [DOI] [PubMed] [Google Scholar]